Abstract
Objective:
Patients with HIV infection have an increased risk of cardiovascular disease compared with uninfected individuals. Antiretroviral therapy with atazanavir (ATV) delays progression of atherosclerosis markers; whether this reduces cardiovascular disease event risk compared with other antiretroviral regimens is currently unknown.
Design:
Population-based, noninterventional, historical cohort study conducted from 1 July 2003 through 31 December 2015.
Setting:
Veterans Health Administration hospitals and clinics throughout the United States.
Participants:
Treatment-naive patients with HIV infection (N = 9500).
Antiretroviral exposures:
Initiating antiretroviral regimens containing ATV, other protease inhibitors, nonnucleoside reverse transcriptase inhibitors (NNRTIs), or integrase strand transfer inhibitors (INSTIs).
Main outcome/effect size measures:
Incidence rates of myocardial infarction (MI), stroke, and all-cause mortality within each regimen. ATV versus other protease inhibitor, NNRTI, or INSTI covariate-adjusted hazard ratios by using Cox proportional hazards models and inverse probability of treatment weighting.
Results:
Incidence rates for MI, stroke, and all-cause mortality with ATV-containing regimens (5.2, 10.4, and 16.0 per 1000 patient-years, respectively) were lower than with regimens containing other protease inhibitors (10.2, 21.9, and 23.3 per 1000 patient-years), NNRTIs (7.5, 15.9, and 17.5 per 1000 patient-years), or INSTIs (13.0, 33.1, and 21.5 per 1000 patient-years). After inverse probability of treatment weighting, adjusted hazard ratios (95% confidence intervals) for MI, stroke, and all-cause mortality with ATV-containing regimens versus all non-ATV-containing regimens were 0.59 (0.41–0.84), 0.64 (0.50–0.81), and 0.90 (0.73–1.11), respectively.
Conclusion:
Among treatment-naive HIV-infected patients in the Veterans Health Administration initiating ATV-containing regimens, risk of both MI and stroke were significantly lower than in those initiating regimens containing other protease inhibitors, NNRTIs, or INSTIs.
Keywords: atazanavir, cardiovascular disease, HIV, myocardial infarction, stroke
Introduction
Among patients with HIV infection, estimated life expectancy at age 20 has increased from 36.1 additional years during 1996–1999 [1] to 53.1 during 2008–2011 [2] due to progressive improvements in antiretroviral therapy (ART) regimens and earlier diagnosis and treatment [3,4]. Despite these improvements, a significant gap remains compared with the uninfected population for whom life expectancy at 20 years in 2011 was estimated at an additional 59.5 years [5]. This gap is likely due to a corresponding increase in chronic comorbidities, for which the increasingly aging HIV-infected population is at greater risk [6,7].
Numerous large cohort studies have shown that morbidity and mortality from cardiovascular disease are more than 50% greater in HIV-infected individuals than in uninfected individuals. In most studies, this difference persists even after adjustment for differences in traditional risk factors such as smoking, hypertension, dyslipidemia, and diabetes mellitus [8–15].
Reasons for the increased cardiovascular risk among patients with HIV infection are multifactorial, including a greater prevalence of traditional risk factors such as smoking [16], an increase in calcified and noncalcified atherosclerotic plaques [17], HIV-associated oxidative stress [18], and ART-associated proatherogenic effects such as dyslipidemia, body fat redistribution, and insulin resistance [19,20]. In addition, HIV-associated immune activation and inflammation [20–22], as indicated by increased markers such as high-sensitivity C-reactive protein (hs-CRP) [23,24], IL-6 [23–25], and D-dimer [23,24], has been associated with an increase in cardiovascular risk in some but not all studies [26].
Although ART reduces the overall risk of cardiovascular disease compared with untreated HIV infection [27], certain nucleoside reverse transcriptase inhibitors (NRTIs), such as abacavir and didanosine, and protease inhibitors such as indinavir, ritonavir-boosted lopinavir [28], and in preliminary findings, ritonavir-boosted darunavir (DRV/r) [29], have been associated with an increased relative risk.
There has been interest in studying the impact of atazanavir (ATV) on markers of cardiovascular disease. ATV inhibits uridine diphosphate glucuronosyltransferase 1A1, which can often lead to hyperbilirubinemia caused by reduced bilirubin glucuronidation and excretion [30]. This hyperbilirubinemia is generally asymptomatic but can lead to jaundice/scleral icterus in 5–10% of patients, which is reversible upon discontinuation [31]. Bilirubin is a potent antioxidant that suppresses the oxidation of lipids and impacts various other aspects of the atherosclerotic process [32]. Epidemiological studies of individuals with Gilbert syndrome, a condition associated with chronic low-level hyperbilirubinemia, suggest that these individuals have an approximately 50% lower risk of cardiovascular disease compared with the general population [33].
In some studies, treatment with ritonavir-boosted ATV (ATV/r) has been shown to be associated with favorable effects on surrogates of cardiovascular disease, such as carotid intima–media thickness (cIMT) progression [34–37] and other functional biomarkers [38–40]. However, the clinical significance of these relative cIMT [e.g. ATV/r versus DRV/r changes of −4.7 (at common carotid artery) to −6.2 μm/year (at carotid bifurcation)] [35] and biomarker improvements with ATV/r is unclear, as there are very limited data examining the impact of ATV/r on the risk of cardiovascular events, such as myocardial infarction (MI) and stroke. The only data to date are from the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study, which suggested that ATV/r was not associated with an increased risk of MI or stroke, although no significant decrease in risk was found [29,41].
To further explore the relative impact of ATV on the risk of MI and stroke compared with other antiretroviral agents, we conducted a historical cohort study of HIV-infected treatment-naive patients in the US national Veterans Health Administration (VHA) database who initiated ATV-containing regimens versus those who initiated non-ATV-containing regimens.
