Abstract
A clear visualization of the operative field is of critical importance in endoscopic surgery. During surgery the endoscope lens can get fouled by body fluids (eg, blood), ground substance, rinsing fluid, bone dust, or smoke plumes, resulting in visual impairment. As a result, surgeons spend part of the procedure on intermittent cleaning of the endoscope lens. Current cleaning methods that rely on manual wiping or a lens irrigation system are still far from ideal, leading to longer procedure times, dirtying of the surgical site, and reduced visual acuity, potentially reducing patient safety. With the goal of finding a solution to these issues, a literature review was conducted to identify and categorize existing techniques capable of achieving optically clean surfaces, and to show which techniques can potentially be implemented in surgical practice. The review found that the most promising method for achieving surface cleanliness consists of a hybrid solution, namely, that of a hydrophilic or hydrophobic coating on the endoscope lens and the use of the existing lens irrigation system.
Keywords: biomedical engineering, flexible endoscopy, gynecologic laparoscopy, interventional endoscopy, neurosurgery, NOTES, SILS, single-site surgery
1. Introduction
Endoscopic vision can be obstructed due to fogging or soiling of the lens. Surgeons spend about 3% of their time during laparoscopic Nissen fundoplications on cleaning the endoscope lens.1 This equals about 6 cleaning events per hour.2 Schoofs and Gossot3 found that soiling of the endoscope lens during thoracoscopic procedures was considered troublesome by 68% of the surgeons. Contamination of the endoscope lens thus may disrupt the flow of the operation.1 Unnecessary disruptions and distractions may result in errors in judgment and technique, potentially causing patient injury.4-7 In addition, disturbances may lengthen the procedure time and may increase treatment costs, essentially decreasing surgical efficiency.8,9
In endoscopic surgery, lens contamination typically consists of body fluids (eg, blood), intercellular gel-like material (ie, ground substance), bone dust, and smoke plumes generated by surgical cautery devices.1 Surgical smoke consists of small (<500 nm) and large (>500 nm) particles.10 Small particles are produced by the nucleation of vapors as they cool, whereas large particles develop due to entrainment of tissue. High concentrations of small particles are most responsible for the deterioration of laparoscopic vision.10
Two commonly used techniques for cleaning the endoscope lens during surgery are manual wiping and the use of a lens irrigation system. The manual wiping method involves rubbing the endoscope lens against a nearby soft organ or, more often, requires a full withdrawal of the endoscope and a subsequent wipe of the lens with a gauze or clean cloth soaked in either a defogging solution or distilled water. Typically, before reinsertion, a final wipe with dry gauze is required to dry the lens. Moreover, due to a temperature difference between patient and operating room, condensation frequently occurs after reinsertion, leading to impaired vision.11-13 Therefore, cleaning the lens using the manual wiping method may require multiple steps.
The second method makes use of a hollow sheath wherein the endoscope is inserted. Examples of such systems are CLEARVISION II and the lens cleaning sheath for ENDOEYE FLEX.14,15 The hollow sheath adds an irrigation nozzle to the distal end of the endoscope that allows for in situ cleaning. The washing liquid, generally saline or distilled water, flows through the irrigation channel down the endoscope lens. This cleaning method often does not clean the lens satisfactorily.9 Moreover, as a result of irrigation, unwanted fluid buildup in the surgical site may eventually impair vision and further influence the surgical workflow. Another observed impairment is the possible accumulation of a bubble or fluid droplet on the endoscope lens after irrigation. In order to prevent this problem, some devices use a special pump that creates a negative pressure to extract remaining liquid after irrigation (eg, KARL STORZ GmbH & Co KG14).
1.2. Goal
The aim of this article is to identify, categorize, and describe techniques that can achieve optical surface cleanliness, and in doing so provide a comprehensive source of references. Through the categorization, the complete solution space is explored, and the existing and potentially relevant emerging cleaning techniques are presented. Thereafter for each of the techniques, the patents found with a potential application to endoscopes are described. Economic aspects of the presented techniques are outside the scope the presented research.
2. Methods
A literature review was conducted in November 2015 using Web of Science. Non-English literature and patents were excluded. A total of 910 results were found using the following search query:
TI=((*clean* OR wash* OR rins* OR *foul*) AND (*eye* OR *lens* OR *glass))
Through this query, titles were searched for any combination of 2 sets of keywords. A second selection by hand based on title, abstract, and the removal of duplicates yielded 88 significant results.
In addition, a patent search in title and abstract was performed using Espacenet during the same time period, yielding 150 results, using the following search query:
((((clean* OR wash*) OR rins*) OR foul*) AND ((eye* OR lens*) OR glass*)) AND scop*
In addition, the keyword “clean*” was used to search in title and abstract in categories confined by endoscope-related patents, respectively, A61B1/0008 and A61B1/00121. Last, 47 patents were selected from these 3 searches combined based on their potential for use during surgery (in situ). Devices that are intended for cleaning endoscopes out of surgery (eg, sterilization systems) were discarded.
3. Categorization
The ACCREx method was implemented, which involves abstraction, categorization, reflection, reformulation, and extension of search results.16 Consequently, an all-inclusive structure was created from the search results that offers insight into known working principles. Moreover, it identifies gaps in literature with respect to (transparent) surface cleaning methods suitable as solutions to the lens cleaning problem.
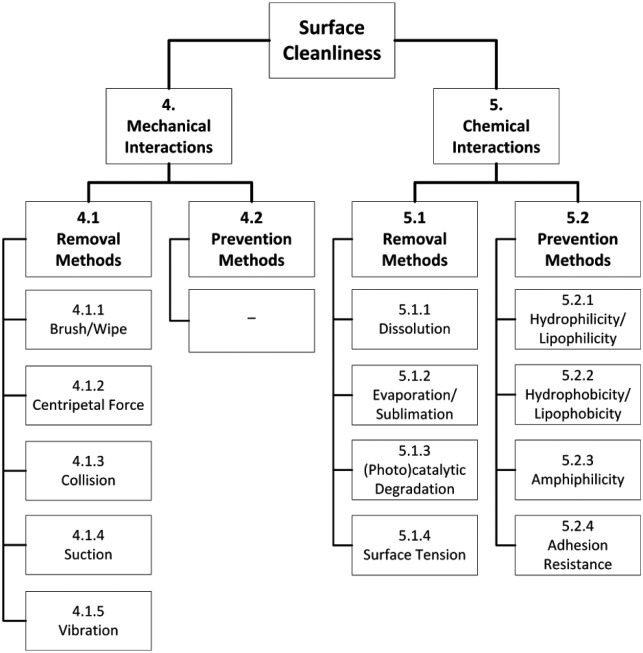
The constructed categorization structure is shown in Figure 1. The first level is Surface Cleanliness, which is lexically defined as “careful to keep or make clean.”17 On a lower level, a distinction between mechanical and chemical interactions is made. Results in Mechanical Interactions rely on forces other than the driving force of chemical reactions to establish surface cleanliness, whereas results in Chemical Interactions rely on chemical reactions or state changes.18 Both are subdivided into removal and prevention methods, where removal methods are techniques that remove settled impurities from a surface and prevention methods block an impurity from reaching and settling on a surface. The abstract methods gathered in this review have been subdivided into 1 of these 4 categories as shown in Figure 1. Mechanical removal methods include Brush/Wipe, Centripetal Force, Collision, Suction, and Vibration. The mechanical prevention methods that were found were thought to be too specific to create general categories. Chemical removal methods include Dissolution, Evaporation/Sublimation, Photo(catalytic) Degradation, and Surface Tension. Last, chemical prevention methods contain Hydrophilicity/Lipo-philicity, Hydrophobicity/Lipophobicity, Amphi philicity, and Adhesion Resistance.
Figure 1.
Schematic showing the ACCREx classification of results. Each box in the first 3 levels represents a category to (further) divide the found methods to achieve surface cleanliness. The lowest level (under Removal and Prevention) is the final categorization of solutions that is sorted in order of discussion and numbered correspondingly to the sections in this article.
Note that it is impossible to create a perfect categorization. Therefore, several solutions that rely on multiple working principles can be and have been placed in more than one category. Moreover, this classification is only used for the purpose of clarity, that is, to gain insight into existing solutions and to find gaps in literature. Last, with respect to many of the techniques identified in patents, literature validating those specific techniques is often lacking. Where available, appropriate references are provided.
4. Mechanical Interactions
In this section, solutions are described that rely on the use of a certain force other than the forces that drive a chemical reaction.18 As previously discussed, this category is divided into removal and prevention methods. First, removal methods will be examined. Second, due to the lack of results in literature, hypothetical mechanical prevention methods and found patents will be described.
4.1. Removal Methods
4.1.1. Brush/Wipe
Brushing or wiping is effective in removing impurities from a surface if its forces are able to overcome particle adhesion forces.19 The major interaction forces are the van der Waals force and the electrostatic double layer force of repulsion, primarily in liquid environments.20 During wiping, particles are removed from the surface by a wiper or cloth. As numerous wipers and types of cloths exist, and possible wiping motions are infinite, this method is highly flexible. Nevertheless, wiper-based cleaning tends to be the most effective for removal of contamination from large surface areas in a controlled fashion.19 One of the simplest methods of cleaning glass is to wash the surface with a detergent and water, followed by wiping with cotton-wool steeped in isopropyl alcohol or another solvent.21
In patent literature 4 main types of devices could be distinguished. The first set of devices is intended to be inserted into the patient through a separate incision, next to the endoscope. Fisher et al22 designed an apparatus consisting of a plastic handle with a 0.89 mm cannula attached. In the hollow cannula at the distal end of the device a wick is located, which can manually be extended and retracted. By extending and moving the wick toward the endoscope lens, it is possible to wipe it.23 Variations include an apparatus with a cloth being disposed between 2 prongs that can be collapsed for insertion into the patient, and expanded to clean the lens.24
The second set of devices centers on wipers that can be attached to the distal end of an endoscope. In this set, devices use a wiper constructed from a super elastic alloy,25 and wipers controlled by an actuator26 or electromagnets.27
The third set of devices rely on sponges that are attached to either the trocar28 or a hollow sheath wherein the endoscope is inserted.29 In this way, the endoscope lens can be wiped while still being inside the trocar30 or patient.31 Variations include comparable systems with an additional fluid channel to provide cleaning fluid to the sponge32 and a trocar with 3 different cleaning components.33
The fourth set of devices consists of those attached to and used inside the patient.34 Cassera et al evaluated a patented device named EndoClear that is attached to the inner abdominal wall at the start of a laparoscopic procedure, allowing the surgeon to wipe the endoscope lens inside the patient. It was found that EndoClear lowers the cleaning time, but does not significantly improve overall procedure time as the device requires a set up and repositioning time.1 EndoClear is the only found commercial solution to significantly differ from the “traditional” cleaning techniques discussed in the introduction.
4.1.2. Centripetal Force
Centripetal force represents accelerated motion directed toward the center of curvature of a body in curvilinear motion. Devices exploiting centripetal forces to clean a surface or to separate substances are known as centrifuges. Each particle is centrifuged at a rate proportional to the applied centrifugal field.35 As smaller particles (<50 µm) adhere to surfaces primarily by van der Waals forces, the force holding a 20 µm particle to a surface may be 10 000 times its weight.36-38 Moreover, for smaller micrometer-size particles total adhesion can exceed the gravitational force by factors greater than 106.36 A relatively large force is therefore required to dislodge a small particle in a centrifuge.
