Abstract
Objective
The aim of this study was to further elucidate the mechanisms of dual-phase technetium-99m methoxyisobutylisonitrile (99mTc-MIBI) parathyroid imaging by exploring the association between early uptake results (EUR), delayed uptake results (DUR), and the retention index (RI) in dual-phase 99mTc-MIBI parathyroid imaging and P glycoprotein (P-gp), multidrug resistance-associated protein 1 (MRP1), and glutathione S-transferase-π (GST-π) expression in hyperparathyroidism (HPT).
Patients and methods
Preoperative dual-phase (early and delayed) 99mTc-MIBI imaging was performed on 74 patients undergoing parathyroidectomy for HPT. EUR, DUR, and RI were calculated. P-gp, MRP1, and GST-π expressions were assessed using immunohistochemistry in resected tissue from HPT and control patients. The association between P-gp, MRP1, and GST-π expressions and EUR, DUR, and RI in HPT was evaluated.
Results
The positive rate of dual-phase 99mT c-MIBI imaging was 91.89% (68/74) and the false-negative rate was 8.11% (6/74). P-gp and GST-π expressions were higher in tissues resected from control compared with HPT patients (47.37 and 81.5%, P<0.05); there was no difference in MRP1. EUR were associated with P-gp and GST-π expressions, and DUR were associated with MRP1 expression. There was a significant difference in MRP1 expression between RI greater than or equal to 0 and RI less than 0. There was no relationship between the sensitivity of dual-phase 99mTc-MIBI imaging and P-gp, MRP1, and GST-π expressions in resected parathyroid tissue. The six false-negative HPT cases consisted of three P-gp (−)/MRP1 (−) tissues, three P-gp (−)/GST-π (−) tissues, and four MRP1 (−)/GST-π (−) tissues.
Conclusion
As P-gp and GST-π expressions were higher in tissues resected from control compared with HPT patients, 99mTc-MIBI may wash out faster from normal parathyroid tissue surrounding the lesion compared with the lesion itself, facilitating detection.
Keywords: glutathione S-transferase-π, hyperparathyroidism, multidrug resistance-associated protein 1, parathyroid scintigraphy, P-glycoprotein, technetium-99m methoxyisobutylisonitrile
Introduction
Hyperparathyroidism (HPT) is a generalized disturbance of calcium and phosphate metabolism that occurs because of the oversecretion of parathyroid hormone (PTH). HPT is characterized by increased serum PTH and calcium levels and decreased serum phosphorus levels. HPT is usually caused by parathyroid adenoma or hyperplastic glands, and rarely by parathyroid carcinomas or parathyroid cysts. In ~85% of cases, primary HPT is attributable to a single benign adenoma 1, and parathyroidectomy is the only effective treatment. In these patients, accurate location of the adenoma is critical for successful surgical intervention. Evidence suggests that B ultrasound, computed tomography (CT), and MRI have little clinical value in imaging before parathyroidectomy, whereas radionuclide scintigraphy facilitates functional imaging of the parathyroid and is considered the superior imaging modality for preoperative parathyroid localization, especially in ectopic HPT 2,3. Technetium-99m methoxyisobutylisonitrile (99mTc-MIBI) was first applied to parathyroid imaging in 1989 4, and in 1992, dual-phase 99mTc-MIBI imaging was used to diagnose HPT 5. Since then, dual-phase 99mTc-MIBI imaging has shown a high detection rate for parathyroid adenoma, with sensitivity and specificity reported at 39–90% and 94%, respectively 5–10.
99mTc-MIBI is a lipophilic cation complex; its uptake by a tissue represents the presence of actively functioning mitochondria, and is therefore a measure of a tissue’s oxidative burden 11. The mechanism of dual-phase 99mTc-MIBI imaging in HPT involves uptake of 99mTc-MIBI by mitochondria-rich oxyphilic cells in parathyroid adenomas 8,12, whereby 99mTc-MIBI tracer uptake has been correlated with oxyphilic cell content, volume of parathyroid lesions, and the functional status of parathyroid adenomas 13,14. Furthermore, 99mTc-MIBI is more rapidly washed out from healthy thyroid tissue than abnormal parathyroid glands, providing good target-to-background activity 5,15,16. Although this evidence provides an explanation for the mechanism of dual-phase 99mTc-MIBI parathyroid imaging, it is likely that other factors are involved. As preoperative localization of abnormal parathyroid glands by dual-phase 99mTc-MIBI imaging may improve patient outcomes and reduce the duration and cost of surgery for HPT, further investigations are warranted 17,18.
