Abstract
Angus bulls (n = 48) were randomly assigned to control (castrated without the application of a postoperative healing agent) or surgical castration followed by either the application of a topical germicide, aluminum powder spray, or liquid bandage. The objective of this study was to determine the efficacy of commercial topical healing agents in improving wound healing and reducing inflammation and secondary infection after surgical castration. Indicators of wound healing included scrotal area temperature (determined by infrared thermography), scrotal circumference, clinical state of the scrotum score, and the wound healing score. Pain sensitivity was measured using a Von Frey anesthesiometer. The healing agents used in this study did not improve indicators of healing such as swelling and healing rate scores or indicators of inflammation including scrotal temperature and circumference of surgical castration lesions. Pain sensation associated with surgical castration was found to last 35 d after the procedure.
Résumé
Usage d’agents cicatrisants topiques sur des blessures scrotales après la castration chirurgicale chez des veaux de boucherie sevrés. Quarante-huit taureaux Angus ont été assignés au hasard à la castration témoin (castration sans l’application d’un agent cicatrisant postopératoire) ou à la castration chirurgicale suivie soit de l’application d’un germicide topique, d’un poudre à l’aluminium en vaporisateur ou d’un pansement liquide dans le but de déterminer l’efficacité des agents cicatrisants topiques commerciaux pour l’amélioration de la guérison des plaies et la réduction de l’inflammation et de l’infection secondaire après la castration chirurgicale. Les indicateurs de cicatrisation des plaies incluaient la température de la région scrotale déterminée par thermographie infrarouge, la circonférence scrotale, le pointage de l’état clinique du scrotum et le pointage de la cicatrisation de la plaie; et la sensibilité à la douleur mesurée à l’aide d’un anesthésiomètre Von Frey. Les agents cicatrisants utilisés dans cette étude n’ont pas amélioré les indicateurs de cicatrisation comme l’enflure et les notes de la rapidité de cicatrisation ou des indicateurs de l’inflammation qui incluaient la température scrotale et la circonférence des lésions de castration chirurgicale. Il a été constaté que la sensation de douleur associée à la castration chirurgicale durait 35 jours après l’intervention.
(Traduit par Isabelle Vallières)
Introduction
Surgical (knife) castration is one of the most commonly employed techniques used in North American beef cattle (1). It is done by creating an incision on the scrotum using a scalpel or Newberry knife to gain access to the testicles so they can be removed by pulling or using an emasculator. This procedure results in an open wound that is susceptible to infection and bleeding (2). In many cow-calf operations calves are castrated before weaning (3); however, some are not castrated until after weaning (4). The Canadian Veterinary Medical Association (CVMA) recommends that castration be performed at the youngest age possible and with the use of pain mitigation (5), although typically castration is performed without pain relief (1).
Tissue repair or wound healing follows 4 phases that overlap: hemostasis, inflammation, proliferation, and tissue remodeling (6). During the inflammation phase prevention of infection plays an important role in healing, as this phase persists as long as required to remove bacteria and exudate from the wound (7).
A study conducted by Mintline et al (8) evaluated the effect of flunixin meglumine administered at the time of castration on wound healing rate, scrotal size, and inflammation; no improvements were reported. Several topical wound healing products are commercially available with recommended use in improving post-castration wound healing in cattle. However, no studies assessing the efficacy of these products for castration wounds have been published. The objective of this work was to evaluate whether selected topical wound healing agents applied directly to the wound immediately after surgical castration would improve healing rate and reduce inflammation, secondary infection, and associated pain.
