Abstract
This retrospective study evaluated the outcomes of dogs with macroscopic pulmonary metastasis of appendicular osteosarcoma (OSA) treated with toceranib. Medical records of 20 dogs with macroscopic pulmonary metastasis of OSA that received toceranib were reviewed. The median dose and duration of toceranib administration were 2.52 mg/kg (range: 2.12 to 2.72 mg/kg) and 60 days (range: 17 to 231 days). The median progression free survival (PFS) and overall survival (OS) were 36 days (range: 17 to 231 days) and 90 days (range: 17 to 433 days), respectively. The clinical benefit rate was 10% (2/20; 1 partial response and 1 stable disease). The longest length of initial pulmonary nodules had significant impact on both PFS (P = 0.01) and OS (P = 0.02). The prognosis for dogs with metastatic OSA was poor with only 10% of dogs showing clinical benefit from toceranib. These results suggest that toceranib may not improve outcome in dogs with macroscopic pulmonary metastasis of OSA.
Résumé
Évaluation rétrospective du traitement avec tocéranib (Palladia) pour l’ostéosarcome appendiculaire métastatique canin. Cette étude rétrospective a évalué les résultats des chiens souffrant de métastase pulmonaire macroscopique de l’ostéosarcome appendiculaire (OSE) traité avec tocéranib. Les dossiers médicaux de 20 chiens atteints de métastase pulmonaire macroscopique d’OSE qui ont reçu tocéranib ont été évalués. La dose médiane et la durée de l’administration de tocéranib étaient de 2,52 mg/kg (étendue de 2,12 à 2,72 mg/kg) et de 60 jours (étendue de 17 à 231 jours). La progression de survie libre (PSL) médiane et la survie totale (ST) étaient de 36 jours (étendue de 17 à 231 jours) et de 90 jours (étendue de 17 à 433 jours), respectivement. Le taux de bienfaits cliniques étaient de 10 % (2/20; 1 réponse partielle et 1 maladie stable). Le plus long intervalle avant l’apparition des nodules pulmonaires initiaux avait un impact important sur la PSL (P = 0,01) et la ST (P = 0,02). Le pronostic pour les chiens atteints d’OSE métastatique était mauvais et seulement 10 % des chiens ont manifesté des bienfaits cliniques lors de l’usage de tocéranib. Ces résultats suggèrent que le tocéranib pourraient ne pas améliorer les résultats cliniques chez les chiens souffrant de métastase pulmonaire macroscopique causée par OSE.
(Traduit par Isabelle Vallières)
Introduction
Osteosarcoma (OSA), the most common primary bone tumor in dogs, can occur at various locations on the body. Appendicular OSA is the most prevalent type, however, comprising up to 85% of cases (1). The reported median survival time for dogs with appendicular OSA is 4 to 5 mo with amputation alone; the addition of adjuvant chemotherapy improves median survival times to 8 to 12 mo (2). Despite the improved overall outcome of this disease with chemotherapy, a positive response to conventional chemotherapy has not been observed in dogs with macroscopic pulmonary metastasis of OSA (3–5). Therefore, a new focus of treatment is shifting from standard cytotoxic approaches to targeted therapeutics in an attempt to suppress the growth of metastatic tumor cells (6–13). Among the various mechanisms associated with the process of tumor metastasis, tumor angiogenesis is known to play an important role in the metastatic process and has been a validated therapeutic target for various tumors (14).
The involvement of vascular endothelial growth factor (VEGF) and its receptor (VEGFR) in tumor angiogenesis has been reported in many studies (15–17). Tumor cells can drive the migration of VEGFR2 expressing circulating endothelial precursors (CEPs) from the bone marrow to the tumor micro-environment through the production of VEGF (18–20). Anti-VEGF/VEGFR therapy has been shown to decrease survival signaling and mobilization of CEPs to the site of tumor growth (21). These findings led to further studies with small molecule receptor tyrosine kinase (RTK) inhibitors such as sunitinib and sorafenib in preclinical settings. Both drugs were found to inhibit the proliferation of OSA cell lines in vitro and sunitinib treatment significantly reduced tumor burden, microvessel density, and pulmonary metastasis in a human xenograft OSA mouse model. These results suggested potential use of these drugs for OSA treatment (22,23).
Vascular endothelial growth factor is detectable in both human and canine OSA, and has been associated with increased malignancy and poor prognosis (24–29). Toceranib, a small molecule RTK inhibitor, has generated interest in veterinary medicine as a potential treatment option for metastatic OSA. Toceranib targets several members of the split-kinase family such as VEGFR, platelet-derived growth factor receptor (PDGFR), Kit, colony stimulating factor-1 receptor (CSF-1R), and Fms Related Tyrosine Kinase 3 (Flt-3) (30–32). A previous study revealed that a 2.4 to 2.9 mg/kg body weight (BW), q48h dose of toceranib significantly increased plasma VEGF, indicating VEGFR2 inhibition, suggesting that this concentration of toceranib may be considered for the treatment of VEGF-driven metastatic tumor (33).
