Abstract
Background
The development of non-animal alternatives for skin sensitization potency prediction is dependent upon the availability of a sufficient dataset whose human potency is well characterized. Previously, establishment of basic categorization criteria for 6 defined potency categories, allowed 131 substances to be allocated into them entirely on the basis of human information.
Objectives
To supplement the original dataset with an extended range of fragrance substances.
Methods
A more fully described version of the original criteria was used to assess 89 fragrance chemicals, allowing their allocation into one of the 6 potency categories.
Results
None of the fragrance substances were assigned to the most potent group, category 1, whereas 11 were category 2, 22 were category 3, 37 were category 4, and 19 were category 5. Although none were identified as non-sensitizing, note that substances in category 5 also do not pass the threshold for regulatory classification.
Conclusions
The combined datasets of >200 substances placed into potency categories solely on the basis of human data provides an essential resource for the elaboration and evaluation of predictive non-animal methods.
The fundamental purpose of toxicological evaluation is to uncover substances that possess properties, rendering them a potential hazard to human health.1 However, the identification of such substances is often meaningless unless the strength of that hazard, often termed potency, is also characterized. With respect to the toxicological hazard known as skin sensitization, the simple identification of hazard has been ensured for many decades, and the key details were well documented.1,2 However, in recent decades, the concept of simultaneously measuring the relative potency of the identified hazard has also become central to the process of assessing the risk of skin sensitization.3–7 It is not germane to the present work to discuss the merits (or otherwise) of the risk assessment itself, save to note that it is well characterized and transparent, such that it is capable of critical scrutiny to move it into a second-generation version.8–10 What is pertinent is that the toxicological predictions of the relative potency of a skin sensitizer are actually meaningful in terms of the species of concern, that is, humans. To meet this challenge, a first publication (in this journal) detailed an approach to the subcategorization of chemicals into 1 of 6 potency classes, solely on the basis of human data, and then reported on the outcome for a total of 131 substances.11 Of these, only a small minority were fragrance chemicals, so that, in an associated follow-up, human data were presented for a small number of additional fragrance chemicals.12 In the present work, we have endeavored to extend the original series more substantially via the addition of information on a larger body of substances used as fragrances. In total, 89 chemicals were assessed because they had sufficient information to permit potency categorization using only human data. However, as a refinement to the previous publication, we have endeavored to offer a clearer explanation of the basis for individual classification, thereby enhancing the categorization outline provided in that original publication.11 It is anticipated that this additional set of substances will further assist those working to produce nonanimal models capable of predicting the relative human potency of newly identified skin sensitizing substances.
MATERIALS AND METHODS
The 89 substances considered are reported in Table 1, along with their chemical abstracts service (CAS) numbers. All materials were of the quality supplied to downstream users by the fragrance industry, thus ensuring that data generated using them were relevant to the real-life situation.
TABLE 1.
Outline of Potency Categorization Guidance
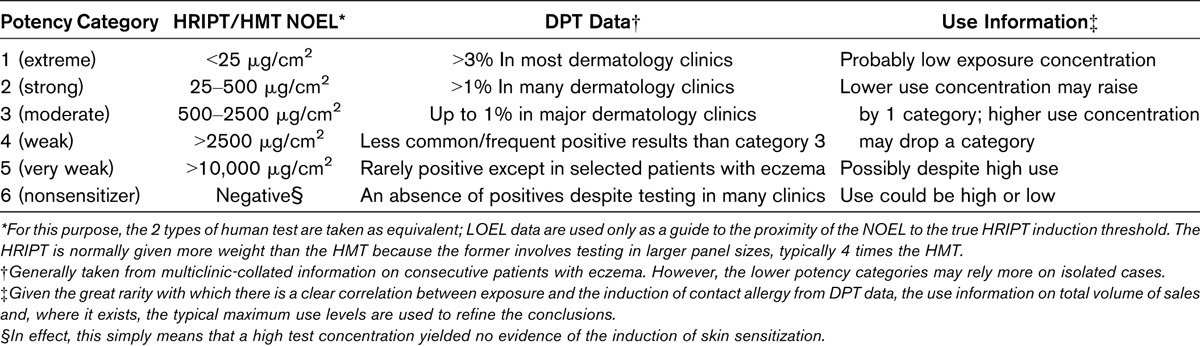
A decision on allocation to a category was achieved using information from experimental human studies, specifically the human repeated insult patch test (HRIPT), conducted according to the protocol previously published, or in a few instances, the human maximization test (HMT) as published by Kligman.13,14 Most data from these sources offer a no-effect level (NOEL), and where multiple data exist, the highest value has been taken. For a few substances, a lowest-effect level (LOEL) has been recorded. Accordingly, it is important to state that no new positive data have been generated for the purpose of this work—all of the LOEL data are derived from historic studies. The authors recognize that the conduct of new human studies to determine an LOEL for the induction of contact allergy is, by definition, unethical. Human repeated insult patch test and HMT studies as conducted by Research Institute for Fragrance Materials are of equivalent sensitivity and thus taken as interchangeable. The limited LOEL data provide a guide concerning the extent to which the NOEL data are close to the true threshold.