Specific to endoscopes, a patent was found that describes a device exploiting centripetal force.39 It consists of a transparent disk and a ring-shaped socket that is placed over the distal end of an endoscope. The ring is provided with rim turbine blades, which allows it to rotate due to one or more jets of pressurized air from a nozzle. As a result, the produced centripetal force should dislodge impurities that may be present on the optical viewing disc. No actual prototype using the patent was found.
4.1.3. Collision
Particle collision plays an important role in a wide range of natural and industrial processes where agglomeration or breakup of particles is essential.40 When particles collide, momentum is exchanged through momentum transfer. Methods that exploit particle collision are sputtering and wet laser cleaning techniques.
Sputtering as a cleaning process relies on a stream of energetic particles bombarding impurities present on the surface. If the energy transferred to the surface particle has a component normal to the surface that is larger than the surface binding energy, the particle is dislodged (ie, becomes sputtered).41 Efficient sputtering methods to clean a glass surface are plasma glow discharge and positive-ion bombardment.21,42 However, preapplied coatings with a thickness of several molecular layers may be removed during sputtering; therefore, this method is less suitable for cleaning coated surfaces.
Cho et al43 described the use of atmospheric pressure radiofrequency glow discharge plasma to remove organic contaminants from Indium Tin Oxide (ITO) glass. Li et al44 cleaned ITO glass using CO2 snow jet spray that relies on high pressure CO2 gas being expanded through a specially designed Venturi nozzle. In this way, a high-velocity stream composed of solid CO2 snow particles, with diameters of up to 100 µm, was generated. By aiming this jet on the polluted surface, impurities were sputtered. This method is believed to be nonabrasive, residue-free, nondestructive, and environmentally friendly as there is no chemical waste. Moreover, the authors claim that CO2 snow jet spray results in a cleaner surface compared to low-frequency ultrasonic cleaning and can therefore replace it. Another method is the use of microbubbles with a diameter of 10 to 100 µm to remove, for example, polymer ink from a glass substrate.45
The lens irrigation system described in the introduction is an example of a technique based on momentum transfer and is described in various patents.46-55 All patents describe the use of a sterilizable or disposable56 hollow sheath wherein the endoscope is inserted. This effectively adds an auxiliary fluid channel and irrigation nozzle near the endoscope lens, and sometimes an additional suction channel to discharge the irrigation fluid.57 Some of the devices can be used to spray both a liquid and a gas,58-60 to, for example, dry the lens after irrigation61-63 or to form a fluid mixture,64-68 while others are only able to spray a gas.69 Other patents describe the use of a flow guide used together with the irrigation system to direct a fluid flow in a more optimal way70-72 or the use of suction to control and subsequently discharge the fluid.73 Another patent describes a cylindrically shaped balloon with a plurality of small holes on one side.74 The balloon has to be inserted into the endoscope’s working channel. By advancing the balloon out and filling it with a liquid, it expands while irrigating the lens through its small holes directed toward the endoscope lens.
A second category exploiting collision is laser cleaning, which can be divided into dry and wet cleaning techniques. Dry laser cleaning techniques rely on a laser disintegrating impurities directly through evaporation or sublimation. Therefore, these will be further discussed under chemical removal methods. In contrast, most wet laser cleaning techniques heat up a fluid or a metal plate inside a fluid container, thereby generating a turbulent bubble flow that is directed upwards to the surface of the liquid.75 When an unclean object is submerged in the container, and positioned directly into the bubble flow, impurities present on its surface are dislodged similar to sputtering. Contrary to dry laser cleaning techniques, most wet laser cleaning techniques keep the temperature rise of the object’s surface below 30°C since the fluid also acts as a coolant.75
Weng and Tsai75 studied 2 laser-induced wet cleaning techniques that were able to remove 0.5 µm particles from glass substrates. The first technique involved using a Nd:YAG laser to heat up a metal plate in water to create a bubble flow. The second method involved a CO2 laser that directly generated a turbulent bubble flow in the water to clean a glass substrate. In both methods the laser shone through the glass surface, which created a bubble flow on the side opposite of the laser itself, thus defining these methods as so-called backside laser cleaning techniques. Logically, these methods only work if the surface is transparent enough, that is, allows for the transmittance of the laser beam’s wavelength. Additionally, a type of laser has to be chosen that emits the proper wavelength, such that its energy is being absorbed by the medium (eg, water or a metal plate) to create the bubble flow.
Another type of a wet laser cleaning technique is steam laser cleaning, which relies on a liquid film, having a thickness in the order of micrometers, being deposited on the surface just before flash heating with a laser.76 The laser causes the film to be super-heated rapidly, followed by a rapid explosive vaporization. A strong acoustic pressure pulse that is generated then removes small particles down to 0.1 µm from the surface.77
4.1.4. Suction
Suction works due to a pressure difference in a gas or fluid. A common example exploiting suction is a vacuum cleaner. Surgeons frequently use suction to clean their surgical sites from blood and debris. No articles were found that describe the use of suction to clean a surface. Nevertheless, a patent was found that describes the use of suction in combination with a dental mirror.78
4.1.5. Vibration
Ultrasonic agitation is a method to remove gross as well as sub-micrometer impurities from a surface by using vibrations in a detergent. Stowers79 removed small yet larger than 5 µm contaminant particles from glass with an efficiency of 69% to 92%. This cleaning method can be divided into low- and high-frequency agitation. At relatively lower frequencies (20-100 kHz), large cavitation bubbles are generated. Cavitation blasts away impurities from the contaminated surface and therefore assists in removing gross particles. However, high cavitation energies can damage the surface and therefore careful control is required.21 High frequencies can also be used to realize a more gentle cleaning method to save brittle surfaces: cavitation bubbles are smaller, and implosions are less intense. As a result, more time is required for cleaning surfaces than when using low-frequency agitation. In an optimized single process, one would employ low-frequency ultrasound to remove larger particles and high-frequency ultrasound to remove sub-micrometer particles.80 Note that smooth surfaces can roughen with increasing exposure to detergents.81
An endoscope-specific patent was found that describes the use of vibrating elements located at the distal end of the scope in combination with the previously described lens irrigation system.82 During irrigation with the washing liquid, the elements vibrate and may cause cavitation bubbles to enhance the cleaning ability of the liquid. One variant uses an ultrasonic resonating hollow sheath wherein the endoscope is inserted, thereby causing the impurities present on the instrument’s surface to be agitated.83 This can be exploited to clean the endoscope lens.
4.2. Prevention Methods
In the literature, no articles describing mechanical prevention methods were found. However, the following hypothetical concepts could be thought of. A device that is able to (automatically) move the surface that is required to stay clean out of the trajectory of an approaching impurity, possibly imitating the flipping mirror in a single-lens reflex camera. Moreover, another plausible method involves a movable shield to let the impurity land on, instead of on the surface beneath it. This resembles an eyelid protecting the eye from foreign bodies.
Specific to endoscopes, a patent was found describing an apparatus that positions a clear movable film strip over the endoscope lens.84 One side of the film is facing the endoscope lens, while the other side is covering the lens and therefore prevents adhesion of impurities. When impurities land on the film, the strip can be moved such that the endoscopic field of view becomes clear again. A similar patent describes the same principle with pre-folded films in a meandering configuration that replaces the top film automatically when it is removed.85
5. Chemical Interactions
In this section, solutions are described that rely on either state changes or the forces that drive a chemical reaction.18 As discussed previously, solutions are divided into removal and prevention methods. First, the removal methods will be examined. Thereafter, prevention methods will be described.
5.1. Removal Methods
5.1.1. Dissolution
The process of dissolution is a widely used method to clean surfaces. For example, vapor degreasing is an effective process for removing molecular contamination from glass surfaces.21 Vapor degreasing relies on solvent vapors condensing on the surface that dissolve impurities and removes them by dripping off.86 Tests indicate that effective cleaning can be obtained within a time interval of about 2 minutes.21 When using isopropyl alcohol, vapor degreasing achieves higher surface cleanliness than ultrasonic cleaning, and almost provides results comparable to those obtained by ionic bombardment.21 Still, vapor degreasing fails to remove gross contaminants, and should therefore be used with another technique (eg, a detergent washing process) to remove these.21
5.1.2. Evaporation/Sublimation
Dry laser cleaning, or laser ablation, exploits the phenomena of evaporation or sublimation to clean surfaces. Traditional dry laser cleaning may cause damage, especially if the surface to be treated is brittle or has a low melting point, and therefore typically a pulsed laser beam or laser shock cleaning is used.87 Laser shock cleaning relies on shock waves generated by a laser in a gaseous environment to remove small particles and can leave the optical and surface properties unharmed.87 Kumar et al87 studied the effects of dry laser shock cleaning on glass surfaces. They found that a Nd:YAG laser can remove radioactive uranium dioxide (UO2) particles efficiently. Römich et al88 used a pulsed laser to remove crusts from aged glass and found that irradiation at higher energies (200 pulses of ~2.0 J/cm2) irreversibly damaged the glass.
5.1.3. (Photo)catalytic Degradation
Photocatalytic degradation or photocatalytic oxidation (PCO) is the chemical decomposition of carbon molecules (eg, organic impurities) by using photons and a photocatalyst. In PCO reactions, metal oxide semiconductors (eg, TiO2 and ZnO) are often used as photocatalysts.89 TiO2 is inexpensive, chemically stable, and biologically inert (ie, biocompatible). Moreover, it has a high mechanical toughness, photocatalytic activity (PCA), and a favorable redox potential.90-95
Many self-cleaning materials are based on TiO2 thin film coatings and are thus able to clean themselves through PCO reactions. However, as a thin film coating can get poisoned by reaction intermediates there are still practical limitations.96 Moreover, during the often necessary thermal treatment, sodium ions from the glass substrate migrate to the TiO2 film.97 This is highly detrimental to the PCA of the film. Other factors that influence PCA are film composition, atomic to nanoscale roughness, bulk and surface (nano)structure, as well as hydroxyl and impurity concentrations.98-100 As the technology is still in its infancy, individual effects of the aforementioned factors are not yet well understood.101
Besides properties of the thin film itself affecting PCA, the environment plays an important role: H2O and O2 molecules are required for photocatalytic degradation to function. However, as water tends to adhere to the active sites of the coating, the relative humidity of air is important. Increasing humidity levels decreases the PCA due to competition between water and organic pollutants for active sites.102,103 Nevertheless, an increase in temperature is expected to increase the PCA due to a higher photodecomposition rate and other thermal effects (eg, evaporation of pollutants).104-106 Cedillo-Gonzalez et al97 noted that at room humidity (ca. 63%), which would generally be detrimental to the PCA, increasing the temperature increases the PCA. They believe this is due to water molecules releasing from the surface. Even so, TiO2 coatings work best in environments with low humidity and relatively high (>30°C) temperatures. Tests show that the thin films generally have a good chemical resistance to common household cleaning agents as they maintained their PCA when treated with boiling or deionized water, 5% isopropanol, or a detergent.97
TiO2 thin film coatings can be made by using several techniques, including spin-coating, sol-gel dip coating, electrospinning, chemical vapor deposition, sputtering, and screen-printing.107-111 Common substrates that are used for these techniques are mainly glass and other high-temperature–resistant materials as the temperature required to crystallize TiO2 is in the range of hundreds of degrees Celsius.112,113 Moreover, as TiO2 photocatalytically degrades organic molecules, caution is advised when using such coatings in conjunction with organic polymers. A solution to protect a polymer substrate would be to use a barrier layer before application of a TiO2 thin film. Suitable layers that have been applied include silica (SiO2), ZrO2, and polyurethane (PU).90,107,114,115 Likewise, sacrificial protective layers can be used.116
Another effect of TiO2 thin films is that they alter the surface reflection of transparent glass due to their refractive index. The minimum reflection of an ideal homogenous single-layer coating is dependent on the refractive indices of the coating, medium, and substrate.117-119 Yoldas117 found that the reflection at the boundary of air-glass is 4.3%, while that of air-TiO2 is between 18.6% and 21.9%.120 Moreover, increasing the thickness of TiO2 thin films leads to a decreased transparency.121-123 Paz et al115 noted that organic impurities of 50 nm thickness already scatter light and cause glare on automotive windshields.