Materials and methods
Angus bull calves (n = 48) 4 to 5 mo of age [body weight (BW) 187 kg ± 4.9 kg standard deviation (SD)] were managed according to the principles and guidelines of the Canadian Council on Animal Care and the procedures were approved by the local animal care committees at the Lethbridge Research Centre (ACC # 1523) and University of Calgary (AC15–0135) (9). Calves were transported approximately 30 km from a local ranch after weaning and groups of 12 calves were allocated to 1 of 4 feedlot pens (21 m × 27 m) at the Agriculture and Agri-Food Canada Lethbridge Research Centre, Lethbridge, Alberta, Canada. Animals were blocked by BW and allotted to 1 of the 4 treatments: control (CT, surgical castration without the application of a post-operative wound healing agent), or surgical castration followed by either the application of a topical germicide (GR, Blue-Kote; Dr. Naylor Blu-Kote, Morris, New York, USA), aluminum powder spray (AL, Aluspray; Vétoquinol, Buckingham, UK), or liquid bandage (LB, Champion seal; KeriCure, Tampa, Florida, USA). Treatments were mixed within each pen (4 treatments per pen, 3 animals per treatment). Pens had a centrally located water system and a concrete apron (2.4 m × 24.5 m) directly in front of the feed bunk. Calves were fed a total mixed ration (TMR) ad libitum. Two diets were used to gradually increase the percentage of silage during the study. During the first 3 d after arrival, calves were fed a diet consisting of 47% chopped grass hay, 30% barley silage, 20% dry rolled barley, and 3% vitamin-mineral supplement (as fed basis), once daily. The percentage of chopped grass hay was reduced to 10% between days 4 and 7 after arrival and barley silage was gradually increased to 67%. On day 9 after arrival, hay was removed from the ration and calves remained on this diet until the end of the trial (77% barley silage, 20% dry rolled barley, and 3% vitamin-mineral supplement). Calves were provided with fresh water ad libitum.
On the day of castration (day 0) all calves received anesthesia via an epidural injection of xylazine (Rompun 20 mg/mL injectable; Bayer Health, Toronto, Ontario), 0.07 mg/kg BW, 20 min before castration (10). The scrotum of each bull calf was cleaned with disinfectant wipes (Wet Ones, Antibacterial hand wipes; Edgewell Operations, St. Louis, Missouri, USA) immediately before surgical castration. The scrotum was opened using a Newberry knife inserted into the side of the scrotum, closed and pulled down to make a large side-to-side incision across the entire scrotum and dividing the septum. With the testes descended partially below the bottom of the opened scrotum, one testis was extended downward and the cord was isolated as far up as possible. The sterilized emasculator was then used to crimp the cord then held in place for 30 to 60 s to crush the blood vessels. The process was repeated for the remaining testicle. Immediately after castration, each healing agent was applied according to the manufacturer’s specifications.
Animals were weighed on day −1 and BW records were used to block treatment groups on the day of castration. Animals were also weighed on days 0, 1, 2, 5, and 7, and weekly thereafter until day 49 to calculate average daily gain (ADG).
Inflammation was measured using scrotal temperature and scrotal circumference. Thermographic images of the scrotal area were obtained before scrotal manipulation with a Flir i60 infrared camera (Flir systems, Burlington, Ontario) that was held 1 m away from the scrotum of all calves on days −1, 0 (day of castration), 1, 2, 5, 7, and weekly until the end of the experiment, when calves were restrained in the same positon in the squeeze chute to maintain a consistent focus and view of the image. The images were then processed with ThermaCam QuickView 1.3 (Flir systems) which recorded the maximum and average temperatures. Scrotal circumference was measured using a scrotal tape (Reliabull; Lane Manufacturing, Denver, Colorado, USA) on days −1, 0 (day of castration), 1, 2, 5, 7, and weekly until the end of the study (8). The clinical state of the scrotum was scored on a 5-point scale, modified from Molony et al (11): 0 — No swelling, inflammation or infection visible; 1 — Increasing degrees of swelling without obvious erythema; 2 — Increasing degree of swelling with obvious erythema but without pus; 3 — Increasing degree of swelling with presence of pus; and 4 — Inflammatory response with presence of pus with intervention needed.
Wound healing or incision state was assessed following a 5-point scale, also on days −1, 0, 1, 2, 5, 7, and weekly until the end of the study:
The incision ran the length of the scrotum and tissue was exposed in this area. The incision had exudate, either wet or dry. Scabbing was uncommon but may have been present in isolated locations at the edges or across the center of the wound;
The incision was greater than approximately 3/4 the length of the scrotum and scabbing was present. The incision may have had exudate either wet or dry;
The incision was scabbed or open and was less than 3/4 of the scrotum. The incision site may also have had exudate, either wet or dry;
The wound/incision site was less than 1/4 of the scrotum. A small scab or discoloration was present at the center of the scrotum/wound site. This wound may have had exudate, wet or dry; and
The incision site was no longer visible, there was no tissue exposed anywhere on the scrotum. There was no scabbing and or dried exudate (8).
Castration wounds were considered completely healed when no swelling, inflammation, or infection was observed, and when there was no opening along the incision site.
