Abstract
A blinded randomized controlled trial was used to evaluate a multi-modal therapeutic regime for treatment of beef bulls infected with Campylobacter fetus subsp. venerealis (Cfv). Treatment included 2 doses of a commercially available monovalent vaccine and long-acting oxytetracycline applied twice at a 2-week interval with treatment completed 2 weeks before post-treatment observation. Fifteen confirmed Cfv infected bulls were randomly allocated to control (n = 8) or treatment groups (n = 7). Preputial scrapings were collected each week from before infection to 11 weeks following the last treatment. When the polymerase chain reaction (PCR) results for both culture and preputial scrapings were interpreted in parallel, there were no significant differences between treated and untreated bulls. Regardless of the type of diagnostic testing considered, treatment with 2 label doses of this regime did not stop shedding of Cfv in all treated bulls and is, therefore, not recommended as an effective management strategy.
Résumé
Évaluation de l’oxytétracycline d’action prolongée et d’un vaccin monovalent commercial pour maîtriser l’infection par Campylobacter fetus ssp. venerealis chez les taureaux de boucherie. Un essai clinique randomisé à l’insu a été utilisé pour évaluer un régime thérapeutique multimodal pour le traitement des taureaux de boucherie infectés par Campylobacter fetus ssp. venerealis (Cfv). Le traitement a inclus deux doses d’un vaccin monovalent disponible dans le commerce et de l’oxytétracycline d’action prolongée appliquée deux fois à un intervalle de 2 semaines et le traitement a été complété deux semaines avant l’observation post-traitement. Quinze taureaux présentant une infection confirmée par Cfv ont été assignés au hasard au groupe témoin (n = 8) ou au groupe de traitement (n = 7). Des grattages de la surface du prépuce ont été prélevés à chaque semaine à partir du moment avant l’infection jusqu’à 11 semaines suite au dernier traitement. Lorsque les résultats de l’amplification en chaîne par polymérase (ACP) pour les cultures et les biopsies de surface du prépuce ont été interprétés en parallèle, il n’y avait aucune différence significative entre les taureaux traités et les taureaux non traités. Sans égard au type de test diagnostique considéré, le traitement à l’aide de deux doses recommandées sur l’étiquette n’a pas freiné l’excrétion de Cfv chez tous les taureaux traités et n’est donc pas recommandé comme une stratégie de gestion efficace.
(Traduit par Isabelle Vallières)
Introduction
Embryonic deaths and abortions in the North American cow-calf herd result in substantial production losses and economic hardship for producers (1). Venereal pathogens, such as bovine genital campylobacterosis (BGC), are an important cause of reproductive loss (2). The impact of BGC among western Canadian cow-calf herds was thought to be limited; however, recent studies suggest that BGC is present and an important cause of reproductive failure (3,4).
The causative agent of BGC, Campylobacter fetus subsp. venerealis (Cfv), also referred to as “vibrio” stemming from previous nomenclature (2,5–9), has been conclusively linked to fetal losses. The economic losses are often compounded since the full extent of the problem is not recognized until the herd is pregnancy checked in the fall or until the subsequent calving season (10–13). Campylobacter fetus subsp. venerealis is primarily maintained and transmitted by chronically infected bulls that serve as asymptomatic carriers which harbor the bacteria within folds or crypts in the prepuce (11,13,14).
The 2 most common options for control of BGC in infected herds include vaccination and test and removal of infected bulls (14). While commercial vaccines for BGC are available to producers in Canada there is limited information on their effectiveness (15). As vaccination alone is often not considered sufficient to manage an outbreak, testing and culling of infected bulls is routinely recommended. Test and cull procedures, however, have a substantial cost associated with premature loss of high value animals; especially, considering the price of breeding bulls. The effectiveness of testing and removal of infected bulls is also limited by the clinical sensitivity and specificity of the diagnostic tests suitable for use under field conditions (16).
Some researchers have reported the potential for treating carrier bulls using either vaccination with a monovalent oil-based vaccine or repeated antibiotic therapy (17–22). To date, many of the published antimicrobial treatment protocols use antimicrobial products that are not commercially available in Canada (17,21). Furthermore, many of the previous studies either lacked an appropriate control group or used autogenous vaccines (18–20,22). None of the published studies have provided strong evidence supporting a treatment option that eliminates shedding in infected bulls.
The objective of this study was to use a randomized controlled trial to determine whether treatment with a combination of a long-acting antimicrobial and a commercially available Campylobacter fetus vaccine would induce bacterial clearance in carrier bulls for the duration of a breeding season lasting three 21-day cycles. The authors chose a combination therapy regime, vaccination and treatment with long-acting oxytetracycline, in order to minimize the number of treatment groups given the limited number of study subjects available. If a favorable outcome is found for the combined treatment, further study would be indicated to determine whether vaccination or antimicrobial therapy alone is sufficient for bacterial clearance. Long-acting oxytetracycline was used because of its wide availability in Canada and the USA, its limited use in human health care, and a report from research in Argentina (23). The authors hypothesize that the combination of antimicrobial treatment and vaccination will not result in the clearance of Campylobacter fetus subsp. venerealis from the prepuce of carrier bulls.
Materials and methods
Experimental infection
All animal procedures were performed in accordance with the Canadian Council on Animal Care and approved by the University of Saskatchewan Animal Research Ethics Board (Protocol # 20150025). Twenty (n = 20) cull beef bulls were obtained either by purchase from a local auction or from a local community pasture bull battery (Table 1). Preputial scrapings were collected from all bulls before the start of the experiment and tested by culture and direct real-time polymerase chain reaction (RT-PCR) to confirm that the bulls were test negative for Cfv.