Photocatalysis requires photons with a certain wavelength or wavelength range, depending on the type of metal oxide semiconductor used. Most of the photocatalytic materials respond to UV light. TiO2 coatings can be doped with metallic ions to increase their PCA.124,125 Murugan et al126 used Ni and Fe to obtain PCA in the visible light region. As PCO reactions are slow, disintegrating impurities on the surface is a process taking hours. Examples of timescales found in literature include 300 minutes for a fingerprint (containing 0.5-1.5 nmol/cm2 palmitic acid127), and 140 hours to remove 98 wt% of 5.28 nm thick crystalline fluoranthene or 48 days of outdoor solar exposure under diffuse sunlight.128-130 The thickness of organic layers oxidizing on TiO2-coated surfaces was found to be 0.2 to 5 µm/day.115,127,131
Aside from PCO, catalytic oxidation exists that, instead of being driven by photons, depends on a catalyst and heat to disintegrate organic contaminants. Contrary to photocatalytic thin films, most existing coatings are not transparent and only work at temperatures between 200°C and 350°C. Verhelst et al132 described a transparent catalytic coating that removed up to 98 wt% of a 0.6 µm-thick layer of a fatty contaminant in 40 minutes at 250°C.
5.1.4. Surface Tension
Surface tension is the tendency of a liquid to acquire the least amount of surface area possible. It can be measured as the energy that is required to increase the surface area of a liquid by a unit of area. Surfactants are substances that lower the surface tension between 2 liquids or between a liquid and a solid. Together with their intrinsic properties, they are able to soak up fatty acids by forming micelles. Classic cleaning formulations contain, in addition to surfactants, alkaline builders (eg, silicates, condensed phosphates), chelating agents, and several other substances.133 Zoller133 studied several surfactant-based formulations in water of medium hardness (0.0001% CaCO3) on their ability to clean different types of glass lenses. He found that a relatively high level of condensed phosphates, mainly sodium triphosphate, and chelating agents are essential to the formulation’s results.
Saponins are a category of surfactants and produce a soap-like foaming when shaken in aqueous solutions.134,135 They are found in particular abundance in various plant species and are contained in soaps and detergents.9,136 Moreover, saponins have a wide range of beneficial biological activity, such as being anti-inflammatory, antiviral, and antiparasitic.137 Recently, studies reported oolong tea to exhibit surfactant properties due to saponins being present.137,138 As a result, oolong tea can be used to clean oil soiling.
Ito et al139 decided to use oolong tea as a lens-cleansing solution in colonoscopies. Komazawa et al9 further examined the effects of using oolong tea as a washing solution in transnasal esophagogastroduodenoscopy. They compared oolong tea, barley tea (contains less saponin than oolong tea), and distilled water as lens-cleansing solutions. It was found that oolong tea improved the lens-cleansing effect in small-caliber endoscopes compared to barley tea and distilled water. Moreover, the used volume was significantly smaller, and the procedure time shorter for oolong tea compared to the other 2 groups.140 However, Yoshida et al141 found that the effects of oolong tea were limited when used in endoscopic submucosal dissection. Therefore, they developed a solution based on 2 types of nonionic surfactants and pharmaceutical additives (eg, solubilizing agents) that reduces lens cloudiness compared to standard lens cleaner (SL cleaner; Sugiken, Tokyo, Japan).
5.2. Prevention Methods
Several chemical prevention methods exist that rely on interactions near or on the surface of the object that is to be kept clean. Most found solutions are based on coatings that prevent impurities from adhering to the surface allowing impurities to simply slide off. Traditionally, many of these coatings exploit the hydrophilic or hydrophobic effect to keep a surface clean. Hydrophilic surfaces have an affinity for water whereas hydrophobic surfaces lack this affinity, thus repelling water. Analogously, a surface can be lipophilic or lipophobic: respectively having an affinity for fats, oils, lipids, and nonpolar solvents or lacking this affinity.
The degree of wetting, or the wettability, which is the ability of a liquid to maintain contact with a solid surface, is determined by the interaction between adhesive and cohesive forces. These forces are respectively exerted by the surface and by the liquid within itself. As a result, the water contact angle (WCA), a static phenomenon, is determined by this force balance. The WCA is the angle at which the liquid-vapor interface meets the solid-liquid interface. By measuring this angle, a surface can be defined as either being hydrophilic (WCA <90°) or hydrophobic (WCA >90°). Two special types of surfaces can be distinguished, namely, superhydrophilic (typical WCA <10°) and superhydrophobic (WCA >150°) surfaces.
5.2.1. Hydrophilicity/Lipophilicity
Hydrophilic coatings are often used in combination with photocatalytic degradation TiO2 coatings that exhibit a PCA and simultaneously exhibit hydrophilic effects that increase after having been irradiated by light (ie, photo-induced hydrophilicity). However, to compare solutions with each other, both effects are described separately here. Since many articles describe different varieties of the method(s) or the coating(s) they have used, and often a comparison is involved, only the most suitable solution(s) for endoscopes (in the sense of highest transparency) of each article is shown in Table 1.
Table 1.
Summary of Found (Super)hydrophilic surfaces In Literaturea.
| Reference | Treatment Method | Material | Substrate | WCA Substrate (°) | WCA After Treatment (°) | WCA (°) After Irradiation, t (minutes) | Film Thickness (nm) | Transmittance (%) | Wavelength (nm) |
|---|---|---|---|---|---|---|---|---|---|
| 128 | e-Beam evaporation | ZrO2/3 pairs of SiO2/TiO2 | Glass | 10 | ~0, t = 300.05 | 420 | 99 | 405 | |
| 90 | Spin coating | TiO2 (multiple layers) | HDPE | 46.7 | 0 | ~0, t = 1440 | |||
| 90 | Spin coating | TiO2 (multiple layers) | Borosilicate glass (BK7, SCHOTT) | 21.1 | 0 | ~0, t = 1440 | |||
| 146 | Spin coating | Niobia nanosheets | Soda-lime glass | 42 | <5, t = 80 | ||||
| 147 | GLAD/dry etching | Nanocone arrays | Borosilicate glassb | ~63 | 5 | >94.2 | 330-1800 | ||
| 108 | Electrospinning | Nanocrystalline TiO2 + DEA | Glass | 56.0 ± 1 | 2 ± 1 | >85 | 350-800 | ||
| 143 | Aqueous electrostatic layer-by-layer assembly | 120 TiO2/SiO2 bilayers | Glass | 125 | ~0, t = 60 | 2421 (avg.) | 93.5 (avg.) | 400-800 | |
| 148 | Sol-gel dip coating | TiO2 | ITO glass | 27 | 12, t = 30 | 30.1 (avg.) | |||
| 149 | Combined sol-gel dip coating | Glass | 89.5 | 23.5, t = 15 | 75 | 94.8 | 550 | ||
| 150 | Dip coating in 2-propanol | TiO2 | PET | <5, t = 90 | 76-95 | 400-800 | |||
| 151 | Dip coating in water | PtCl-TiO2 | PMMA | 136 | 6.1 | <5, t = 120 | 61-92 | 400-800 | |
| 109 | DC reactive magnetron sputtering | TiO2-x-Nx/TiO2 bilayer | ZnO-coated (h = 80 nm) soda-lime glass | 25 ± 1.5 | 14 ± 1, t = 60 | 830 | >71 | 400-1000 | |
| 152 | Sol-gel dip coating | TiO2 | Float glass | 39 ± 11 | 55 ± 6 | 0, t = 1440 | 300-400 | >80 | 380-1000 |
| 152, 153 | TiO2 | Pilkington Activ | 70 ± 6 | 0, t = 5760 | ~15 | >76 | 380-1000 | ||
| 154 | ICP-RIE | Nanostructures | Borosilicate glassb | 61 | 32 | 200 | >92 | 400-1000 | |
| 155 | Sol-gel dip coating | TiO2-Cu (1%) | Glass | 45.8 | 17.4, t = 30 | ~1000 | >72 | 2500-25 000 | |
| 156 | Ultrasonic nebulization | PEG/TiO2 hydrosol | Glass | <5 | 0, t = 0.17 | ~1.0 | |||
| 144 | ICP-RIE | Nanostructures | Borosilicate glassb | 62 | 21 | 400 | >89 | 400-900 | |
| 157 | PVD/MPII | TiO2-Fe | Glass | 9.95 | |||||
| 158 | Solvent-based spin coating, RIE | Nanostructures | Borosilicate glassb | 58.9 | 12.6 | 108 | >93.5 | 400-1800 | |
| 159 | Magnetron sputtering | TiO2 | Soda-lime glass | 18 | 5, t = 20 | >65 | 400-1100 | ||
| 160 | Reactive magnetron sputtering | SiO2/TiO2 bilayer | Quartz glass | 3.29 | 46.44 | >63 | 400-1000 | ||
| 161 | Hydrothermal synthesis | NH4OH | glass | <5 | ~100 | 98.4 | 500 |
Abbreviations: HDPE, high-density polyethylene; GLAD, glancing angle deposition technique; DEA, diethanolamine; ICP, inductively coupled plasma; RIE, reactive ion etching; PVD, physical vapor deposition; MPII, metal plasma ion implantation.
The material after a “+” sign in the material column indicates that the material is added to the precursor solution. A “−” sign indicates that the original substance is doped by the material that follows. Some entries are intentionally left blank as the literature did not provide information on those aspects.
BOROFLOAT 33, Schott.
While most photo-induced hydrophilic coatings operate under UV irradiation, some solutions are able to function under visible light. To achieve this and to improve material characteristics, thin films are often doped, or implanted, with other particles to change structural parameters.142 Another method to alter the behavior of a typical TiO2 thin film is the use of bilayers (ie, closely packed double layers of molecules). For example, Lin et al143 used 120 bilayers to obtain a photocatalytic surface under visible light. However, the mechanical integrity is often poor due to the absence of polyelectrolytes that glue the multilayers together.143 Furthermore, hydrophilic surfaces can be created with nanostructures through microelectromechanical system (MEMS) fabrication techniques. Verma et al144 observed that the WCA decreased with an increase in nanostructure height and thus surface roughness. According to Wenzel’s law, a rougher surface indeed increases the wetting characteristics of intrinsically hydrophilic surfaces.145
5.2.2. Hydrophobicity/Lipophobicity
As pure TiO2 thin films exhibit hydrophilic behavior, most hydrophobic surfaces are based on either nanostructures or impure coatings. Contrary to discussed hydrophilic surfaces, artificial hydrophobic surfaces often do not exhibit any PCA, that is, no photocatalytic degradation occurs on the surface. Moreover, the hydrophobic effects of these surfaces are not dependent on light (ie, are not photo-induced). An important estimation parameter for the self-cleaning property of a hydrophobic surface is the sliding angle or hysteresis.162 It is defined as the critical angle of the surface where a water droplet begins to slide off.163 When the sliding angle is small, the water droplet is more inclined to roll off the surface, greatly benefitting the self-cleaning ability of the surface.164 Among the best optimized hydrophobic surfaces known to date are the leafs of the lotus (Nelumbo nucifera) and taro (Colocasia esculenta).165,166 The surfaces found that rely on hydrophobicity are summarized in Table 2.
Table 2.