Tissue expression of multidrug-resistant proteins, such as P glycoprotein (P-gp) and multidrug resistance-associated protein 1 (MRP1), may play a role in the mechanism of dual-phase 99mTc-MIBI parathyroid imaging. Multidrug-resistant proteins are members of the ATP-binding cassette transporter family, which move drugs and their metabolites across cell membranes 7. In-vivo and in-vitro studies 19–21 show that 99mTc-MIBI is a suitable transport substrate for P-gp 22,23 and MRP1 20, and overexpression of P-gp or MRP1 can result in an efflux of 99mTc-MIBI from tumor cells and lead to false-negative results in dual-phase 99mTc-MIBI imaging. However, currently, the association between P-gp and MRP1 expression and false-negative results in dual-phase 99mTc-MIBI imaging is controversial 7,19,24–26.
The glutathione S-transferase (GST) system is involved in the metabolism of various cytotoxic agents and increased drug resistance 26,27. GST is a multifunctional protein and GST-π is predominantly expressed in tumors. To the authors’ knowledge, there are no reports on the association between 99mTc-MIBI efflux and GST-π expression in the parathyroid.
The aim of this study was to further elucidate the mechanisms of dual-phase 99mTc-MIBI parathyroid imaging by exploring the association between early uptake results (EUR), delayed uptake results (DUR), and the retention index (RI) in dual-phase 99mTc-MIBI parathyroid imaging and P-gp, MRP1, and GST-π expressions in HPT.
Patients and methods
Study design
Patients diagnosed with HPT undergoing a parathyroidectomy at our hospital from 2011 to 2015 were eligible for this study. Control tissues were obtained from patients who had their parathyroid glands resected during thyroidectomy for thyroid cancer. Control patients had no history or clinical symptoms of parathyroid disease, and normal serum levels of PTH, calcium, and phosphorus. Dual-phase 99mTc-MIBI parathyroid scintigraphy (planar and SPECT/CT) was performed in HPT patients before parathyroidectomy. The presence of P-gp, MRP1, and GST-π proteins was investigated in resected HPT and control parathyroid tissues by immunohistochemistry. The protocol for this study was approved by the Ethics Committee of the Firth Affiliated Hospital, School of Medicine, Xi’an Jiaotong University (Xi’an, China), and written informed consent was obtained from all participants.
Dual-phase 99mTc-MIBI parathyroid and SPECT/CT imaging
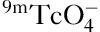 was obtained from a commercial 99Mo/99mTc generator (Beijing Atom High Tech Co. Ltd, Beijing, China). MIBI was obtained from the Jiangsu Atom Medicine Research Institute, Jiangyuan Pharmaceutical Factory (Wuxi, China). Labeling was performed according to the manufacturer’s instructions. Labeling efficiency was more than 95%. Overall, 740–1110 MBq (20–30 mCi) 99mTc-MIBI was injected intravenously into all HPT patients. After 15 min, dual-phase planar static imaging of the cervical and thoracic area in the anterior view was performed (early phase) using a large-field gamma camera (Siemens, Erlangen, Germany) with a low-energy high-resolution collimator, a 20% energy window centered at a 140-keV photopeak, a 128×128 matrix size, a 1.45 zoom factor, and 500 k counts/view. The delayed phase was performed 120 min after the 99mTc-MIBI injection. SPECT/CT fusion tomography was performed 30 min after the 99mTc-MIBI injection using a Symbia T16 scanner (Siemens, Erlangen, Germany).
was obtained from a commercial 99Mo/99mTc generator (Beijing Atom High Tech Co. Ltd, Beijing, China). MIBI was obtained from the Jiangsu Atom Medicine Research Institute, Jiangyuan Pharmaceutical Factory (Wuxi, China). Labeling was performed according to the manufacturer’s instructions. Labeling efficiency was more than 95%. Overall, 740–1110 MBq (20–30 mCi) 99mTc-MIBI was injected intravenously into all HPT patients. After 15 min, dual-phase planar static imaging of the cervical and thoracic area in the anterior view was performed (early phase) using a large-field gamma camera (Siemens, Erlangen, Germany) with a low-energy high-resolution collimator, a 20% energy window centered at a 140-keV photopeak, a 128×128 matrix size, a 1.45 zoom factor, and 500 k counts/view. The delayed phase was performed 120 min after the 99mTc-MIBI injection. SPECT/CT fusion tomography was performed 30 min after the 99mTc-MIBI injection using a Symbia T16 scanner (Siemens, Erlangen, Germany).
Immunohistochemistry stain
All resected HPT and control parathyroid tissues were immediately fixed in 10% formalin, embedded in paraffin, and sliced into 4 μm sections. Primary antibodies were the mouse anti-human monoclonal antibodies C494 (anti-P-gp), QCRL1 (anti-MRP1, mouse no. ab3369; Abcam, Shanghai, China), and 353-10 (anti-GST-π, EIivision super kit; Fuzhou Maixin Biotechnology Development Co. Ltd., Fuzhou, China). P-gp, MRP1, and GST-π proteins were detected using the EIivision super kit, according to the manufacturer’s instructions. Positive controls were tumor tissue samples known to stain positive for P-gp, MRP1, or GST-π; negative controls were represented by replacing the primary antibody with PBS.