Methods
Patients and study design
This was a population-based, historical cohort study of treatment-naive HIV-infected patients newly initiating ART in VHA hospitals and clinics from 1 July 2003 through 31 December 2015. The VHA represents the largest integrated healthcare delivery system in the United States, with more than 33 000 HIV-infected individuals receiving care at 150 hospitals and 850 clinics throughout the United States. The VHA collects data on utilization (e.g. pharmacy records and inpatient/outpatient encounters), clinical parameters (e.g. vital signs, laboratory results, and radiology reports), and demographics (e.g. age, sex, and race/ethnicity). Data for this study were extracted from several VHA datasets (Supplemental Table S1) hosted in the Veterans Affairs Informatics and Computing Infrastructure (VINCI) environment, which links relevant data by using the VHA's scrambled social security number, a unique anonymized patient identifier, as well as data from the Centers for Medicare and Medicaid Services (CMS). The study was conducted in accordance with International Society for Pharmacoepidemiology Guidelines for Good Pharmacoepidemiology Practices and applicable regulatory requirements. The University of Utah Institutional Review Board and the Salt Lake City VA Health Care System Office of Research and Development approved this study.
Inclusion criteria
The cohort of interest included all treatment-naive HIV-infected patients diagnosed between 1 July 2003 and 31 December 2015, who received their first ART regimen while in the VHA healthcare system during the study period. Diagnosis codes from the 9th and 10th Revisions of the International Classification of Diseases (ICD-9/10) were used to identify patients with HIV infection (for a list of codes, refer to Supplemental Table S2). The index date was defined as the date of the first prescription for initial ART with a protease inhibitor (ATV, darunavir, lopinavir, saquinavir, indinavir, nelfinavir, fosamprenavir, and tipranavir), an integrase strand transfer inhibitor (INSTI; raltegravir, elvitegravir, and dolutegravir), or a nonnucleoside reverse transcriptase inhibitor (NNRTI; efavirenz, rilpivirine, and nevirapine), and two or more other agents of the NRTI class.
Exclusion criteria
A validated algorithm [42] with a sensitivity of 86% and positive predictive value of 87% [43] was used to exclude patients with evidence of prior ART received outside of the VHA, which included the following criteria: exposure to any antiretroviral agents during a 1-year period before the index date (the preindex period); patients whose index ART regimen was a ‘salvage’ regimen (i.e. composed of both a protease inhibitor and an NNRTI or composed of five or more agents); and patients whose HIV RNA levels before the index date were low enough (<500 copies/ml) to suggest prior antiretroviral exposure.
Patients not demonstrating a pattern of receiving routine care from the VHA (i.e. no inpatient or outpatient encounter for at least 6 months before the index date) were also excluded.
Outcomes and covariates
Outcomes of interest included MI, stroke (ischemic, including transient ischemic attack, and hemorrhagic), and all-cause mortality. Diagnosis codes from ICD-9 and ICD-10 were used to identify MIs and strokes, including 410 (ICD-9) and I21–I22 (ICD-10) for MI and 430–434 (ICD-9) and I60–I64 (ICD-10) for stroke, based on the condition algorithms published by the CMS Chronic Conditions Data Warehouse [44] (refer to Supplemental Table S3, for further information). The use of ICD-9 and ICD-10 diagnostic codes from administrative data identifies cases of acute MI and stroke with over 90% accuracy [45,46]. Deaths were identified from the VHA Vital Status files, which contain death records for patients from sources such as the Veterans Benefits Administration, Social Security Administration, Medicare, and Veterans Affairs National Cemetery Administration.
To control for selection bias and confounding by indication, analyses were adjusted for relevant variables that were selected on the basis of extensive literature search as well as prior clinical knowledge of ART and potential associations with treatment and/or outcomes. Within the preindex period, baseline patient characteristics (age, sex, race, BMI, and marital status) and HIV-related prognostic indices (CD4+ cell count and HIV RNA level) were collected most proximal to each patient's index date. Comorbid diagnoses known to be associated with outcomes of interest and/or possibly ART choice were also collected during the preindex period. These included a previous history of coronary artery disease/cerebrovascular disease (MI, stroke, heart failure, percutaneous coronary intervention, coronary artery bypass graft, and angina), hepatitis, dyslipidemia, statin use, diabetes, hypertension, alcohol abuse, illicit drug use, psychiatric disorder, and smoking. Medication exposures, including hepatitis treatments, antidyslipidemic agents, antihyperglycemic agents, antihypertensives, and abacavir, were also collected during the preindex period. Identification of comorbidities was based on both diagnosis codes and treatments in which applicable (Supplemental Table S4).
Statistical analysis
Patient characteristics and covariates at baseline were reported by using means and standard deviations for continuous variables and frequencies and proportions for categorical variables. Differences in baseline variables were compared overall and by treatment group by using standardized mean differences (SMDs), with SMDs outside of the bounds of ±0.1 indicating meaningful differences [47].
To control for confounding by indication and selection bias, patient groups were weighted to balance differences in covariates through the use of inverse probability of treatment weighting (IPTW). IPTW is an application of propensity scores that provides superior covariate balance, can be used with variable follow-up time, and uses all observations in the data set [48]. The propensity scores used to compute the inverse probability weights were estimated by using logistic regression with ATV as the dependent variable and observed baseline characteristics, likely to influence ART selection and the outcomes of interest, as the independent variables. Inverse probability weights were calculated as 1/propensity scores in patients who received ATV and 1/(1 − propensity scores) in patients who did not. In addition to the primary analysis by using IPTW, a number of sensitivity analyses were performed, including by using matching weights, truncated IPTW, stabilized weights, doubly robust analysis, IPTW with imputation of missing data, IPTW with adjustment for calendar year, and IPTW with adjustment for guideline changes. For further details of these procedures, see the Supplemental Digital Content (Statistical methods for covariate adjustment).
Crude incidence rates of MI, stroke, and death from any cause per 1000 patient-years of exposure and associated exact 95% confidence intervals (CIs) were calculated in the unweighted cohort assuming a Poisson distribution.