Summary of Found (Super)hydrophobic Surfaces in the Literaturea.
| Reference | Method | Material | Substrate | WCA Substrate (°) | WCA (°) | Sliding Angle (°) | Film Thickness (nm) | Transmittance (%] | Wavelength (nm) |
|---|---|---|---|---|---|---|---|---|---|
| 178 | PECVD | Carbon nanotubes | Glass | 141 | 0-40 | 300-1100 | |||
| 179 | Sol-gel dip coating | SNPs/PTFE | Soda-lime glass | 169 ± 2 | ≤2 | 200 | >94.9 | 500-750 | |
| 179 | sol-gel dip coating | SNPs/silica aerogel/PTFE | soda-lime glass | 158 ± 2 | <5 | 255 | ~99 | 650-750 | |
| 162 | Sol-gel dip coating | MTMS/TEOS | Glass | 30 ± 1 | 135 ± 2 | <5 | 730.8 | ||
| 164 | CCVD | SiO2 | Glass | 109 | 170 | <5 | >94 | 400-700 | |
| 95 | Sol-gel dip coating | TiO2 + DDA | Glass | 35 ± 4 | 155.5 ± 1.2 | 10 | >85 | 350-800 | |
| 180 | NIL + ICP | Nanosized patterns | Glass | 156/122 (oil) | 200-300 | >94.5 | 400-700 | ||
| 181 | Layer-by-layer assembly | PDDA/SiO2 nanoparticles | Soda-lime glass | 104 | 130 | 26 | 120 | >91.5 | 400-800 |
| 182 | Vertically oriented rutile nanorods | Glass | 118 | >70 | 520-800 | ||||
| 183 | Spin-and-spray assembly | Solid SNPs, stearic acid, POTS | Fresnel lenses | 151.5 ± 1.5 | <5 | >97.5 | 400-800 |
Abbreviations: PECVD, plasma enhanced chemical vapor deposition, SNPs, silica nanoparticles; PTFE, polytetrafluoroethylene; MTMS, methyltrimethoxysilane; TEOS, tetraethoxysilane; CCVD, combustion chemical vapor deposition; DDA, dodecylamine; NIL, nanoimprint lithography; ICP, inductively coupled plasma dry etching; PDDA, poly(diallyladimethylammoniumchloride); POTS, 1H,1H,2H,2H-perfluorooctyltriethoxysilane.
The material after a “+” sign in the material column indicates that the material is added to the precursor solution. Some entries are intentionally left blank as the literature did not provide information on those aspects.
5.2.3. Amphiphilicity
A molecule is said to be amphiphilic when it possesses both (1) hydrophilic and (2) lipophilic (or hydrophobic) properties. When used as a removal method, a common example of a fluid having these properties is a detergent, which allows fatty molecules (eg, food residue) to dissolve in water. The hydrophilic end of a detergent molecule then binds with water molecules, while the other end of the molecule binds with the fatty impurity, forming a micelle.
Besides detergents, amphiphilic behavior can be introduced as a prevention method by using a coating. André et al167 used spermine on a glass surface to create durable, homogenous, and thin SnO2 films on glass. On exposure to sunlight the glass surface becomes hydrophilic. However, when the surface is not exposed to light, it becomes hydrophobic. This effect of light-driven behavior acting as a switch can be exploited to get desired surface properties in various settings.
5.2.4. Adhesion Resistance
Adhesion resistance is the ability of a material to selectively resist impurities adhering to its surface. Although many objects found in nature exhibit this anti(bio)fouling ability, the effect can also be achieved artificially by altering surface characteristics (eg, surface roughness). Methods to alter the surface finish include UV irradiation, deposition of thin film coatings, and polymerization techniques. For example, Kougo et al168 sputtered semitransparent metal layers on glass to inhibit biofilm formation. Halfpenny et al169 radiated the surface of a silica substrate with UV light, resulting in the number of particles with a diameter of 0.3 µm adhering to the surface being 80% less. Zhang et al170 describe the superlow fouling properties of glass slides grafted with zwitterionic polymers via atom-transfer radical-polymerization. Xu et al171 used zwitterionic polymers to develop anti-biofouling contact lenses. The final 2 solutions created surfaces that resist the protein adsorption, and the adhesion of mammalian cells or bacteria.
Part of the adhesion resistance solutions are applied in marine environments, for example, to keep the hull of a ship free from impurities that increase the wave-making resistance. Moreover, solutions are used to keep underwater cover glasses of sensors free from biofouling. Booth et al172 found that PMMA combined with Irgarol 1051, an algaecide, has the best optical transparency, impact, and mechanical properties after 6 months in a marine environment. They conclude that this polymeric material is favored for its anti-biofouling properties. Dineshram et al173 also conducted research in a marine environment to study the (macro)biofouling of metal oxide coatings on glass substrates. They deposited silica, TiO2, and niobia (Nb2O5) on glass and kept the substrates underwater for 15 days. Nonetheless, these coatings did not prevent organisms from adhering, and no significant adhesion resistance compared to the control substrate was found.
To prevent biofouling in marine environments, Strahle et al174 designed a reusable plastic antifouling ring that slips over transmissometer lenses. The ring can be used to dispense antifoulant into the water in front of the lens for 4 months to retard macrofaunal growth (ie, barnacles, hydroids, and tunicates) without obstructing the light path. Although these rings significantly reduced macrofaunal growth, other biological and sediment fouling continued to block the light path.174
Besides artificial solutions, the peculiar eye surface of a green crab is probably antifouling.175 Greco et al175 used atomic force microscopy to obtain a new insight into the morphology of the crab eye’s surface and its characteristics. They describe 3 key requirements to prevent biofouling adhesion: (1) cells/organisms must remain above topographical features and must not be able to settle between them, (2) they must not be able to contact and settle their entire structure on a single feature, and (3) if they bridge 2 features, then they must not be able to contact the intervening floor. Concretely, a suitable surface is one with many dense peaks.
Another branch of anti-biofouling is the use of so-called polymer brushes. A polymer brush is a chain of polymers that is attached to a surface with only one of its ends, thus resembling a brush filament. These are found to be useful in biomedical applications as they prevent adsorption of specific proteins such as bovine serum albumin, usually used as a nutrient in cell culture, and collagen type I, the most abundant protein in mammals.176,177
6. Discussion
Going back to the problem of the endoscope lens in surgery, it is important, in terms of design and implementation requirements, for cleaning methods to be
Nondestructive
Nonabrasive (to lens and patient)
Biocompatible
Safe for both patient and surgeon
Fast: The time to achieve surface cleanliness should be as short as possible, preferably while maintaining visual acuity of the operative field
Residue-free, at least on the surface of the endoscope lens
Easy to use for the surgical team
Preferably not be exceeding the current endoscope sheath diameter significantly as this brings less maneuverability and potential incompatibilities with existing equipment
Not impairing vision (eg, be transparent), especially for methods that rely on altering the surface
Reposable/disposable or sterilizable (eg, resistant to high temperatures)
Cost-effective
In addition, technical and practical feasibility of solutions are equally important. Nonetheless, as previously described techniques may improve over time, currently unfeasible solutions may eventually become applicable to endoscopy. The economic aspects of the presented techniques and lens-cleaning solutions were kept outside the scope, as there is a lack of literature in this regard.
6.1. Mechanical Interactions
Starting with the mechanical removal methods, brushing, or wiping is very flexible as possible modes are infinite. A downside of using this method is that it adds to the dimensions of the endoscope. Surgical sites inside the body are often spatially limited, which makes a solution such as EndoClear in its current form not applicable for every endoscopic procedure. Patents describing wipers are more promising for use in a confined space, although they have to be made sterilizable or disposable. The same holds for altered trocars with cleaning systems inside. A downside of these systems is the lack of vision during cleaning, and that no experimental data exist on their performance. In addition, these solutions might significantly add to the diameter of the endoscope sheath. Last, some procedures such as those performed though natural orifices may not require a trocar and thus make a trocar with an inner cleaning system not universally applicable as a solution.
Cleaning methods exploiting centripetal force have the downside of having relatively rapid moving parts with a potential to cause harm when dislodged. For example, the device consisting of a rotating disk39 should be implemented with caution as the ring could cause significant injury when unintentionally dislodged inside the patient. Moreover, centrifugal devices might add to the overall diameter of the endoscope.
Methods relying on collision often need an additional system to be able to generate a jet of particles or a laser beam. The impurities that are dislodged by sputtering may cause harm to the patient as these particles move off the surface at high velocities. Principles relying on Plasma Glow Discharge would require a plasma to function, of which procedural risks to the human are less extensively documented compared to other energetic treatment techniques.184 Wet laser cleaning techniques rely on a medium, a fluid, to clean a surface and thus introduce new problems such as how to establish the medium inside a cavity. Steam laser cleaning is more favorable in this respect, but adds the dangers of super-heated fluids inside the body with acoustic pressure pulses. Another aspect is the potential reflection of the laser beam that could cause damage.185 Last, due to localized heating, the risk of thermal burns is present.
Suction techniques are widely used in surgery and offer potential as a means to clean the endoscope lens. However, when incorporated into the endoscope, this method may add to the total dimensions due to an extra channel. Considering no studies were found regarding the effectiveness of cleaning a surface using suction, it may not clean the lens satisfactorily. Yet with respect to sterilization, suction tubes are widely available and adaptable for use in a suitable solution.
Ultrasonic agitation that brings cavitation bubbles inside the patient may be undesirable as it could cause damage and possible undesired vibrations in the surgical environment when the appropriate measures (such as shielding) are not taken. Another downside is the requirement of a medium, similar to wet laser cleaning. The device82 exploiting vibrating elements while using the already implemented lens irrigation system to clean the lens could be a feasible solution, but its effectiveness is unknown.
The patents84,85 relying on a movable clear film strip covering the lens as a mechanical prevention method offer potential. Possible issues here might be regarding sterilization, increased dimensions, and the removal of discarded film. In addition, no data are available on the effectiveness of these devices.
6.2. Chemical Interactions
Dissolution was found to be a chemical removal method that relies on solvents. Most (organic) solvents can lead to a sudden loss of consciousness when inhaled, or even death.186 Moreover, health hazards associated with solvent exposure include toxicity, cancer, organ damage, respiratory impairment, and dermatitis.187,188 This bioincompatibility makes dissolution unsuited for use inside the patient to clean the endoscope lens as it can cause harm to both the patient and the surgical team.
Evaporation or sublimation by use of a laser may cause damage to the endoscope lens, especially if the surface to be treated is brittle or has a low melting point. Furthermore, the risks involved with laser use in surgery include the laser beam potentially reflecting off of surfaces and, due to localized heating, thermal burns that potentially cause injury.185 Moreover, surgical smoke produced by the laser is a biohazard: it is considered a toxin, can be carcinogenic, and can even spread viruses.189-193 The implementation of a laser cleaning system could interfere with the surgeon’s field of view, depending on the wavelength and the exposure time. Overall, due to the associated health risks, the use of laser cleaning techniques inside the patient may be cumbersome and not the optimal solution.
Photocatalytic degradation is an interesting method, since TiO2 is chemically stable and biocompatible. However, possible wear of the thin film could turn out to be a downside after a certain amount of time. Certainly, the temperature requirements of these coatings is of importance with respect to sterilization and their use during surgery. In laparoscopic procedures the temperature in the abdominal cavity is often lower than human body temperature,2,194 causing PCO coatings to perform suboptimal. Nevertheless, the main downside of this cleaning method is the amount of time that is required for organic contamination to be disintegrated: the least amount of time reported is 5 hours, which is too long for intraoperative use. Hence, unless the time required for photocatalytic degradation can be reduced to, for example, a few seconds, this method is unsuited for cleaning the endoscope lens in situ. Coatings exploiting catalytic oxidation share this downside and in addition have the disadvantage of generally being nontransparent and requiring even higher temperatures.