Evaluation of dual-phase 99mTc-MIBI imaging
Data were evaluated using visual and semiquantitative analysis. For visual evaluation, dual-phase 99mTc-MIBI images were interpreted independently by two experienced nuclear medicine physicians who were blinded both to the surgical results and to histopathologic diagnoses. A positive dual-phase 99mTc-MIBI parathyroid image for HPT was defined as a focal concentration of 99mTc-MIBI in the early phase, which became increasingly concentrated or showed a fixed concentration in the delayed phase. For semiquantitative analysis of dual-phase 99mTc-MIBI positive imaging, a region of interest was defined manually on the lesion in early and delayed imaging; an identical background region of interest was drawn over the deltoid muscle on the contralateral side. The uptake ratio and RI were calculated using the formulae: uptake ratio=mean lesion count/mean contralateral tissue count, RI=(DUR−EUR)/EUR 27,28. No semiquantitative analysis was carried out for 99mTc-MIBI negative imaging 29.
Scoring of immunoreactivity
Positive P-gp or MRP1 expression was observed on the cell membrane and/or the cytoplasm (Fig. 1), whereas GST-π was present in the cytoplasm and nucleus (Fig. 2). Immunohistochemical staining was evaluated independently by two investigators using the intensity and the degree of stain. Semiquantification was performed under a transmission electron microscope by randomly selecting 10 high-power fields and counting 100 cells in each field. The intensity of nuclear stain was scored as 0, achromatic; 1, light yellow; 2, brownish yellow; or 3, brown. The degree of nuclear stain was scored as 1 when 0–25% of the nuclei were stained, 2 when 26–75% of the nuclei were stained, and 3 when 76% or more of the nuclei were stained 30. Both parameters were summed to obtain a final score: less than or equal to 4 was considered a negative result and greater than or equal to 5 was considered a positive result.
Fig. 1.
P-gp expression in normal parathyroid (a) and primary hyperparathyroidism (b). P-gp, P-gylcoproetin.
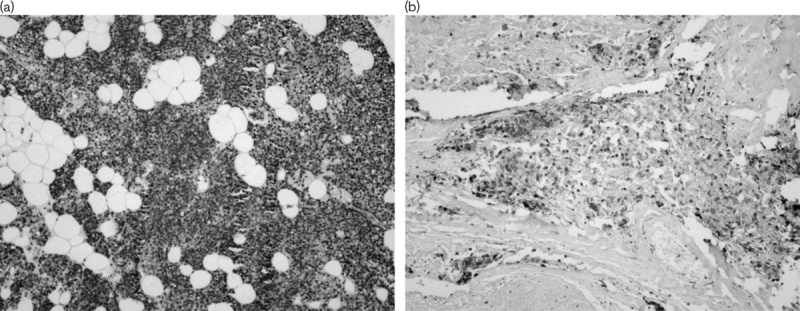
Fig. 2.
GST-π expression in normal parathyroid (a) and primary hyperparathyroidism (b). GST-π, glutathione S-transferase-π.
Statistical analysis
Data were analyzed using SPSS, version 18.0 (SPSS Inc., Chicago, Illinois, USA). Continuous data are expressed as mean±SD. Between-sample differences were evaluated using a group-design t-test; differences between multiple samples in the group design were assessed using single-factor analysis of variance (one-way analysis of variance). The χ2-statistic was used to compare categorical variables. Statistical significance was set at P less than 0.05.
Results
A total of 74 patients diagnosed with HPT undergoing a parathyroidectomy at our hospital were included in this study. Among these patients, there were 28 men and 46 women; patients’ mean age was 48.6±15.6 (range: 10–77) years. Preoperative dual-phase 99mTc-MIBI parathyroid scintigraphy (planar and SPECT/CT) was performed to locate the lesion causing HPT. Postoperative pathology was as follows: parathyroid adenoma: 23 cases, parathyroid hyperplasia: 46 cases, parathyroid carcinoma: four cases, and parathyroid cyst: one case. The control group included 39 samples of normal parathyroid tissue. Among the control patients, there were 13 men and 26 women. The mean age of the patients in the control group was 40.15±12.91 (range: 15–68) years.
99mTc-MIBI imaging
The positive rate of dual-phase 99mTc-MIBI imaging was 91.89% (68/74) and the false-negative rate was 8.11% (6/74). Using RI greater than or equal to 0 as the semiquantitative threshold for the presence of HPT in the 68 positive cases in dual-phase 99mTc-MIBI imaging, the true-positive and false-negative rate was 67.65% (46/68) and 32.35% (22/68), respectively.