Table 1.
Outline of the timeline for infection, treatment and post-treatment monitoring of Campylobacter fetus subsp. venerealis in beef bulls
| Week | 20 — Negative naïve bulls and 3 — Infected bulls | |||||
|---|---|---|---|---|---|---|
| −2 | Infection period | Preputial scraping and infection protocol | Baseline testing and inoculation of naïve bulls | |||
| −1 | Preputial scraping and infection protocol | |||||
| 15 — Infected bulls (8 — Bulls culled) | ||||||
| 8 — Treatment bulls | 7 — Control bulls | Random allocation to groups | ||||
| 0 | Treatment period | Vaccine, antibiotic, Preputial scraping | Preputial scraping | Treatment 1 | ||
| 1 | Preputial scraping | Preputial scraping | ||||
| 2 | Vaccine, antibiotic, Preputial scraping | Preputial scraping | Treatment 2 | |||
| 3 | Preputial scraping | Preputial scraping | ||||
| 8 — Treatment Bulls | 7 — Control Bulls | Start of observation period | ||||
| 4 | Post-treatment monitoring period 3 estrous cycles or (equivalent to 63 day breeding period) | Preputial scraping | Preputial scraping | Day 1 | Period 1 | |
| 5 | Preputial scraping | Preputial scraping | Day 7 | |||
| 6 | Preputial scraping | Preputial scraping | Day 14 | |||
| 7 | Preputial scraping | Preputial scraping | Day 21 | Day 1 | ||
| Preputial scraping | Preputial scraping | Period 2 | Day 7 | |||
| 9 | Preputial scraping | Preputial scraping | Day 14 | |||
| 10 | Preputial scraping | Preputial scraping | Day 1 | Period 3 | Day 21 | |
| 11 | Preputial scraping | Preputial scraping | Day 7 | |||
| 12 | Preputial scraping | Preputial scraping | Day 14 | |||
| 13 | Preputial scraping | Preputial scraping | Day 21 | |||
Three previously infected (PI) bulls, owned by the Western College of Veterinary Medicine (Saskatoon, Canada), were used as a source of organisms to infect the naïve bulls (16). Preputial scrapings were collected from the 3 PI bulls followed by isolation and recovery of Cfv isolates. Pure cultures of Cfv from the 3 previously infected bulls were expanded on Skirrow agar plates to generate sufficient inoculum to infect the naïve bulls. Pure Cfv cultures grown from the PI bulls were harvested into warmed phosphate buffered saline (PBS; 20 mM phosphate, 150 mM NaCl) and diluted to an optical density (OD600) of 0.4. Immediately before transfer, 2.5 mL of this inoculum was loaded into a series of 10-mL sterile plastic syringes with 1 syringe for each bull. Each of the 20 naïve bulls were infected by placing the inoculum directly into the prepuce using an AI pipette, then 6 mL of air was used to flush the remaining inoculum through the AI pipette. This technique was modified from Bier et al (24). The procedure was repeated 1 wk later, so each of the 20 naïve bulls was inoculated twice (Table 1). At the end of the infection protocol, 12 of the 20 bulls were determined by culture and PCR to be positive for Cfv. Together with the 3 PI bulls, a cohort of 15 infected bulls was available at the start of treatment.
The 15 infected bulls were randomized into either the control (n = 8) or treatment (n = 7) group within blocks based on source (auction, community pasture, or previously infected). The bulls were confirmed positive based on laboratory testing immediately before their second infective dose of Cfv at week -1 and their positive status was re-evaluated with a sample collected immediately before treatment at week 0. The treatment group was vaccinated with a monovalent oil-based Cfv commercial vaccine (Vibrin; Zoetis Canada, Kirkland, Quebec), 2 mL and injected with long-acting oxytetracycline (Liquamycin LA-200; Zoetis Canada), 1 mL/10 kg body weight (BW), after collection of a preputial sample. Both the vaccine and antibiotic were administered subcutaneously twice at a 2-wk interval (Table 1). The vaccine and antibiotic were placed on contralateral sides of each bull’s neck at each treatment.
The person who collected the preputial scrapings and the laboratory personnel were blinded to the treatment status of the bulls. Vaccination and injection with long-acting oxytetracycline were administered by other researchers and veterinarians from the WCVM teaching hospital. All treatment records were maintained by a research assistant.
Preputial samples were collected from all bulls each week of the study both before and during treatment and for 11 wk following the last treatment (Table 1). One aliquot from the sample was designated for culture and a second was tested using direct RT-PCR.
Preputial sample collection
The procedure for collecting preputial scrapings was adapted from Guerra et al (16). The sample was obtained from the prepuce of each bull using a 63.5-cm plastic uterine infusion pipette attached to a 20-mL syringe. The pipette was repeatedly inserted its full length into the prepuce in a back and forth fashion such that the preputial lining was scraped at least 10 times while at the same time approximately 15 mL of suction was applied by the syringe. The resulting sample was immediately transferred into a screw top vial containing 2 mL of PBS. The sample was maintained at 20°C for transport to the laboratory (25). A new sterile syringe and pipette and new latex gloves were used for each sample (Table 1).
