Abstract
Recent approaches to reducing radiation exposure during CT examinations typically utilize automated dose modulation strategies on the basis of lower tube voltage combined with iterative reconstruction and other dose-saving techniques. Less clearly appreciated is the potentially substantial role that iodinated contrast media (CM) can play in low-radiation-dose CT examinations. Herein we discuss the role of iodinated CM in low-radiation-dose examinations and describe approaches for the optimization of CM administration protocols to further reduce radiation dose and/or CM dose while maintaining image quality for accurate diagnosis. Similar to the higher iodine attenuation obtained at low-tube-voltage settings, high-iodine-signal protocols may permit radiation dose reduction by permitting a lowering of mAs while maintaining the signal-to-noise ratio. This is particularly feasible in first pass examinations where high iodine signal can be achieved by injecting iodine more rapidly. The combination of low kV and IR can also be used to reduce the iodine dose. Here, in optimum contrast injection protocols, the volume of CM administered rather than the iodine concentration should be reduced, since with high-iodine-concentration CM further reductions of iodine dose are achievable for modern first pass examinations. Moreover, higher concentrations of CM more readily allow reductions of both flow rate and volume, thereby improving the tolerability of contrast administration.
INTRODUCTION
CT is an indispensable diagnostic imaging technique whose use and range of applications have increased dramatically in recent years, primarily due to its widespread availability, ease-of-use and cost-effectiveness relative to other diagnostic procedures.1 However, while rapid, ongoing technical innovations have led to marked improvements in image quality and diagnostic performance, the increasing utilization of CT has raised considerable concern over the risks associated with exposure to ionizing radiation, particularly with regard to the risk of radiation-induced cancer.2–11 Overall, radiation exposure from CT examinations in Europe accounts for roughly 60% of the total radiation dose from all radiographic procedures12 while the Lifetime Attributable Risk of radiation-induced cancer from CT scanning has been estimated to range from around 0.7%3 to 1.5–2.0%.4
Although the “as low as reasonably achievable”13,14 or, more appropriately for radiographic procedures, the “as low as diagnostically acceptable” principle is the widely invoked “rule-of-thumb” approach to minimize patient exposure to radiation, it is, as the term indicates, merely a principle rather than a scientific or technical innovation. In looking to reduce radiation exposure to patients, manufacturers of scanners have introduced various automated dose modulation strategies on the basis of lower tube voltage (kVp) and/or tube current (mAs) combined with iterative reconstruction (IR) and other dose-saving approaches to image acquisition.1 Such techniques have been shown to permit considerable radiation dose savings.
Less clearly appreciated is the potentially substantial role that iodinated contrast media (CM) can play in low-radiation-dose CT examinations. The relative attenuation of iodinated contrast material is increased at lower kVp,15 resulting in higher contrast enhancement than that obtained at higher kVp for a similar amount of administered CM. Several studies across a range of applications have exploited this increased attenuation at low kVp to emphasize the possibilities for reducing both CM dose and radiation dose when utilizing low-kVp protocols.16–22 However, very few studies have focused on the possibilities for further radiation dose reduction using optimized CM injection protocols. Moreover, considerable debate exists as to the optimal approach to lowering the CM dose when utilizing low-kVp protocols.
Herein we discuss the role of iodinated CM in low-radiation-dose examinations and describe approaches for the optimization of CM administration protocols to further reduce radiation dose and/or CM dose while maintaining image quality for accurate diagnosis.
Fundamentals of low-tube-voltage examinations in CT
The number of X-ray photons delivered in CT depends on the tube current (mA) and exposure time (s) and increases linearly with increasing mAs. The number of photons is directly proportional to the applied radiation dose.23 Conversely, the kVp determines the energy of the photons delivered. A common misconception is that the lower energy of the delivered photons is the reason for the radiation dose reduction potential of low-kVp examinations in CT. However, according to the recommendations of the International Commission on Radiological Protection, the weighting factor given to all sparsely ionizing radiations is equivalent (i.e. scored as “one”), implying that the biological effect of X-ray photons is independent of their energy. Moreover, the absorption of the low-energy photons typically delivered in medical examinations is higher than that of higher energy photons, especially in superficial body regions, meaning that the radiation dose applied to a patient should be even higher at low kVp. Softer X-rays are potentially more biologically damaging and thus have a larger radiation risk than hard X-rays because lower energy X-rays deposit a larger proportion of the dose in the body in the form of track-end electrons.23 The actual reason the radiation dose is lower at low kVp is that the number of photons generated by the CT tube is considerably lower at low kVp than at higher kVp when the same mAs value is used.24 This is primarily for technical reasons, such as the lower effectiveness of the tube at low kV (the X-ray tube output is proportional to kVp2 and thus a reduction from 120 to 100 kVp will result in (100/120)2 = 0.69, i.e. about 69% of the photons compared with that emitted at 120 kVp) and stronger filtering of low-kV photons.
Knowing this, it is evident that increased image noise (N) is a consequence of imaging at low kVp. Image noise is proportional to the denominator of the square root of the number of X-ray photons (#p) and is thus directly dependent on the number of photons delivered:24
| (1) |
The lower the number of photons delivered, the greater the image noise. Thus, protocols that utilize a low-kVp setting with unchanged mAs will deliver a reduced radiation dose but will suffer from increased image noise. A compensatory increase in mAs is thus required to overcome the greater noise. However, quantifying the optimum increase in mAs for an individual patient can be challenging. The resulting low practicability and lack of standardized guidelines may have been reasons for the reluctance among radiologists to use low-kVp protocols.25
In practice, approaches to mAs compensation in low-kVp examinations focus on maintaining either a constant image noise (N) or a constant signal-to-noise ratio (SNR).
Constant image noise
If the approach chosen is to maintain a constant N, then the potential for radiation dose reduction at lower tube potentials is limited or non-existent.26 This has been demonstrated in studies on phantoms in which noise was shown to increase substantially on low-kV images, especially for phantoms with larger diameters.27 Moreover, significant photon-starvation artefacts appear due to the decreased penetration capability of the lower energy photons and electronic noise. Even for a 25-cm phantom which represents the attenuation of a very small adult, a 29% increase in dose [relative volume CT dose index (CTDIvol)] was required at 80-kV examination in order to match the noise of a 120-kV examination.26 In other words, the required mAs compensation would have to be high even in small adults so as to swamp any perceived benefit of reduced kV if mAs compensation is based on matching image noise. For this reason, image reconstruction techniques such as IR have been developed to compensate for image noise.