Indications concerning potential categorization were modified by information derived from a survey of diagnostic patch test (DPT) data from published clinical literature, with the existence of such information typically being indicated by the recording of a patch test concentration.15 Particular account also was taken of the important and comprehensive review of fragrance allergy already completed by the European Commission independent advisory body, the Scientific Committee on Consumer Safety (SCCS).16
To assist in understanding the process of potency categorization solely on the basis of human data, an outline of the criteria used is provided in Table 1. It is worth reinforcing here the key point that larger NOEL values equate to lower skin sensitization potency. Thus, where there are multiple values, unless there is compelling information to suggest a different strategy, the higher value should always be used. The converse argument would always apply to LOEL values, where the smaller value must be adopted. As always, the final decision on a category will have considered all of the available evidence. This includes DPT data, where this exists, judged against the use volume information. Diagnostic patch test data can be taken from the clinical literature and, for some of the materials here, from the SCCS review already mentioned.16
RESULTS
The outcome of the analysis on this set of fragrance substances is contained in Table 2. None of the substances were allocated to the highest, category 1, although for 2 materials, trans-2-hexenal and methyl 2-nonynoate, the decision was borderline, and so this is discussed in more detail later. Ultimately, along with 9 others, they were assigned to category 2. For the remainder, 22 were assigned to category 3, 37 were assigned to category 4, and 19 were assigned to category 5. None were assigned to category 6, the true nonsensitizers. To facilitate the understanding of the rationale, several of these are discussed to provide an exposition of how the criteria described in Table 1 and the previous publication are applied.10 None of the substances was regarded as entirely nonsensitizing; thus, category 6 was not represented.
TABLE 2.