Weighted Cox proportional hazards regression models were employed to calculate covariate-adjusted hazard ratios for cardiovascular outcomes associated with ATV-containing regimens compared with those receiving other protease inhibitor-containing regimens, NNRTI-containing regimens, INSTI-containing regimens, and non-ATV-containing regimens overall. Within this survival analysis framework, patients were censored on the first date of any 30-day gap in the index ART regimen (to limit confounding caused by multiple exposures to different antiretroviral regimens over time), the last encounter in the VHA system, or at the end of the study period. To confirm successful weighting, the SMDs for baseline characteristics between treatment groups were calculated again in the weighted cohort and graphically compared with those in the unweighted cohort. Weighted SMD values within ±10% and closer to zero demonstrate covariate balance between treatment groups [47].
Formal sample size and power calculations were not considered to be necessary as the large number of patients available within the VHA system were regarded as sufficient to detect meaningful differences in event rates for MI and stroke.
Results
Patients
The cohort selection process and attrition from patients not meeting study criteria are described in the PRISMA flow diagram (Fig. 1). Of the final cohort of 9500 patients, 1529 (16.1%) were receiving an ATV-containing regimen (82.6% boosted and 17.4% unboosted) and 7971 (83.9%) a non-ATV-containing regimen. Of these latter patients, 2053 (21.6%) were receiving protease inhibitor-containing regimens with a protease inhibitor other than ATV (mostly lopinavir), 5307 (55.9%) an NNTRI-containing regimen (mostly efavirenz), and 611 (6.4%) an INSTI-containing regimen. Of the 9500 patients, the distribution receiving NRTIs was 6784 (71.4%) for tenofovir disoproxil fumarate, 5930 (62.4%) for emtricitabine, 3454 (36.4%) for lamivudine, 2029 (21.4%) for zidovudine, 1299 (13.7%) for abacavir, 394 (4.2%) for stavudine, and 351 (3.7%) for didanosine, with most patients being on two of these NRTIs.
Fig. 1.
Patient selection according to eligibility criteria.
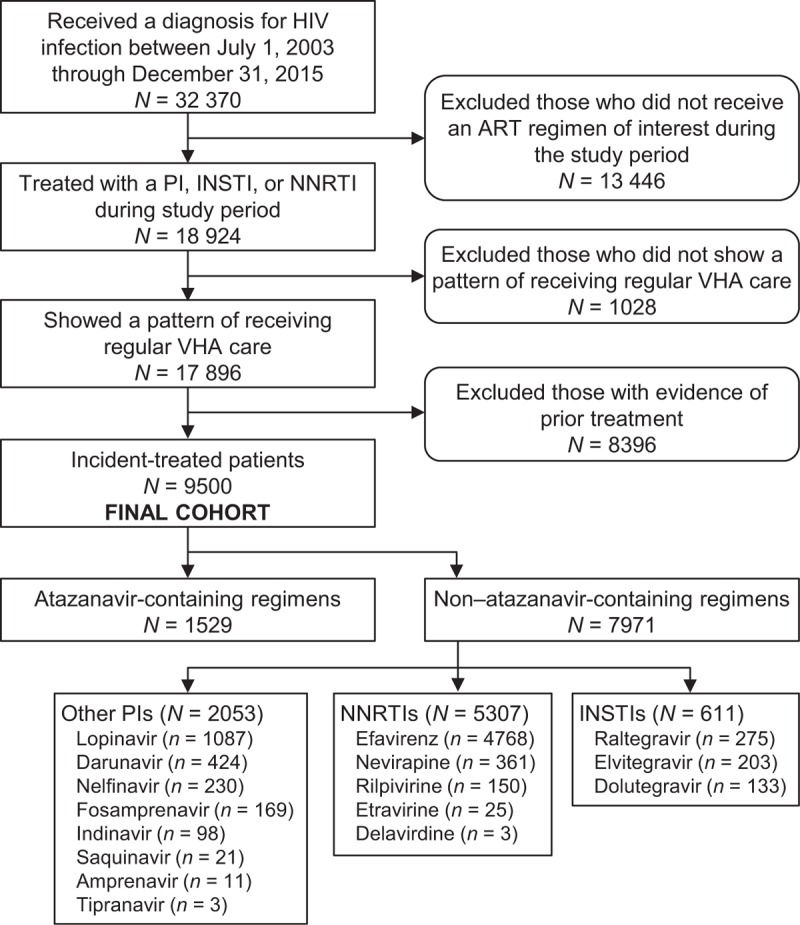
ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; VHA, Veterans Health Administration.
Mean age at ART initiation was 50 years, 93% were men, 56.3% were African-American, 26.9% had a CD4+ cell count less than 200 cells/μl, 18.6% had an HIV RNA more than 100 000 copies/ml, 10.4% had a prior history of cardiovascular disease, 35.0% had hypertension, 13.3% had diabetes mellitus, 28.2% used tobacco, and 11.5% were receiving statins. Although most baseline and demographic characteristics before weighting appeared balanced across treatment groups (Table 1), and the majority of unadjusted SMDs in baseline covariates were within ±0.1 (the threshold commonly considered as representing meaningful differences), a number of differences across treatment groups were evident (Fig. 2 and Supplemental Fig. S1). Thus, male patients, patients of African-American origin, patients with mildly impaired renal function, and patients with psychiatric disorder were overrepresented in those receiving ATV-containing versus other protease inhibitor-containing regimens (Supplemental Fig. S1A). Patients receiving abacavir in the index regimen, patients with a diagnosis of and/or receiving treatment for viral hepatitis, and patients with psychiatric disorder were overrepresented in those receiving ATV-containing versus NNRTI-containing regimens (Supplemental Fig. S1B). Patients of African-American origin, patients with mild-to-moderately impaired renal function, and patients with a diagnosis of and/or receiving treatment for viral hepatitis were overrepresented in those receiving ATV-containing versus INSTI-containing regimens (Supplemental Fig. S1C). Patients with multiple risk factors for cardiovascular disease (e.g. tobacco use, diabetes, hypertension, and dyslipidemia) were overrepresented in those receiving INSTI-containing versus ATV-containing regimens (Supplemental Fig. S1C). After applying IPTW, SMDs for all baseline covariates were balanced [i.e. within ±0.1 (Fig. 2)]. A visual examination of the survival functions revealed that the plots for each exposure were parallel and without overlap, confirming that the proportional hazards assumption was met for all comparisons.