Constant signal-to-noise ratio
The alternative approach is to maintain a constant SNR, in which the signal S is defined as the signal from the iodinated CM in the body. The X-ray absorption of iodine increases with decreasing effective kVp as long as the effective energy remains above the κ-edge (33.2 KeV) of iodine.15 Thus, the signal S will be higher in low-kV CT examinations with the same amount of contrast applied. This is one of the fundamental roles of iodine in low-radiation-dose examinations. Owing to this higher signal, higher noise can also be accepted when maintaining a constant SNR because:
| (2) |
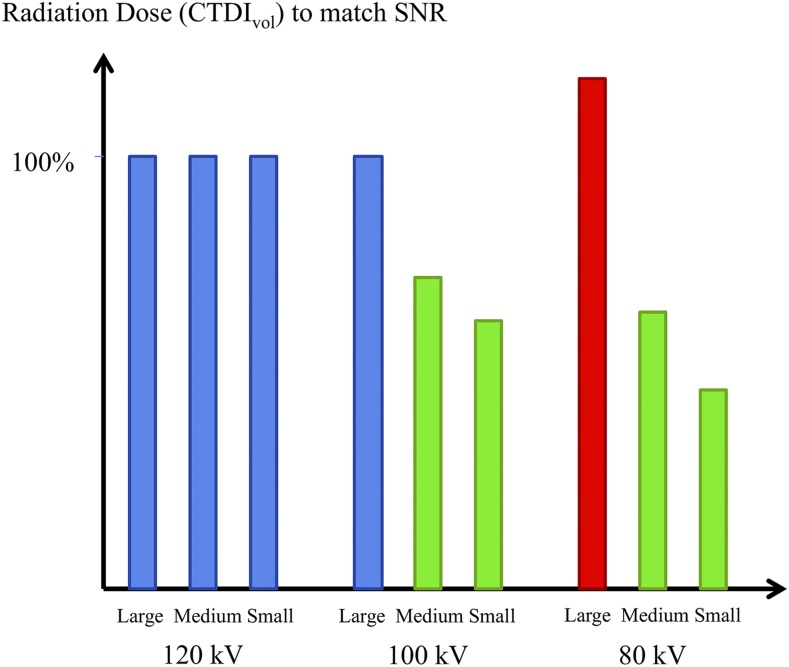
Thus, when the SNR is kept constant at reduced kVp, only a moderate compensatory increase in mAs is needed. However, the indication and size of the patient should still be considered. Phantom measurements have shown that the CTDIvol needed for identical SNR relative to that at 120 kV is 46% at 80 kV and 62% at 100 kV for a small adult phantom. This implies a tremendous reduction in radiation dose. However, for a large patient (45-cm diameter phantom), 18% more dose (CTDIvol) is needed at 80 kV relative to that at 120 kV in order to match the iodine SNR.15 Thus, even when the choice is to maintain a constant SNR, a low-kV protocol may not be feasible for radiation dose reduction in all patients (Figure 1). Importantly, these considerations apply not only to single-energy low-kVp examinations but also to dual-energy or spectral imaging examinations.
Figure 1.
The volume CT dose index (CTDIvol) values needed to match the signal-to-noise ratio (SNR) obtained in water phantoms with three tube potentials and three phantom sizes (25-, 35- and 45-cm diameter). Unlike at 120 kV, 80 and 100 kV allow for a radiation dose reduction in small- and medium-size phantoms. However, with the large-size phantom, 80 kV would require a higher radiation dose to match SNR than 120 kV (based on values provided by Yu26).
One option to optimizing tube potential is to use patient weight or size-based kV-mAs tables and to manually adjust values for a specific patient. However, a less complex approach is automated kVp selection in which radiation exposure is individually determined on the basis of effective attenuation values measured by the scanner.28
The appropriate choice of kV is invariably determined by the clinical task to be performed. For the evaluation of highly iodine-enhanced vessels or structures, the iodine contrast-to-noise ratio (CNR) may be the most appropriate metric to use. Thus, the potential of low-kVp protocols for radiation dose reduction is highest for indications such as CT angiography (CTA) and acquisitions during the arterial phase (e.g. for detection of arterially vascularized liver tumours). For these indications, low kVp and higher iodine signal not only have the potential to reduce radiation dose but also to improve image quality. This applies similarly to all organs with considerable parenchymal enhancement in the portal-venous phase such as in the liver, kidney and pancreas. Zamboni et al29 found a higher conspicuity of pancreatic adenocarcinoma when using low kVp, whereas Ramgren et al30 used highly concentrated CM and low kVp to improve image quality in intracranial CTA.
On the other hand, for non-enhancing or poorly enhancing soft-tissue structures, matching noise may be more appropriate. Since iodine does not provide a higher signal in these cases, dose reduction with low kV is generally limited. Many diagnostic tasks, such as routine contrast-enhanced abdominal CT examinations, fall somewhere between these two scenarios. Image quality indices such as the “noise-constrained iodine CNR” have been proposed to quantify different levels of image quality for different diagnostic tasks.31 AutokV tools (e.g. CarekV; Siemens Healthcare) employ these principles; the strength of the setting “noise vs CNR constraints” for an individual examination is defined by the user (settings from non-enhanced CT to highly iodine-enhanced CT, e.g. in CTA) and the software automatically determines the optimal kVp and adapted mAs based on a fixed-reference mAs.
In summary, the role of iodine in radiation dose reduction using low kVp is fundamental. Iodine attenuation is higher at low kVp, and thus SNR can be kept constant with reduced radiation exposure. The greatest potential of low kVp for reducing exposure is mainly limited to highly iodine-enhancing examinations such as CTA or arterial phase imaging.
AN ADDITIONAL OPTION TO REDUCE RADIATION DOSE WITH IODINE
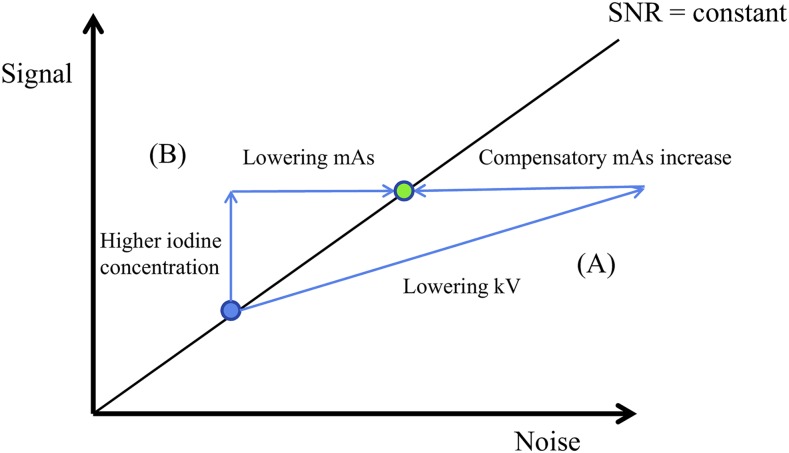
An alternative and supplemental approach to reducing the radiation dose in CT is through increasing the iodine concentration in the target structures to be examined. Based on a similar SNR = constant approach to that utilized in low-kV examinations, the use of a higher iodine concentration means that higher noise can be accepted due to the higher iodine signal. The increased noise in this scenario is realized by lowering the mAs which in turn implies reduced radiation dose (Figure 2). To note, however, is that this approach is different from and in addition to the approaches utilized in the automated tube current modulation tools that are provided for CT scanners. These tools can further reduce the overall applied radiation dose by modifying a reference mAs over scanning time and space32 while the “high iodine concentration—low mAs” concept allows an overall lowering of the reference mAs.
Figure 2.
Two options to reduce the radiation dose while keeping a constant signal-to-noise ratio (SNR). (A) Low-tube-voltage approach: lowering kV increases iodine signal but at the same time increases noise; a compensatory increase in mAs is needed to achieve the same SNR (green dot); (B) starting from the same standard examination (standard kV, blue dot), increasing the local iodine concentration provides higher signal and allows for a reduction of the mAs to match the SNR at the identical level.
Despite the fact that this concept seems simple and straightforward, awareness of and experience with this approach to radiation dose reduction is somewhat limited. Nevertheless, several studies have demonstrated its validity. Szucs-Farkas et al33 demonstrated its potential in chest phantom studies, noting that a similar CNR to that achieved with a 300-mg-iodine/ml CM concentration could be achieved with a 400-mg-iodine/ml CM concentration but at 18–40% lower CTDIvol depending on the phantom size and applied kVp.