The use of surfactants to lower the surface tension and subsequent soaking up of impurities is a promising method. The research into oolong tea yielded favorable results for certain applications. However, using oolong tea as a washing liquid for the lens irrigation system does not change the disadvantages of the current irrigation system of fluid buildup in the surgical working area. Moreover, some surgeons favored a mixed solution of surfactants over the use of oolong tea. Decisively, more research is required into oolong tea and other natural surfactants as a washing solution for the lens irrigation system.
The chemical prevention methods, which yielded the most results in literature, are also the most promising solutions. Adhesion resistant surfaces have the potential to achieve surface cleanliness in endoscope lenses as they fulfill most of the listed requirements. A downside might be the low resistance to wear and sterilization procedures (eg, see Fraise et al195 for endoscope cleaning procedures). The specificity that might be present is another downside: antifouling surfaces only provide adhesion resistance to a limited number of impurities. This method may therefore not be flexible enough, and more research is therefore required.
Hydrophilic coatings may not be ideal stand-alone solutions as the coatings only work for watery impurities. In addition, many hydrophilic coatings are photo-induced, requiring minutes to hours of irradiation before the surface becomes hydrophilic and then functions for a limited amount of time when irradiation is absent. The aspect of irradiation time can possibly be countered by starting to irradiate the surface ahead of surgery, but the prolonging hydrophilicity property of these surfaces during surgery is unknown.
Most hydrophobic coatings are not photo-induced, giving them an advantage over typical photo-induced hydrophilic coatings. In combination with the lens irrigation system, the watery impurities can easily slide off the lens surface. This mimics leafs found in nature, mainly the lotus leaf, which clean themselves when it is raining or when dew is present. Advantages of hydrophobic coatings include the high transparency and the ease of application. However, data on the durability and temperature resistance of these coatings in surgery is not available.
An amphiphilic coating would combine hydrophilic and hydrophobic properties into one solution by using, for example, the switching behavior based on exposure to light. For example, amphiphilicity could be exploited for endoscope lens cleaning by allowing the lens to be hydrophilic while applying a liquid. The created liquid film is maintained as the hydrophilic surface allows complete wetting. Whenever impurities land onto the surface of the lens, and thus touch the liquid film, the lens could be transformed into a hydrophobic surface. As a result, the liquid film could roll off the surface taking impurities along. Yet similar to photocatalytic degradation, the photo-induced switching behavior from one state to the other is a matter of hours. Therefore, at this moment, the benefits of using the amphiphilic coating as opposed to just a hydrophilic or hydrophobic coating are questionable.
6.3. Hybrid Solutions
The most optimal solutions can be obtained by combining methods to create hybrids. For example, a hybrid consisting of a surfactant (eg, oolong tea) as a washing solution for the lens irrigation system and a hydrophobic endoscope lens. In this way, oolong tea soaks up the impurities (eg, fatty acids), while the hydrophobic coating lets the solution easily slide off, leaving no residue on the lens. Another promising hybrid is that of the current lens irrigation system and a hydrophilic coating on the lens. The hydrophilic lens would cause a film of water to remain on its surface similar to a tear film found in mammalian eyes. The irrigation system could then mimic the production of tears and simulate the function of an eyelid with, for example, bursts of water. Both proposed hybrids likely increase the cleaning capabilities as compared to current lens cleaning systems.
7. Conclusion
During endoscopic surgery it is of critical importance that the surgeon maintains a clear visualization of the operative field. However, throughout surgery the endoscope lens gets fouled by blood, ground substance, and other impurities, resulting in visual impairment. As manual wiping and the use of a lens irrigation system are still far from ideal, this literature review was conducted with the goal of finding a solution suitable for implementation in endoscopic instrumentation to achieve lens surface cleanliness, thereby providing optimal visual acuity to the surgeon. Certain found techniques are unsuitable for this goal, based on the specified requirements regarding safety and cleaning speed. In conclusion, the application of a hydrophilic or hydrophobic coating to the endoscope lens and the use of the current lens irrigation system as a hybrid is likely the most promising method to achieve surface cleanliness in endoscope lenses.
Footnotes
Author Contributions: Study concept and design: D. Kreeft, E. A. Arkenbout, P. W. J. Henselmans, W. R. van Furth
Acquisition of data: D. Kreeft, E. A. Arkenbout, P. W. J. Henselmans
Analysis and interpretation: D. Kreeft, E. A. Arkenbout, P. W. J. Henselmans, P. Breedveld
Study supervision: E. A. Arkenbout, P. W. J. Henselmans, W. R. van Furth, P. Breedveld
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been supported by the Dutch Technology Foundation STW, which is part of the Netherlands Organization for Scientific Research (NWO), and which is partly funded by the Ministry of Economic Affairs (STW Project 12137).
References
- 1. Cassera MA, Goers TA, Spaun GO, Swanstrom LL. Efficacy of using a novel endoscopic lens cleaning device: a prospective randomized controlled trial. Surg Innov. 2011;18:150-155. [DOI] [PubMed] [Google Scholar]
- 2. Champion JK, Williams M. Prospective randomized trial of heated humidified versus cold dry carbon dioxide insufflation during laparoscopic gastric bypass. Surg Obes Relat Dis. 2006;2:445-449. [DOI] [PubMed] [Google Scholar]
- 3. Schoofs J, Gossot D. A neglected but frustrating ergonomic issue: the thoracoscopic trocar. Minim Invasive Ther Allied Technol. 2004;13:133-137. [DOI] [PubMed] [Google Scholar]
- 4. Wiegmann DA, ElBardissi AW, Dearani JA, Daly RC, Sundt TM., 3rd Disruptions in surgical flow and their relationship to surgical errors: an exploratory investigation. Surgery. 2007;142:658-665. [DOI] [PubMed] [Google Scholar]
- 5. Healey A, Sevdalis N, Vincent C. Measuring intra-operative interference from distraction and interruption observed in the operating theatre. Ergonomics. 2006;49:589-604. [DOI] [PubMed] [Google Scholar]
- 6. Zheng B, Martinec DV, Cassera MA, Swanstrom LL. A quantitative study of disruption in the operating room during laparoscopic antireflux surgery. Surg Endosc. 2008;22:2171-2177. [DOI] [PubMed] [Google Scholar]
- 7. Sutton E, Youssef Y, Meenaghan N, et al. Gaze disruptions experienced by the laparoscopic operating surgeon. Surg Endosc. 2010;24:1240-1244. [DOI] [PubMed] [Google Scholar]
- 8. Geryane MH, Hanna GB, Cuschieri A. Time-motion analysis of operation theater time use during laparoscopic cholecystectomy by surgical specialist residents. Surg Endosc. 2004;18:1597-1600. [DOI] [PubMed] [Google Scholar]
- 9. Komazawa Y, Amano Y, Yuki M, et al. Oolong tea is useful for lens cleansing in transnasal small-caliber esophagogastroduodenoscopy. Endoscopy. 2010;42:104-108. [DOI] [PubMed] [Google Scholar]
- 10. Weld KJ, Dryer S, Ames CD, et al. Analysis of surgical smoke produced by various energy-based instruments and effect on laparoscopic visibility. J Endourol. 2007;21:347-351. [DOI] [PubMed] [Google Scholar]
- 11. Macia I, Gossot D. Maintaining a clear vision during long-lasting thoracoscopic procedures. Interact Cardiovasc Thorac Surg. 2010;11:522-524. [DOI] [PubMed] [Google Scholar]
- 12. Runia AJ, Zengerink JF, Mannaerts GH. Easy cleaning of the scope’s lens in a syringe to prevent condensation during laparoscopic surgery. Surg Endosc. 2009;23:2849-2850. [DOI] [PubMed] [Google Scholar]
- 13. Van Deurzen DF, Mannaerts GH, Jakimowicz JJ, Cuschieri A. Prevention of lens condensation in laparoscopic surgery by lens heating with a thermos flask. Surg Endosc. 2005;19:299-300. [DOI] [PubMed] [Google Scholar]
- 14. CLEARVISION® II: For intraoperative cleaning of the front lens [press release]. 2015. https://www.karlstorz.com/cps/rde/xbcr/karlstorz_assets/ASSETS/3061780.pdf
- 15. LENS CLEANING SHEATH: Clears debris from the lens surface with quick and easy operation during endoscopic surgery [press release]. 2016. https://www.olympus-europa.com/medical/en/medical_systems/contact___support/mediacentre/media_detail_91456.jsp
- 16. Breedveld P, Herder J, Tomiyama T. Teaching creativity in mechanical design. Paper presented at: 4th World Conference on Design Research (IASDR2011); October 2011; Delft, Netherlands. [Google Scholar]
- 17. Cleanliness [definition 1]. http://dictionary.reference.com/browse/cleanliness. Accessed April 28, 2017.