In the setting of metastatic canine OSA, a retrospective multi-institutional study showed that 11/23 (47.8%) dogs experienced clinical benefit including 1 partial response and 10 stable disease (no progression nor new lesion for at least 10 wk) with toceranib treatment (13). The reported median duration of treatment for the 11 dogs that experienced clinical benefit was 24 wk, which suggested potential use of toceranib for the treatment of macroscopic metastatic OSA. In a recent randomized prospective clinical trial (12), however, the addition of toceranib to metronomic piroxicam/cyclophosphamide therapy following limb amputation and adjuvant carboplatin chemotherapy failed to show any improvement in the outcome of dogs with appendicular OSA. This study raised a question regarding the benefit of toceranib treatment for microscopic metastatic OSA. The efficacy of toceranib treatment, however, is difficult to evaluate solely based on these studies because standardization of treatment and recheck protocols were lacking in the retrospective study while most dogs were removed from the prospective study once metastasis was present.
Conclusive data to support the use of toceranib for metastatic canine OSA has not been established. Therefore, the purpose of the current study was to evaluate the efficacy of toceranib treatment for macroscopic pulmonary metastasis of appendicular OSA in dogs. A secondary goal was to identify prognostic factors in this subset of the population.
Materials and methods
Case selection
Electronic and hard copy medical records from the Ontario Veterinary College from October 2011 to June 2016 were reviewed. Dogs that were cytologically or histologically diagnosed with appendicular OSA, had pulmonary metastasis confirmed by thoracic radiographs, and received toceranib treatment were included. For response evaluation, only dogs with follow-up thoracic radiographs were included.
Treatment protocol
Following confirmation of pulmonary nodules by 3-view thoracic radiographs, dogs were prescribed toceranib at a target dose of 2.5 mg/kg BW, PO, 3 times per week on Monday, Wednesday, and Friday (MWF). Monthly 3-view thoracic radiographs were recommended to monitor tumor response. The primary endpoint of toceranib treatment was the time when evidence of disease progression was observed. All dogs had baseline complete blood (cell) counts (CBC) and biochemistry before toceranib treatment. To monitor for toxicity during toceranib treatment, CBCs were performed every 2 wk for the first month and repeated monthly; serial biochemistry panels were performed monthly.
Medical records review
Information recorded included signalment, thoracic radiography results, histology or cytology results, primary tumor location, date of diagnosis of pulmonary metastasis, number and size (recorded as longest length) of pulmonary metastases, previous treatments, dose and duration of toceranib treatment, and adverse effects. Follow-up information was documented including monitoring pulmonary metastases via radiographs to determine progression-free survival (PFS) and overall survival (OS) (Table 1). Progression-free survival was defined as the time from the first toceranib treatment to progression of the disease or death from any cause. Overall survival was defined as the time from the first toceranib treatment to death from any cause. Progression-free survival and OS were obtained from the medical records or by contacting the referring veterinarians and/or the owners. Toxicities were graded according to the Veterinary Cooperative Oncology Group-Common Terminology Criteria for Adverse Events criteria (34).
Table 1.
Variables used for Cox regression analysis of progression free survival (PFS) and overall survival (OS)
| Variables | Number of dogs | P-values | |
|---|---|---|---|
|
| |||
| PFS | OS | ||
| Age | 20 | 0.82 | 0.40 |
| Body weight | 20 | 0.01 | 0.11 |
| Concurrent metronomic treatment | 6 | 0.94 | 0.49 |
| Dose of toceranib | 20 | 0.84 | 0.05 |
| Duration of toceranib treatment | 20 | 0.11 | 0.03 |
| Longest length of initial pulmonary nodules | 20 | 0.01 | 0.02 |
| Gender | 0.16 | 0.39 | |
| Castrated male | 8 | ||
| Spayed female | 11 | ||
| Intact female | 1 | ||
| Number of initial pulmonary nodules | 0.33 | 0.72 | |
| Low | 9 | ||
| Intermediate | 6 | ||
| High | 5 | ||
| Previous treatment | 0.35 | 0.38 | |
| Surgery alone | 4 | ||
| Surgery and carboplatin | 10 | ||
| Surgery, carboplatin, and metronomic treatment | 3 | ||
| Others | 3 | ||
| Tumor location | 0.31 | 0.44 | |
| Thoracic limbs | 11 | ||
| Pelvic limbs | 9 | ||
Response to therapy
Modified response evaluation criteria in solid tumor (RECIST) was used for assessment of response to toceranib treatment (35). Response to therapy was defined as: complete response (CR), resolution of all target and non-target lesions and no new lesions; partial response (PR), at least 30% decrease in the longest diameter of the target lesions, no progression of non-target lesions and no new lesions; stable disease (SD), decrease in the longest diameter of target lesions of < 30% or increase of target lesions < 20%, no progression of non-target lesions and no new lesions for at least 10 wk; or progressive disease (PD), > 20% increase in the longest diameter of target lesions, progression of non-target lesions and identification of new lesions. Clinical benefit (CB) was defined as CR, PR, or SD.