In clinical studies, this benefit has been demonstrated for CTA of the aorta, the pulmonary arteries and myocardial perfusion CT.34–38 One advantage is that the limitations associated with low-kVp examinations do not apply to this approach. Thus, large body habitus, for which a low-kVp protocol is typically not a feasible approach to reduce radiation dose (Figure 1), is generally not a limitation for the high iodine concentration–low mAs concept. Furthermore, low kV is a limitation in some other applications since the maximum tube output (mAs) is technically limited at lower kVp. The required compensatory mAs increase for low kV may thus not always be feasible. This is especially the case at high helical pitch. Therefore, when a fast scanning speed and a short scan time are desired, a low tube potential may not be appropriate, even for small patients.26 The high iodine concentration–low mAs approach, however, would again not be a limitation in these cases.
The low kV and “low mAs–high iodine concentration” options can be combined to maximize the radiation dose reduction potential. Iezzi et al34 showed that the radiation dose could be reduced by as much as 74% when reducing kVp from 120 to 80, at the same time increasing the iodine signal by using 400 mg iodine/ml concentration instead of 300 mg iodine/ml. It can also be combined with automatic kVp selection tools. In this case, the reference mAs should be set at a lower level when using a contrast injection protocol that provides higher signal. Schwarz et al35 demonstrated that autokV tools can work more effectively in choosing the lower kVp option more frequently when high iodine concentration (IC)/low mAs is used.
Limitations of both the low-kV and low-mAs/high-iodine-signal approaches—especially when both approaches are combined—are that streaking and dark shadow artefacts are potentially more prevalent in high-signal structures.26 On the other hand, broadening the window width can compensate for image noise and thus further improve image quality at high-contrast examinations.39,40
THE IMPORTANCE OF IODINE DELIVERY RATE
The “low-mAs/high-iodine-signal” approach requires a higher iodine concentration in the relevant body structures to be examined. Two injection parameters influence iodine enhancement in CT: the total iodine dose (D) and the iodine delivery rate (IDR).41–43
| (3) |
| (4) |
Dose D is the relevant parameter that determines maximum enhancement in venous phase examinations while the IDR crucially influences the maximum enhancement in first pass examinations such as CTA, arterial phase imaging and perfusion CT.41–43 It also has benefits in parenchymal phases although to a lesser degree.44 Since first pass examinations are precisely the highly iodine-enhancing examinations in which the potential for radiation dose reduction is the greatest, when designing optimum contrast injection protocols for radiation dose reduction, it is the IDR that is the most crucial parameter. This has been borne out by several studies that have utilized optimized contrast injection protocols using high flow rates and high iodine concentrations to increase IDR in the context of radiation dose reduction.34–38 Crucially, it is important to bear in mind that such protocols do not imply the injection of more iodine but merely that a faster IDR is needed.43
On the other hand, an increase in iodine dose is potentially an option for radiation dose reduction in venous phase examinations, although in these low-iodine-enhancing examinations, the potential to reduce radiation dose using the SNR = constant approach (either by lowering kVp or by high IC/low mAs) is more limited. Nevertheless, lower radiation exposure and improved image quality using the “high IC/low mAs” concept has been demonstrated for portal-venous phase abdominal CT.45
CONTRIBUTION OF ITERATIVE RECONSTRUCTION
IR is a technique for reducing radiation exposure that utilizes an alternative image reconstruction algorithm to filtered back projection to reduce noise without impairing signal.46–51 The combination of low kVp and IR is now an established approach in modern CT. Importantly, IR can be combined with the high IC/low mAs approach and other techniques to further reduce radiation dose. In principle, IR has three potential benefits (Figure 3).
Figure 3.
Three options when combining iterative reconstruction (IR) and low kVp. (A) Increased image quality due to a higher signal-to-noise ratio (SNR) (all blue arrows, end at blue dot). (B) Additional reduction in radiation dose by lowering mAs (green arrow to green dot, to match SNR of that obtained at low kV if no IR was used). (C) Contrast dose reduction to obtain signal, image noise and SNR as before (red arrow to red dot; identical situation compared with standard).
Improved image quality
Without any modification, the higher SNR can be used to improve image quality and the visualization of small enhancing structures (e.g. small vessels in CTA or arterially enhancing lesions) (Figure 3a). Studies have shown that low kVp in the absence of IR can lead to an increase in image quality.29,30 Clearly, if IR were to be utilized, a further reduction of noise and thus an increase in SNR would be possible, resulting in increased image quality.
Lowered radiation exposure
The mAs can be further reduced to obtain a SNR that is similar to that which would otherwise have been obtained had low kV been used without IR (Figure 3b), thus IR is used to additionally reduce radiation dose, giving SNR values that are similar to the above-mentioned “low kV alone” and low-mAs/high-iodine-signal approaches (Figure 2). This has been demonstrated in several studies.52–55
Reduced iodine dose
The third option is to reduce the contrast dose to obtain the same signal S, the same noise N and the same SNR as with the standard kV and without IR (Figure 3c). Numerous studies addressing low contrast volume and low iodine concentration suggest that this approach is widely used.16–22,56–73
Of note, these three options can be combined to give a low kV + IR CT protocol that provides a lower radiation dose but which keeps the SNR higher than that of standard protocols, thereby combining radiation dose reduction with image quality improvement. The limitations of the constant SNR approach in low-iodine-enhancing examinations (e.g. routine venous phase abdominal CT) do not apply in this setting since the noise is also kept low. However, strong kVp reductions may still be susceptible to photon-starvation artefacts, especially in larger patients.
In general, the relative benefits of increased image quality, lower radiation exposure, and contrast dose reduction have to be weighed against each other, especially in first pass examinations where all options are feasible. The decision should depend on the individual clinical situation. Although radiation dose reduction may be more important in younger, healthier patients, the opposite may be true for contrast dose reduction. Safety and tolerability of contrast injections thus become part of the decision-making process.
HOW TO REDUCE CONTRAST DOSE
As evident from Equation (3) above, a reduction of contrast volume and/or a reduction of the iodine concentration will result in a reduced iodine dose. However, although several studies have focused on the potential value of reduced iodine concentration in conjunction with low-kV settings,56–68 numerous factors clearly favour reduced contrast volume rather than lower concentration as a means to reduce the total administered contrast dose.16–23,69–73
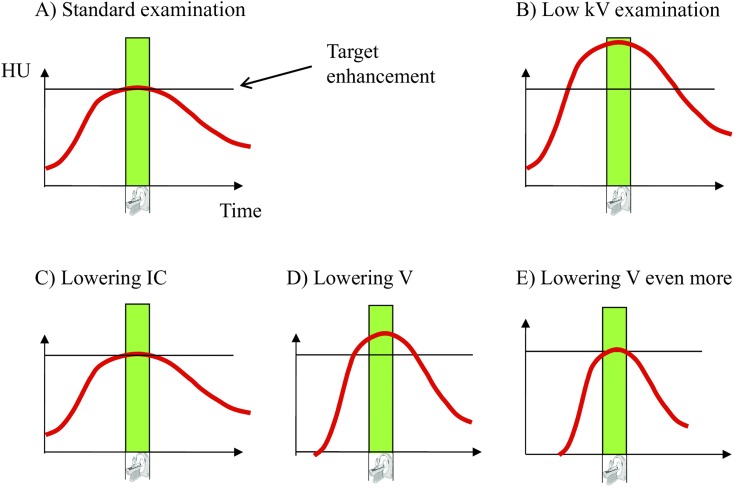
The CT applications with the highest potential for low-kV/low-radiation-dose protocols are CTA and perfusion imaging in which the signal during the first pass of CM is crucially influenced by the IDR. As noted above, the potential for iodine reduction in a low-kVp setting derives from the considerably higher attenuation of iodine at low kV (Figure 4a,b). However, a drawback of reducing the iodine concentration for these applications is that the IDR would also be reduced for a similar given injection rate. Although this would result in a lower CM dose, both the contrast bolus shape and maximum enhancement would remain essentially unchanged (Figure 4c), meaning that the opportunity for further contrast (or radiation) dose reduction is lost. A benefit of maintaining a high iodine concentration and reducing only the volume administered for the same injection rate is that the IDR remains high resulting in greater enhancement (Figure 4d) and potentially improved image quality. An additional benefit is that the contrast and/or radiation dose can be reduced still further (i.e. by further reductions in administered CM volume or mAs, respectively).