Table 1.
Unadjusted demographic and baseline characteristics.
| Characteristic | ATV, n = 1529 | Non-ATVa, n = 7971 | Other PIb, n = 2053 | NNRTIc, n = 5307 | INSTId, n = 611 |
| Age (years) | 50 ± 9.6 | 50 ± 10 | 50 ± 9.4 | 50 ± 10 | 50 ± 13 |
| Men | 1471 (96.2) | 7356 (92.3) | 1762 (85.8) | 5015 (94.5) | 579 (94.8) |
| Married | 101 (6.6) | 623 (7.8) | 140 (6.8) | 419 (7.9) | 64 (10.5) |
| Race/ethnicity | |||||
| White | 463 (30.3) | 2441 (30.6) | 600 (29.2) | 1627 (30.7) | 214 (35.0) |
| Black | 929 (60.8) | 4419 (55.4) | 1024 (49.9) | 3078 (58.0) | 317 (51.9) |
| Hispanic | 76 (5.0) | 482 (6.0) | 125 (6.1) | 312 (5.9) | 45 (7.4) |
| Asian | 9 (0.6) | 64 (0.8) | 16 (0.8) | 38 (0.7) | 10 (1.6) |
| Other | 11 (0.7) | 43 (0.5) | 7 (0.3) | 33 (0.6) | 3 (0.5) |
| Missing | 41 (2.7) | 523 (6.6) | 281 (13.7) | 220 (4.1) | 22 (3.6) |
| BMI (kg/m2) | 26 ± 9.6 | 26 ± 10.0 | 25 ± 9.4 | 26 ± 10.0 | 27 ± 13.0 |
| CD4+ cell count (cells/μl) | |||||
| <200 | 441 (28.8) | 2119 (26.6) | 602 (29.3) | 1401 (26.4) | 116 (19.0) |
| 200–299 | 233 (15.2) | 1186 (14.9) | 245 (11.9) | 869 (16.4) | 72 (11.8) |
| 300–399 | 183 (12.0) | 976 (12.2) | 183 (8.9) | 720 (13.6) | 73 (11.9) |
| 400–499 | 97 (6.3) | 633 (7.9) | 101 (4.9) | 470 (8.9) | 62 (10.1) |
| ≥500 | 194 (12.7) | 1154 (14.5) | 257 (12.5) | 739 (13.9) | 158 (25.9) |
| Missing | 381 (24.9) | 1904 (23.9) | 665 (32.4) | 1109 (20.9) | 130 (21.3) |
| HIV RNA (copies/ml) | |||||
| <10 000 | 505 (33.0) | 2371 (29.7) | 617 (30.1) | 1521 (28.7) | 233 (38.1) |
| 10 000–100 000 | 441 (28.8) | 2207 (27.7) | 421 (20.5) | 1610 (30.3) | 176 (28.8) |
| >100 000 | 275 (18.0) | 1489 (18.7) | 366 (17.8) | 1015 (19.1) | 108 (17.7) |
| Missing | 308 (20.1) | 1905 (23.9) | 649 (31.6) | 1162 (21.9) | 94 (15.4) |
| eGFR (ml/min) | |||||
| Stage 1, ≥90 | 477 (31.2) | 2332 (29.3) | 365 (17.8) | 1839 (34.7) | 128 (20.9) |
| Stage 2, 60–89 | 316 (20.7) | 1467 (18.4) | 238 (11.6) | 1169 (22.0) | 60 (9.8) |
| Stage 3, 30–59 | 42 (2.7) | 156 (2.0) | 21 (1.0) | 131 (2.5) | 4 (0.7) |
| Stage 4, 15–29 | 1 (0.1) | 6 (0.1) | 3 (0.1) | 3 (0.1) | 0 (0.0) |
| Stage 5, <15 | 5 (0.3) | 58 (0.7) | 24 (1.2) | 34 (0.6) | 0 (0.0) |
| Missing | 688 (45.0) | 3953 (49.6) | 1402 (68.3) | 2132 (40.2) | 419 (68.6) |
| Comorbid medical diagnoses | |||||
| Alcohol abuse | 358 (23.4) | 1728 (21.7) | 401 (19.5) | 1173 (22.1) | 154 (25.2) |
| Angina | 17 (1.1) | 102 (1.3) | 20 (1.0) | 73 (1.4) | 9 (1.5) |
| Bone disease | 10 (0.7) | 61 (0.8) | 13 (0.6) | 42 (0.8) | 6 (1.0) |
| Chronic kidney disease | 132 (8.6) | 655 (8.2) | 174 (8.5) | 402 (7.6) | 79 (12.9) |
| Diabetes mellitus diagnosis | 203 (13.3) | 1057 (13.3) | 238 (11.6) | 707 (13.3) | 112 (18.3) |
| Dyslipidemia diagnosis | 214 (14.0) | 1189 (14.9) | 248 (12.1) | 790 (14.9) | 151 (24.7) |
| Heart failure diagnosis | 34 (2.2) | 256 (3.2) | 52 (2.5) | 171 (3.2) | 33 (5.4) |