Statistical analysis
Kaplan-Meier survival curves were generated for median PFS and OS. All 20 dogs were included in PFS analysis while 1 dog still alive at the end of the study was censored from OS analysis. A Cox proportional hazards univariate analysis was used on variables including signalment, body weight, primary tumor location, previous treatment, dose and duration of toceranib treatment, longest length and number of pulmonary nodules, and concurrent metronomic treatment. In order to evaluate the impact of previous treatments, dogs were assigned to 1 of 4 treatment groups for analysis; surgery alone (n = 4), surgery and carboplatin (n = 10), surgery and carboplatin followed by metronomic treatment (n = 3), and others [radiation (n = 1); surgery and doxorubicin (n = 1); no therapy (n = 1)]. For further analysis of the number of initial pulmonary nodules, dogs were assigned to one of 3 groups based on the number of pulmonary nodules (low < 3, intermediate > 3 to ≤ 5, high > 5). For all analyses, a P-value < 0.05 was deemed significant. Statistical software (IBM SPSS Statistics version 23 software for Windows; SPSS, Chicago, Illinois, USA) was used for statistical analysis.
Results
A total of 29 dogs received toceranib treatment for macroscopic pulmonary metastasis of appendicular OSA during the study period. Nine dogs were excluded due to absence of follow-up thoracic radiographs (n = 6) or non-confirmation of diagnosis by cytology or histology (n = 3). The 20 remaining dogs consisted of 14 breeds including Rottweiler (n = 3), Labrador retriever (n = 2), doberman pinscher (n = 2), golden retriever (n = 2), mixed-breed (n = 2), and 1 each of American pit bull terrier, Australian shepherd, border collie, cocker spaniel, Scottish deer-hound, Dogue de Bordeaux, English bulldog, great Dane, and mastiff. The median body weight was 31.6 kg (range: 13.4 to 61.2 kg), and the median age was 6.8 y (range: 1.7 to 13.2 y). There were 11 spayed females, 1 intact female, and 8 castrated males. The primary tumor was located in the humerus (n = 5), tibia (n = 5), femur (n = 4), radius (n = 2), scapula (n = 2), ulna (n = 1), and radius/ulna (n = 1).
The median dose and duration of toceranib were 2.52 mg/kg BW (range: 2.12 to 2.72 mg/kg) and 60 d (range: 17 to 231 d), respectively. During the course of treatment, all dogs received toceranib 3 times/week on MWF. Nineteen of the 20 dogs received other treatments prior to toceranib treatment: surgery (limb amputation, n = 15; scapulectomy, n = 1; acetabulectomy, n = 1; ulnar ostectomy, n = 1), chemotherapy (carboplatin, n = 13; doxorubicin, n = 1; metronomic cyclophosphamide, n = 3), and/or radiation therapy (n = 2). Of the 13 dogs receiving carboplatin, 3 dogs also received metronomic cyclophosphamide following carboplatin before starting the toceranib treatment. Six dogs were treated with toceranib and concurrent metronomic treatments (chlorambucil, n = 2; cyclophosphamide, n = 4), while 2 dogs received concurrent radiation therapy. Two dogs received follow-up chemotherapy after the toceranib treatment was discontinued (doxorubicin, n = 1; metronomic cyclophosphamide, n = 1). Other medications used include pamidronate (n = 1 before toceranib; n = 4 with toceranib), non-steroidal anti-inflammatory drugs (n = 6 with toceranib), and prednisone (n = 2 with toceranib; n = 4 after toceranib).