Figure 4.
Illustration of contrast dose reduction in first pass CT examinations. (a) Contrast enhancement over time compared with acquisition time (green bar) in a standard setting. (b) Use of low kV increases iodine enhancement when the same injection protocol is used. (c) Iodine concentration (IC) is reduced to obtain the same enhancement level as in the standard examination. (d) When volume V is reduced instead of IC, a sharper contrast bolus will be injected (due to higher IDR); with the same amount of contrast dose reduction compared with (c) (in grams of iodine), a higher enhancement level is obtained. (e) Thus, more contrast dose reduction is feasible when lowering volume V instead of IC when targeting the same enhancement level. HU, Hounsfield unit.
To note is that the injection duration is shorter when the volume administered is reduced. For most first pass examinations using newer scanner technologies, this is an additional benefit because the bolus duration should mimic the scanning duration.73 An unnecessarily long injection leads to a waste of CM41 because the CM administered after the acquisition of data does not contribute to data acquisition. Unfortunately, this principle is often overlooked in clinical practice. Multidetector CT has dramatically short image acquisition times, typically of just a few seconds.74,75 Therefore, the necessarily higher volumes associated with administration of CM containing lower concentrations of iodine are increasingly inappropriate. The use of low iodine concentration to reduce iodine dose does not utilize the potential for contrast injection optimization (Figure 4a,c), whereas the use of high iodine concentration and lower volume more practically permits the optimization of injection protocols (Figure 4d,e).
First pass examinations which cover large body areas such as peripheral CTA not only require that the width of the contrast bolus matches the acquisition over time but also that it matches over space. Otherwise, the bolus may be overridden if the acquisition time is too fast or venous overlay may occur if the scan is too slow.75 Since the individual variation in bolus “transportation” can be considerable and may not be known a priori, a relatively slow table speed and broadening of the contrast bolus is required. In such situations, the above-mentioned option to optimize the bolus geometry is limited.
SAFETY AND TOLERABILITY ASPECTS OF IODINATED CONTRAST ADMINISTRATION IN CT
For all CT examinations, a reduction of the iodine concentration generally means that the injected volume and flow rate will be higher than if a higher iodine concentration is used at the same contrast dose and IDR. This touches on issues related to tolerability and safety.
Apart from serious allergoid or anaphylactoid reactions, which can occur after administration of only small amounts of any CM,76 the most relevant side effect of iodinated CM which possibly has a correlation with dose is contrast-induced nephropathy,77 more recently referred to as contrast-induced acute kidney injury. Although the existence of contrast-induced nephropathy/contrast-induced acute kidney injury in contrast-enhanced CT is currently a matter of debate,78–84 patients with renal impairment or an elevated risk of renal impairment are nevertheless still likely to benefit most from a reduction of contrast dose.77
Apart from iodine dose, also injection volume and flow rate can influence safety and tolerability. Although maintaining a high flow rate is beneficial in terms of IDR and the potential for radiation and/or CM dose reduction, for many patients, particularly elderly patients or patients with poor venous condition, a high flow rate may be difficult to achieve, inappropriate or potentially harmful. In such cases, administration of a higher concentration CM at a reduced flow rate is potentially advantageous in reducing intolerability while maintaining a sufficiently high IDR.43 Likewise, a reduced total volume of administered CM is beneficial in terms of lowered cardiac preload, a factor that may be especially crucial in critically ill patients.
It has been stated that high IC and newer scanner generations are mutually compatible and that sporadic failure would be unpreventable if using low IC due to the fact that, in most cases, a patient's cardiac output is not known prior to scan initiation.84 Studies have confirmed this suggestion. A study comparing low- and high-concentration CM at identical flow rate (i.e. resulting in a higher IDR with the high iodine concentration) found no differences in the rate of adverse events.85 Conversely, at identical IDR, Mühlenbruch86 found a significantly higher rate of general patient discomfort when using a lower iodine concentration. Specifically, significantly greater patient discomfort was noted with a CM containing 300 mgI/ml than with CM containing 370 or 400 mgI/ml despite relatively moderate injection rates.
The possibility that high flow rate is associated with greater patient discomfort was reinforced by Andreini et al87 who demonstrated increased heat sensation, higher post-examination heart rate and an increased number of premature heartbeats for an identical volume (80 ml) of iodixanol-320 injected at 6.2 ml s−1 compared with 5.0 ml s−1. Again, these findings suggest that the possibility to lower the injection rate while maintaining a sufficiently high IDR for diagnosis is a potential advantage for high-concentration CM over low-concentration CM in terms of patient tolerability.
A potentially confounding factor is that solution viscosity increases when the iodine concentration is increased. However, there is no evidence that a higher viscosity of the contrast agent formulation reduces tolerability or safety.86,87 On the other hand, high viscosity can be a technical challenge since the required injection pressure increases with viscosity. With the highest iodine concentration (400 mg I/ml) flow rates of up to 6 ml s−1 are feasible using commercially available injectors.88 Of note, administration of CM containing 300 mg I/ml does not deliver a higher maximum IDR since 8 ml s−1 was the maximum injection rate achievable with this lower iodine concentration giving the same maximum IDR of 2.4 gI/s. Warming the CM reduces viscosity, and therefore allows for higher injection rates. Clinical observation suggests that patients are often more comfortable if the CM is warmed before administration.89
More relevant than CM formulation viscosity is the viscosity of the iodinated molecules themselves, and it is important not to confuse the two. Contrast agents formulated at high iodine concentrations have, by definition, minimally viscous molecules because it is their low viscosity that facilitates the preparation of more highly concentrated formulations.90 Conversely, the more viscous molecules (e.g. molecular dimers such as iodixanol which have the highest viscosity of all non-ionic products) cannot be formulated at high concentrations. Any considerations or concerns regarding the viscosity of the ingredients should therefore not impact the choice of the iodine concentration but rather the choice of the contrast agent product itself.