| History of CAD/CVD | 149 (9.7) | 841 (10.6) | 185 (9.0) | 576 (10.9) | 80 (13.1) |
| Hypertension diagnosis | 533 (34.9) | 2795 (35.1) | 597 (29.1) | 1931 (36.4) | 267 (43.7) |
| Illicit drug abuse | 211 (13.8) | 891 (11.2) | 224 (10.9) | 600 (11.3) | 67 (11.0) |
| Myocardial infarction | 9 (0.6) | 42 (0.5) | 8 (0.4) | 31 (0.6) | 3 (0.5) |
| PCI/CABG | 2 (0.1) | 20 (0.3) | 3 (0.1) | 16 (0.3) | 1 (0.2) |
| Psychiatric disorder | 674 (44.1) | 2960 (37.1) | 741 (36.1) | 1891 (35.6) | 328 (53.7) |
| Stroke | 15 (1.0) | 89 (1.1) | 22 (1.1) | 52 (1.0) | 15 (2.5) |
| Tobacco use | 432 (28.3) | 2247 (28.2) | 489 (23.8) | 1541 (29.0) | 217 (35.5) |
| Tuberculosis | 18 (1.2) | 101 (1.3) | 26 (1.3) | 70 (1.3) | 5 (0.8) |
| Viral hepatitis diagnosis | 486 (31.8) | 2128 (26.7) | 570 (27.8) | 1400 (26.4) | 158 (25.9) |
| Concomitant medications | |||||
| Abacavir (in index regimen) | 319 (20.9) | 980 (12.3) | 395 (19.2) | 453 (8.5) | 132 (21.6) |
| Antidiabetic agentse | 145 (9.5) | 822 (10.3) | 177 (8.6) | 553 (10.4) | 92 (15.1) |
| Heart failure treatments | 79 (5.2) | 490 (6.1) | 110 (5.4) | 332 (6.3) | 48 (7.9) |
| Hypertension treatments | 540 (35.3) | 2842 (35.7) | 608 (29.6) | 1969 (37.1) | 265 (43.4) |
| Methadone | 30 (2.0) | 104 (1.3) | 30 (1.5) | 68 (1.3) | 6 (1.0) |
| Other dyslipidemia treatment | 183 (12.0) | 1098 (13.8) | 213 (10.4) | 751 (14.2) | 134 (21.9) |
| Statins | 147 (9.6) | 949 (11.9) | 171 (8.3) | 650 (12.2) | 128 (20.9) |
| Viral hepatitis treatments | 139 (9.1) | 470 (5.9) | 204 (9.9) | 235 (4.4) | 31 (5.1) |
Data are mean ± SD or n (%). ATV, atazanavir; CABG, coronary artery bypass graft; CAD, coronary artery disease; CVD, cerebrovascular disease; eGFR, estimated glomerular filtration rate; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PCI, percutaneous coronary intervention; PI, protease inhibitor.
aNon-ATV includes all other study antiretroviral therapies, including darunavir, lopinavir, saquinavir, indinavir, nelfinavir, fosamprenavir, tipranavir, efavirenz, rilpivirine, nevirapine, raltegravir, elvitegravir, and dolutegravir.
bOther PI includes darunavir, lopinavir, saquinavir, indinavir, nelfinavir, fosamprenavir, and tipranavir.
cNNRTI includes efavirenz, rilpivirine, and nevirapine.
dINSTI includes raltegravir, elvitegravir, and dolutegravir.
eOral or injectable.
Fig. 2.
Baseline demographics: verification that inverse probability of treatment weighting achieves baseline covariate balance between regimens.
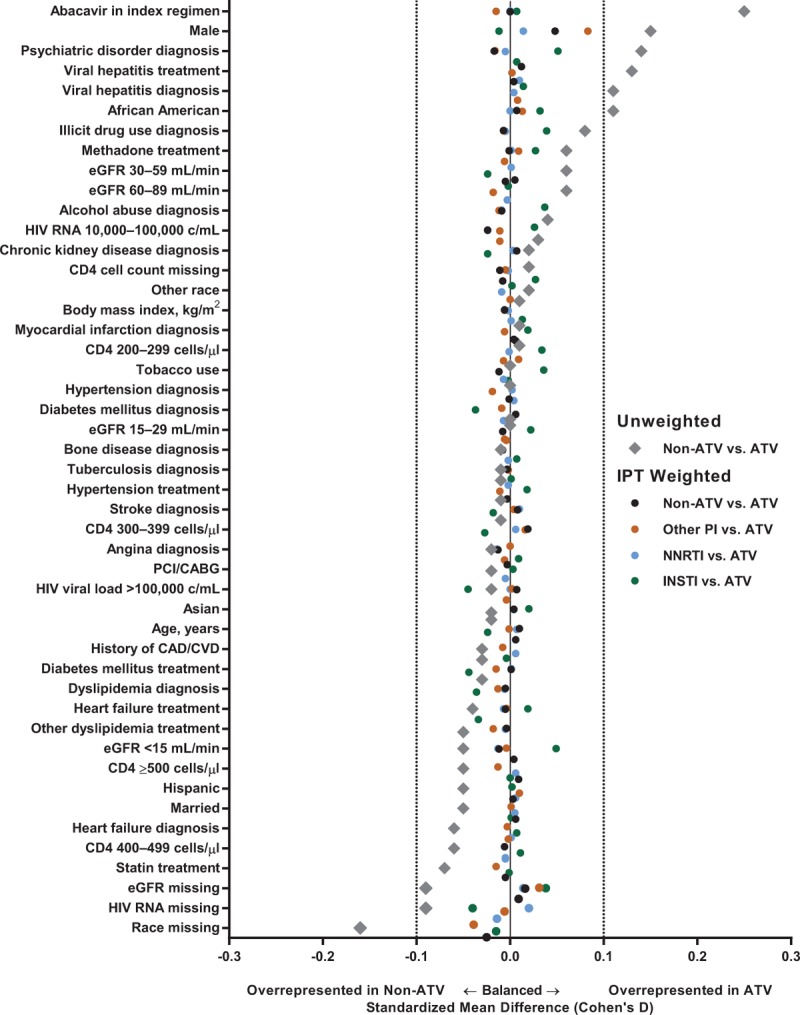
ATV, atazanavir; CAD/CVD, coronary artery disease/cerebrovascular disease; eGFR, estimated glomerular filtration rate; INSTI, integrase strand transfer inhibitor; IPT, inverse probability of treatment; NNRTI, nonnucleoside reverse transcriptase inhibitor; PCI/CABG, percutaneous coronary intervention/coronary artery bypass graft; PI, protease inhibitor.