Pain sensitivity was assessed using a Von Frey anesthesiometer (electronic von Frey anesthesiometer with rigid tip; 0 to 1000 g; IITC-Life Science Instruments, Woodland Hills, California, USA) in 2 areas, directly on the wound and on the skin surrounding the wound on the same days that wound healing rate was measured (12,13). To measure pain sensitivity, calves were standing in the chute unrestrained; the tip of the anesthesiometer was then placed on the surface of the scrotum and pressure was gradually exerted against the scrotum until the calves responded (tail flick, kick, and step forward or backwards). The tip of the anesthesiometer was immediately withdrawn and the maximum pressure elicited was recorded. Greater values indicated less pain sensitivity.
With calf as the experimental unit, all data including BW, scrotal temperature from thermographic images, scrotal circumference, and pain sensitivity were analyzed using a mixed-effect model (SAS 9.4; SAS Institute, Cary, North Carolina, USA) with repeated measures. Healing agent (HA), day, and their interactions (HA × day) were included as fixed effects, and calf within pen as a random effect. Time point was considered a repeated factor and was subjected to 3 variance-covariance structures: compound symmetry, autoregressive order one, and unstructured. The covariance structure that minimized Schwarz’s Bayesian information criterion was considered the most desirable analysis. Results are reported as least square means and a post-hoc test (PDIFF option of SAS) was used to compare the adjusted means.
Clinical state of the scrotum and wound healing score were analyzed with a Wilcoxon-Mann-Whitney test (SAS 9.4; SAS Institute) to evaluate the effect of HA on healing time (d). Medians and 95% distribution free confidence limits were calculated with univariate procedure (SAS 9.4; SAS Institute).
For all analyses, significance was declared at P ≤ 0.05 and tendencies were discussed at 0.05 < P ≤ 0.10.
Results
No treatment differences were observed on maximum (P = 0.18) or average (P = 0.15) temperatures of the scrotum (Table 1) and scrotal circumferences (P = 0.60; Table 1) were similar among treatments for 49 d after castration. The time to reach each wound or incision healing score (Z < 2.96; P > 0.10) and clinical state of the scrotum (Z < 4.72; P > 0.10) did not differ among healing agents over the 49-day study.
Table 1.
Least squares means of scrotal temperature, pain sensitivity, and scrotal circumference of recently weaned beef calves assessed for 49 d after surgical castration without the application of a postoperative healing agent (CT) or with the application of a topical germicide (GR), aluminium powder spray (AL), or liquid bandage (LB)
| Treatment | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Item | CT | GR | AL | LB | SEM | HA | day | HA × day |
| Average scrotal temperature (°C) | 27.8 | 28.5 | 27.5 | 28.1 | 0.33 | 0.15 | < 0.001 | 0.81 |
| Maximum scrotal temperature (°C) | 33.4 | 33.8 | 32.6 | 33.2 | 0.38 | 0.18 | < 0.001 | 0.14 |
| Pain sensitivity skina (g) | 534 | 452 | 554 | 502 | 30.8 | 0.10 | < 0.001 | 0.09 |
| Pain sensitivity wounda (g) | 430 | 400 | 478 | 451 | 36.5 | 0.47 | < 0.001 | 0.96 |
| Scrotal circumference (cm) | 15.7 | 15.6 | 16.0 | 15.6 | 0.24 | 0.57 | < 0.001 | 0.60 |
Maximum pressures exerted using a Von Frey anesthesiometer.
HA — healing agent effect; day — assessed on days 1, 2, 4, 7, and weekly until day 49; CT — control; GR — topical germicide; AL — aluminum powder spray; LB — liquid bandage; SEM — standard error of the mean
There were no differences in pain sensitivity among calves before castration. When the testicles were intact (measurement taken on days −1 and 0 before castration) the mean pressure on the skin required to obtain a response from the calves was 637 g ± 53.9 g. Although no differences were observed among treatments on days 1 and 2, pressure thresholds were reduced (P < 0.001) to 442 g ± 52.9 g and 331.1 g ± 53.9 g, for days 1 and 2, respectively, compared with days −1 and 0. Values of pain sensitivity remained lower and similar (P > 0.10) to the pain values on days 1 and 3 until day 14, when values started to increase (P < 0.001). On day 42, when wounds were almost completely healed (no swelling, inflammation or infection, and almost complete closure of the incision), pressure threshold values increased (P < 0.001) to values similar to those observed on day −1 (708.1 g ± 53.9 g).