CONCLUSION
The role of iodine is fundamental in low-radiation-dose CT. The higher iodine attenuation obtained at low-kVp settings allows for a reduction in radiation exposure while maintaining SNR. Similarly, high-iodine-signal protocols may permit radiation dose reduction by permitting a lowering of mAs. This is particularly feasible in first pass examinations where high-iodine signal can be achieved by injecting iodine more rapidly. The combination of recent technical developments, principally relating to low kV and IR, permits further reductions of radiation exposure, increases diagnostic image quality and/or allows for a reduction of the iodine dose. In an optimum contrast injection protocol, the volume of CM administered rather than the iodine concentration should be reduced, as with high-iodine-concentration CM further reductions of iodine dose are achievable for modern first pass examinations. Moreover, when necessary (e.g. in elderly patients), higher concentration CM more readily permit a reduction of flow rate and volume, thereby improving the tolerability of contrast administration.
Contributor Information
Andrik J Aschoff, Email: Andrik.Aschoff@klinikum-kempten.de.
Carlo Catalano, Email: carlo.catalano@uniroma1.it.
Miles A Kirchin, Email: miles.kirchin@bracco.com.
Martin Krix, Email: martin.krix@bracco.com.
Thomas Albrecht, Email: Thomas.Albrecht@vivantes.de.
REFERENCES
- 1.Lee TY, Chhem RK. Impact of new technologies on dose reduction in CT. Eur J Radiol 2010; 76: 28–35. doi: https://doi.org/10.1016/j.ejrad.2010.06.036 [DOI] [PubMed] [Google Scholar]
- 2.National Research Council. Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 3.Sodickson A, Baeyens PF, Andriole KP. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology 2009; 251: 175–84. doi: https://doi.org/10.1148/radiol.2511081296 [DOI] [PubMed] [Google Scholar]
- 4.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med 2007; 357: 2277–84. doi: https://doi.org/10.1056/nejmra072149 [DOI] [PubMed] [Google Scholar]
- 5.Griffey RT, Sodickson A. Cumulative radiation exposure and cancer risk estimates in emergency department patients undergoing repeat or multiple CT. AJR Am J Roentgenol 2009; 192: 887–92. doi: https://doi.org/10.2214/ajr.08.1351 [DOI] [PubMed] [Google Scholar]
- 6.Picano E. Risk of cancer from diagnostic X-rays. Lancet 2004; 363: 1909–10. doi: https://doi.org/10.1016/S0140-6736(04)16373-3 [DOI] [PubMed] [Google Scholar]
- 7.Berrington de González A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet 2004; 363: 345–51. doi: https://doi.org/10.1016/s0140-6736(04)15433-0 [DOI] [PubMed] [Google Scholar]
- 8.Fazel R, Krumholz HM, Wang Y, Ross JS, Chen J, Ting HH, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med 2009; 361: 849–57. doi: https://doi.org/10.1056/nejmoa0901249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Einstein AJ, Fazel R, Krumholz HM, Wang Y, Ross JS, et al. Cumulative exposure to ionizing radiation from diagnostic and therapeutic cardiac imaging procedures: a population-based analysis. J Am Coll Cardiol 2010; 56: 702–11. doi: https://doi.org/10.1016/j.jacc.2010.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorfman AL, Fazel R, Einstein AJ. Use of medical imaging procedures with ionizing radiation in children: a population-based study. Arch Pediatr Adolesc Med 2011; 165: 458–64. doi: https://doi.org/10.1001/archpediatrics.2010.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meer AB, Basu PA, Baker LC, Atlas SW. Exposure to ionizing radiation and estimate of secondary cancers in the era of high-speed CT scanning: projections from the Medicare population. J Am Coll Radiol 2012; 9: 245–50. doi: https://doi.org/10.1016/j.jacr.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 12. Medical Radiation Exposure of the European Population. [Accessed 25 January 2017]. Available from: https://ec.europa.eu/energy/sites/ener/files/documents/RP180.pdf.
- 13.Sodhi KS, Krishna S, Saxena AK, Sinha A, Khandelwal N, Lee EY. Clinical application of “Justification” and “Optimization” principle of ALARA in pediatric CT imaging: how many children can be protected from unnecessary radiation? Eur J Radiol 2015; 84: 1752–7. doi: https://doi.org/10.1016/j.ejrad.2015.05.030 [DOI] [PubMed] [Google Scholar]
- 14.McCollough CH, Primak AN, Braun N, Kofler J, Yu L, Christner J. Strategies for reducing radiation dose in CT. Radiol Clin North Am 2009; 47: 27–40. doi: https://doi.org/10.1016/j.rcl.2008.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waaijer A, Prokop M, Velthuis BK, Bakker CJ, de Kort GA, van Leeuwen MS. Circle of Willis at CT angiography: dose reduction and image quality—reducing tube voltage and increasing tube current settings. Radiology 2007; 242: 832–9. doi: https://doi.org/10.1148/radiol.2423051191 [DOI] [PubMed] [Google Scholar]
- 16.Hunsaker AR, Oliva IB, Cai T. Contrast opacification using a reduced volume of iodinated contrast material and low peak kilovoltage in pulmonary CT angiography: objective and subjective evaluation. AJR Am J Roentgenol 2010; 195: W118–24. doi: https://doi.org/10.2214/ajr.09.3342 [DOI] [PubMed] [Google Scholar]
- 17.Szucs-Farkas Z, Christe A, Megyeri B, Rohacek M, Vock P, Nagy EV, et al. Diagnostic accuracy of computed tomography pulmonary angiography with reduced radiation and contrast material dose: a prospective randomized clinical trial. Invest Radiol 2014; 49: 201–8. [DOI] [PubMed] [Google Scholar]
- 18.Cho ES, Chung TS, Ahn SJ, Chong K, Baek JH, Suh SH. Cerebral computed tomography angiography using a 70 kVp protocol: improved vascular enhancement with a reduced volume of contrast medium and radiation dose. Eur Radiol 2015; 25: 1421–30. doi: https://doi.org/10.1007/s00330-014-3540-z [DOI] [PubMed] [Google Scholar]
- 19.Oda S, Utsunomiya D, Awai K, Takaoka H, Nakaura T, Katahira K, et al. Indirect computed tomography venography with a low-tube-voltage technique: reduction in the radiation and contrast material dose—a prospective randomized study. J Comput Assist Tomogr 2011; 35: 631–6. doi: https://doi.org/10.1097/rct.0b013e31822a563d [DOI] [PubMed] [Google Scholar]
- 20.Nakaura T, Nakamura S, Maruyama N. Low contrast agent and radiation dose protocol for hepatic dynamic CT of thin adults at 256-detector row CT: effect of low tube voltage and hybrid iterative reconstruction algorithm on image quality. Radiology 2012; 264: 445–54. doi: https://doi.org/10.1148/radiol.12111082 [DOI] [PubMed] [Google Scholar]