Culture and PCR diagnostics
The culture protocol was adapted from Chaban et al (25). Fresh preputial scrapings were transported to the laboratory within 2 h of collection. Upon arrival, 300 μL aliquots were spread onto a sterile 0.65 μm mixed cellulose ester membrane filter (Millipore; Billerica, Massachusetts, USA) and placed on Skirrow (Campylobacter-selective) agar plates (Oxoid, Nepean, Ontario). After incubation of the plates for 30 min at 37°C, filter-side up, the membranes were removed and the plates were incubated for 48 h in microaerophilic conditions using GasPak EZ Campy Pouch System (BO Diagnostics, Mississauga, Ontario). Colonies with the morphology consistent with Campylobacter spp. were Gram-stained for verification then subcultured for 48 h under the same conditions to produce pure Campylobacter colonies for DNA extraction and PCR analysis.
Real-time PCR was completed weekly on both direct preputial DNA samples and DNA from pure Cfv colonies. The DNA was released from 200 μL prepuce scraping in PBS solution using direct heat lysis with minor alterations (4). The preputial samples were centrifuged for 5 min at 12 000 × g and the supernatant discarded (26). The preputial pellet was then re-suspended in 100 μL sterile water before heat lysis at 95°C for 10 min. Finally, samples were diluted 1:10 in sterile water before analysis using both conventional and real-time quantitative PCR (27).
The RT-PCR mixture was created using SYBR green (iQ SYBR green supermix; Bio-Rad, Mississauga, Ontario), 400 nM of each primer, and 2 μL of dilute lysate in a final volume of 25 μL. All samples were run in duplicate on a thermocycler (iCycler/MyIQ; Bio-Rad) as previously described (4) with a primer set targeting Cfv (VenSF and VenSR) (4). Each test included 1 template and positive controls, also in duplicate. Melt-curve analysis was used to indicate infection status; the lower detection limit was 103 copies. The resulting data were analyzed using commercial software (iQ5 Optical System Software, Bio-Rad). Samples with a melt curvature signature comparable to the positive control, peak signal of 78.5°C ± 0.5°C [mean ± standard deviation (SD)], and threshold cycle (Ct) value < 35 were considered positive.
Identification of the Cfv organisms and pure Cfv colonies at both the species and subspecies levels was confirmed with a series of conventional PCR tests of pure sub-cultured colony DNA lysate. In the first step, the highly conserved insertion gene, ISCfe1, was used for subspecies identification (28). A second multiplex PCR was used to confirm the species (i.e. Campylobacter fetus) and provide additional evidence to differentiate between subspecies (i.e., C. fetus fetus versus C. fetus venerealis) as per Hum et al. (29). The carbon starvation gene (cstA) identifies the species level, while the plasmid stabilization protein gene (parA) provides additional information to differentiate the subspecies. The PCR products were resolved by gel electrophoresis (110 V, 40 min) on a 1.5% agarose gel stained with ethidium bromide (Fisher Scientific, Ottawa, Ontario) and visualised under UV light. Positively identified PCR products were purified using QIAquick PCR Purification Kit (Qiagen, Valencia, California, USA), and sequenced through Macrogen Inc. (Seoul, Korea). Sequence results were aligned using the Staden Software Package 2-1.0.0b9. A BLAST search was completed for consensus sequences on the National Center for Biotechnology Information (NCBI) website: (http://www.ncbi.nlm.nih.gov) for verification of identity. Conventional PCR and PCR product sequence analysis was performed 4 times throughout the trial.
Whole genome sequencing
Due to some controversy in the literature regarding the specificity of the available PCR primers (14), we definitively verified the identity of the isolates used in the infection trial by submitting DNA from the Cfv isolates for whole genome sequencing (WGS) through Macrogen (South Korea) at the conclusion of the study. Isolates were selected and expanded in triplicate on Skirrow plates as described. Each plate was washed with 1.5 mL PBS then pelleted by centrifugation for 10 min at 21 000 × g. The DNA was extracted using a modified salting out procedure (30) then combined in various quantities until the desired concentration of 1 μg per sample was achieved. Quality and quantity were rechecked using a Nanodrop 2000 and a Qubit Fluorometer 3.0 (Thermofisher Scientific). Additional quality tests included running 10 μL DNA on 1% agarose gel at 90 V for 1 h to monitor for shearing and assure high quality samples were submitted.
Campybacter fetus DNA of adequate quality was sequenced from isolates from 4 of the artificially infected bulls. The FASTA sequences resulting from the analysis were uploaded to the NCBI nucleotide site and a BLAST search was undertaken for highly similar sequences (megablast) using default settings to confirm the identity of isolates as Cfv (Appendix 1 — available on request from the corresponding author).
Statistical analysis
All data were entered into a spreadsheet and analyzed using Stata 13 for Windows (StataCorp LP, College Station, Texas, USA). Criteria for whether bulls were positive or negative were adapted from Guerra et al (16). All bulls were considered positive before treatment if they had at least 2 positive weekly tests at the time of treatment. A bull was considered negative if it had 4 consecutive negative tests (16).
Sampling times and results in the observation period following treatment were summarized into three 21-day periods representative of three 21-day estrous cycle periods which constitute an industry recommended 63-day breeding season (Table 1). The first 21-day cycle, which was also the first observation period, began on Week 4 or 2 wk after the final treatment (Week 2). The start of the post-treatment observation period (OP) was set assuming the final treatment would require at least 2 wk to be effective and, therefore, if recommended for field use the series of treatments would be completed at least 2 wk before commencement of a breeding season.
The sample collected on Day 21 of Period 1 (or Week 7) represented the status of the bull at the end of Period 1. The sample collected on Day 21 of Period 1 was also considered to represent the bull’s status for Day 1 of Period 2. An outline of the treatment and subsequent sampling periods is shown in Table 1. For a bull to have been considered test negative at the end of a complete 21-day OP, it needed to have a negative test on all 4 test days linked to the period (Days 1, 7, 14, and 21). If the bull was positive on any 1 test day during the respective period, it was considered test positive for that period.