The most common adverse effects (AE) were gastrointestinal (GI), consisting of grade 3 GI AE in 3 dogs and grade 1 or 2 GI AE in 4 dogs. Three dogs had changes in the dose of toceranib during the treatment; the dose of toceranib was reduced in 2 dogs (2.61 to 2.24 mg/kg BW and 2.61 mg/kg BW to 2.23 mg/kg BW) with grade 3 GI AE; the dose was increased in 1 dog (2.29 mg/kg BW to 2.72 mg/kg BW) due to the progression of pulmonary metastasis. Grade 1 alanine aminotransferase (ALT) and alkaline phosphatase (ALP) elevations were found in 1 and 5 dogs, respectively. Only 1 dog had grade 3 neutropenia during the course of toceranib treatment. However, no clinical signs were associated with the neutropenia and the neutrophil count returned to normal in 2 wk without any supportive treatment or delay of toceranib treatment. This dog received a 2.6 mg/kg BW dose of toceranib for 27 d. The reasons for discontinuation of toceranib were GI AEs (n = 7), death (n = 6), progression of pulmonary metastasis (n = 4), or lameness (n = 3).
All dogs had pulmonary metastasis confirmed by thoracic radiographs that were reviewed by a radiologist. The median time from the diagnosis of OSA to development of pulmonary metastasis was 113 d (range: 0 to 691 d). Three dogs had pulmonary metastasis at the time of OSA diagnosis. The median longest length of pulmonary nodules was 12 mm (range: 5 to 99 mm). Based on the number of initial pulmonary nodules on radiographs, there were 9, 6, and 5 dogs in low, intermediate, and high groups, respectively.
Four dogs had postmortem evaluation, which confirmed the presence of metastatic OSA in the lungs. Three of these dogs also had metastasis in multiple organs including kidney, pleura, pericardium, myocardium, small intestine, subcutaneous tissue, and skeletal muscle.
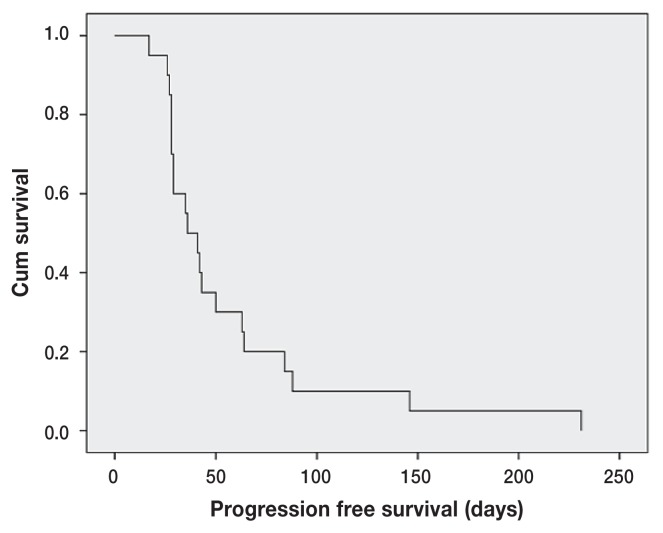
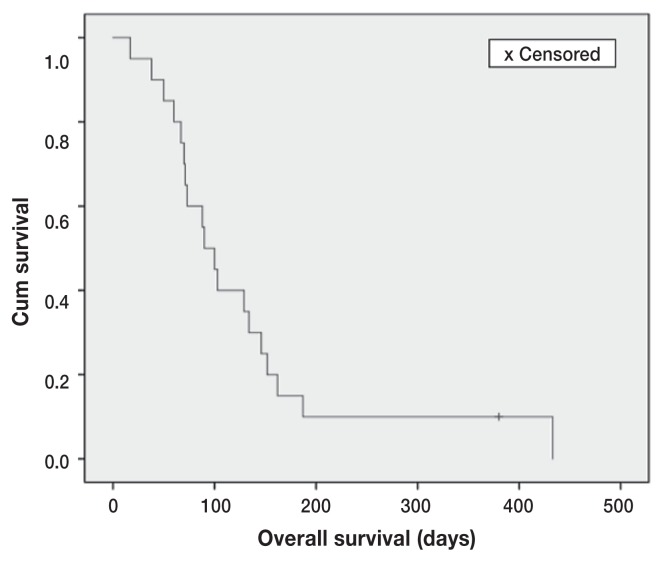
The median PFS and OS for toceranib treatment were 36 d (range: 17 to 231 d) and 90 d (range: 17 to 433 d), respectively (Figures 1 and 2). One dog still alive at the time of analysis was censored from the OS analysis (day 380). There was 1 PR and 1 SD resulting in a clinical benefit (CB) rate of 10% (2/20). The duration of CB in the 2 dogs was 56 d for the PR and 288 d for the SD. Of the prognostic factors evaluated (Table 1), the longest length of initial pulmonary nodules had a significant impact on both PFS (P = 0.01, HR = 1.041) and OS (P = 0.02, HR = 1.087) (Tables 2, 3). Body weight had a significant impact on PFS (P = 0.01, HR = 0.951) (Table 2). The duration of toceranib treatment had a significant impact on OS (P = 0.03, HR = 0.985) (Table 3). No statistical significance was found in the other analyzed variables.