List of Substances and HPC Conclusions
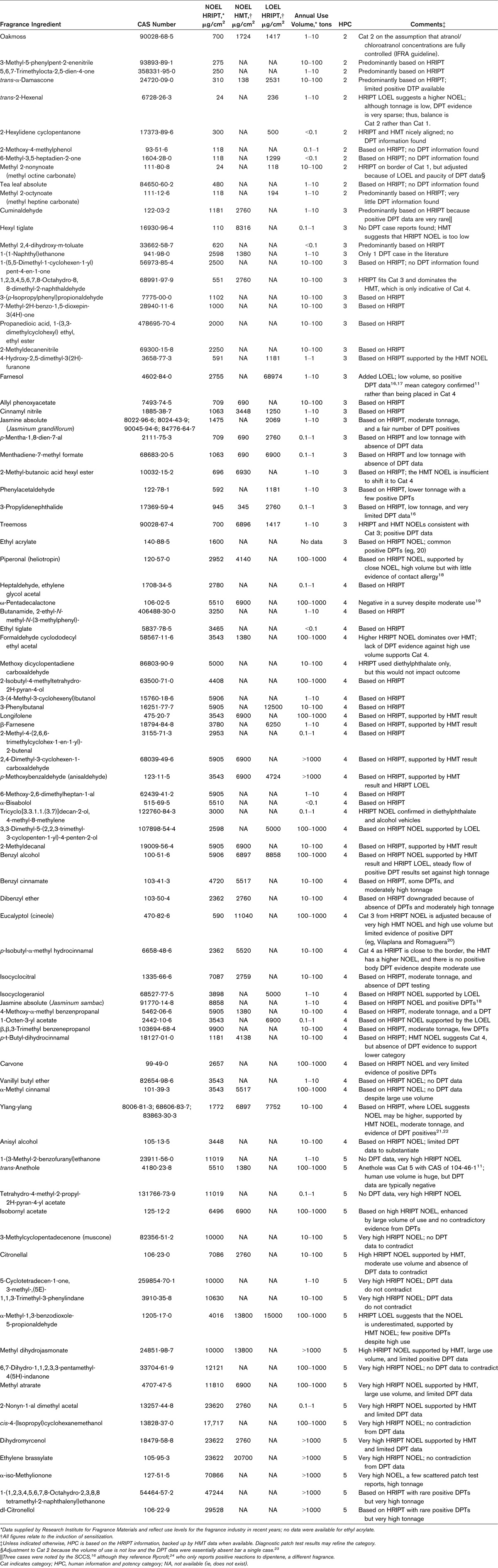
For a first example, trans-2-hexenal is considered. It has an HRIPT NOEL of 24 μg/cm2, which is only less than the threshold for category 1 (Table 1). However, the HRIPT LOEL is almost 10-fold higher, suggesting that the true NOEL is higher than the category 1 threshold. There is no HMT information to add to the mix; the remaining source of information for consideration is therefore DPT data. In this case, it is very sparse. A patch test concentration of 1% is suggested.15 However, a search on PubMed reveals an absence of any data, an outcome consistent with the conclusions of a European Commission advisory body report.16 Consequently, the decision must be that trans-2-hexenal is most appropriately placed into category 2. A similar logic was applied to methyl 2-nonynoate, supported by the occurrence of only a single positive patch test reaction in the literature.23
In comparison, the next example, farnesol, is somewhat less clear-cut. The HRIPT NOEL is close to category 3, but it is clearly in category 4. However, it is a well-known human contact allergen that is used in routine diagnostic testing as a component of fragrance mix II.17,18 The frequency of positive patch tests for a fragrance component that has rather low use volume was regarded as sufficient evidence to elevate farnesol into category 3.
1,2,3,4,5,6,7,8-Octahydro-8,8-dimethyl-2-naphthaldehyde was placed into category 3 on the basis of the view that the HRIPT, which in this case involved only more than 100 volunteers, would not be overridden by the HMT, which used only a quarter of the number and recorded an NOEL that was not too far from the category 3/4 border. Had the HMT value been much higher, as was the case with ylang-ylang, then the decision might have been different. However, in this latter case, the fact that the HRIPT NOEL was not as low (ie, relatively close to the category 3/4 border) and that the HMT is in category 4, together with the availability of HRIPT LOEL data well into category, made the final placement of ylang-ylang into category 4 a simple decision. It is worth noting that the moderate volume of use and occasional clinical evidence of positive reactions from normal use of ylang-ylang are also perfectly consistent with category 4.
For the final example, consider formaldehyde cyclododecyl ethyl acetal. This substance was placed into category 4, although the HMT NOEL suggested category 3. However, all of these studies involve a single dose level, so we do not know whether testing in the HMT at a higher concentration might also have proven negative and delivered a higher NOEL. That this would likely be the case is suggested by the HRIPT NOEL, which is clearly in category 4. There are no DPT data to contradict this categorization decision.