- 18. Evans MG, Polanyi M. Inertia and driving force of chemical reactions. T Faraday Soc. 1938;34(1):0011-0023. [Google Scholar]
- 19. Mittal K, Jaiswal R. Particle Adhesion and Removal. New York, NT: John Wiley; 2015. [Google Scholar]
- 20. Visser J. Particle adhesion and removal: a review. Particulate Sci Technol. 1995;13:169-196. [Google Scholar]
- 21. Putner T. Methods of cleaning glass by vapour degreasing and ultrasonically agitated solvents. Br J Appl Phys. 1959;10:332-336. [Google Scholar]
- 22. Fisher YL, Ciardella AP, Chang B, Solomon L. Disposable intraocular endoscope lens cleaner. Retina. 1997;17:265. [PubMed] [Google Scholar]
- 23. Fisher Y, inventor. Retractable wipe for cleaning endoscopic surgical devices. US patent US20090105543 A1. 1996. [Google Scholar]
- 24. Guru K, Chowriappa A, inventors. Lens cleaning instrument for surgical procedures performed in an enclosed cavity. US patent WO2014145063. 2014. [Google Scholar]
- 25. Kessler J, Kunze J, Rouse E, Green G, Subramanian H, inventors. Light source and lens cleaner for laparoscopic surgery. US patent US20090240111. 2009. [Google Scholar]
- 26. Clark CA, Fleming AI, O’Prey C, inventors. Endoscope wiper blade cleaner. Patent AU2011236022. 2011. [Google Scholar]
- 27. Zarate H, Lucio R, Kilpatrick J, Desai N, inventors. Endoscopic lens cleaner. US patent US8460180. 2013. [Google Scholar]
- 28. Cercone RJ, Adams MK, Gatto DL, Valentine DR, inventors. Endoscope cleaning and defogging apparatus. US patent US5274874. 1994. [Google Scholar]
- 29. Gomez RA, Heck SL, inventors. Minimally invasive apparatus and method for cleaning endoscope lenses. US patent US20140215736. 2014. [Google Scholar]
- 30. Hurst R, inventor. System and method for cleaning a scope during a surgical procedure. US patent US20140246051. 2014. [Google Scholar]
- 31. O’Prey C, Scott VA, Fleming AI, inventors. Thoracic scope port cleaner. US patent US20130150670. 2013. [Google Scholar]
- 32. Haig FM, O’Prey C, inventors. Thoracic scope port sponge cleaner. US patent US20130150674. 2013. [Google Scholar]
- 33. Nguyen HT, inventor. Multi-purpose trocar with lens cleaner. US patent US20140171739. 2014. [Google Scholar]
- 34. Gordin U, Heftman G, Sobol M, Schvartzer A, Szold A, inventors. Lens cleaning device, system and method for surgical procedures. US patent US20090250081. 2009. [Google Scholar]
- 35. Wilson K, Walker JM. Principles and Techniques of Practical Biochemistry. Cambridge, England: Cambridge University Press; 2000. [Google Scholar]
- 36. Bowling RA. An analysis of particle adhesion on semiconductor surfaces. J Electrochem Soc. 1985;132:2208-2214. [Google Scholar]
- 37. Corn M. The adhesion of solid particles to solid surfaces. I. A review. J Air Pollut Control Assoc. 1961;11:523-528. [DOI] [PubMed] [Google Scholar]
- 38. Corn M. The adhesion of solid particles to solid surfaces. II. J Air Pollut Control Assoc. 1961;11:566-575. [DOI] [PubMed] [Google Scholar]
- 39. Swallert SA, inventor. Rotable lens cleaning device. US patent US6258025. 2001. [Google Scholar]
- 40. Meyer CJ, Deglon DA. Particle collision modeling—a review. Miner Eng. 2011;24:719-730. [Google Scholar]
- 41. Behrisch R, Wittmaack K. Sputtering by Particle Bombardment. Vol 3 Berlin, Germany: Springer; 1983. [Google Scholar]
- 42. Holland L. Ionic bombardment cleaning of glass. Nature. 1958;181:1451-1452. [Google Scholar]
- 43. Cho JH, Kim JW, Kim KS, Lee WY, Kim SH, Choi WY. LCD glass cleaning by atmospheric pressure glow discharge plasma. Key Eng Mater. 2005;297-300:2351-2355. [Google Scholar]
- 44. Li JJ, Qi T, Li SL, Zhao G. Cleaning of ITO glass with carbon dioxide snow jet spray. Paper presented at: Proc. SPIE 6722, 3rd International Symposium on Advanced Optical Manufacturing and Testing Technologies: Advanced Optical Manufacturing Technologies, 67224I; November 14, 2007. doi: 10.1117/12.783731. [DOI] [Google Scholar]
- 45. Matsuura K, Ogawa S, Kasaki S, Koyama K, Kodama M, Yanase S. Cleaning polymer ink from a glass substrate using microbubbles generated by a hydrogen bubble method. Separation Purification Technol. 2015;142:242-250. [Google Scholar]
- 46. Wiita BE, Teets MJ, Wiita GD, inventors. Lens cleaning apparatus. Patent EP0664101. 1995. [Google Scholar]
- 47. Mondschein R, inventor. Laparoscope cleaning system. US patent US20080200765. 2013. [Google Scholar]
- 48. Weisenburgh II William B, Shurtleff CJ, Hess CJ, et al. , inventors. Apparatus for keeping clean a distal scope end of a medical viewing scope. US patent US20090234193. 2009. [Google Scholar]
- 49. Hartoumbekis E, Rockrohr B, Fischvogt G, inventors. Surgical instrument cleaning arrangement. US patent US20130041230. 2015. [Google Scholar]
- 50. Mowlai-Ashtiani A, inventor. Endoscope lens cleaning device. US patent US20140318582. 2014. [Google Scholar]
- 51. Kinoshita K, inventor. Cleaning device for an observation window of an endoscope. US patent US4281646. 1981. [Google Scholar]
- 52. Wiita BE, Teets MJ, Wiita GD, inventors. Lens cleaning means for invasive viewing medical instruments. US patent US5313934. 1994. [Google Scholar]
- 53. Murdoch MJ, inventor. Laparoscopic telescope lens cleaner and protector. US patent US5400767. 1995. [Google Scholar]
- 54. Proch FS, Ireland P, inventors. Endoscope cleaning and irrigation sheath. US patent US6126592. 2000. [Google Scholar]
- 55. Smith AC, Peters GF, inventors. Method and apparatus for cleaning an endoscope lens. US patent US6447446. 2002. [Google Scholar]
- 56. Grice G, Miles J, inventors. Disposable scope cleaner and method of using same. US patent US20060293559. 2006. [Google Scholar]
- 57. Kato DT, inventor. Automated laparoscopic lens cleaner. US patient US6354992. 2002. [Google Scholar]
- 58. Poll WL, Huddleston MJ, Crisafulli CM, Landis A, Drach GP, inventors. Systems and methods for optimizing and maintaining visualization of a surgical field during the use of surgical scopes. US patent US20150005582. 2015. [Google Scholar]
- 59. Ito T, inventor. Endoscopic device including cleaning mechanism. US patent US20150073218. 2015. [Google Scholar]
- 60. Sasaki L, inventor. Laparoscopic lens cleaner. US patent US8047215. 2011. [Google Scholar]
- 61. Sasaki LS, inventor. Laparoscopic lens cleaner. US patent US20070282253. 2011. [Google Scholar]
- 62. Poll WL, Huddleston MJ, Post WJ, Ward TJ, Crisafulli CM, Landis A, inventors. Device for maintaining visualization with surgical scopes. US patent US20080319266. 2015. [Google Scholar]
- 63. Kawanishi T, inventor. Observation window cleaning device for endoscope. US patent US20070225566. 2007. [Google Scholar]
- 64. Fujimoto R, inventor. Endoscope cleaning sheath, and endoscope apparatus and endoscope comprising the cleaning sheath. US patent US20080188715. 2011. [Google Scholar]
- 65. Miyamoto S, inventor. Endoscope, distal end cap-equipped endoscope and endoscope cleaning sheath. US patent US20090253964. 2009. [Google Scholar]
- 66. Fujimoto R, inventor. Endoscope cleaning sheath. US patent US20110230716. 2014. [Google Scholar]
- 67. Miyamoto S, inventor. Endoscope, distal end cap-equipped endoscope and endoscope cleaning sheath. US patent US20120046524. 2012. [Google Scholar]
- 68. Miyamoto S, Suzuki A, inventors. Endoscope, distal end cap-equipped endoscope and endoscope cleaning sheath. US patent US20130204088. 2013. [Google Scholar]
- 69. Shalman M, inventor. Endoscope with cleaning optics. US patent US20040220452. 2008. [Google Scholar]
- 70. James AG, Chen J, Wills AA, inventors. Flow guide for an endoscope. European patent GB2481727. 2012. [Google Scholar]
- 71. James AG, Chen J, Wills AA, inventors. Flow guide for cleaning the end surface of an endoscope. European patent GB2474309. 2011. [Google Scholar]
- 72. Kawano H, inventor. Cleaning device for cleaning view window of endoscope. US patent US6409657. 2002. [Google Scholar]
- 73. Weisenburgh II William B, Hess CJ, Nobis RH, Griffith DB, Trusty RM, inventors. Apparatus and methods for cleaning the lens of an endoscope. US patent US20130217970. 2015. [Google Scholar]
- 74. Maguire MD, inventor. Endoscope cleansing catheter and method of use. US patent US5337730. 1994. [Google Scholar]
- 75. Weng TS, Tsai CH. Laser-induced backside wet cleaning technique for glass substrates. Appl Phys A. 2014;116:597-604. [Google Scholar]
- 76. Tam AC, Park HK, Grigoropoulos CP. Laser cleaning of surface contaminants. Appl Surf Sci. 1998;127:721-725. [Google Scholar]
- 77. Tam AC, Leung WP, Zapka W, Ziemlich W. Laser-cleaning techniques for removal of surface particulates. J Appl Phys. 1992;71:3515-3523. [Google Scholar]
- 78. Emmons DL, III, inventor. Dental mirror with mirror-cleaning suction. US patent US8133052. 2012. [Google Scholar]
- 79. Stowers IF. Advances in cleaning metal and glass surfaces to micron-level cleanliness. J Vacuum Sci Technol. 1978;15:751-754. [Google Scholar]
- 80. Cleaning Technologies Group. Frequently asked questions: ultrasonic cleaning. http://www.ctgclean.com/ultrasonic-cleaning-faq.php#ten. Accessed May 14, 2015.
- 81. Barnes AS, Pantano CG, Conzone SD. Surface cleaning and etching of rare-earth-doped phosphate glass. Paper presented at: International Symposium on Optical Science and Technology (International Society for Optics and Photonics); 2001: pp. 115-125. [Google Scholar]
- 82. McCaffrey NJ, inventor. Electromechanical in-situ cleaning of optical elements. US patent US20080188714. 2008. [Google Scholar]
- 83. Pal D, Peterson T, inventors. Method and apparatus for cleaning medical instruments and the like. Patent WO0053341. 2000. [Google Scholar]
- 84. Newton JP, IV, inventor. Method and apparatus for cleaning the field of view of an endoscopic lens. US patent US20120178995. 2012. [Google Scholar]
- 85. Bahls T, Froehlich FA, inventors. Camera system and method for cleaning a camera. US patent US20150216401. 2015. [Google Scholar]
- 86. Mertens JA. Vapor degreasing with chlorinated solvents. Metal Finishing. 1999;97(5):56-64. [Google Scholar]
- 87. Kumar A, Prasad M, Bhatt RB, et al. Laser shock cleaning of radioactive particulates from glass surface. Opt Lasers Eng. 2014;57:114-120. [Google Scholar]
- 88. Römich H, Mottner P, Hildenhagen J, Dickmann K, Hettinger G, Bornschein F. Comparison of cleaning methods for stained glass windows. Springer Proc Phys. 2005;100:157-161. [Google Scholar]
- 89. Zhao J, Yang XD. Photocatalytic oxidation for indoor air purification: a literature review. Building Environ. 2003;38:645-654. [Google Scholar]
- 90. Kasanen J, Suvanto M, Pakkanen TT. Self-cleaning, titanium dioxide based, multilayer coating fabricated on polymer and glass surfaces. J Appl Polym Sci. 2009;111:2597-2606. [Google Scholar]
- 91. Carp O, Huisman CL, Reller A. Photoinduced reactivity of titanium dioxide. Prog Solid State Chem. 2004;32:33-177. [Google Scholar]
- 92. Brunella MF, Diamanti MV, Pedeferri MP, Di Fonzo F, Casari CS, Bassi AL. Photocatalytic behavior of different titanium dioxide layers. Thin Solid Films. 2007;515:6309-6313. [Google Scholar]
- 93. Linsebigler AL, Lu GQ, Yates JT. Photocatalysis on TiO2 surfaces—principles, mechanisms, and selected results. Chem Rev. 1995;95:735-758. [Google Scholar]
- 94. Hagfeldt A, Gratzel M. Light-induced redox reactions in nanocrystalline systems. Chem Rev. 1995;95:49-68. [Google Scholar]
- 95. Kartini I, Santosa SJ, Febriyanti E, Nugroho OR, Yu H, Wang LZ. Hybrid assembly of nanosol titania and dodecylamine for superhydrophobic self-cleaning glass. J Nanoparticle Res. 2014;16(7). doi: 10.1007/s11051-014-2514-z. [DOI] [Google Scholar]