Figure 1.
Kaplan-Meier progression free survival (PFS) curve for dogs with macroscopic pulmonary metastasis of appendicular OSA treated with toceranib (n = 20). The median PFS was 36 days.
Figure 2.
Kaplan-Meier overall survival (OS) curve for dogs with macroscopic pulmonary metastasis of appendicular OSA treated with toceranib (n = 20). One dog alive at the end of study was censored (+ mark). The median OS was 90 days.
Table 2.
Hazard ratios (HRs) and confidence intervals (CI) of the variables that have significant impact on progression free survival (PFS) in Cox regression analysis
| 95% Cl | ||||
|---|---|---|---|---|
|
|
||||
| Variables | HR | Lower | Upper | P-value |
| Body weight | 0.951 | 0.915 | 0.990 | 0.01 |
| Longest length of initial pulmonary nodules | 1.041 | 1.008 | 1.075 | 0.01 |
Table 3.
Hazard ratios (HRs) and confidence intervals (CI) of the variables that have significant impact on overall survival (OS) in Cox regression analysis
| 95% Cl | ||||
|---|---|---|---|---|
|
|
||||
| Variables | HR | Lower | Upper | P-value |
| Duration of toceranib treatment | 0.985 | 0.973 | 0.998 | 0.03 |
| Longest length of initial pulmonary nodules | 1.087 | 1.014 | 1.164 | 0.02 |
Discussion
The improvement in median survival times of dogs with appendicular OSA by the use of adjuvant chemotherapy has demonstrated that this is a chemosensitive disease in its early stages. However, the proportion of dogs cured or with long-term survival (> 2-year survival) remains intractably low at ~20% in spite of the adjuvant use of diverse approaches of chemotherapy (36,37). Furthermore, most OSA patients die of pulmonary metastasis, and chemotherapy has not been proven effective to delay progression of macroscopic metastases after their development. Ogilvie et al (3) evaluated single agent chemotherapy for macroscopic metastatic OSA in 45 dogs and found 1 dog achieved partial response for 21 d and the remaining dogs had a median survival time of 61 d without an objective response (3). They concluded that the chemotherapy agents used in the study were ineffective for the treatment of measurable meta-static OSA in the dog. A similar poor prognosis was shown in retrospective studies by Boston et al (4) and Batschinski et al (5) in which median survival time was reported as 76 d and 95 d, respectively. The latter studies corroborated the lack of efficacy of conventional chemotherapy against macroscopic pulmonary metastasis of OSA. These outcomes are comparable with the outcome herein, which suggests that macroscopic metastatic OSA is resistant to chemotherapy, including the kinase inhibitor toceranib in this study.
The poor outcome of the present study with 36 d of PFS contrasts with the previous study by London et al (13), which had a CB rate of 47.8% and a median PFS of 24 wk in the dogs that experienced CB. A possible explanation for this difference might be more frequent toceranib treatment in the previous study. In that study, 10/23 dogs received the drug every other day, whereas all dogs in the present study were treated on MWF schedule. Another potential difference between the 2 studies could be the schedule of the follow-up radiographs. The schedule of rechecks was not clearly defined in the previous study and unlikely standardized due to the nature of a multi-institutional retrospective study. Thus, it is possible that less frequent rechecks with thoracic radiographs might delay the detection of progression, which might have falsely increased the median PFS. It is also important to note that the previous study solicited the cases by the e-mail-based forum (American College of Veterinary Internal Medicine Oncology Listserve). This method might have caused a selection bias in the process by highlighting the minority of cases that had good responses to the toceranib treatment.
The longest length of initial pulmonary nodules was a prognostic factor identified for both PFS (P = 0.01, HR = 1.041) and OS (P = 0.02, HR = 1.087). These results could be explained by the fact that the longest length of the pulmonary nodules might correlate with tumor burden in lungs and suggest a more advanced stage of metastasis. Another factor that may affect tumor burden in the lungs is the number of pulmonary nodules, as a heavy tumor burden can be present if the number of small nodules is high. However, the number of initial pulmonary nodules did not have an impact on either PFS or OS in the present study, which suggests that the longest length of pulmonary nodules might be a better representation of the overall tumor burden in the lungs. However, the lack of correlation between the number of pulmonary nodules and the outcome of this study might be the result of type II error due to the small sample size. The magnitude of the pulmonary metastatic burden in the dogs from the 2 studies could also have affected the results. This hypothesis could not be verified at this time since the information used to compare the magnitude of pulmonary metastasis, such as measurements and numbers of pulmonary nodules, was absent in the previous study. However, given that the addition of toceranib to metronomic chemotherapy/piroxicam adjuvant treatment in a previous study did not improve the outcome in microscopic metastatic OSA, it is possible that the magnitude of pulmonary metastatic burden might not impact the outcome with toceranib treatment. In the present study, 6/20 dogs received concurrent metronomic treatments during the toceranib treatment; however, it was not found to have a significant impact in the evaluation of prognostic factors.