- 21.Luo S, Zhang LJ, Meinel FG, Zhou CS, Qi L, McQuiston AD, et al. Low tube voltage and low contrast material volume cerebral CT angiography. Eur Radiol 2014; 24: 1677–85. doi: https://doi.org/10.1007/s00330-014-3184-z [DOI] [PubMed] [Google Scholar]
- 22.Noda Y, Kanematsu M, Goshima S, Kondo H, Watanabe H, Kawada H, et al. Reduction of iodine load in CT imaging of pancreas acquired with low tube voltage and an adaptive statistical iterative reconstruction technique. J Comput Assist Tomogr 2014; 38: 714–20. doi: https://doi.org/10.1097/rct.0000000000000106 [DOI] [PubMed] [Google Scholar]
- 23.Chadwick KH, Leenhouts HP. Risks from ionising radiation. In: Radiation dose from multidetector CT. Tack D, Kalra MK, Gevenois PA, eds. 2nd edn. Berlin, Germany: Springer; 2012. [Google Scholar]
- 24.Edyvean S, Lewis M, Britten A. Radiation dose metrics and the effect of CT scan protocol parameters. In: Radiation dose from multidetector CT. Tack D, Kalra MK, Gevenois PA, eds. 2nd edn. Berlin, Germany: Springer; 2012. [Google Scholar]
- 25.Niemann T, Henry S, Faivre JB. Clinical evaluation of automatic tube voltage selection in chest CT angiography. Eur Radiol 2013; 23: 2643–51. doi: https://doi.org/10.1007/s00330-013-2887-x [DOI] [PubMed] [Google Scholar]
- 26.Yu L. Optimization of tube potential. In: Radiation dose from multidetector CT. Tack D, Kalra MK, Gevenois PA, eds. 2nd edn. Berlin, Germany: Springer; 2012. [Google Scholar]
- 27.Guimarães LS, Fletcher JG, Harmsen WS, Yu L, Siddiki H, Melton Z, et al. Appropriate patient selection at abdominal dual-energy CT using 80 kV: relationship between patient size, image noise, and image quality. Radiology 2010; 257: 732–42. doi: https://doi.org/10.1148/radiol.10092016 [DOI] [PubMed] [Google Scholar]
- 28.Winklehner A, Goetti R, Baumueller S, Karlo C, Schmidt B, Raupach R, et al. Automated attenuation-based tube potential selection for thoracoabdominal computed tomography angiography: improved dose effectiveness. Invest Radiol 2011; 46: 767–73. doi: https://doi.org/10.1097/rli.0b013e3182266448 [DOI] [PubMed] [Google Scholar]
- 29.Zamboni GA, Ambrosetti MC, Guariglia S, Cavedon C, Pozzi Mucelli R. Single-energy low-voltage arterial phase MDCT scanning increases conspicuity of adenocarcinoma of the pancreas. Eur J Radiol 2014; 83: e113–17. doi: https://doi.org/10.1016/j.ejrad.2013.12.022 [DOI] [PubMed] [Google Scholar]
- 30.Ramgren B, Björkman-Burtscher IM, Holtås S, Siemund R. CT angiography of intracranial arterial vessels: impact of tube voltage and contrast media concentration on image quality. Acta Radiol 2012; 53: 929–34. doi: https://doi.org/10.1258/ar.2012.120218 [DOI] [PubMed] [Google Scholar]
- 31.Yu L, Li H, Fletcher JG, McCollough CH. Automatic selection of tube potential for radiation dose reduction in CT: a general strategy. Med Phys 2010; 37: 234–43. doi: https://doi.org/10.1118/1.3264614 [DOI] [PubMed] [Google Scholar]
- 32.Raman SP, Johnson PT, Deshmukh S. CT dose reduction applications: available tools on the latest generation of CT scanners. J Am Coll Radiol 2013; 10: 37–41. doi: https://doi.org/10.1016/j.jacr.2012.06.025 [DOI] [PubMed] [Google Scholar]
- 33.Szucs-Farkas Z, Verdun FR, von Allmen G, Mini RL, Vock P. Effect of X-ray tube parameters, iodine concentration, and patient size on image quality in pulmonary computed tomography angiography: a chest-phantom-study. Invest Radiol 2008; 43: 374–81. [DOI] [PubMed] [Google Scholar]
- 34.Iezzi R, Santoro M, Marano R, Di Stasi C, Dattesi R, Kirchin M, et al. Low-dose multidetector CT angiography in the evaluation of infrarenal aorta and peripheral arterial occlusive disease. Radiology 2012; 263: 287–98. doi: https://doi.org/10.1148/radiol.11110700 [DOI] [PubMed] [Google Scholar]
- 35.Schwarz F, Grandl K, Arnoldi A, Kirchin MA, Bamberg F, Reiser MF, et al. Lowering radiation exposure in CT angiography using automated tube potential selection and optimized iodine delivery rate. AJR Am J Roentgenol 2013; 200: W628–34. doi: https://doi.org/10.2214/ajr.12.9635 [DOI] [PubMed] [Google Scholar]
- 36.Heusch P, Lanzman RS, Aissa J. Evaluation of a high iodine delivery rate in combination with low tube current for dose reduction in pulmonary computed tomography angiography. J Thorac Imaging 2014; 29: 293–7. doi: https://doi.org/10.1097/rti.0000000000000099 [DOI] [PubMed] [Google Scholar]
- 37.Kröpil P, Lanzman RS, Walther C. Dose reduction and image quality in MDCT of the upper abdomen: potential of an adaptive post-processing filter. Rofo 2010; 182: 248–53. doi: https://doi.org/10.1055/s-0028-1109835 [DOI] [PubMed] [Google Scholar]
- 38.Kim SM, Cho YK, Choe YH. Adenosine-stress dynamic myocardial perfusion imaging using 128-slice dual-source CT in patients with normal body mass indices: effect of tube voltage, tube current, and iodine concentration on image quality and radiation dose. Int J Cardiovasc Imaging 2014; 2(Suppl. 30): 95–103. [DOI] [PubMed] [Google Scholar]
- 39.Singh S, Kalra MK. Scan parameters and CT radiation dose. In: Radiation dose from multidetector CT. Tack D, Kalra MK, Gevenois PA, eds. 2nd edn. Berlin, Germany: Springer; 2012. [Google Scholar]
- 40.Husstedt H, Prokop M, Becker H. Window width as a dosage-relevant factor in high-contrast structures in CT. Rofo 1998; 168: 139–43. doi: https://doi.org/10.1055/s-2007-1015198 [DOI] [PubMed] [Google Scholar]
- 41.Bae KT. Intavenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010; 256: 32–61. doi: https://doi.org/10.1148/radiol.10090908 [DOI] [PubMed] [Google Scholar]
- 42.Fleischmann D. CT angiography: injection and acquisition technique. Radiol Clin North Am 2010; 48: 237–47. doi: https://doi.org/10.1016/j.rcl.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 43.Faggioni L, Gabelloni M. Iodine concentration and optimization in computed tomography angiography: current issues. Invest Radiol 2016; 51: 816–22. doi: https://doi.org/10.1097/rli.0000000000000283 [DOI] [PubMed] [Google Scholar]
- 44.Fenchel S, Fleiter TR, Aschoff AJ, van Gessel R, Brambs HJ, Merkle EM. Effect of iodine concentration of contrast media on contrast enhancement in multislice CT of the pancreas. Br J Radiol 2004; 77: 821–30. doi: https://doi.org/10.1259/bjr/19527646 [DOI] [PubMed] [Google Scholar]
- 45.Watanabe H, Kanematsu M, Miyoshi T, Goshima S, Kondo H, Moriyama N, et al. Improvement of image quality of low radiation dose abdominal CT by increasing contrast enhancement. AJR Am J Roentgenol 2010; 195: 986–92. doi: https://doi.org/10.2214/ajr.10.4456 [DOI] [PubMed] [Google Scholar]