A Wilcoxon rank-sum test was used to evaluate the differences in weight between the bulls in the treatment and control groups. The differences in Cfv infection between the treated and untreated groups were estimated for each period using exact logistic regression. Odds ratios and exact 95% confidence intervals (CI) were calculated to compare the odds of control bulls being positive for Cfv compared with the treated bulls. A P-value < 0.05 was considered statistically significant. The differences for control versus treated bulls were determined for 21-day cycle results summarized from weekly culture results confirmed with PCR, direct RT-PCR of preputial aspirates, and combined results for culture and prepuce RT-PCR. The joint interpretation of both culture and direct RT-PCR represented parallel interpretation of the 2 tests and was evaluated to optimize the sensitivity of the analysis.
Results
Of the 15 Cfv infected bulls used in the project, 5 were 3 y old and 2 were 5 y old. Age records were not available for the remaining 8 bulls, but physical appearance and size suggested they were at least 3 y of age. The bulls ranged in weight from 827 kg to 1102 kg with an average weight of 953 kg and a median weight of 939 kg. There was no difference in weights (P = 0.5) between the bulls assigned to the treatment and control groups. The 15 bulls included 8 Black Angus, 3 Simmental, 2 Red Angus, and 2 Charolais. None of the bulls required antibiotic treatment for other reasons during the study.
All 15 bulls were culture and PCR-positive on at least 2 weekly samples after inoculation of the naïve bulls with Cfv and before the 2 treatment sets were complete. Whole genome sequences of Campylobacter isolates from 4 of the artificially infected bulls provided definitive confirmation that the organisms used for artificial infections were Cfv (Appendix 1). The best match was to Cfv str. 84-112, complete genome GenBank: HG004426.1 (29,30).
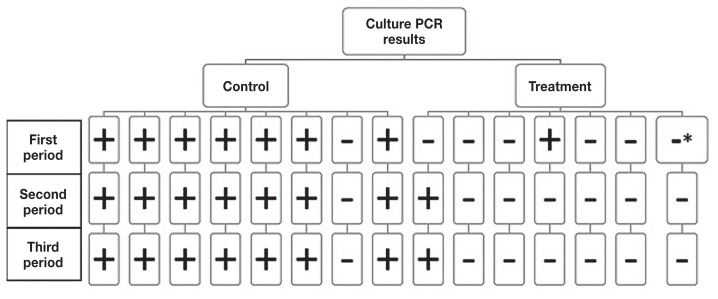
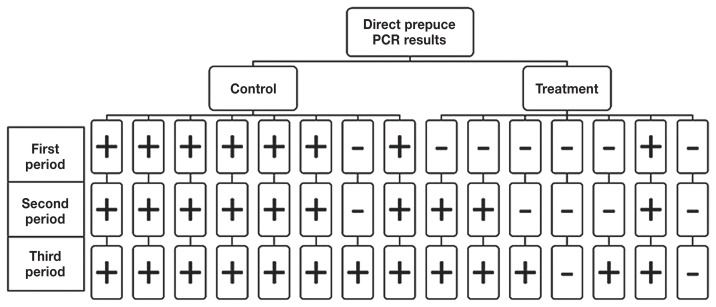
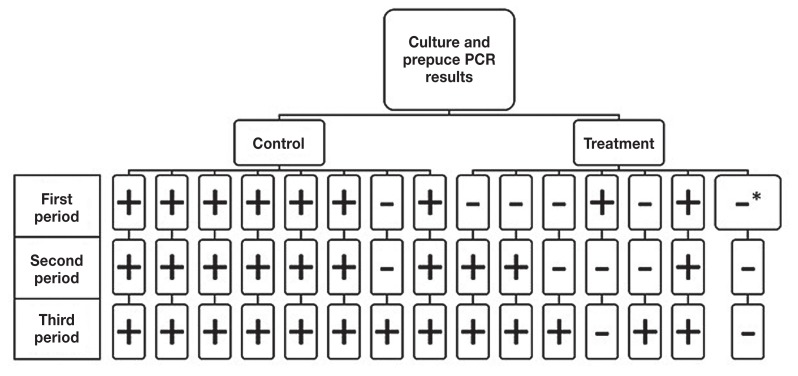
Individual bull test results during the post-treatment observation period (OP) differed between the preputial scrapings cultured and verified with PCR (Figure 1) compared with scrapings analyzed by direct RT-PCR (Figure 2). The results of the 2 tests were then combined to facilitate parallel interpretation (Figure 3) and optimize sensitivity.
Figure 1.
Culture PCR results summarized for individual bulls beginning 2 wk after the second of 2 treatments with injectable oxytetracycline and 2 doses of monovalent Campylobacter fetus vaccine. Bulls were reported as positive if they had at least 1 positive test result on day 1, 7, 14, or 21 for each of the subsequent three 21-day observation periods. A bull was reported as negative for a period if he was test negative on all 4 samples considered part of that period.
* Results were negative for direct prepuce PCR for 2 of 4 samples and 2 samples were undetermined due to fungal overgrowth.
Figure 2.
Direct prepuce PCR results summarized for individual bulls beginning 2 wk after the second of 2 treatments with injectable oxytetracycline and 2 doses of monovalent Campylobacter fetus vaccine. Bulls were reported as positive if they had at least 1 positive test result on day 1, 7, 14, or 21 for each of the subsequent three 21-day observation periods. A bull was reported as negative for a period if he was test negative on all 4 samples considered part of that period.