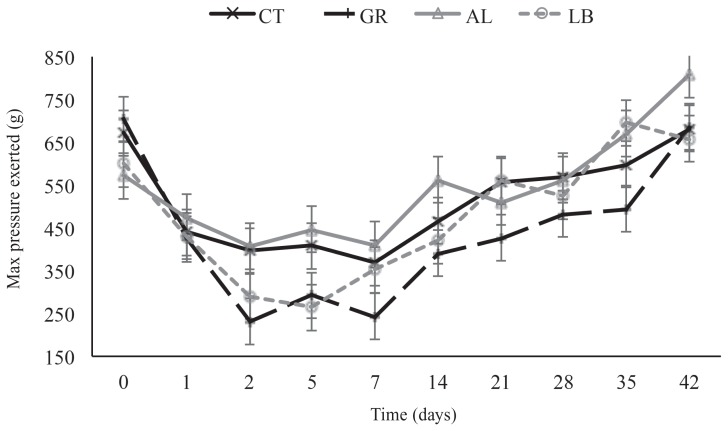
A HA × day interaction (P < 0.09) was observed in pain sensitivity on the surrounding skin from 48 h until day 21 post-castration (Figure 1). Calves assigned to the GR treatment exhibited the greatest pain sensitivity (P < 0.10) on days 2, 5, 7 and 21 compared to CT calves, and on days 2, 5, 7, 14, and 42 (P < 0.10) compared to AL calves. The calves in the LB group did not differ from those in the other treatments except on day 42 (P = 0.05) during which they exhibited more pain sensitivity than did AL calves. However, on days 2 and 5, the pressure threshold was numerically closer to that of GR calves than to those of CT and AL calves. No differences (P = 0.48) were observed in pain sensitivity on the wound.
Figure 1.
Pain sensitivity as maximum pressure (g) exerted with an anesthesiometer in relation to time (d) post-castration without the application of a post-operative healing agent (CT) or with the application of a topical germicide (GR), aluminium powder spray (AL), or liquid bandage (LB) on recent weaned beef calves.
No treatment differences were observed for final BW (P = 0.87) or ADG (P = 0.86; Table 2).
Table 2.
Least squares means of body weight and average daily gain of recently weaned beef calves after surgical castration without the application of a post-operative healing agent (CT) or with the application of a topical germicide (GR), aluminium powder spray (AL), or liquid bandage (LB)
| Treatment | P-value | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Item | CT | GR | AL | LB | SEM | HA |
| Initial BW, kg | 185 | 187 | 188 | 187 | 4.91 | 0.98 |
| Final BW (d 49), kg | 245 | 248 | 245 | 246 | 2.75 | 0.87 |
| ADG (d 49), kg/d | 1.20 | 1.25 | 1.19 | 1.20 | 0.056 | 0.87 |
HA — healing agent effect; ADG — average daily gain; CT — control; GR — topical germicide; AL — aluminum powder spray; LB — liquid bandage; SEM — standard error of the mean.
Discussion
Although many studies have assessed various pain mitigation strategies during and after castration (10,14–16) few studies have assessed strategies to improve wound healing and reduce the pain associated with the wound (8). Healing is a complex event involving multiple interactions of different tissue structures, a large number of biochemical substances and infiltrating cell types (17). In addition, there are several other factors that could affect the rate of wound healing such as animal age or environmental conditions. For example, surgically castrated calves have been shown to spend more time standing after the surgery due to the pain caused by the procedure (18). As calves in the present study were housed in feedlot pens rather than on pasture, it is likely that the open castration wound came into contact with the dirt/manure floor and increased the risk of contamination and infection of the wounds. This may have reduced the effect of the healing agents and future studies should compare pasture-housed versus feedlot housed calves. Complete healing of skin wounds normally occurs within 6 to 8 wk (19) according to 4 overlapping phases: hemostasis, inflammation, repair or proliferation, and remodeling (6,20). It is important to note that wound healing after castration not only involves the skin but also healing of the spermatic cords. In the present study, based on the clinical state of the scrotum, the inflammation phase lasted approximately 14 d after surgery, while the healing of the incision primarily occurred after 35 d. Molony et al (11) reported that swelling was completely gone 9 d after surgical castration in 5- to 7-day-old calves. However, in a study of 2- to 4-month-old beef calves by Stafford et al (21), swelling was gone 14 d after castration, and the wound was completely healed after 48 d (21). Similarly, Mintline et al (8) reported the most pronounced increase in healing occurred between 21 and 35 d after castration in calves castrated at 25 d of age. Based on the results of these studies and the present study, older calves seem to take longer to heal than younger calves; therefore, age may play a large role in the rate of healing after surgical castration.