The decision to place a substance into category 5 typically was prompted by an NOEL value in excess of 10,000 μg/cm2 together with an absence of DPT data that would contradict this decision—a reasonable body of positive evidence, particularly if used volumes were not very high, would elevate a substance to category 4. However, in a couple of instances (trans-anethole and isobornyl acetate), NOEL values a little lower than 10,000 μg/cm2, associated with category 4, have been combined with knowledge of a very high volume of use (for many years) and an absence of DPT results to associate the materials with category 5.16
DISCUSSION
Predictive toxicology is only of value if genuine human hazards are correctly identified, characterized, and assessed. It has long been recognized that in vivo methods have valuable predictive value regarding skin sensitization hazards.2,25,26 More recently, integrated testing strategies involving nonanimal models have been presented as performing to a similar standard.27–29 However, the characterization and assessment of identified skin sensitization hazards, particularly with respect to their relative potency, remains a weakness.30,31 Only the LLNA (and specifically the derived EC3 value) offered an estimation of relative skin sensitization potency with some basis for demonstrating its correlation with human data.32–34 The challenge of developing integrated testing strategies with nonanimal assays is outside the scope of this article, but for those engaged in such work, an essential need is a substantial catalog of chemicals categorized on the basis of their relative potency in humans. A first effort in this respect involving 131 chemicals has already been offered.11 The data in the present publication extend this work with a further 89 substances, with the small overlap meaning that the total data set now totals well more than 200 materials. This combined data set offers a broad distribution into 6 potency categories, with most substances in the more difficult to predict intermediate, lower-potency, categories 3 to 5 (see Fig. 1). It is our view that, taken together, these comprise a valuable basis for the continued development of nonanimal approaches to the prediction of human skin sensitization potency.
Figure 1.
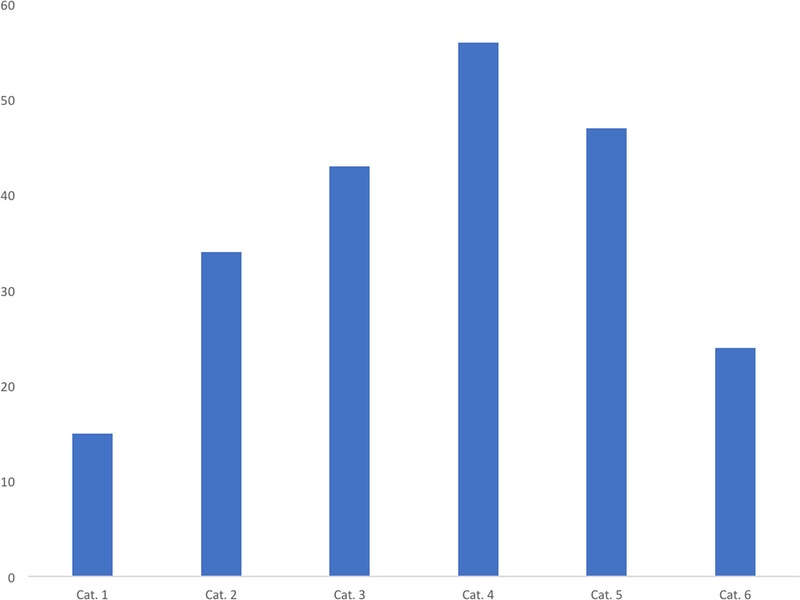
How the potency categories are populated. This figure combines the information from the first publication11 with the present data to show the number of substances placed into each of the 6 categories.
To complete this discussion, it is essential to remind the reader of significant caveats not least that much of the categorization depends on judgment. It is hoped that the reader might regard this as expert judgment, but even then, the human data on which this is based are not of the standard on which toxicological assessment would normally be founded. Human repeated insult patch test and HMT data, the primary drivers of conclusions on potency categorization, are derived from small populations of healthy volunteers. Note that the volunteers are healthy and not recruited from a specially sensitive subpopulation that impacts relative potency indications. The DPT data represent ad hoc collations of information from dermatology clinics whose original purpose was to assist in a correct diagnosis for individual patient care. All of these sources contain imperfections and uncertainties that cannot, and never will, be eliminated, thus “caveat emptor”—the use of the data must also involve an acceptance by the user that these categorizations are the best that can be achieved. Any nonanimal assay-integrated testing strategy that achieves a predictive accuracy against this data set of more than 90% is, by definition, likely to be flawed as a result of overfitting to imperfect data.