Figure 3.
Combination of culture and direct prepuce PCR results summarized for individual bulls beginning 2 wk after the second of 2 treatments with injectable oxytetracycline and 2 doses of monovalent Campylobacter fetus vaccine. Bulls were reported as positive if they had at least 1 positive test result on day 1, 7, 14, or 21 for each of the subsequent three 21-day observation periods. A bull was reported as negative for a period if he was test negative on all 4 samples considered part of that period.
* Results were negative for direct prepuce PCR for 2 of 4 samples and 2 samples were undetermined due to fungal overgrowth.
One control bull was negative based on preputial scraping culture PCR throughout the three 21-day post-treatment OP (Figure 1); however, the bull did test positive once in the final of the three 21-day periods with the direct RT-PCR test (Figure 2).
Only 2 of the 7 treated bulls had at least 1 culture positive preputial scraping confirmed by PCR during the OP (Figure 1). One bull’s preputial scraping culture tested PCR positive once on Day 14 of the first 21-day period and then never tested positive again. The second bull’s preputial scraping culture tested PCR positive on Day 14 of the second 21-day period and Day 21 of the third 21-day period.
For the direct RT-PCR on the preputial scraping only 2 of the 7 treated bulls remained negative throughout the three 21-day post-treatment observation periods (Figure 2). In the first 21-day period there was only 1 direct RT-PCR test positive bull; however, it was not the same bull that had a positive culture result. Three bulls had a positive direct RT-PCR result during the second 21-day period and 4 of 7 bulls had a positive RT-PCR result during the third 21-day OP.
When the results of both culture and direct preputial RT-PCR were considered in parallel [i.e., the bulls that were positive in either of the 2 tests were considered test-positive (Figure 3)] all untreated, control bulls were positive at least once during the OP and all but 1 of the treated bulls were also positive at least once.
If only the preputial cultures confirmed with PCR were considered (Figure 1), the odds that control bulls would test positive for Cfv were 27.2 times higher than for the treated bulls (P = 0.02) in all three 21-day post-treatment observation periods (Table 2). When only the results of the direct RT-PCR test were evaluated (Figure 2), control bulls were 27.2 times more likely to be positive for Cfv (P = 0.02), but only in the first 21-day period (Table 2).
Table 2.
Exact logistic regression results summarizing the odds that a bull would test positive at least once by culture, direct PCR, or either culture or direct PCR for Campylobacter fetus subsp. venerealis for the control group compared to the treated group. Odds ratios were estimated for each of the three 21-day observation periods following treatment. The first observation cycle started 2 weeks after the second of 2 treatments with a monovalent Campylobacter fetus vaccine and injectable oxytetracycline (n = 7 treated bulls and 8 untreated bulls)
| 21-day period | % Treated bulls Cfv positive | % Untreated bulls Cfv positive | Odds ratio | 95% CI | P-value |
|---|---|---|---|---|---|
| 1st period: | |||||
| Culture and PCR | 14% (1/7) | 88% (7/8) | 27.2 | (1.50–2194) | 0.02 |
| Direct preputial PCR | 14% (1/7) | 88% (7/8) | 27.2 | (1.50–2194) | 0.02 |
| Combined culture and direct preputial PCR | 29% (2/7) | 88% (7/8) | 13.6 | (0.86–942) | 0.07 |
| 2nd period: | |||||
| Culture and PCR | 14% (1/7) | 88% (7/8) | 27.2 | (1.50–2194) | 0.02 |
| Direct preputial PCR | 43% (3/7) | 88% (7/8) | 7.9 | (0.50–523) | 0.20 |
| Combined culture and direct preputial PCR | 43% (3/7) | 100% (8/8) | 7.9 | (0.50–523) | 0.20 |
| 3rd period: | |||||
| Culture and PCR | 14% (1/7) | 88% (7/8) | 27.2 | (1.50–2194) | 0.02 |
| Direct preputial PCR | 71% (5/7) | 88% (7/8) | 3.1 | (0.22–Inf) | 0.40 |
| Combined culture and direct preputial PCR | 71% (5/7) | 100% (8/8) | 3.1 | (0.22–Inf) | 0.40 |
When the results of both culture and the direct PCR test were considered in parallel, there was no significant difference in the odds of a positive test result for any of the 21-day post-treatment OP, although the difference between treated and untreated bulls in the first 21-day period (P = 0.07) (Table 2).
No serious side effects were observed in any of the 7 treated bulls. However, 5 bulls developed injection site swellings from either the vaccine (2 bulls) or long-acting oxytetracycline (3 bulls). The swellings were small and resolved with no long-term complications.
Discussion
The main objective of this study was to determine if a multimodal treatment approach, using a combination of vaccination and long-acting antimicrobial therapy, would reduce or eliminate shedding of Cfv in chronically infected carrier bulls. Two treatments with long-acting oxytetracycline at label doses and a commercial monovalent bacterin did not eliminate BGC in all study bulls. There was also no significant difference between the proportion of treated and untreated bulls that tested positive when the results of both culture and direct RT-PCR were considered. The 2 test results were combined to optimize the sensitivity of the testing protocol detection of BGC post-treatment because of the severe consequences of false negatives.