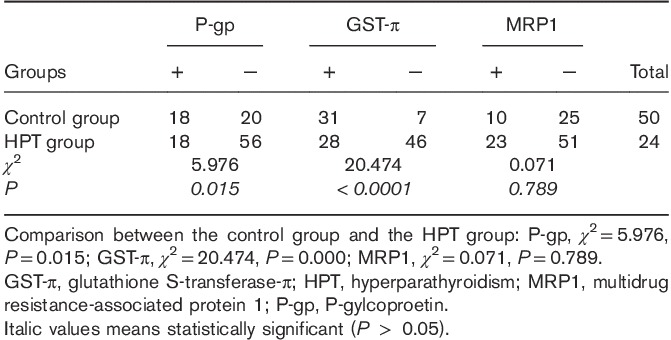
P-gp, GST-π, and MRP1 expressions
P-gp, MRP1, and GST-π were detected in parathyroid tissues resected from the control and HPT groups (Table 1). The rate of P-gp [control: 47.37% (18/38) vs. HPT: 24.32% (18/74)] and GST-π positive expression [control: 81.58% (31/38) vs. HPT: 37.84% (28/74)] were significantly lower in tissues resected from the HPT group compared with the control group (χ2=5.976, P=0.015 and χ2=20.474, P<0.001). There was no significant difference in the rate of MRP1-positive expression [control: 28.57% (10/35) vs. HPT: 31.08% (23/74)] (χ2=0.071, P=0.789). In the control group, the rate of GST-π-positive expression was significantly higher than the rate of P-gp-positive (χ2=10.0, P=0.002) or MRP1-positive (χ2=21.9, P<0.0001) expression, but there was no significant difference in the rate of P-gp-positive and MRP1-positive expressions (χ2=2.75 P=0.097). There were no significant differences in the rates of P-gp-positive, MRP1-positive, and GST-π-positive expressions in the HPT group (χ2=3.173, P=0.205).
Table 1.
P-gp, MRP1, and GST-π expressions in hyperparathyroidism and control tissues
RI in 99mTc-MIBI imaging and P-gp, MRP1, and GST-π expressions in HPT
The correlations between EUR and DUR in dual-phase 99mTC-MIBI imaging and P-gp, MRP1, and GST-π expressions in parathyroid tissue resected from the HPT group are shown in Table 2. EUR were significantly higher in the parathyroid of HPT patients with resected tissues positive for P-gp or GST-π expression than those negative for P-gp or GST-π expression. The relationships between dual-phase 99mTc-MIBI imaging and P-gp/MRP1, P-gp/GST-π, and GST-π/MRP1 expressions in HPT are shown in Tables 3–5, respectively. The six false-negative cases consisted of three P-gp (−)/MRP1 (−) tissues, three P-gp (−)/GST-π (−) tissues, and four MRP1 (−)/GST-π (−) tissues. The relationship between RI in dual-phase 99mTc-MIBI imaging and the expression of P-gp, MRP1, and GST-π in resected tissues is shown in Table 6. There was a significant difference in MRP1 expression between the RI greater than or equal to 0 and the RI less than 0 groups.
Table 2.
Relationship between early uptake results, delayed uptake results, and retention index and P-gp, MRP1, and GST-π expressions in hyperparathyroidism
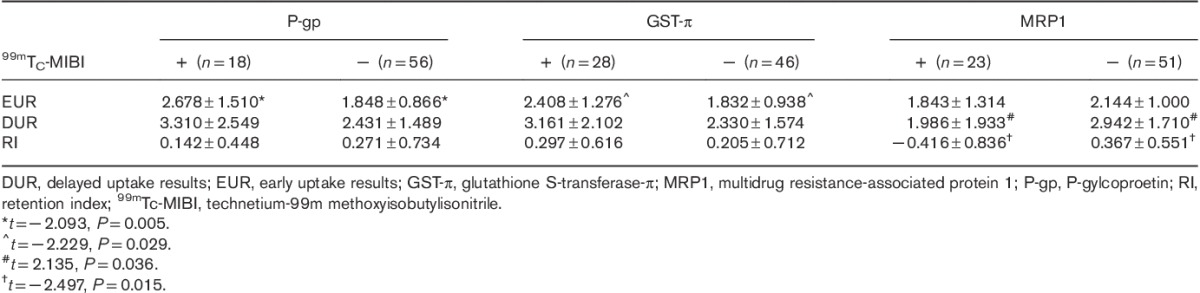
Table 3.
Relationship between dual-phase 99mTc-MIBI imaging and P-gp and MRP1 expressions in hyperparathyroidism
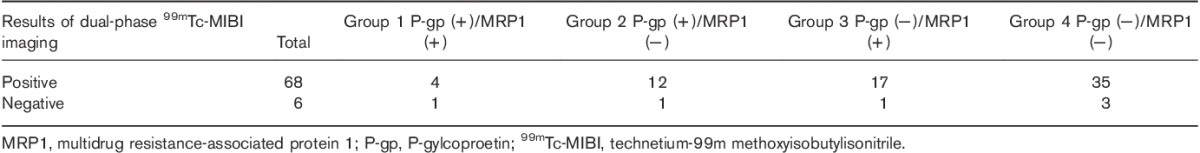
Table 5.