- 96. Ibusuki T, Takeuchi K. Toluene oxidation on UV-irradiated titanium dioxide with and without O2, NO2 or H2O at ambient temperature. Atmos Environ. 1986;20:1711-1715. [Google Scholar]
- 97. Cedillo-Gonzalez EI, Ricco R, Montorsi M, Montorsi M, Falcaro P, Siligardi C. Self-cleaning glass prepared from a commercial TiO2 nano-dispersion and its photocatalytic performance under common anthropogenic and atmospheric factors. Building Environ. 2014;71:7-14. [Google Scholar]
- 98. Ohtani B, Ogawa Y, Nishimoto S. Photocatalytic activity of amorphous-anatase mixture of titanium(IV) oxide particles suspended in aqueous solutions. J Phys Chem B. 1997;101:3746-3752. [Google Scholar]
- 99. Yu JG, Zhao XJ, Du JC, Chen WM. Preparation, microstructure and photocatalytic activity of the porous TiO2 anatase coating by sol-gel processing. J Sol-Gel Sci Technol. 2000;17:163-171. [Google Scholar]
- 100. Paz Y, Heller A. Photo-oxidatively self-cleaning transparent titanium dioxide films on soda lime glass: the deleterious effect of sodium contamination and its prevention. J Mater Res. 1997;12:2759-2766. [Google Scholar]
- 101. Nam HJ, Amemiya T, Murabayashi M, Itoh K. Photocatalytic activity of sol-gel TiO2 thin films on various kinds of glass substrates: the effects of Na+ and primary particle size. J Phys Chem B. 2004;108:8254-8259. [Google Scholar]
- 102. Raillard C, Hequet V, Le Cloirec P, Legrand J. Kinetic study of ketones photocatalytic oxidation in gas phase using TiO2-containing paper: effect of water vapor. J Photochem Photobiol A. 2004;163:425-431. [Google Scholar]
- 103. Coronado JM, Zorn ME, Tejedor-Tejedor I, Anderson MA. Photocatalytic oxidation of ketones in the gas phase over TiO2 thin films: a kinetic study on the influence of water vapor. Appl Catalysis B. 2003;43:329-344. [Google Scholar]
- 104. Obee TN, Hay SO. Effects of moisture and temperature on the photooxidation of ethylene on Titania. Environ Sci Technol. 1997;31:2034-2038. [Google Scholar]
- 105. Costacurta S, Dal Maso G, Gallo R, Guglielmi M, Brusatin G, Falcaro P. Influence of temperature on the photocatalytic activity of sol-gel TiO2 films. ACS Appl Mater Interfaces. 2010;2:1294-1298. [DOI] [PubMed] [Google Scholar]
- 106. Zorn ME, Tompkins DT, Zeltner WA, Anderson MA. Catalytic and photocatalytic oxidation of ethylene on titania-based thin-films. Environ Sci Technol. 2000;34:5206-5210. [Google Scholar]
- 107. Sam ED, Urgen M, Tepehan FZ, Gunay V. Self cleaning photoactive TiO2 coatings on SLS glasses by sol-gel dip coating. Key Eng Mater. 2004;264-268:407-410. [Google Scholar]
- 108. Li F, Li QM, Kim H. Spray deposition of electrospun TiO2 nanoparticles with self-cleaning and transparent properties onto glass. Appl Surface Sci. 2013;276:390-396. [Google Scholar]
- 109. Nejand BA, Sanjabi S, Ahmadi V. Sputter deposition of high transparent TiO2-xNx/TiO2/ZnO layers on glass for development of photocatalytic self-cleaning application. Appl Surface Sci. 2011;257:10434-10442. [Google Scholar]
- 110. Luo ZK, Xu J, Yang H, Gao JW. Self cleaning glasses using TiO2 coated films developed in China. J Sol-Gel Sci Technol. 2008;47:217-218. [Google Scholar]
- 111. Wang YH, Lu L, Yang HX, Che QD. Development of high dispersed TiO2 paste for transparent screen-printable self-cleaning coatings on glass. J Nanoparticle Res. 2013;15(1):1384. [Google Scholar]
- 112. Aarik J, Aidla A, Mandar H, Uustare T, Kukli K, Schuisky M. Phase transformations in hafnium dioxide thin films grown by atomic layer deposition at high temperatures. Appl Surface Sci. 2001;173(1-2):15-21. [Google Scholar]
- 113. Sonawane RS, Hegde SG, Dongare MK. Preparation of titanium(IV) oxide thin film photocatalyst by sol-gel dip coating. Mater Chem Phys. 2003;77:744-750. [Google Scholar]
- 114. Schmidt H, Naumann M, Muller TS, Akarsu M. Application of spray techniques for new photocatalytic gradient coatings on plastics. Thin Solid Films. 2006;502:132-137. [Google Scholar]
- 115. Paz Y, Luo Z, Rabenberg L, Heller A. Photooxidative self-cleaning transparent titanium-dioxide films on glass. J Mater Res. 1995;10:2842-2848. [Google Scholar]
- 116. Lejnieks J, Mourran A, Tillmann W, Keul H, Moller M. Thin film of poly(acrylic acid-co-allyl acrylate) as a sacrificial protective layer for hydrophilic self cleaning glass. Materials. 2010;3:3369-3384. [Google Scholar]
- 117. Yoldas BE. Investigations of porous oxides as an antireflective coating for glass surfaces. Appl Opt. 1980;19:1425-1429. [DOI] [PubMed] [Google Scholar]
- 118. Holland L. Vacuum Deposition of Thin Films. London, England: Chapman & Hall; 1970. [Google Scholar]
- 119. Macleod HA. Thin-Film Optical Filters. Boca Raton, FL: CRC Press; 2001. [Google Scholar]
- 120. Zhang XT, Sato O, Taguchi M, Einaga Y, Murakami T, Fujishima A. Self-cleaning particle coating with antireflection properties. Chem Mater. 2005;17:696-700. [Google Scholar]
- 121. Jung SC, Kim SJ, Imaishi N, Cho YI. Effect of TiO2 thin film thickness and specific surface area by low-pressure metal–organic chemical vapor deposition on photocatalytic activities. Appl Catalysis B. 2005;55:253-257. [Google Scholar]
- 122. Kao M, Chen H, Young S, Kung C, Lin C. The effects of the thickness of TiO2 films on the performance of dye-sensitized solar cells. Thin Solid Films. 2009;517:5096-5099. [Google Scholar]
- 123. Shin I, Seo H, Son MK, Kim JK, Prabakar K, Kim HJ. Analysis of TiO2 thickness effect on characteristic of a dye-sensitized solar cell by using electrochemical impedance spectroscopy. Curr Appl Phys. 2010;10:S422-S424. [Google Scholar]
- 124. Sakthivel S, Shankar M, Palanichamy M, Arabindoo B, Bahnemann D, Murugesan V. Enhancement of photocatalytic activity by metal deposition: characterisation and photonic efficiency of Pt, Au and Pd deposited on TiO2 catalyst. Water Res. 2004;38:3001-3008. [DOI] [PubMed] [Google Scholar]
- 125. Anpo M, Takeuchi M. The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J Catalysis. 2003;216:505-516. [Google Scholar]
- 126. Murugan K, Subasri R, Rao TN, Gandhi AS, Murty BS. Synthesis, characterization and demonstration of self-cleaning TiO2 coatings on glass and glazed ceramic tiles. Prog Organic Coatings. 2013;76:1756-1760. [Google Scholar]
- 127. Romeas V, Pichat P, Guillard C, Chopin T, Lehaut C. Degradation of palmitic (hexadecanoic) acid deposited on TiO2-coated self-cleaning glass: kinetics of disappearance, intermediate products and degradation pathways. New J Chem. 1999;23:365-373. [Google Scholar]
- 128. Hong HG, Kim YJ. Self-cleaning effect of solid immersion lens using photocatalyst TiO2 film for near-field recording. Japan J Appl Phys. 2008;47:5939-5943. [Google Scholar]
- 129. Medina-Valtierra J, Campos-Reyna SJ, Frausto-Reyes C, Calixto S, Ramirez-Ortiz J. Self-cleaning test of doped anatase-coated glass plates. Int J Chem Reactor Eng. 2007;5. [Google Scholar]
- 130. Medina-Valtierra J, Ramirez-Ortiz J, Frausto-Reyes C. Self-cleaning tests of Zn-Fe/anatase-coated glass plates under direct and diffuse sunlight. Int J Chem Reactor Eng. 2011;9. [Google Scholar]
- 131. Heller A. Chemistry and applications of photocatalytic oxidation of thin organic films. Acc Chem Res. 1995;28:503-508. [Google Scholar]
- 132. Verhelst J, Decroupet D, De Vos D. Catalytic self-cleaning coatings for thermal oxidation of organic deposits on glass. Catalysis Sci Technol. 2013;3:1579-1590. [Google Scholar]
- 133. Zoller U. Optimization of an industrial-chemical cleaning process for glass lenses. J Am Oil Chem Soc. 1983;60:628-633. [Google Scholar]
- 134. Urum K, Pekdemir T. Evaluation of biosurfactants for crude oil contaminated soil washing. Chemosphere. 2004;57:1139-1150. [DOI] [PubMed] [Google Scholar]
- 135. Urum K, Grigson S, Pekdemir T, McMenamy S. A comparison of the efficiency of different surfactants for removal of crude oil from contaminated soils. Chemosphere. 2006;62:1403-1410. [DOI] [PubMed] [Google Scholar]
- 136. Song S, Zhu L, Zhou W. Simultaneous removal of phenanthrene and cadmium from contaminated soils by saponin, a plant-derived biosurfactant. Environ Pollut. 2008;156:1368-1370. [DOI] [PubMed] [Google Scholar]
- 137. Guclu-Ustundag O, Mazza G. Saponins: properties, applications and processing. Crit Rev Food Sci. 2007;47:231-258. [DOI] [PubMed] [Google Scholar]
- 138. Lee VS, Chen CR, Liao YW, Tzen JT, Chang CI. Structural determination and DPPH radical-scavenging activity of two acylated flavonoid tetraglycosides in oolong tea (Camellia sinensis). Chem Pharm Bull. 2008;56:851-853. [DOI] [PubMed] [Google Scholar]
- 139. Ito S, Suda H, Maetani I. Use of oolong tea for colonoscopy (author’s English translation, in Japanese). Gastroenterol Endosc. 2007;49:3000-3001. [Google Scholar]
- 140. Komazawa Y, Amano Y, Yuki M, Fukuhara H, Kazumori H, Shizuku T. Oolong tea from a jet nozzle is a useful washing solution for lens cleansing of transnasal small-caliber esophagogastroduodenoscope. Gastrointest Endosc. 2009;69:Ab200. [DOI] [PubMed] [Google Scholar]
- 141. Yoshida N, Naito Y, Hirose R, et al. Risk of lens cloudiness during colorectal endoscopic submucosal dissection and ability of a novel lens cleaner to maintain and restore endoscopic view. Dig Endosc. 2015;27:609-617. [DOI] [PubMed] [Google Scholar]
- 142. Popovic M, Novakovic M, Mitric M, Zhang K, Bibic N. Structural, optical and electrical properties of argon implanted TiN thin films. Int J Refract Metals Hard Mater. 2015;48:318-323. [Google Scholar]
- 143. Lin SH, Wu YN, Lin YC, Yang YM. Design and fabrication of antireflective nanoparticulate thin films with superhydrophilic self-cleaning properties on glass substrate. J Taiwan Inst Chem Eng. 2011;42:852-859. [Google Scholar]
- 144. Verma LK, Sakhuja M, Son J, et al. Self-cleaning and antireflective packaging glass for solar modules. Renewable Energy. 2011;36:2489-2493. [Google Scholar]
- 145. Wenzel RN. Resistance of solid surfaces to wetting by water. Ind Eng Chem. 1936;28:988-994. [Google Scholar]
- 146. Katsumata K, Okazaki S, Cordonier CE, Shichi T, Sasaki T, Fujishima A. Preparation and characterization of self-cleaning glass for vehicle with niobia nanosheets. ACS Appl Mater Interfaces. 2010;2:1236-1241. [DOI] [PubMed] [Google Scholar]
- 147. Leem JW, Yu JS, Heo J, et al. Nanostructured encapsulation coverglasses with wide-angle broadband antireflection and self-cleaning properties for III-V multi-junction solar cell applications. Solar Energy Mater Solar Cells. 2014;120:555-560. [Google Scholar]
- 148. Lin T, Zhang X. Coated hydrophilic thin film on glasses using freeze drying-assisted sol-gel technique for self-cleaning building materials. Adv Mater Res. 2011;150-151:1484-1487. [Google Scholar]
- 149. Miao L, Su LF, Tanemura S, et al. Cost-effective nanoporous SiO2-TiO2 coatings on glass substrates with antireflective and self-cleaning properties. Appl Energy. 2013;112:1198-1205. [Google Scholar]
- 150. Nakata K, Sakai M, Ochiai T, Murakami T, Takagi K, Fujishima A. Antireflection and self-cleaning properties of a moth-eye-like surface coated with TiO2 particles. Langmuir. 2011;27:3275-3278. [DOI] [PubMed] [Google Scholar]
- 151. Nakata K, Terashima C, Fujishima A. Moth-eye-structured antireflective poly(methyl methacrylate) film with visible-light-responsive self-cleaning ability. Chem Lett. 2014;43:1511-1513. [Google Scholar]
- 152. Piispanen M, Hupa L. Comparison of self-cleaning properties of three titania coatings on float glass. Appl Surf Sci. 2011;258:1126-1131. [Google Scholar]
- 153. Pilkington. Pilkington Activ: questions and answers. http://www.pilkington.com/en-gb/uk/householders/types-of-glass/self-cleaning-glass/brochures. Accessed May 15, 2015.