When the results of each diagnostic test were observed separately, there was a significant reduction in the likelihood that a treated bull would test positive based on culture for all 3 post-treatment 21-day observation periods and for direct RT-PCR of the preputial samples for the first 21-day OP (Table 2). One potential argument for considering the results of culture alone would be that culture definitively identifies bulls shedding live organisms; whereas direct RT-PCR could potentially identify DNA from nonviable organisms that would not be a risk for transmission. However, in the current study the observed frequency of RT-PCR positive bulls increased throughout the trial which is consistent with what we would expect with increasing time after treatment. If the RT-PCR results are correct and the preputial cultures were less sensitive than RT-PCR during the post-treatment monitoring phase, the lower sensitivity may have been due to death of Cfv during transport to the laboratory and decreasing environmental temperature during collection in September and October.
The clinical specificity of bacterial culture (100%) is higher than that of RT-PCR (85%) under field conditions in western Canada (16), suggesting the potential for false positive RT-PCR tests. However, recent field data suggest that the difference in the risk of false positives might not be as large as previously reported especially in situations with high pretest probability of infection (31). Previous studies reported similar sensitivities for bacterial culture using the methods described herein and RT-PCR (82.3% and 85.4%, respectively) (16). The published estimates of clinical sensitivity for culture, however, were based on samples delivered to the laboratory substantially faster than in the present study (<30 min). Also, all samples for that study were collected in June and July in an insulated barn, potentially leading to higher culture sensitivity than that in the present study. Previous studies have shown that the sensitivity of the RT-PCR is resilient to the time delays and transport conditions seen herein (32).
Another argument for considering the treatment as potentially successful would be the tendency for a difference between treatments in the first 21-day post-treatment OP when the results of both culture and RT-PCR were considered. The failure to detect a significant result was very likely a reflection of limited study power. The precision of the estimates of effect for all testing methods was affected by the small number of bulls in this study. However, regardless of the statistical significance and precision of effect estimates, treatment success was not considered adequate in the present study given that 29% (first cycle), 43% (second cycle), and 71% (third cycle) of treated bulls were positive on either culture or direct PCR during post-treatment OP designed to simulate a typical breeding season. There was no complete cycle when all of the treated bulls were negative by culture or direct RT-PCR. The high potential cost of having even 1 bull remain infectious warrants a conservative interpretation of the findings.
There is relatively little good published evidence against which to compare the results of the present study; despite historical use of topical streptomycin ointments for the treatment of vibrio-infected cattle (33). Vasquez et al (18) reported on the therapeutic efficacy of the Cfv bacterin used in the present study. Of the 10 bulls used in their 1983 study, 6 were initially vaccinated twice at a 4-week interval and 4 were kept as controls. The 6 original vaccinates tested negative by week 8 post-vaccination and the 4 controls were positive. The 4 controls were then vaccinated and 2 of these bulls infected heifers with Cfv. The authors determined that 2 of 10 vaccinated bulls remained chronically infected with Cfv and therefore did not recommend vaccination alone for treatment of Cfv. Based on this finding, we chose combination therapy with both vaccination and antimicrobials for the current study. The bulls in the present study were followed for 11 wk after vaccination compared with 8 wk in the earlier study. Other limitations of the Vasquez et al (18) study were the smaller sample size and lack of blinding.
The remaining identified published studies of vaccine trials in bulls used autogenous or experimental rather than commercial vaccines (18,19,22,34,35). Most previous studies did not use a negative control, and therefore the infections may have resolved spontaneously. Anecdotally, spontaneous recovery occurs in young bulls infected with Cfv (17). One bull in the control group for the present study tested positive twice before treatment but only once by PCR after treatment at the very end of the OP. Either this bull failed to completely spontaneously clear the experimental infection (likely) or the experimental infection did not establish in this bull and it was re-exposed later through bull-to-bull contact (less likely) (13).
Although a recent review reported that antimicrobial treatments for Cfv are impractical and of limited efficacy (14), 2 previous studies suggested some potential for antimicrobial use in management of positive bulls. The first study observed bulls that were infected by both Cfv and Tritrichomonas foetus and were treated with subcutaneous and intramuscular dimetridazole chlorhydrate (DCL) (21). Ten naturally infected bulls were confirmed positive by fluorescent antibody technique (FAT) and culture. Of the 10 bulls, 4 were infected by Cfv alone, 3 were infected by Cfv and Campylobacter fetus subsp. fetus (Cff ), and 3 were infected by Cff alone. All 10 bulls were negative for Cfv and Cff by FAT and culture at each of 4 bi-weekly samplings after treatment. However, the authors acknowledged that the negative test results could be biased by sporadic resolution as no control animals were used. This previous study also used DCL, which is not allowed for use in food producing animals in Canada.
In addition to DCL, treatment with long-acting oxytetracycline and fluoroquinolone has been reported for bulls naturally infected with Cff in Argentina (23). While this paper did not directly address Cfv infection, it was the motivation for the choice of antibiotic in the present study. The Argentinian study reported 5 of 5 of bulls were negative for Cff by culture and FAT 30 d after 2 treatments 72 h apart with long-acting oxytetracycline (0.10 mL/kg, ½ IM and ½ SC) (23). Only 1 of 7 bulls treated with fluoroquinolone was negative at 30 d. There was no negative control group. The preferred antibiotic for the present study was long-acting oxytetracycline because of its cost relative to other options, limited use in human health, wide availability in Canada, and due to its performance in the previous study.