Relationship between dual-phase 99mTc-MIBI imaging and GST-π and MRP1 expressions in hyperparathyroidism
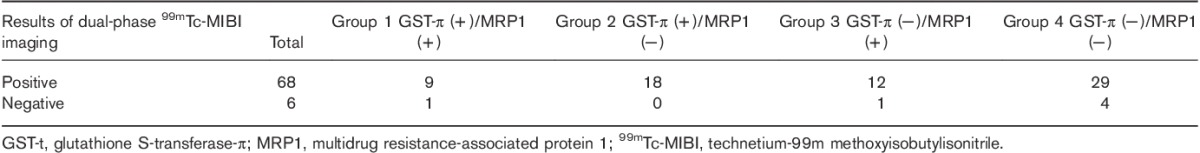
Table 6.
Relationship between retention index and P-gp, MRP1, and GST-π expressions in hyperparathyroidism
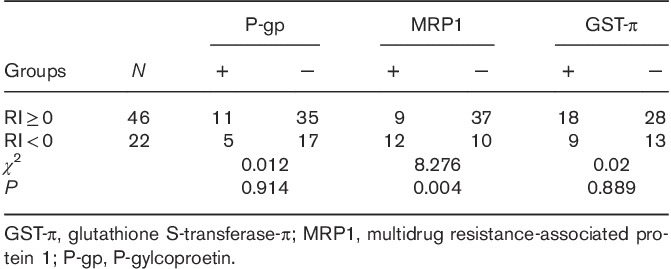
Table 4.
Relationship between dual-phase 99mTc-MIBI imaging and P-gp and GST-π expressions in hyperparathyroidism
Discussion
The aim of this study was to further elucidate the mechanisms of dual-phase 99mTc-MIBI parathyroid imaging by exploring the association between EUR, DUR, and RI and P-gp, MRP1, and GST-π expressions in HPT. Findings showed that the positive rate of dual-phase 99mTc-MIBI imaging for detecting parathyroid lesions in our patient population was 91.89% (68/74) and the false-negative rate was 8.11% (6/74). P-gp, MRP1, and GST-π expressions were low in tissues resected from patients with HPT. EUR in dual-phase 99mTc-MIBI imaging were associated with P-gp and GST-π expressions, and DUR were associated with MRP1 expression. There was no relationship between the sensitivity of dual-phase 99mTc-MIBI imaging and P-gp, MRP1, and GST-π expressions in resected parathyroid tissue.
These findings may further explain the mechanism of localization of parathyroid lesions by dual-phase 99mTc-MIBI imaging. Our results showed that the expressions of P-gp, MRP1, and GST-π were lower in tissues resected from patients with HPT than in control tissues. This suggests that 99mTc-MIBI may wash out faster from the normal parathyroid tissue surrounding the lesion compared with the lesion itself, facilitating detection.
The current study showed no relationship between the sensitivity of dual-phase 99mTc-MIBI imaging and P-gp, MRP1, and GST-π expressions in resected parathyroid tissue. There are many factors that influence the diagnosis and location of parathyroid lesions in HPT by dual-phase 99mTc-MIBI imaging, including the size of the parathyroid gland, blood flow in the parathyroid adenoma, and the secretory function and the activity of the parathyroid cells 14,31–35. In addition, autoimmune thyroid disease 16 and nonsteroidal anti-inflammatory drugs 36 may reduce the sensitivity of Tc-99m MIBI parathyroid scintigraphy. Kao et al. 37 reported that eight parathyroid adenomas with significant P-gp or MRP expression were not detected by 99mTc-MIBI imaging, but focal 99mTc-MIBI uptake could be found in 39 parathyroid adenomas negative for both P-gp and MRP expressions. The authors concluded that the sensitivity of 99mTc-MIBI parathyroid imaging for localizing parathyroid adenomas was limited by the presence of P-gp or MRP. Although several studies reported similar results 34,38, in accordance with our findings, others showed that P-gp or MRP1 expression was not related to the uptake of 99mTc-MIBI in parathyroid tumors 19,26,39 (Fig. 3).
Fig. 3.