Furthermore, castration at younger ages facilitates management, and reduces stress and risk of disease due to reduced testicular development (22,23). The CVMA recommends that castration be performed in animals as young as possible to reduce the adverse effects of the procedure. However, there is still a large percentage of cattle castrated at weaning and later in life (1). In addition, when castration is performed in recently weaned calves, cell types involved in the healing process (mainly leukocyte subsets) may be reduced due to the immunosuppressive effect associated with weaning stress, making calves more susceptible to infection and morbidity (24,25). When infection occurs during the inflammation phase of the healing process, the wound may become chronic (7) as the extended inflammation does not allow the proliferation and remodeling phase to proceed normally, thereby extending the healing time (26). In the present study, the application of healing agents did not reduce or prolong the inflammation phase most likely because we did not observe chronic wounds. In addition, the application of healing agents did not reduce healing time; thereby, not making the wound healing process in each phase faster. The wound healing agents tested in this study were selected based on their ability to facilitate wound drainage. However, as observed by the greater values of pain sensitivity from days 2 to 14, and the numerically greater clinical state values on days 2 and 5, and from days 28 to 35, the AL spray may not have facilitated drainage from the scrotum resulting in the formation of scar tissue in the spermatic cords.
Despite the lack of differences in the healing process, we observed that calves had sensitive skin (measured with the Von Frey anesthesiometer) until 35 d after castration when inflammation had declined (as observed by clinical state of the scrotum score < 2) and the incision was less than 3/4 of the scrotum (as observed by wound healing score < 3). These findings indicate that calves continue to feel pain during the inflammation phase and pain is not reduced until the remodeling phase of the wound is achieved.
Our results indicate that the topical wound healing agents used in this study did not improve wound healing. However, information regarding healing time and the length of pain sensation associated with these types of wounds was documented. Further studies on castration methods (both surgical and band techniques) and wound healing according to age, breed, types of incision, and environmental factors are needed to develop strategies to reduce healing time and secondary infections in castrated beef calves.
Acknowledgments
The authors appreciate the invaluable help of Agriculture and Agri-Food Canada research feedlot staff, our technicians Randy E Wilde and Fiona Brown, and Dr. Cassandra Tucker for help in the statistical analysis. We are very thankful for the funding provided by the Beef Cattle Research Council through the Canadian Beef Industry Science Cluster. This article is Lethbridge Research Centre contribution # 16054 CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Coetzee JF, Nutsch AL, Barbur LA, Bradburn RM. A survey of castration methods and associated livestock management practices performed by bovine veterinarians in the United States. BMC Vet Res. 2010;6:12. doi: 10.1186/1746-6148-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner AS, McIlwraith CW. Techniques in Large Animal Surgery. 4th ed. Philadelphia, Pennsylvania: Lea and Febiger; 2013. [Google Scholar]
- 3.Moggy MA, Pajor EA, Thurston WE, et al. Management practices associated with pain in cattle on western Canadian cow-calf operations: A mixed methods study. J Anim Sci. 2017;95:958–969. doi: 10.2527/jas.2016.0949. [DOI] [PubMed] [Google Scholar]
- 4.Warnock TM, Thrift TA, Irisk M, et al. Effect of castration technique on beef calf performance, feed efficiency, and inflammatory response. J Anim Sci. 1995;90:2345–2352. doi: 10.2527/jas.2011-4511. [DOI] [PubMed] [Google Scholar]
- 5.Canadian Veterinary Medical Association. Castration of cattle, sheep, and goats. Position statement. [Last accessed July 31, 2017]. Available from: https://www.canadianveterinarians.net/documents/castration-cattle-sheep-goats.