- 154. Sakhuja M, Son J, Yang H, Bhatia CS, Danner AJ. Outdoor performance and durability testing of antireflecting and self-cleaning glass for photovoltaic applications. Solar Energy. 2014;110:231-238. [Google Scholar]
- 155. Sangchay W, Sikong L, Kooptarnond K, Jin W. Photocatalytic and self-cleaning properties of TiO2-Cu thin films on glass substrate. Mech Eng Mater. 2012;152-154:409-413. [Google Scholar]
- 156. Shi JJ, Yang EL. Non-UV driven self-cleaning and anti-fogging glasses prepared by ultrasonic nebulization of TiO2 hydrosol. Chem Eng Mater Properties. 2012;549:674-678. [Google Scholar]
- 157. Weng KW, Huang YP. Preparation of TiO2 thin films on glass surfaces with self-cleaning characteristics for solar concentrators. Surface Coatings Technol. 2013;231:201-204. [Google Scholar]
- 158. Yeo CI, Kang EK, Lee SK, Song YM, Lee YT. Efficiency enhancement of III-V triple-junction solar cell using nanostructured bifunctional coverglass with enhanced transmittance and self-cleaning property. IEEE Photonics J. 2014;6(3):1-9. [Google Scholar]
- 159. Zhao XJ, Zhao QN, Yu JG, Liu BS. Development of multifunctional photoactive self-cleaning glasses. J Non-Crystalline Solids. 2008;354:1424-1430. [Google Scholar]
- 160. Zhu W, Tong DL, Xu JB, Liu Y, Ma J. Multifunctional composite multilayer coatings on glass with self-cleaning, hydrophilicity and heat-insulating properties. Thin Solid Films. 2012;526:201-211. [Google Scholar]
- 161. Zhang JF, Zhu Y, Li ZH, Zhou W, Zheng JC, Yang DQ. Preparation of anti-reflection glass surface with self-cleaning and anti-dust by ammonium hydroxide hydrothermal method. Materials Express. 2015;5:280-290. [Google Scholar]
- 162. Ganbavle VV, Bangi UKH, Latthe SS, Mahadik SA, Rao AV. Self-cleaning silica coatings on glass by single step sol-gel route. Surface Coatings Technol. 2011;205:5338-5344. [Google Scholar]
- 163. Miwa M, Nakajima A, Fujishima A, Hashimoto K, Watanabe T. Effects of the surface roughness on sliding angles of water droplets on superhydrophobic surfaces. Langmuir. 2000;16:5754-5760. [Google Scholar]
- 164. Jiang YD, Smalley Y, Paris H, Hunt AT. Hypertransparent nanostructured superhydrophobic self-cleaning coatings on glass substrates. Clean Technol. 2008. http://www.ct-si.org/publications/proceedings/pdf/2008/694.pdf. Accessed April 28, 2017.
- 165. Wagner P, Furstner R, Barthlott W, Neinhuis C. Quantitative assessment to the structural basis of water repellency in natural and technical surfaces. J Exp Bot. 2003;54:1295-1303. [DOI] [PubMed] [Google Scholar]
- 166. Solga A, Cerman Z, Striffler BF, Spaeth M, Barthlott W. The dream of staying clean: lotus and biomimetic surfaces. Bioinspir Biomim. 2007;2:S126-S134. [DOI] [PubMed] [Google Scholar]
- 167. André R, Natalio F, Tahir MN, Berger R, Tremel W. Self-cleaning antimicrobial surfaces by bio-enabled growth of SnO2 coatings on glass. Nanoscale. 2013;5:3447-3456. [DOI] [PubMed] [Google Scholar]
- 168. Kougo T, Kanematsu H, Wada N, Hihara T, Minekawa M, Fujita Y. Metal coated glasses by sputtering and their microfouling properties. Irago Conference 2013. 2014;1585:160-163. [Google Scholar]
- 169. Halfpenny DR, Kane DM, Lamb RN, Gong B. Creation of adhesion resistant silica glass surfaces with ultraviolet laser cleaning. Paper presented at: Conference on Optoelectronic and Microelectronic Materials and Devices; 1996; Piscataway, NJ. [Google Scholar]
- 170. Zhang Z, Chao T, Chen S, Jiang S. Superlow fouling sulfobetaine and carboxybetaine polymers on glass slides. Langmuir. 2006;22:10072-10077. [DOI] [PubMed] [Google Scholar]
- 171. Xu LN, Ma PP, Yuan B, et al. Anti-biofouling contact lenses bearing surface-immobilized layers of zwitterionic polymer by one-step modification. RSC Advances. 2014;4:15030-15035. [Google Scholar]
- 172. Booth C, Wheeler P, Hancock J, Ximenes R, Patterson DE. Optical behavior of antibiofouling additives in environment-friendly coverglass materials for bio-sensors and solar panels. Polym Adv Technol. 2009;20:626-630. [Google Scholar]
- 173. Dineshram R, Subasri R, Somaraju KR, et al. Biofouling studies on nanoparticle-based metal oxide coatings on glass coupons exposed to marine environment. Colloids Surfaces B Biointerfaces. 2009;74:75-83. [DOI] [PubMed] [Google Scholar]
- 174. Strahle WJ, Perez CL, Martini MA, IEEE. Antifouling leaching technique for optical lenses. Paper presented at: OCEANS ’94. “Oceans Engineering for Today’s Technology and Tomorrow’s Preservation.” Proceedings; 1994. doi: 10.1109/OCEANS.1994.364133. [DOI] [Google Scholar]
- 175. Greco G, Lanero TS, Torrassa S, et al. Microtopography of the eye surface of the crab Carcinus maenas: an atomic force microscope study suggesting a possible antifouling potential. J R Soc Interface. 2013;10:20130122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Kitano H, Kondo T, Kamada T, Iwanaga S, Nakamura M, Ohno K. Anti-biofouling properties of an amphoteric polymer brush constructed on a glass substrate. Colloids Surfaces B Biointerfaces. 2011;88:455-462. [DOI] [PubMed] [Google Scholar]
- 177. Moore E, Delalat B, Vasani R, McPhee G, Thissen H, Voelcker NH. Surface-initiated hyperbranched polyglycerol as an ultralow-fouling coating on glass, silicon, and porous silicon substrates. ACS Appl Mater Interfaces. 2014;6:15243-15252. [DOI] [PubMed] [Google Scholar]
- 178. Bu IYY, Oei SP. Hydrophobic vertically aligned carbon nanotubes on Corning glass for self cleaning applications. Appl Surf Sci. 2010;256:6699-6704. [Google Scholar]
- 179. Camargo KC, Michels AF, Rodembusch FS, Kuhn MF, Horowitz F. Visible and near infrared, wide-angle, anti-reflection coatings with self-cleaning on glass. Opt Mater Express. 2012;2:969-977. [Google Scholar]
- 180. Kim YD, Shin JH, Cho JY, Choi HJ, Lee H. Nanosized patterned protective glass exhibiting high transmittance and self-cleaning effects for photovoltaic systems. Phys Stat Sol A Appl Mater Sci. 2014;211:1822-1827. [Google Scholar]
- 181. Li XY, He JH, Liu WY. Broadband anti-reflective and water-repellent coatings on glass substrates for self-cleaning photovoltaic cells. Mater Res Bull. 2013;48:2522-2528. [Google Scholar]
- 182. Mu Q, Li Y, Wang H, Zhang Q. Self-organized TiO2 nanorod arrays on glass substrate for self-cleaning antireflection coatings. J Colloid Interface Sci. 2012;365:308-313. [DOI] [PubMed] [Google Scholar]
- 183. Zhou G, He JH, Gao LJ, Ren TT, Li T. Superhydrophobic self-cleaning antireflective coatings on Fresnel lenses by integrating hydrophilic solid and hydrophobic hollow silica nanoparticles. RSC Advances. 2013;3:21789-21796. [Google Scholar]
- 184. van de Berg NJ, van den Dobbelsteen JJ, Jansen FW, Grimbergen CA, Dankelman J. Energetic soft-tissue treatment technologies: an overview of procedural fundamentals and safety factors. Surg Endosc. 2013;27:3085-3099. [DOI] [PubMed] [Google Scholar]
- 185. Kim A, Adamson G. Laparoscopic Laser Injury. Prevention and Management of Laparoscopic Surgical Complications. Miami, FL: Society of Laparoendoscopic Surgeons; 1999. [Google Scholar]
- 186. Clark DG, Tinston DJ. Acute inhalation toxicity of some halogenated and non-halogenated hydrocarbons. Hum Toxicol. 1982;1:239-247. [DOI] [PubMed] [Google Scholar]
- 187. Dick FD. Solvent neurotoxicity. Occup Environ Med. 2006;63:221-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Lynge E, Anttila A, Hemminki K. Organic solvents and cancer. Cancer Causes Control. 1997;8:406-419. [DOI] [PubMed] [Google Scholar]
- 189. Barrett WL, Garber SM. Surgical smoke: a review of the literature. Is this just a lot of hot air? Surg Endosc. 2003;17:979-987. [DOI] [PubMed] [Google Scholar]
- 190. Ulmer BC. The hazards of surgical smoke. AORN J. 2008;87:721-734. [DOI] [PubMed] [Google Scholar]
- 191. Bigony L. Risks associated with exposure to surgical smoke plume: a review of the literature. AORN J. 2007;86:1013-1020. [DOI] [PubMed] [Google Scholar]
- 192. de Boorder T, Verdaasdonk R, Klaessens J. The visualization of surgical smoke produced by energy delivery devices: significance and effectiveness of evacuation systems. Proc SPIE. 2007;6440:6440R. doi: 10.1117/12.701308. [DOI] [Google Scholar]
- 193. Sawchuk WS, Weber PJ, Lowy DR, Dzubow LM. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49. [DOI] [PubMed] [Google Scholar]
- 194. Ott DE. Laparoscopic hypothermia. J Laparoendosc Surg. 1991;1(3):127-131. [DOI] [PubMed] [Google Scholar]
- 195. Fraise AP, Lambert PA, Maillard JY. Russell, Hugo & Ayliffe’s Principles and Practice of Disinfection, Preservation & Sterilization. New York, NY: John Wiley; 2008. [Google Scholar]