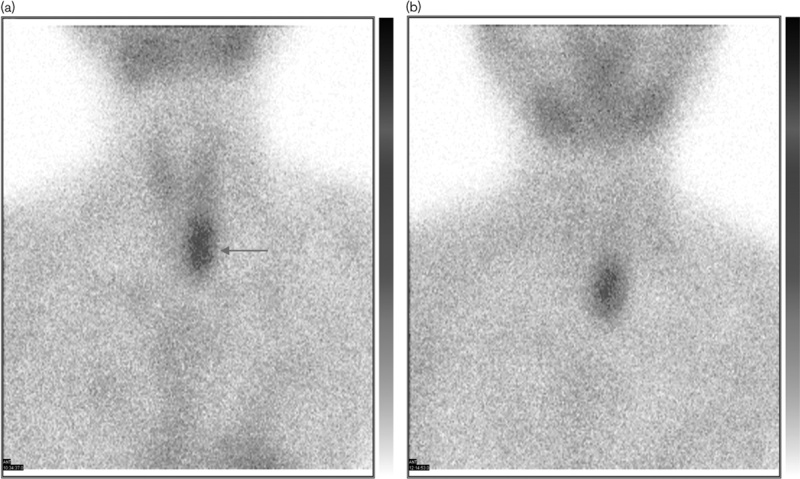
P-gp (+)/MRP1 (+)/GST-π (+), 99mTc-MIBI imaging 15 min (a) and 2 h (b). EUR=2.07, DUR=2.03. DUR, delayed uptake results; EUR, early uptake results; GST-π, glutathione S-transferase-π; MRP1, multidrug resistance-associated protein 1; P-gp, P-gylcoproetin; 99mTc-MIBI, technetium-99m methoxyisobutylisonitrile. Arrow shows parathyroid tumor.
The results of this study indicate that EUR in dual-phase 99mTc-MIBI imaging were associated with P-gp and GST-π expression, and DUR were associated with MRP1 expression. The data suggest that P-gp, MRP1, and GST-π play temporally distinct roles in 99mTc-MIBI efflux. In the early phase, the washout of 99mTc-MIBI is influenced by P-gp and GST-π, whereas washout in the delayed phase is influenced by MRP1. Furthermore, there were no significant differences in P-gp or GST-π expression and RI in dual-phase 99mTc-MIBI imaging. This may be because low positive expression of P-gp and GST-π in parathyroid lesions of HPT patients has little influence on 99mTc-MIBI efflux. However, these data should be interpreted with caution as the determination of the lesion boundary when identifying the region of interest was subjective, which may introduce some inaccuracy when calculating the RI.
Conclusion
P-gp, MRP1, and GST-π expressions were low in tissues resected from patients with HPT compared with control tissues. This may further explain the mechanism of localization of parathyroid lesions by dual-phase 99mTc-MIBI imaging. 99mTc-MIBI may wash out faster from the normal parathyroid tissue surrounding the lesion compared with the lesion itself, facilitating detection. However, our findings suggest that there is no relationship between the sensitivity of dual-phase 99mTc-MIBI imaging and P-gp, MRP1, and GST-π expressions in resected parathyroid tissue from patients with HPT.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
Footnotes
*Jianjun Xue and Yan Liu contributed equally to the writing of this article.
References
- 1.Fraker DL, Harsono H, Lewis R. Minimally invasive parathyroidectomy: benefits and requirements of localization, diagnosis, and intraoperative PTH monitoring. long-term results. World J Surg 2009; 33:2256–2265. [DOI] [PubMed] [Google Scholar]
- 2.Phillips CD, Shatzkes DR. Imaging of the parathyroid glands. Semin Ultrasound CT MR 2012; 33:123–129. [DOI] [PubMed] [Google Scholar]
- 3.Hindié E, Zanotti-Fregonara P, Tabarin A, Rubello D, Morelec I, Wagner T, et al. The role of radionuclide imaging in the surgical management of primary hyperparathyroidism. J Nucl Med 2015; 56:737–744. [DOI] [PubMed] [Google Scholar]
- 4.Coakley AJ, Kettle AG, Wells CP, O'Doherty MJ, Collins RE. 99Tcm sestamibi – a new agent for parathyroid imaging. Nucl Med Commun 1989; 10:791–794. [DOI] [PubMed] [Google Scholar]
- 5.Taillefer R, Boucher Y, Potvin C, Lambert R. Detection and localization of parathyroid adenomas in patients with hyperparathyroidism using a single radionuclide imaging procedure with technetium-99m-sestamibi (double-phase study). J Nucl Med 1992; 33:1801–1807. [PubMed] [Google Scholar]
- 6.Rao VV, Chiu ML, Kronauge JF, Piwnica-Worms D. Expression of recombinant human multidrug resistance P-glycoprotein in insect cells confers decreased accumulation of technetium-99m-sestamibi. J Nucl Med 1994; 35:510–515. [PubMed] [Google Scholar]
- 7.Sun SS, Shiau YC, Lin CC, Kao A, Lee CC. Correlation between P-glycoprotein (P-gp) expression in parathyroid and Tc-99m MIBI parathyroid image findings. Nucl Med Biol 2001; 28:929–933. [DOI] [PubMed] [Google Scholar]
- 8.Piñero A, Rodriguez JM, Martinez-Barba E, Canteras M, Stiges-Serra A, Parrilla P. Tc99m-sestamibi scintigraphy and cell proliferation in primary hyperparathyroidism: a causal or casual relationship? Surgery 2003; 134:41–44. [DOI] [PubMed] [Google Scholar]