- 46.Winklehner A, Karlo C, Puippe G, Schmidt B, Flohr T, Goetti R, et al. Raw data-based iterative reconstruction in body CTA: evaluation of radiation dose saving potential. Eur Radiol 2011; 21: 2521–6. doi: https://doi.org/10.1007/s00330-011-2227-y [DOI] [PubMed] [Google Scholar]
- 47.Den Harder AM, Willemink MJ, De Ruiter QM. Dose reduction with iterative reconstruction for coronary CT angiography: a systematic review and meta-analysis. Br J Radiol 2016; 89: 20150068. doi: https://doi.org/10.1259/bjr.20150068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scholtz JE, Wichmann JL, Hüsers K, Albrecht MH, Beeres M, Bauer RW, et al. Third-generation dual-source CT of the neck using automated tube voltage adaptation in combination with advanced modeled iterative reconstruction: evaluation of image quality and radiation dose. Eur Radiol 2016; 26: 2623. doi: https://doi.org/10.1007/s00330-015-4099-z [DOI] [PubMed] [Google Scholar]
- 49.Kaul D, Kahn J, Huizing L, Wiener E, Grupp U, Böning G, et al. Reducing radiation dose in adult head CT using iterative reconstruction—a clinical study in 177 patients. Rofo 2016; 188: 155–62. [DOI] [PubMed] [Google Scholar]
- 50.Bodelle B, Isler S, Scholtz JE. Benefits of sinogram-affirmed iterative reconstruction in 0.4 mSv ultra-low-dose CT of the upper abdomen following transarterial chemoembolisation: comparison to low-dose and standard-dose CT and filtered back projection technique. Clin Radiol 2016; 71: e11–15. doi: https://doi.org/10.1016/j.crad.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 51.Vaishnav JY, Jung WC, Popescu LM, Zeng R, Myers KJ. Objective assessment of image quality and dose reduction in CT iterative reconstruction. Med Phys 2014; 41: 071904. doi: https://doi.org/10.1118/1.4881148 [DOI] [PubMed] [Google Scholar]
- 52.den Harder AM, Willemink MJ, de Ruiter QM, Schilham AM, Krestin GP, Leiner T, et al. Achievable dose reduction using iterative reconstruction for chest computed tomography: a systematic review. Eur J Radiol 2015; 84: 2307–13. doi: https://doi.org/10.1016/j.ejrad.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 53.Patino M, Fuentes JM, Singh S, Hahn PF, Sahani DV. Iterative reconstruction techniques in abdominopelvic CT: technical concepts and clinical implementation. AJR Am J Roentgenol 2015; 205: W19–31. doi: https://doi.org/10.2214/ajr.14.13402 [DOI] [PubMed] [Google Scholar]
- 54.Naoum C, Blanke P, Leipsic J. Iterative reconstruction in cardiac CT. J Cardiovasc Comput Tomogr 2015; 9: 255–63. doi: https://doi.org/10.1016/j.jcct.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 55.Cademartiri F, Mollet NR, Runza G. Influence of intracoronary attenuation on coronary plaque measurements using multislice computed tomography: observations in an ex vivo model of coronary computed tomography angiography. Eur Radiol 2005; 15: 1426–31. doi: https://doi.org/10.1007/s00330-005-2697-x [DOI] [PubMed] [Google Scholar]
- 56.Wichmann JL, Hu X, Kerl JM. 70 kVp computed tomography pulmonary angiography: potential for reduction of iodine load and radiation dose. J Thorac Imaging 2015; 30: 69–76. doi: https://doi.org/10.1097/rti.0000000000000124 [DOI] [PubMed] [Google Scholar]
- 57.Sun G, Hou YB, Zhang B. Application of low tube voltage coronary CT angiography with low-dose iodine contrast agent in patients with a BMI of 26–30 kg/m2. Clin Radiol 2015; 70: 138–45. doi: https://doi.org/10.1016/j.crad.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 58.Yuan R, Shuman WP, Earls JP, Hague CJ, Mumtaz HA, Scott-Moncrieff A, et al. Reduced iodine load at CT pulmonary angiography with dual-energy monochromatic imaging: comparison with standard CT pulmonary angiography—a prospective randomized trial. Radiology 2012; 262: 290–7. doi: https://doi.org/10.1148/radiol.11110648 [DOI] [PubMed] [Google Scholar]
- 59.Kanematsu M, Goshima S, Miyoshi T, Kondo H, Watanabe H, Noda Y, et al. Whole-body CT angiography with low tube voltage and low-concentration contrast material to reduce radiation dose and iodine load. AJR Am J Roentgenol 2014; 202: W106–16. doi: https://doi.org/10.2214/ajr.13.10720 [DOI] [PubMed] [Google Scholar]
- 60.Cho ES, Chung TS, Oh DK. Cerebral computed tomography angiography using a low tube voltage (80 kVp) and a moderate concentration of iodine contrast material: a quantitative and qualitative comparison with conventional computed tomography angiography. Invest Radiol 2012; 47: 142–7. doi: https://doi.org/10.1097/rli.0b013e31823076a4 [DOI] [PubMed] [Google Scholar]
- 61.Cho ES, Yu JS, Ahn JH. CT angiography of the renal arteries: comparison of lower-tube-voltage CTA with moderate-concentration iodinated contrast material and conventional CTA. AJR Am J Roentgenol 2012; 199: 96–102. doi: https://doi.org/10.2214/ajr.11.7450 [DOI] [PubMed] [Google Scholar]
- 62.Zheng M, Liu Y, Wei M, Wu Y, Zhao H, Li J. Low concentration contrast medium for dual-source computed tomography coronary angiography by a combination of iterative reconstruction and low-tube-voltage technique: feasibility study. Eur J Radiol 2014; 83: e92–9. doi: https://doi.org/10.1016/j.ejrad.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 63.Zhang WL, Li M, Zhang B, Geng HY, Liang YQ, Xu K, et al. CT angiography of the head-and-neck vessels acquired with low tube voltage, low iodine, and iterative image reconstruction: clinical evaluation of radiation dose and image quality. PLoS One 2013; 8: e81486. doi: https://doi.org/10.1371/journal.pone.0081486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng M, Wu Y, Wei M, Liu Y, Zhao H, Li J. Low-concentration contrast medium for 128-slice dual-source CT coronary angiography at a very low radiation dose using prospectively ECG-triggered high-pitch spiral acquisition. Acad Radiol 2015; 22: 195–202. doi: https://doi.org/10.1016/j.acra.2014.07.025 [DOI] [PubMed] [Google Scholar]
- 65.Yin WH, Lu B, Gao JB, Li PL, Sun K, Wu ZF, et al. Effect of reduced X-ray tube voltage, low iodine concentration contrast medium, and sinogram-affirmed iterative reconstruction on image quality and radiation dose at coronary CT angiography: results of the prospective multicenter REALISE trial. J Cardiovasc Comput Tomogr 2015; 9: 215–24. doi: https://doi.org/10.1016/j.jcct.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 66.Shin HJ, Kim SS, Lee JH, Park JH, Jeong JO, Jin SA, et al. Feasibility of low-concentration iodinated contrast medium with lower-tube-voltage dual-source CT aortography using iterative reconstruction: comparison with automatic exposure control CT aortography. Int J Cardiovasc Imaging 2016; 32: 53–61. doi: https://doi.org/10.1007/s10554-015-0816-6 [DOI] [PubMed] [Google Scholar]