Footnotes
A.M.A., R.P., and D.O.B. are all full-time employees of the Research Institute for Fragrance Materials. D.A.B. was compensated for his work in the preparation of this manuscript.
The authors have no funding or conflicts of interest to declare.
REFERENCES
- 1.Ballantyne B, Myers T, Syversen T. General and Applied Toxicology. 3rd ed Chichester, UK: Wiley; 2009. [Google Scholar]
- 2.Thyssen JP, Giménez-Arnau E, Lepoittevin JP, et al. The critical review of methodologies and approaches to assess the inherent skin sensitization potential (skin allergies) of chemicals. Parts I and II. Contact Dermatitis 2012;66(Suppl 1):11–24. [DOI] [PubMed] [Google Scholar]
- 3.Kimber I, Basketter DA. Contact sensitization: a new approach to risk assessment. Hum Ecol Risk Assess 1997;3:385–395. [Google Scholar]
- 4.Basketter DA, Lea LJ, Cooper K, et al. A comparison of statistical approaches to the derivation of EC3 values from local lymph node assay dose responses. J Appl Toxicol 1999;19:261–266. [DOI] [PubMed] [Google Scholar]
- 5.Basketter DA, Gerberick F, Kimber I. The local lymph node assay EC3 value: status of validation. Contact Dermatitis 2007;57:70–75. [DOI] [PubMed] [Google Scholar]
- 6.Gerberick GF, Robinson MK, Felter S, et al. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis 2001;45:333–340. [DOI] [PubMed] [Google Scholar]
- 7.Api AM, Basketter DA, Cadby PA, et al. Dermal sensitization quantitative risk assessment (QRA) for fragrance ingredients. Regul Toxicol Pharmacol 2008;52:3–23. [DOI] [PubMed] [Google Scholar]
- 8.SCCS. (2008) Opinion on dermal sensitisation quantitative risk assessment. Available at: https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_135.pdf. Accessed December 29, 2016.
- 9.Basketter D, Safford B. Skin sensitization quantitative risk assessment: a review of underlying assumptions. Regul Toxicol Pharmacol 2016;74:105–116. [DOI] [PubMed] [Google Scholar]
- 10.IDEA Project. Final report on the QRA2. September 2016. Available at: http://www.ideaproject.info/uploads/Modules/Documents/qra2-dossier-final--september-2016.pdf. Accessed December 29, 2016.
- 11.Basketter DA, Alépée N, Ashikaga T, et al. Categorization of chemicals according to their relative human skin sensitizing potency. Dermatitis 2014;25:11–21. [DOI] [PubMed] [Google Scholar]
- 12.Api AM, Basketter D, Lalko J. Correlation between experimental human and murine skin sensitization induction thresholds. Cut Ocul Toxicol 2015;34:298–302. [DOI] [PubMed] [Google Scholar]
- 13.Politano VT, Api AM. The research institute for fragrance materials' human repeated insult patch test protocol. Regul Toxicol Pharmacol 2008;52:35–38. [DOI] [PubMed] [Google Scholar]
- 14.Kligman AM. The identification of contact allergens by human assay: III. The Maximization Test: a procedure for screening and rating contact sensitizers. 1966. J Invest Dermatol 1989;92:151S. [DOI] [PubMed] [Google Scholar]
- 15.De Groot AC. Patch Testing. 3rd ed Wapserveen, the Netherlands: Acdegroot Publishing; 2008. [Google Scholar]
- 16.Scientific Committee on Consumer Safety, 2012. Opinion on fragrance allergens in cosmetic products. Adopted at the 15th Plenary Meeting, 26–27 June, 2012. Available at: http://ec.europa.eu/health//sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_102.pdf. Accessed December 21, 2016.