- 9.Gotthardt M, Lohmann B, Behr TM, Bauhofer A, Franzius C, Schipper ML, et al. Clinical value of parathyroid scintigraphy with technetium-99m methoxyisobutylisonitrile: discrepancies in clinical data and a systematic metaanalysis of the literature. World J Surg 2004; 28:100–107. [DOI] [PubMed] [Google Scholar]
- 10.Treglia G, Sadeghi R, Schalin-Jäntti C, Caldarella C, Ceriani L, Giovanella L, et al. Detection rate of 99mTc-MIBI single photon emission computed tomography (SPECT)/CT in preoperative planning for patients with primary hyperparathyroidism: a meta-analysis. Head Neck 2015; 38:E2159–E2172. [DOI] [PubMed] [Google Scholar]
- 11.Giovanella L, Campenni A, Treglia G, Verburg FA, Trimboli P, Ceriani L, et al. Molecular imaging with 99mTc-MIBI and molecular testing for mutations in differentiating benign from malignant follicular neoplasm: a prospective comparison. Eur J Nucl Med Mol Imaging 2016; 43:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bénard F, Lefebvre B, Beuvon F, Langlois MF, Bisson G. Rapid washout of technetium-99m-MIBI from a large parathyroid adenoma. J Nucl Med 1995; 36:241–243. [PubMed] [Google Scholar]
- 13.Pons F, Torregrosa JV, Vidal-Sicart S, Sabater L, Fuster D, Fernández-Cruz L, et al. Preoperative parathyroid gland localization with technetium-99m sestamibi in secondary hyperparathyroidism. Eur J Nucl Med 1997; 24:1494–1498. [DOI] [PubMed] [Google Scholar]
- 14.Melloul M, Paz A, Koren R, Cytron S, Feinmesser R, Gal R. 99mTc-MIBI scintigraphy of parathyroid adenomas and its relation to tumour size and oxyphil cell abundance. Eur J Nucl Med 2001; 28:209–213. [DOI] [PubMed] [Google Scholar]
- 15.O'Doherty MJ, Kettle AG, Wells P, Collins RE, Coakley AJ. Parathyroid imaging with technetium-99m-sestamibi: preoperative localization and tissue uptake studies. J Nucl Med 1992; 33:313–318. [PubMed] [Google Scholar]
- 16.Hwang SH, Rhee Y, Yun M, Yoon JH, Lee JW, Cho A. Usefulness of SPECT/CT in parathyroid lesion detection in patients with thyroid parenchymal 99mTc-sestamibi retention. Nucl Med Mol Imaging 2017; 51:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenz JI, Jacobs JM, Op de Beeck B, Huyghe IA, Pelckmans PA, Moreels TG. Acute necrotizing pancreatitis as first manifestation of primary hyperparathyroidism. World J Gastroenterol 2010; 16:2959–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bae EH, Kim HS, Kim MJ, Kang YU, Kim YH, Kim CS, et al. Hypercalcemia in a patient with polycythemia vera. Chonnam Med J 2012; 48:128–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorna FH, Hollema H, Hendrikse HN, Bart J, Brouwers AH, Plukker JT. P-gp and MRP1 expression in parathyroid tumors related to histology, weight and 99mTc-sestamibi imaging results. Exp Clin Endocrinol Diabetes 2009; 117:406–412. [DOI] [PubMed] [Google Scholar]
- 20.Hendrikse NH, Franssen EJ, van der Graaf WT, Vaalburg W, de Vries EG. Visualization of multidrug resistance in vivo. Eur J Nucl Med 1999; 26:283–293. [DOI] [PubMed] [Google Scholar]
- 21.Hendrikse NH. Monitoring interactions at ATP-dependent drug efflux pumps. Curr Pharm Des 2000; 6:1653–1668. [DOI] [PubMed] [Google Scholar]
- 22.Arbab AS, Koizumi K, Toyama K, Araki T. Uptake of technetium-99m-tetrofosmin, technetium-99m-MIBI and thallium-201 in tumor cell lines. J Nucl Med 1996; 37:1551–1556. [PubMed] [Google Scholar]
- 23.Ballinger JR, Bannerman J, Boxen I, Firby P, Hartman NG, Moore MJ. Technetium-99m-tetrofosmin as a substrate for P-glycoprotein: in vitro studies in multidrug-resistant breast tumor cells. J Nucl Med 1996; 37:1578–1582. [PubMed] [Google Scholar]
- 24.Gupta Y, Ahmed R, Happerfield L, Pinder SE, Balan KK, Wishart GC. P-glycoprotein expression is associated with sestamibi washout in primary hyperparathyroidism. Br J Surg 2007; 94:1491–1495. [DOI] [PubMed] [Google Scholar]