Overall mean follow-up time was 13 months (range, 9.1 months for INSTIs to 15.1 months for NNRTIs) (Table 2).
Table 2.
Observation (follow-up) time and time to event by outcomes of interest and treatment group.
| Observation time (days) | Time to event (days) | ||||||
| Outcome | Exposure | N | Mean (SD) | Median (Q1, Q3) | n | Mean (SD) | Median (Q1, Q3) |
| MI | All ARVs | 9500 | 394 (626) | 145 (59, 409) | 80a | 489 (622) | 243 (67, 690) |
| ATV | 1529 | 370 (598) | 144 (61, 363) | 8 | 363 (440) | 180 (46, 637) | |
| Non-ATV | 7971 | 399 (631) | 146 (59, 419) | 72 | 503 (640) | 250 (77, 690) | |
| Other PIs | 2053 | 279 (450) | 116 (51, 282) | 16 | 598 (821) | 249 (128, 821) | |
| NNRTI | 5307 | 459 (704) | 161 (61, 511) | 50 | 502 (610) | 256 (53, 701) | |
| INSTI | 611 | 276 (336) | 145 (66, 361) | 6 | 260 (223) | 233 (98, 345) | |
| Stroke | All ARVs | 9500 | 390 (622) | 144 (59, 404) | 170b | 403 (593) | 144 (28, 575) |
| ATV | 1529 | 367 (601) | 143 (60, 357) | 16 | 163 (215) | 72 (18, 280) | |
| Non-ATV | 7971 | 394 (626) | 144 (58, 416) | 154 | 428 (615) | 151 (31, 691) | |
| Other PIs | 2053 | 277 (451) | 113 (51, 281) | 34 | 249 (323) | 104 (31, 357) | |
| NNRTI | 5307 | 454 (698) | 160 (60, 509) | 105 | 521 (696) | 175 (37, 799) | |
| INSTI | 611 | 271 (326) | 144 (64, 360) | 15 | 186 (284) | 41 (8, 289) | |
| All-cause mortality | All ARVs | 9500 | 397 (630) | 147 (59, 413) | 190c | 501 (632) | 204 (70, 698) |
| ATV | 1529 | 373 (604) | 144 (61, 364) | 25 | 609 (864) | 127 (63, 894) | |
| Non-ATV | 7971 | 402 (635) | 147 (59, 428) | 165 | 484 (591) | 210 (71, 694) | |
| Other PIs | 2053 | 282 (455) | 116 (51, 284) | 37 | 410 (561) | 119 (55, 515) | |
| NNRTI | 5307 | 463 (708) | 164 (61, 516) | 118 | 523 (616) | 270 (88, 722) | |
| INSTI | 611 | 278 (339) | 146 (66, 365) | 10 | 302 (293) | 165 (84, 453) | |
N = number of patients in each treatment group; n = number of events in each treatment group. ARV, antiretroviral; ATV, atazanavir; INSTI, integrase strand transfer inhibitor; MI, myocardial infarction; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; Q1, first quartile; Q3, third quartile.
aA total of 8692 patients were censored due to a 30-day gap in medication and 728 were administratively censored at the end of study period.
bA total of 8627 patients were censored due to a 30-day gap in medication and 703 were administratively censored at the end of study period.
cA total of 8575 patients were censored due to a 30-day gap in medication and 735 were administratively censored at the end of study period.
Cardiovascular outcomes
Crude incidence rates for MI, stroke, and all-cause mortality with ATV-containing regimens (5.2, 10.4, and 16.0 per 1000 patient-years, respectively) were lower than with regimens containing other protease inhibitors (10.2, 21.9, and 23.3 per 1000 patient-years), NNRTIs (7.5, 15.9, and 17.5 per 1000 patient-years), or INSTIs (13.0, 33.1, and 21.5 per 1000 patient-years) (Fig. 3).
Fig. 3.
Unadjusted incidence rates and inverse probability of treatment weighting adjusted hazard ratios of cardiovascular disease events.
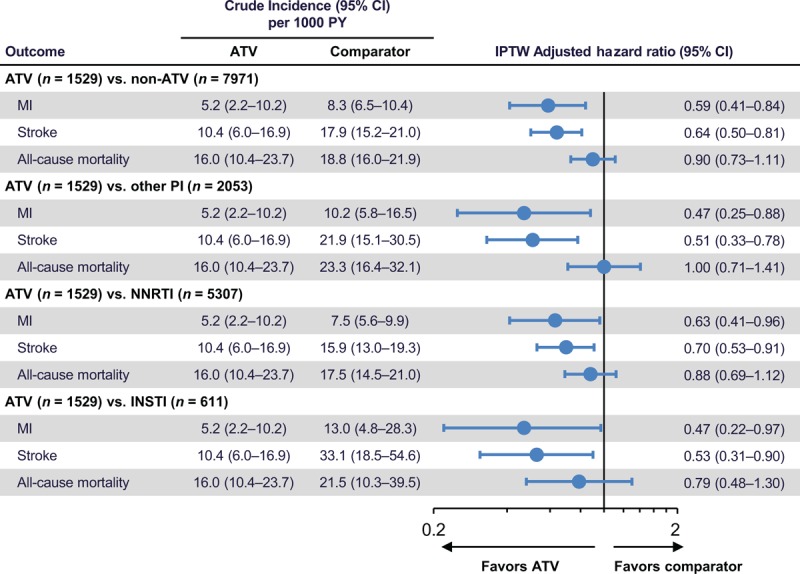
ATV, atazanavir; CI, confidence interval; INSTI, integrase strand transfer inhibitor; IPTW, inverse probability of treatment weighting; MI, myocardial infarction; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PY, patient-years.