- 17.Nardelli A, Carbonez A, Drieghe J, et al. Results of patch testing with fragrance mix 1, fragrance mix 2, and their ingredients, and Myroxylon pereirae and colophonium, over a 21-year period. Contact Dermatitis 2013;68:307–313. [DOI] [PubMed] [Google Scholar]
- 18.Frosch PJ, Johansen JD, Menné T, et al. Further important sensitizers in patients sensitive to fragrances: II. Reactivity to essential oils. Contact Dermatitis 2002;47:279–287. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura M, Ishihara M, Itoh M, et al. Results of patch tests conducted on cosmetic ingredients between 1979 and 1982. Skin Research 1984;26:945–954. [Google Scholar]
- 20.Vilaplana J, Romaguera C. Allergic contact dermatitis due to eucalyptol in an anti-inflammatory cream. Contact Dermatitis 2000;43:118. [PubMed] [Google Scholar]
- 21.Warshaw EM, Maibach HI, Taylor JS, et al. North American Contact Dermatitis Group patch test results: 2011–2012. Dermatitis 2015;26:49–59. [DOI] [PubMed] [Google Scholar]
- 22.de Groot AC, Schmidt E. Essential oils, part VI: sandalwood oil, ylang-ylang oil, and jasmine absolute. Dermatitis 2017;28:14–21. [DOI] [PubMed] [Google Scholar]
- 23.English JS, Rycroft RJ. Allergic contact dermatitis from methyl heptine and methyl octine carbonates. Contact Dermatitis 1988;18:174–175. [DOI] [PubMed] [Google Scholar]
- 24.Rycroft RJ. Allergic contact dermatitis from dipentene in honing oil. Contact Dermatitis 1980;6:325–329. [DOI] [PubMed] [Google Scholar]
- 25.Botham PA, Basketter DA, Maurer T, et al. Skin sensitization—a critical review of predictive test methods in animals and man. Food Chem Toxicol 1991;29:275–286. [DOI] [PubMed] [Google Scholar]
- 26.Gerberick GF, Ryan CA, Kimber I, et al. Local lymph node assay: validation assessment for regulatory purposes. Am J Contact Dermatitis 2000;11:3–18. [DOI] [PubMed] [Google Scholar]
- 27.Basketter D, Ashikaga T, Casati S, et al. Alternatives for Skin sensitisation testing and assessment. Regul Toxicol Pharmacol 2015;73:660–666. [DOI] [PubMed] [Google Scholar]
- 28.Urbisch D, Mehling A, Guth K, et al. Assessing skin sensitization hazard in mice and men using non-animal test methods. Regul Toxicol Pharmacol 2015;71:337–351. [DOI] [PubMed] [Google Scholar]
- 29.van der Veen JW, Rorije E, Emter R, et al. Evaluating the performance of integrated approaches for hazard identification of skin sensitizing chemicals. Regul Toxicol Pharmacol 2014;69:371–379. [DOI] [PubMed] [Google Scholar]
- 30.Adler S, Basketter DA, Creton S, et al. Alternative (non-animal) methods for cosmetics testing: current status and future prospects—2010. Arch Toxicol 2011;85:367–485. [DOI] [PubMed] [Google Scholar]
- 31.Leist M, Hasiwa N, Rovida C, et al. Consensus report on the future of animal-free systemic toxicity testing. ALTEX 2014;31:341–356. [DOI] [PubMed] [Google Scholar]
- 32.Schneider K, Akkan Z. Quantitative relationship between the local lymph node assay and human skin sensitization assays. Regul Toxicol Pharmacol 2004;39:245–255. [DOI] [PubMed] [Google Scholar]
- 33.Basketter DA, Clapp C, Jefferies D, et al. Predictive identification of human skin sensitization thresholds. Contact Dermatitis 2005;53:260–267. [DOI] [PubMed] [Google Scholar]
- 34.Basketter DA, McFadden JP. Cutaneous allergies. In: Dietert RR, Luebke RW. eds. Immunotoxicity, Immune Dysfunction and Chronic Disease. New York, NY: Humana Press; 2012:103–126. [Google Scholar]