There were other factors affecting PFS or OS in this study. Body weight was found to impact PFS (P = 0.01, HR = 0.951). The reason for this finding is not clear. It is possible that the dogs’ body condition scores (BCS) affected the toceranib doses for each patient, as weight-based prescriptions tend to overexpose obese patients (38). This speculation could not be confirmed because BCS was not consistently recorded. The duration of toceranib treatment also had a positive impact on OS (P = 0.03, HR = 0.985). This result should be interpreted with caution, as dogs with longer survival were likely to have received toceranib for longer periods of time.
The most common toxicity was GI AE (7/20, 35%), characterized by inappetence, vomiting, diarrhea, or a combination of these. However, due to the presence of systemic OSA and the retrospective nature of the study, it is not possible to differentiate these adverse events from signs related to disease progression although OSA is less likely the cause of the GI signs given the usual metastatic pattern of OSA (39). Hematologic toxicity was rare. Grade 3 neutropenia occurred in 1 dog (1/20) and it was resolved in 2 wk without any further supportive treatments or drug holidays. No other hematologic toxicities were observed. This AE profile of common GI and rare hematologic toxicities is consistent with the previous report of the study evaluating the efficacy of toceranib in dogs with solid malignancies. In that study, GI toxicity was commonly seen (diarrhea 51.8%; anorexia 35.3%; vomiting 18.8%), neutropenia was only seen in 10.6% of patients (13). This similarity in toxicity profiles might be explained by the similar toceranib doses that were used between the previous (median doses between 2.67 to 2.87 mg/kg BW) and present studies (median dose: 2.52 mg/kg BW). Alterations in biochemical parameters were noted in 5 dogs (5/20). The most common biochemical toxicity was grade 1 ALP elevation (4/20) followed by ALT elevation (1/20). The clinical relevance of these abnormalities was not investigated. Transient liver enzyme elevations are consistent with the results of a study in which transient grade 1 ALT elevation was noted in 7 dogs (7/40) and grade 1 and grade 3 ALP elevations were observed in 10 dogs (10/40) and 1 dog (1/40), respectively (33). No dogs in that study were reported to have developed clinical evidence of hepatotoxicity. Three dogs had their toceranib treatment discontinued due to progression or new development of lameness. The lameness might be associated with an adverse effect of the toceranib treatment as lameness has been reported to be an adverse effect in previous studies (30,31,33). However, the lameness in 2 of the dogs was suspected to be due to the progression of OSA because the lameness occurred in the same limb that had been previously diagnosed with primary OSA. The other dog might have had the lameness as a true adverse effect from the toceranib treatment, but other possibilities such as metastasis cannot be ruled out as no further evaluation was performed at that time.
Some limitations exist in the present study because of its retrospective design with a relatively small number of patients and absence of standardization of treatments. The statistical power for this study is weak because each group in the Cox analysis had limited sample sizes, which increases the chance of type II error. Due to the small number of patients some valuable analyses could not be performed. For example, the effects of follow-up chemotherapy could not be evaluated because only 2 dogs received follow-up chemotherapy after the toceranib treatment failed. However, positive effects of follow-up chemotherapy are not expected given that no study has demonstrated chemosensitizer effects of toceranib and the general prognosis of this disease with conventional chemotherapy is poor. Similarly, the prognostic value of ALP elevation was not analyzed since only 2 dogs had ALP elevation before the toceranib treatment. The lack of complete information in this study also caused limitations; possible adverse effects including proteinuria and hypertension might have been overlooked because urinalysis and blood pressure measurement were not routinely conducted during the toceranib treatment. A lack of full staging tests before toceranib treatment and a lack of antemortem biopsy or postmortem evaluation meant that some of the pulmonary nodules might not have been due to OSA. Another limitation is that thoracic radiographs were used to measure the pulmonary nodules. According to the recommendation from the RECIST working group, thoracic radiograph measurement of lesions surrounded by pulmonary parenchyma is acceptable, but not preferable as the measurement represents a summation of densities. In conjunction with poor identification of new lesions within the thorax on radiograph as compared with CT, CT is a preferable modality for detection or measurement of pulmonary nodules. However, availability and cost of CT scan pose limitations in use of this modality for regular staging tests and recheck evaluations. The findings with radiographs in this study might be practical and relevant in many clinical settings. The other limitation was the various toceranib doses that were used in this study and the fact that 6/20 dogs received < 2.4 mg/kg BW on the MWF schedule. A previous study showed dogs dosed with > 2.4 mg/kg BW toceranib achieved a plasma concentration predicted to produce effective target inhibition (33). Given the study result, it is possible that the 6 dogs might not have achieved sufficient plasma concentrations to show efficacy of the treatment. This limitation could be addressed by setting up a minimum required dose of toceranib of 2.4 mg/kg BW in future studies.