After IPTW, adjusted hazard ratios (95% CIs) indicated a significantly reduced risk of both MI and stroke with ATV-containing regimens versus regimens containing other protease inhibitors [0.47 (0.25–0.88) and 0.51 (0.33–0.78), respectively], NNRTIs [0.63 (0.41–0.96) and 0.70 (0.53–0.91)], or INSTIs [0.47 (0.22–0.97) and 0.53 (0.31–0.90)] (Fig. 3). Hemorrhagic strokes were very uncommon (14 overall, and none in the ATV group); thus, results for stroke largely reflect risk for ischemic stroke and transient ischemic attack. The risk of all-cause mortality was not significantly reduced with ATV-containing regimens versus other comparator regimens.
Sensitivity analyses
To support our primary analysis, a number of sensitivity analyses were performed as outlined in the Supplementary Digital Content section entitled ‘Statistical methods for covariate adjustment’. The results of these sensitivity analyses are shown in Supplemental Table S5. Truncated IPTW and IPTW with missing data imputed produced hazard ratios that were qualitatively and quantitatively similar to the primary analysis. For the matching weights analysis, results were qualitatively similar to the primary analysis, but the smaller sample sizes entailed by the use of matching weights resulted in CIs that crossed unity. For the stabilized weights analysis, hazard ratios were qualitatively similar to the primary analysis but significant only for the ATV versus non-ATV comparison for MI and stroke and for the ATV versus other protease inhibitor comparison for stroke. The doubly robust and guideline era-adjusted analyses showed significant reductions in risk of stroke for ATV versus non-ATV, ATV versus other protease inhibitor, and ATV versus NNRTI comparisons, and significant reductions in risk of MI for the ATV versus non-ATV comparison (doubly robust analysis) and ATV versus other protease inhibitor comparison (both doubly robust and guideline era-adjusted analyses).
Discussion
In this population of primarily older men, ATV-containing regimens were associated with an adjusted 41% reduced risk of MI and 36% reduced risk of stroke compared with non-ATV-containing regimens. This significant reduction in MI and stroke risk in the primary analysis was consistently observed across all comparisons between regimens containing ATV and other protease inhibitors, NNRTIs, or INSTIs. No between-regimen differences in all-cause mortality were observed. Of note, the non-ATV comparison arm contained mostly contemporary antiretroviral agents, with 75% of the third agents used being either recommended or alternative agents for treatment-naive individuals from the 2016 guidelines of the Department of Health and Human Services [3]. In addition, this population had a high prevalence of cardiovascular risk factors (i.e. 93% men, 56% black racial origin, 36% receiving antihypertensive therapy, 28% smokers, 25% dyslipidemia treatment, 22% alcohol abuse, 13% diabetes, and 10% history of cardiovascular disease).
Sensitivity analyses largely supported the results of the primary analysis with some differences. The majority of analyses found a significant difference between ATV versus other protease inhibitors for MI and stroke and versus NNRTIs for stroke (Supplemental Table S5).
The crude MI incidence rates overall (7.8 per 1000 patient-years) and in all arms (5.2–13.0 per 1000 patient-years) were higher than those found in previous analyses of Veterans Affairs populations: 3.7 per 1000 patient-years in Bedimo et al.[49] and 2.8 per 1000 patient-years in Desai et al.[50]. This is likely as these earlier analyses collated data on cardiovascular events solely through the VHA Clinical Case Registry, whereas the current analysis obtained data from multiple sources hosted within the VINCI environment as well as data from the CMS. Therefore, the incidence rates established in the current analysis are likely to be more representative of the true underlying rate in this high-risk population. The overall incidence rate of MI in the current analysis was also higher than in other non-Veterans Affairs populations, such as the D:A:D study, which was 2.3 per 1000 patient-years for 1999–2015 [51]. This difference may reflect that the D:A:D study population, with the exception of smoking frequency, had lower proportions of cardiovascular risk factors than the current population (74% men, 7% black racial origin, 9% hypertension, 6% diabetes, and <1% history of cardiovascular disease) [51].
The overall crude stroke incidence rate (16.8 per 1000 patient-years) was also higher than those found in a previous Veterans Affairs analysis (11.7 per 1000 patient-years [49]) and in the D:A:D study (1.7 per 1000 patient-years for 1999–2015 [51]). Of interest, the rate of stroke was also higher than that for MI in the Bedimo et al.[49] Veterans Affairs analysis, whereas it was lower than that for MI in the D:A:D analysis [51]. Taken together, this would suggest a specific cardiovascular risk profile in Veterans Affairs populations of higher risk of stroke than MI, which requires further investigation.
To the best of our knowledge, the current study is the first to demonstrate a reduced risk of cardiovascular disease events with ATV-containing regimens versus non-ATV-containing regimens. The D:A:D study did not find a significantly increased risk of cardiovascular events with ATV [29,41], and the Centers for AIDS Research Network of Integrated Clinical Systems cohort found a nonsignificant trend toward a reduced risk of atherosclerotic events with ATV compared with other protease inhibitors (hazard ratio 0.78; 95% CI: 0.42–1.45) [52]. The data from the current study provide further support to the hypothesis that hyperbilirubinemia-associated delayed cIMT progression [34–36] and reduction in biomarkers [38,40], and indeed hyperbilirubinemia itself [32,53], may be cardioprotective in HIV-infected patients. Three studies that compared atherosclerotic progression as measured by changes in cIMT between ATV/r and various other antiretroviral agents found a slower progression with ATV/r [34–36]. Recent analyses from one of these studies, AIDS Clinical Trials Group (ACTG) A5260s, suggested that the slower progression of cIMT with ATV/r versus DRV/r and raltegravir is mediated by the effect of bilirubin on reduction in lipid and glucose biomarkers, including oxidized lipoproteins, and potentially via its effect on markers of inflammation [40]. Significantly greater reductions in hs-CRP versus DRV/r and in D-dimer versus raltegravir were observed in a separate A5260s analysis [39]. Similar findings were observed in ACTG 5202, in which ATV-induced hyperbilirubinemia was inversely correlated with soluble receptor of TNF-α receptor type II and soluble vascular cellular adhesion molecule-1 [54], and in a prospective observational study demonstrating reduction in oxidized lipoproteins with ATV/r versus efavirenz, which was correlated with ATV-induced changes in serum bilirubin [38].