- 67.Kanematsu M, Goshima S, Kawai N. Low-iodine-load and low-tube-voltage CT angiographic imaging of the kidney by using bolus tracking with saline flushing. Radiology 2015; 275: 832–40. doi: https://doi.org/10.1148/radiol.14141457 [DOI] [PubMed] [Google Scholar]
- 68.Lu GM, Luo S, Meinel FG, McQuiston AD, Zhou CS, Kong X, et al. High-pitch computed tomography pulmonary angiography with iterative reconstruction at 80 kVp and 20 mL contrast agent volume. Eur Radiol 2014; 24: 3260–8. doi: https://doi.org/10.1007/s00330-014-3365-9 [DOI] [PubMed] [Google Scholar]
- 69.Viteri-Ramírez G, García-Lallana A, Simón-Yarza I. Low radiation and low-contrast dose pulmonary CT angiography: comparison of 80 kVp/60 ml and 100 kVp/80 ml protocols. Clin Radiol 2012; 67: 833–9. [DOI] [PubMed] [Google Scholar]
- 70.Xia W, Wu JT, Yin XR, Wang ZJ, Wu HT. CT angiography of the neck: value of contrast medium dose reduction with low tube voltage and high tube current in a 64-detector row CT. Clin Radiol 2014; 69: e183–9. doi: https://doi.org/10.1016/j.crad.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 71.Kalva SP, Sahani DV, Hahn PF, Saini S. Using the K-edge to improve contrast conspicuity and to lower radiation dose with a 16-MDCT: a phantom and human study. J Comput Assist Tomogr 2006; 30: 391–7. doi: https://doi.org/10.1097/00004728-200605000-00008 [DOI] [PubMed] [Google Scholar]
- 72.Kidoh M, Nakaura T, Nakamura S, Namimoto T, Nozaki T, Sakaino N, et al. Contrast material and radiation dose reduction strategy for triple-rule-out cardiac CT angiography: feasibility study of non-ECG-gated low kVp scan of the whole chest following coronary CT angiography. Acta Radiol 2014; 55: 1186–96. doi: https://doi.org/10.1177/0284185113514886 [DOI] [PubMed] [Google Scholar]
- 73.Heiland S, Erb G, Ziegler S, Krix M. Where contrast agent concentration really matters—a comparison of CT and MRI. Invest Radiol 2010; 45: 529–37. doi: https://doi.org/10.1097/rli.0b013e3181ea703d [DOI] [PubMed] [Google Scholar]
- 74.Meyer BC, Klein S, Krix M, Aschoff AJ, Wacker FK, Albrecht T. Comparison of a standard and a high-concentration contrast medium protocol for MDCT angiography of the lower limb arteries. Rofo 2012; 184: 527–34. doi: https://doi.org/10.1055/s-0031-1299412 [DOI] [PubMed] [Google Scholar]
- 75.Fleischmann D, Rubin GD. Quantification of intravenously administered contrast medium transit through the peripheral arteries: implications for CT angiography. Radiology 2005; 236: 1076–82. doi: https://doi.org/10.1148/radiol.2363041392 [DOI] [PubMed] [Google Scholar]
- 76.Clement O, Webb JA. Acute adverse reactions to contrast media: mechanisms and prevention. In: Contrast media. Safety issues and ESUR guidelines. Thomsen HS, Webb JA, eds. 3rd edn. Berlin, Germany: Springer; 2014. [Google Scholar]
- 77.Thomsen HS, Stacul F, Webb JA. Contrast medium-induced nephropathy. In: Contrast media. Safety issues and ESUR guidelines. Thomsen HS, Webb JA, eds. 3rd edn. Berlin, Germany: Springer; 2014. [Google Scholar]
- 78.Newhouse JH, Kho D, Rao QA, Starren J. Frequency of serum creatinine changes in the absence of iodinated contrast material: implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol 2008; 191: 376–82. doi: https://doi.org/10.2214/ajr.07.3280 [DOI] [PubMed] [Google Scholar]
- 79.Katzberg RW, Lamba R. Contrast-induced nephropathy after intravenous administration: fact or fiction? Radiol Clin North Am 2009; 47: 789–800. doi: https://doi.org/10.1016/j.rcl.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 80.Katzberg RW, Newhouse JH. Intravenous contrast medium-induced nephrotoxicity: is the medical risk really as great as we have come to believe? Radiology 2010; 256: 21–8. doi: https://doi.org/10.1148/radiol.10092000 [DOI] [PubMed] [Google Scholar]
- 81.McDonald RJ, McDonald JS, Newhouse JH, Davenport MS. Controversies in contrast material-induced acute kidney injury: closing in on the truth? Radiology 2015; 277: 627–32. doi: https://doi.org/10.1148/radiol.2015151486 [DOI] [PubMed] [Google Scholar]
- 82.Tao SM, Wichmann JL, Schoepf UJ, Fuller SR, Lu GM, Zhang LJ. Contrast-induced nephropathy in CT: incidence, risk factors and strategies for prevention. Eur Radiol 2016; 26: 3310–8. doi: https://doi.org/10.1007/s00330-015-4155-8 [DOI] [PubMed] [Google Scholar]
- 83.Hinson JS, Ehmann MR, Fine DM, Fishman EK, Toerper MF, Rothman RE, et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med 2017; 16: 31388–9. [DOI] [PubMed] [Google Scholar]
- 84.Hofmann MH. Contrast agent application and protocols. In: Multislice CT. Reiser MF, Becker CR, Nikolaou K, Glazer G, eds. 3rd edn. Berlin, Germany: Springer; 2009. [Google Scholar]
- 85.Setty BN, Sahani DV, Ouellette-Piazzo K, Hahn PF, Shepard JA. Comparison of enhancement, image quality, cost, and adverse reactions using 2 different contrast medium concentrations for routine chest CT on 16-slice MDCT. J Comput Assist Tomogr 2006; 30: 818–22. doi: https://doi.org/10.1097/01.rct.0000229999.30897.3b [DOI] [PubMed] [Google Scholar]
- 86.Mühlenbruch G, Behrendt FF, Eddahabi MA, Knackstedt C, Stanzel S, Das M, et al. Which iodine concentration in chest CT? A prospective study in 300 patients. Eur Radiol 2008; 18: 2826–32. doi: https://doi.org/10.1007/s00330-008-1080-0 [DOI] [PubMed] [Google Scholar]
- 87.Andreini D, Pontone G, Mushtaq S, Bartorelli AL, Conte E, Bertella E, et al. Coronary stent evaluation with coronary computed tomographic angiography: comparison between low-osmolar, high-iodine concentration iomeprol-400 and iso-osmolar, lower-iodine concentration iodixanol-320. J Cardiovasc Comput Tomogr 2014; 8: 44–51. doi: https://doi.org/10.1016/j.jcct.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 88.Knollmann F, Schimpf K, Felix R. Iodine delivery rate of different concentrations of iodine-containing contrast agents with rapid injection. Rofo 2004; 176: 880–4. [DOI] [PubMed] [Google Scholar]
- 89.Thomsen HS. Iodine-based contrast medium temperature and adverse reactions. In: Contrast media. Safety issues and ESUR guidelines. Thomsen HS, Webb JA, eds. 3rd edn. Berlin, Germany: Springer; 2014. [Google Scholar]
- 90.Gallotti A, Uggeri F, Favilla A, Cabrini M, de Haën C. The chemistry of iomeprol and physico-chemical properties of its aqueous solutions and pharmaceutical formulations. Eur J Radiol 1994; 1(Suppl. 18): S1–12. [DOI] [PubMed] [Google Scholar]