The previous studies that observed treatment response of Cfv infected bulls relied on culture or fluorescent antibody screening for their diagnostic testing. While culture has been the gold standard for many years, its sensitivity is problematic especially when lag times exist between sampling and arrival at the laboratory (25). Our study used PCR techniques directly on preputial scrapings in addition to culture. Use of techniques such as direct prepuce PCR is important in western Canada because access to laboratory facilities is limited by distance and transport time. The development of direct prepuce PCR could improve test sensitivity as it is not impacted by transport times up to 96 h or to moderate temperature changes (32). The sensitivity of the direct PCR also improves with repeated sampling employed in this study (16). The specificity of diagnosis for the agent used in the experimental infection and the isolates recovered during the trial was assured with conventional PCR using multiple primers followed by whole genome sequencing for a subset of isolates.
The differences in results between the RT-PCR of the direct prepuce samples and culture results observed in this study might be attributed to the limited sensitivity of culture under field conditions (14,25) or the limited sensitivity and specificity of the direct RT-PCR (16). While samples were collected as quickly as possible and efforts were made to hold the samples at a constant temperature and then deliver them directly to the laboratory, there is the potential for loss of viability due to the time lag and temperature fluctuations between collection and plating for culture, given the susceptibility of Cfv to adverse environmental conditions (25).
While this study does provide the first randomized, controlled, and blinded trial on combined therapy with commercially licensed products, increasing the number of animals in this study would have resulted in more precise estimates of the effect of combined vaccination and treatment on Cfv status in mature beef bulls. Subsequent studies should consider other commercially licensed long-acting antimicrobials or emerging alternatives to antimicrobial therapy.
Acknowledgments
The authors acknowledge support from the Saskatchewan Agricultural Development Fund, Zoetis Canada, and the Prairie Farm Rehabilitation Administration. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Bellows DS, Ott SL, Bellows RA. Review: Cost of reproductive diseases and conditions in cattle. Prof Anim Sci. 2002;18:26–32. [Google Scholar]
- 2.McCool CJ, Townsend MP, Wolfe SG, et al. Prevalence of bovine venereal disease in the Victoria River District of the Northern Territory: Likely economic effects and practicable control measures. Aust Vet J. 1988;65:153–156. doi: 10.1111/j.1751-0813.1988.tb14445.x. [DOI] [PubMed] [Google Scholar]
- 3.Waldner C, Hendrick S, Chaban B, et al. Application of a new diagnostic approach to a bovine genital campylobacteriosis outbreak in a Saskatchewan beef herd. Can Vet J. 2013;54:373–376. [PMC free article] [PubMed] [Google Scholar]
- 4.Chaban B, Chu S, Hendrick S, Waldner C, Hill JE. Evaluation of a Campylobacter fetus subspecies venerealis real-time quantitative polymerase chain reaction for direct analysis of bovine preputial samples. Can J Vet Res. 2012;76:166–173. [PMC free article] [PubMed] [Google Scholar]
- 5.Givens MD. A clinical, evidence-based approach to infectious causes of infertility in beef cattle. Theriogenology. 2006;66:648–654. doi: 10.1016/j.theriogenology.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Finlay RC, Ruckerbauer GM, Stovell PL. Campylobacter fetus in artificial insemination unit and slaughterhouse bulls in Ontario. Can J Comp Med. 1985;49:231–232. [PMC free article] [PubMed] [Google Scholar]
- 7.Bawa E, Adekeye J, Oyedipe E, Umoh J. Prevalence of bovine campylobacteriosis in indigenous cattle of three states in Nigeria. Trop Anim Health Prod. 1991;23:157–160. doi: 10.1007/BF02356996. [DOI] [PubMed] [Google Scholar]
- 8.Hum S. Bovine abortion due to Campylobacter fetus. Aust Vet J. 1987;64:319–320. doi: 10.1111/j.1751-0813.1987.tb07343.x. [DOI] [PubMed] [Google Scholar]
- 9.van Bergen MAP, Linnane S, van Putten JPM, Wagenaar JA. Global detection and identification of Campylobacter fetus subsp. venerealis. Rev Sci Tech OIE. 2005;24:1017–1026. [PubMed] [Google Scholar]
- 10.BonDurant RH. Venereal diseases of cattle: Natural history, diagnosis, and the role of vaccines in their control. Vet Clin N Am Food Anim Pract. 2005;21:383–408. doi: 10.1016/j.cvfa.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Bondurant RH. Inflammation in the bovine female reproductive tract. J Anim Sci. 1999;77:101–110. doi: 10.2527/1999.77suppl_2101x. [DOI] [PubMed] [Google Scholar]
- 12.Campero CM, Moore DP, Odeón AC, Cipolla AL, Odriozola E. Aetiology of bovine abortion in Argentina. Vet Res Commun. 2003;27:359–369. doi: 10.1023/a:1024754003432. [DOI] [PubMed] [Google Scholar]
- 13.Hoffer MA. Bovine campylobacteriosis: A review. Can Vet J. 1981;22:327–330. [PMC free article] [PubMed] [Google Scholar]