In conclusion, the outcome of dogs with pulmonary metastasis from appendicular OSA treated with toceranib was similar to that of dogs treated with conventional chemotherapy, with short response duration and low CB rate. The prognosis for dogs with metastatic OSA was guarded in the present study with a median OS 90 d. This result suggests that single agent toceranib treatment might not be an effective treatment for macroscopic pulmonary metastasis of appendicular OSA. The longest length of initial pulmonary nodules was a prognostic factor identified for both PFS and MST. Prospective studies using standardized criteria are warranted to evaluate the efficacy of toceranib for the treatment of macroscopic pulmonary metastasis in dogs with appendicular OSA. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Ehrhart NP, Ryan SD, Fan TM. Withrow and MacEwen’s Small Animal Clinical Oncology. Elsevier; 2013. Tumors of the skeletal system; pp. 463–503. [Google Scholar]
- 2.Spodnick GJ, Berg J, Rand WM, et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978–1988) J Am Vet Med Assoc. 1992;200:995–999. [PubMed] [Google Scholar]
- 3.Ogilvie GK, Straw RC, Jameson VJ, et al. Evaluation of single-agent chemotherapy for treatment of clinically evident osteosarcoma metastases in dogs: 45 cases (1987–1991) J Am Vet Med Assoc. 1993;202:304–306. [PubMed] [Google Scholar]
- 4.Boston SE, Ehrhart NP, Dernell WS, Lafferty M, Withrow SJ. Evaluation of survival time in dogs with stage III osteosarcoma that undergo treatment: 90 cases (1985–2004) J Am Vet Med Assoc. 2006;228:1905–1908. doi: 10.2460/javma.228.12.1905. [DOI] [PubMed] [Google Scholar]
- 5.Batschinski K, Dervisis NG, Kitchell BE. Evaluation of ifosfamide salvage therapy for metastatic canine osteosarcoma. Vet Comp Oncol. 2012;12:249–257. doi: 10.1111/j.1476-5829.2012.00355.x. [DOI] [PubMed] [Google Scholar]
- 6.Grignani G, Palmerini E, Dileo P, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: An Italian Sarcoma Group study. Ann Oncol. 2012;23:508–516. doi: 10.1093/annonc/mdr151. [DOI] [PubMed] [Google Scholar]
- 7.Kolb EA, Gorlick R, Reynolds CP, et al. Initial testing (stage 1) of eribulin, a novel tubulin binding agent, by the pediatric preclinical testing program. Pediatr Blood Cancer. 2013;60:1325–1332. doi: 10.1002/pbc.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beristain AG, Narala SR, Di Grappa MA, Khokha R. Homotypic RANK signaling differentially regulates proliferation, motility and cell survival in osteosarcoma and mammary epithelial cells. J Cell Sci. 2012;125(Pt 4):943–955. doi: 10.1242/jcs.094029. [DOI] [PubMed] [Google Scholar]
- 9.Kolb EA, Gorlick R, Billups CA, et al. Initial testing (stage 1) of glembatumumab vedotin (CDX-011) by the pediatric preclinical testing program. Pediatr Blood Cancer. 2014;61:1816–1821. doi: 10.1002/pbc.25099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth M, Linkowski M, Tarim J, et al. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer. 2014;120:548–554. doi: 10.1002/cncr.28461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauchle U, Selvarajah GT, Mol JA, Kirpensteijn J, Verheije MH. Identification of anti-proliferative kinase inhibitors as potential therapeutic agents to treat canine osteosarcoma. Vet J. 2015;205:281–287. doi: 10.1016/j.tvjl.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 12.London CA, Gardner HL, Mathie T, et al. Impact of toceranib/piroxicam/cyclophosphamide maintenance therapy on outcome of dogs with appendicular osteosarcoma following amputation and carboplatin chemotherapy: A multi-institutional study. PLoS One. 2015;10:e0124889. doi: 10.1371/journal.pone.0124889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.London C, Mathie T, Stingle N, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia®) in solid tumours. Vet Comp Oncol. 2011;10:194–205. doi: 10.1111/j.1476-5829.2011.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Onco. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 15.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 16.Shahi PK, Pineda IF. Tumoral angiogenesis: Review of the literature. Cancer Invest. 2009;26:104–108. doi: 10.1080/07357900701662509. [DOI] [PubMed] [Google Scholar]