- 6.Boughton G, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117:12–34. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 7.Harper D, Young A, McNaught CE. The physiology of wound healing. Surgery. 2014;32:445–450. [Google Scholar]
- 8.Mintline EM, Varga A, Banuelos J, et al. Healing of surgical castration wounds: A description and an evaluation of flunixin. J Anim Sci. 2014;92:5659–5665. doi: 10.2527/jas.2014-7885. [DOI] [PubMed] [Google Scholar]
- 9.Canadian Council on Animal Care. CCAC guidelines on: The care and use of farm animals in research, teaching, and testing. Canadian Council on Animal Care; Ottawa, Ontario, Canada: 2009. [Google Scholar]
- 10.González LA, Schwartzkopf-Genswein KS, Caulkett NA, et al. Pain mitigation after band castration of beef calves and its effects on performance, behavior, Escherichia coli, and salivary cortisol. J Anim Sci. 2010;88:802–810. doi: 10.2527/jas.2008-1752. [DOI] [PubMed] [Google Scholar]
- 11.Molony V, Kent JE, Robertson IS. Assessment of acute and chronic pain after different methods of castration of calves. Appl Anim Behav Sci. 1995;46:33–48. [Google Scholar]
- 12.Lomax S, Windsor PA. Topical anesthesia mitigates the pain of castration in beef calves. J Anim Sci. 2013;91:4945–4952. doi: 10.2527/jas.2012-5984. [DOI] [PubMed] [Google Scholar]
- 13.Tucker CB, Mintline EM, Banuelos J, et al. Effect of a cooling gel on pain sensitivity and healing of hot-iron cattle brands. J Anim Sci. 2014;92:5666–5673. doi: 10.2527/jas.2014-7860. [DOI] [PubMed] [Google Scholar]
- 14.Ting STL, Earley B, Crowe MA. Effect of repeated ketoprofen administration during surgical castration of bulls on cortisol, immunological function, feed intake, growth, and behavior. J Anim Sci. 2003;81:1253–1264. doi: 10.2527/2003.8151253x. [DOI] [PubMed] [Google Scholar]
- 15.Pang WY, Earley B, Sweeney T, Crowe MA. Effect of carprofen administration during banding or burdizzo castration of bulls on plasma cortisol, in vitro interferon-γ production, acute-phase proteins, feed intake, and growth. J Anim Sci. 2006;84:351–359. doi: 10.2527/2006.842351x. [DOI] [PubMed] [Google Scholar]
- 16.Repenning PE, Ahola JK, Callan RJ, et al. Effect of pain mitigation and method of castration on behavior and feedlot performance in cull beef bulls. J Anim Sci. 2013;91:4964–4974. doi: 10.2527/jas.2012-6061. [DOI] [PubMed] [Google Scholar]
- 17.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- 18.White BJ, Coetzee JF, Renter DG, Babcock AH, Thomson DU, Andersen D. Evaluation of two-dimensional accelerometers to monitor beef cattle behavior post-castration. Am J Vet Res. 2008;69:1005–1012. doi: 10.2460/ajvr.69.8.1005. [DOI] [PubMed] [Google Scholar]
- 19.Basu S, Shukla V. Complications of wound healing. In: Mani R, Romanelli M, Shukla V, editors. Measurements in Wound Healing Science and Practice. London, United Kingdom: Springer-Verlag; 2012. pp. 109–144. [Google Scholar]
- 20.Schultz GS, Gibson DJ. Measurement of biomarkers for impaired healing in fluids and tissues. In: Mani R, Romanelli M, Shukla V, editors. Measurements in Wound Healing Science and Practice. London, United Kingdom: Springer-Verlag; 2012. pp. 243–258. [Google Scholar]
- 21.Stafford KJ, Mellor DJ, Todd SE, Bruce RA, Ward N. Effect of local anesthesia or local anesthesia plus a non-steroidal anti-inflammatory drug on the acute cortisol response of calves to five different methods of castration. Res Vet Sci. 2002;73:61–70. doi: 10.1016/s0034-5288(02)00045-0. [DOI] [PubMed] [Google Scholar]
- 22.Robertson IS, Kent JE, Molony V. Effect of different methods of castration on behaviour and plasma cortisol in calves of three ages. Res Vet Sci. 1994;56:8–17. doi: 10.1016/0034-5288(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 23.Bretschneider G. Effects of age and method of castration on performance and stress response of beef male cattle. A review. Livest Prod Sci. 2005;97:89–100. [Google Scholar]
- 24.Lynch EM, McGee M, Doyle S, Earley B. Effect of pre-weaning concentrate supplementation on peripheral distribution of leukocytes, functional activity of neutrophils, acute phase protein and behavioural responses of abruptly weaned and housed beef calves. BMC Vet Res. 2012;8:1. doi: 10.1186/1746-6148-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch EM, McGee M, Doyle S, Earley B. Effect of post-weaning management practices on physiological and immunological responses of weaned beef calves. Irish J Agr Food Res. 2011;50:161–174. [Google Scholar]
- 26.Schreml S, Szeimies RM, Prantl L, Landthaler M, Babilas P. Wound healing in the 21st century. J Am Acad Dermatol. 2010;63:866–881. doi: 10.1016/j.jaad.2009.10.048. [DOI] [PubMed] [Google Scholar]