- 25.Ugur O, Bozkurt MF, Hamaloglu E, Sokmensuer C, Etikan I, Ugur Y, et al. Clinicpathologic and radiopharmacokinetic factors affecting gamma probe-guided parathyroidectomy. Arch Surg 2004; 139:1175–1179. [DOI] [PubMed] [Google Scholar]
- 26.Turgut B, Elagoz S, Erselcan T, Koyuncu A, Dokmetas HS, Hasbek Z, et al. Preoperative localization of parathyroid adenomas with technetium-99m methoxyisobutylisonitrile imaging: relationship with P-glycoprotein expression, oxyphilic cell content, and tumoral tissue volume. Cancer Biother Radiopharm 2006; 21:579–590. [DOI] [PubMed] [Google Scholar]
- 27.Xue JJ, Yang AM, Zhang FR, Wang HY, Deng Y, Li JH, et al. Correlation between uptake of 99Tcm-MIBI and expression of multidrug resistant protein in primary lung cancer. Chin J Med Imaging Technol 2006; 22:1090–1094. [Google Scholar]
- 28.Yang A, Xue J, Li X, Yu Y, Deng H, Hu G, et al. Experimental and clinical observations of 99mTc-MIBI uptake correlate with P-glycoprotein expression in lung cancer. Nucl Med Commun 2007; 28:696–703. [DOI] [PubMed] [Google Scholar]
- 29.Kurata S, Ushijima K, Kawahara A, Kaida H, Kawano K, Hirose Y, et al. Assessment of 99mTc-MIBI SPECT(/CT) to monitor multidrug resistance-related proteins and apoptosis-related proteins in patients with ovarian cancer: a preliminary study. Ann Nucl Med 2015; 29:643–649. [DOI] [PubMed] [Google Scholar]
- 30.Wu HS, Liu YC, Kao A, Wang JJ, Ho ST. Using technetium 99m-tetrofosmin parathyroid imaging to detect parathyroid adenoma and its relation to P-glycoprotein expression. Surgery 2002; 132:456–460. [DOI] [PubMed] [Google Scholar]
- 31.Carcangiu ML, Chambers JT, Voynick IM, Pirro M, Schwartz PE. Immunohistochemical evaluation of estrogen and progesterone receptor content in 183 patients with endometrial carcinoma. Part I: clinical and histologic correlations. Am J Clin Pathol 1990; 94:247–254. [DOI] [PubMed] [Google Scholar]
- 32.Leslie WD, Riese KT, Dupont JO, Peterdy AE. Parathyroid adenomas without sestamibi retention. Clin Nucl Med 1995; 20:699–702. [DOI] [PubMed] [Google Scholar]
- 33.Rauth JD, Sessions RB, Shupe SC, Ziessman HA. Comparison of Tc-99m MIBI and TI-201/Tc-99m pertechnetate for diagnosis of primary hyperparathyroidism. Clin Nucl Med 1996; 21:602–608. [DOI] [PubMed] [Google Scholar]
- 34.Pons F, Torregrosa JV, Fuster D. Biological factors influencing parathyroid localization. Nucl Med Commun 2003; 24:121–124. [DOI] [PubMed] [Google Scholar]
- 35.Arbab AS, Ueki J, Koizumi K, Araki T. Effects of extracellular Na+ and Ca2+ ions and Ca2+ channel modulators on the cell-associated activity of 99mTc-MIBI and 99mTc-tetrofosmin in tumour cells. Nucl Med Commun 2003; 24:155–166. [DOI] [PubMed] [Google Scholar]
- 36.Araz M, Çayir D, Erdoğan M, Uçan B, Çakal E. Factors affecting the sensitivity of Tc-99m methoxyisobutylisonitrile dual-phase parathyroid single photon emission computed tomography in primary hyperparathyroidism. Nucl Med Commun 2017; 38:117–123. [DOI] [PubMed] [Google Scholar]
- 37.Kao A, Shiau YC, Tsai SC, Wang JJ, Ho ST. Technetium-99m methoxyisobutylisonitrile imaging for parathyroid adenoma: relationship to P-glycoprotein or multidrug resistance-related protein expression. Eur J Nucl Med 2002; 29:1012–1015. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi S, Yachiku S, Hashimoto H, Kaneko S, Nishihara M, Niibori D, et al. Relation between technetium 99m-methoxyisobutylisonitrile accumulation and multidrug resistance protein in the parathyroid glands. World J Surg 2002; 26:29–34. [DOI] [PubMed] [Google Scholar]
- 39.Bhatnagar A, Vezza PR, Bryan JA, Atkins FB, Ziessman HA. Technetium-99m-sestamibi parathyroid scintigraphy: effect of P-glycoprotein, histology and tumor size on detectability. J Nucl Med 1998; 39:1617–1620. [PubMed] [Google Scholar]