- 17.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asahara T. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–966. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 19.Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 20.Stoelting S, Trefzer T, Kisro J, Steinke A, Wagner T, Peters SO. Low-dose oral metronomic chemotherapy prevents mobilization of endothelial progenitor cells into the blood of cancer patients. In Vivo. 2008;22:831–836. [PubMed] [Google Scholar]
- 21.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar RMR, Arlt MJ, Kuzmanov A, Born W, Fuchs B. Sunitinib malate (SU-11248) reduces tumour burden and lung metastasis in an intratibial human xenograft osteosarcoma mouse model. Am J Cancer Res. 2015;5:2156–2168. [PMC free article] [PubMed] [Google Scholar]
- 23.Mei J, Zhu X, Wang Z. VEGFR, RET, and RAF/MEK/ERK pathway take part in the inhibition of osteosarcoma MG63 cells with sorafenib treatment. Cell Biochem Biophys. 2014;69:151–156. doi: 10.1007/s12013-013-9781-7. [DOI] [PubMed] [Google Scholar]
- 24.Kaya M, Wada T, Akatsuka T, et al. Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res. 2000;6:572–577. [PubMed] [Google Scholar]
- 25.Ohba T, Cates JMM, Cole HA, et al. Autocrine VEGF/VEGFR1 signaling in a subpopulation of cells associates with aggressive osteosarcoma. Mol Cancer Res. 2014;12:1100–1111. doi: 10.1158/1541-7786.MCR-14-0037. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Yang D, Sun Y, et al. Genetic amplification of the vascular endothelial growth factor (VEGF) pathway genes, including VEGFA, in human osteosarcoma. Cancer. 2011;117:4925–4938. doi: 10.1002/cncr.26116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thamm DH, O’Brien MG, Vail DM. Serum vascular endothelial growth factor concentrations and postsurgical outcome in dogs with osteosarcoma. Vet Comp Oncol. 2008;6:126–132. doi: 10.1111/j.1476-5829.2007.00153.x. [DOI] [PubMed] [Google Scholar]
- 28.Wergin MC, Kaser-Hotz B. Plasma vascular endothelial growth factor (VEGF) measured in seventy dogs with spontaneously occurring tumours. In Vivo. 2004;18:15–19. [PubMed] [Google Scholar]
- 29.Bajpai J, Sharma M, Sreenivas V, et al. VEGF expression as a prognostic marker in osteosarcoma. Pediatr Blood Cancer. 2009;53:1035–1039. doi: 10.1002/pbc.22178. [DOI] [PubMed] [Google Scholar]
- 30.London CA, Hannah AL, Zadovoskaya R, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 2003;9:2755–2768. [PubMed] [Google Scholar]
- 31.London CA, Malpas PB, Wood-Follis SL, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15:3856–3865. doi: 10.1158/1078-0432.CCR-08-1860. [DOI] [PubMed] [Google Scholar]
- 32.Pryer NK, Lee LB, Zadovaskaya R, et al. Proof of target for SU11654: Inhibition of KIT phosphorylation in canine mast cell tumors. Clin Cancer Res. 2003;9:5729–5734. [PubMed] [Google Scholar]
- 33.Bernabe L, Portela R, Nguyen S, et al. Evaluation of the adverse event profile and pharmacodynamics of toceranib phosphate administered to dogs with solid tumors at doses below the maximum tolerated dose. BMC Vet Res. 2013;9:190. doi: 10.1186/1746-6148-9-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veterinary cooperative oncology group — Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol. 2016;14:417–446. doi: 10.1111/vco.283. [DOI] [PubMed] [Google Scholar]
- 35.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 36.Frimberger AE, Chan CM, Moore AS. Canine osteosarcoma treated by post-amputation sequential accelerated doxorubicin and carboplatin chemotherapy: 38 cases. J Am Anim Hosp Assoc. 2016;52:149–156. doi: 10.5326/JAAHA-MS-6315. [DOI] [PubMed] [Google Scholar]
- 37.Selmic LE, Burton JH, Thamm DH, Withrow SJ, Lana SE. Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med. 2014;28:554–563. doi: 10.1111/jvim.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pai MP. Drug dosing based on weight and body surface area: Mathematical assumptions and limitations in obese adults. Pharmacotherapy. 2012;32:856–868. doi: 10.1002/j.1875-9114.2012.01108.x. [DOI] [PubMed] [Google Scholar]
- 39.Jeffree GM, Price CH, Sissons HA. The metastatic patterns of osteosarcoma. Br J Cancer. 1975;32:87–107. doi: 10.1038/bjc.1975.136. [DOI] [PMC free article] [PubMed] [Google Scholar]