- 14.Michi AN, Favetto PH, Kastelic J, Cobo ER. A review of sexually transmitted bovine trichomoniasis and campylobacteriosis affecting cattle reproductive health. Theriogenology. 2016;85:781–791. doi: 10.1016/j.theriogenology.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 15.Schurig GG, Duncan JR, Winter AJ. Elimination of genital vibriosis in female cattle by systemic immunization with killed cells or cell-free extracts. J Infect Dis. 1978;188:463–472. doi: 10.1093/infdis/138.4.463. [DOI] [PubMed] [Google Scholar]
- 16.Guerra AG, Chaban B, Hill JE, Waldner CL, Hendrick SH. Clinical sensitivity and specificity of a real-time PCR assay for Campylobacter fetus subsp venerealis in preputial samples from bulls. Am J Vet Res. 2014;75:851–560. doi: 10.2460/ajvr.75.9.851. [DOI] [PubMed] [Google Scholar]
- 17.Truyers I, Luke T, Wilson D, Sargison N. Diagnosis and management of venereal campylobacteriosis in beef cattle. BMC Vet Res. 2014 Nov 27;10:280. doi: 10.1186/s12917-014-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasquez LA, Ball L, Bennett BW, et al. Bovine genital campylobacteriosis (vibriosis): Vaccination of experimentally infected bulls. Am J Vet Res. 1983;44:1553–1537. [PubMed] [Google Scholar]
- 19.Clark BL, Dufty JH, Monsbourgh MJ. Vaccination of bulls against bovine vibriosis. Aust Vet J. 1968;44:530. doi: 10.1111/j.1751-0813.1968.tb09024.x. [DOI] [PubMed] [Google Scholar]
- 20.Allan PJ. A field evaluation of vaccination of bulls against vibriosis. Aust Vet J. 1972;48:72–73. doi: 10.1111/j.1751-0813.1972.tb05131.x. [DOI] [PubMed] [Google Scholar]
- 21.Campero CM, Ballabene NC, Cipolla AC, Zamora AS. Dual infection of bulls with campylobacteriosis and trichomoniasis: Treatment with dimetridazole chlorhydrate. Aust Vet J. 1987;64:320–321. doi: 10.1111/j.1751-0813.1987.tb07344.x. [DOI] [PubMed] [Google Scholar]
- 22.Foscolo CB, Pellegrin AO, Leite RC, Stynen APR, Lage AP. Vaccination of bulls against genital campylobacteriosis: A therapeutic approach. Anim Reprod. 2005;2:122–127. [Google Scholar]
- 23.Campero CM, Cipolla AC, Odriozola E, Medina D, Morsella CG, Saubidet M. Tratamientos sistemicos en toros con infeccion genital a Campylobacter fetus subsp. fetus. Veterinaria Argentina. 1993;10:303–309. [Google Scholar]
- 24.Bier PJ, Hall CE, Duncan JR, Winter AJ. Experimental infections with Campylobacter fetus in bulls of different ages. Vet Microbiol. 1977;2:13–27. [Google Scholar]
- 25.Chaban B, Garcia Guerra A, Hendrick SH, Waldner CL, Hill JE. Isolation rates of Campylobacter fetus subsp venerealis from bovine preputial samples via passive filtration on nonselective medium versus selective medium, with and without transport medium. Am J Vet Res. 2013;74:1066–1069. doi: 10.2460/ajvr.74.8.1066. [DOI] [PubMed] [Google Scholar]
- 26.McMillen L, Fordyce G, Doogan VJ, Lew AE. Comparison of culture and a novel 5′ Taq nuclease assay for direct detection of Campylobacter fetus subsp. venerealis in clinical specimens from cattle. J Clin Microbiol. 2006;44:938–945. doi: 10.1128/JCM.44.3.938-945.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaban B, Musil KM, Himsworth CG, Hill JE. Development of cpn60-based real-time quantitative PCR assays for the detection of 14 Campylobacter species and application to screening of canine fecal samples. Appl Environ Microbiol. 2009;75:3055–3061. doi: 10.1128/AEM.00101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abril C, Vilei EM, Brodard I, Burnens A, Frey J, Miserez R. Discovery of insertion element ISCfe1: A new tool for Campylobacter fetus subspecies differentiation. Clin Microbiol Infect. 2007;13:993–1000. doi: 10.1111/j.1469-0691.2007.01787.x. [DOI] [PubMed] [Google Scholar]
- 29.Hum S, Quinn K, Brunner J, On SL. Evaluation of a PCR assay for identification and differentiation of Campylobacter fetus subspecies. Aust Vet J. 1997;75:827–831. doi: 10.1111/j.1751-0813.1997.tb15665.x. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Platero AM, Valdivia E, Maqueda M, Martinez-Bueno M. Fast, convenient and economical method of isolating genomic DNA for lactic acid bacteria using a modification of the protein “salting-out” procedure. Anal Biochem. 2007;366:102–104. doi: 10.1016/j.ab.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Waldner C, Parker S, Gesy K, Waugh T, Lanigan E, Campbell J. Application of direst polymerase chain reaction assays for Campylobacter fetus subsp. venerealis and Tritrichomonas foetus to screen preputial samples from breeding bulls in cow-calf herds in western Canada. Can J Vet Res. 2017;81:91–99. [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Guerra A, Waldner C, Pellegrin A, et al. Effect of sample pooling and transport conditions on the clinical sensitivity of a real-time polymerase chain reaction assay for Campylobacter fetus subsp. venerealis in preputial samples from bulls. Can J Vet Res. 2016;80:32–39. [PMC free article] [PubMed] [Google Scholar]
- 33.McEntee K. Bovine vibriosis. Iowa State University Vet. 1958;20:14–15. [Google Scholar]
- 34.Bouters R, De Keyser J, Vandeplasshe M, Van Aert A, Brone E, Bonte P. Vibrio fetus infection in bulls: Curative and preventive vaccination. Br Vet J. 1973;129:52–57. doi: 10.1016/s0007-1935(17)36588-0. [DOI] [PubMed] [Google Scholar]
- 35.Yarokhno Y. MSc dissertation. Saskatoon, Saskatchewan: University of Saskatchewan; 2015. Diagnosis and vaccination for bovine genital campylobacteriosis in beef heifers. [Google Scholar]