In addition to careful selection of antiretroviral agents, multifactorial interventions aimed at reducing cardiovascular risk (e.g. smoking cessation and treatment of hypertension and dyslipidemia) are essential components of cardiovascular risk management in patients with HIV infection. Of concern, there is evidence of therapeutic inertia in this domain, with many HIV-infected patients not receiving optimal management of cardiovascular risk factors during routine clinical care [55–57].
The current analysis has limitations. First, average follow-up times were short (approximately 1 year). Average times to first events were longer than average follow-up times for some antiretroviral comparisons and outcomes (Table 2), which may have led to underascertainment of relevant cardiovascular events. The on-treatment nature of the analysis of treatment-naive patients was chosen to limit the confounding effects of other antiretroviral agents on outcomes of interest, but the trade-off for this cleaner comparison was shorter average follow-up times and smaller sample sizes.
Second, a related concern is the possibility of differential censoring given the small number of events and the large proportion of patients who were censored. Most patients (90–92%) were censored when they discontinued the index regimen. However, given that the average follow-up time was close to a year across all regimens, there was little evidence of differential censoring related to discontinuation. A much smaller proportion of patients (8–10%) were administratively censored at the end of the study period while still on treatment. However, given that this proportion was small and consistent across regimens, differential administrative censoring is also unlikely to have resulted in bias.
Third, although IPTW was used to adjust for selection bias and measured confounders, and appeared to achieve balance for all measured covariates (Fig. 2), as with any observational nonrandomized study, the potential for unmeasured confounders and incomplete adjustment for measured confounders cannot be ruled out. For example, on the one hand, multiple cardiovascular risk factors (such as tobacco use, diabetes, hypertension, and dyslipidemia) were more prevalent in patients receiving an INSTI-containing regimen, likely due to channeling bias. On the other hand, abacavir-containing regimens were more often prescribed with ATV than with non-ATV-containing regimens in this population. However, our method of controlling for confounding effectively balanced the observed differences in these measured characteristics.
Fourth, cardiovascular disease events in the VHA are not adjudicated but are based on ICD-9/10 diagnosis and treatment codes, leading to likely underascertainment of events and potential misdiagnosis. However, as the VHA is both the provider and payer, billing is tied more closely to treatments than traditional insurance databases, resulting in less likelihood of coding errors or omissions than in other insurance claims analyses. In addition, even if underascertainment occurred, this is likely to have affected events associated with other antiretroviral agents as well as ATV. Although this could potentially affect crude incidence rates, it should not impact relative comparisons between regimens.
Fifth, a substantial proportion of patients receiving a diagnosis of HIV infection appeared to have never started a combination ART regimen during the study (13 446/32 370; 42.5%) and were excluded from our analysis. However, previous research has indicated that up to 56.6% of US veterans may use both Veterans Affairs and non-Veterans Affairs healthcare services [58]. Thus, a proportion of these patients with HIV infection who did not start ART within the VHA may have done so outside of the VHA.
Finally, this analysis could not differentiate between type 1 (atherosclerotic) and type 2 (supply/demand mismatch) MI. As ATV is hypothesized to specifically impact only type 1 MI, inclusion of both type 1 and type 2 MI within the MI outcome of interest may potentially mask larger treatment differences in type 1 MI. Further longitudinal research by using adjudicated type 1 MI events [59] is warranted to address this question.
To conclude, in the US national VHA database, regimens containing ATV were associated with a significantly lower risk for both MI and stroke compared with those containing other protease inhibitors, NNRTIs, or INSTIs in our primary analysis. Sensitivity analyses were largely consistent with the primary analysis, although some of them suggested that reduction in risk for MI may only be evident for the ATV versus other protease inhibitor comparison and that the risk of stroke may only be reduced for ATV versus NNRTIs and other protease inhibitors and not INSTIs. Further research is warranted to elucidate the mechanism for the slower progression of atherosclerosis and potential reduced risk of cardiovascular disease events with ATV.
Acknowledgements
The study was supported by Bristol-Myers Squibb, the manufacturer of atazanavir. The authors thank Julian Martins of inScience Communications, Springer Healthcare, who provided medical writing assistance, which was funded by Bristol-Myers Squibb.
J.L., A.P.B., L.R., P.S., J.M., and C.R. contributed to the conception and design of the study. J.L., A.P.B., and J.C. contributed to data collection. J.L., A.P.B., and J.C. contributed to data analysis. J.L., A.P.B., L.R., P.S., J.M., and C.R. contributed to data interpretation. J.L., A.P.B., L.R., J.C., P.S., J.M., and C.R. contributed to writing and editing the article.
Conflicts of interest
The study was supported by Bristol-Myers Squibb by a grant to the University of Utah; J.L., A.P.B., and J.C. received some salary support from this grant. J.L., A.P.B., and J.C. declare no intellectual property rights related to this research. Outside of the funded work, over the last 3 years, the following organizations provided research grants to the University of Utah and the following individuals worked on those projects and/or received salary or other types of support that were funded by those grants: Cubist (J.C.); Gilead Sciences, Inc. (J.L., A.P.B., and J.C.); Anolinx LLC (J.L. and J.C.); Skaggs Foundation (J.L. and J.C.); Agency for Healthcare Research and Quality (J.L. and J.C.); Utah Department of Health (J.C.); and the National Heart, Lung, and Blood Institute (NHLBI) (A.P.B.). P.E.S. declares having received honoraria for advisory board membership and consultancy services from AbbVie, Bristol-Myers Squibb, GlaxoSmithKline/ViiV, Gilead Sciences, Janssen, and Merck; his institution has received research grants from Bristol-Myers Squibb, GlaxoSmithKline/ViiV, and Gilead Sciences. L.R., J.M., and C.R. are employees of and own stock in Bristol-Myers Squibb.
Supplementary Material
References
- 1.Antiretroviral Therapy Cohort Collaboration (ART-CC). Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry CP, Jr, Klein DB, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016; 73:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, 2016. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf [Accessed 18 October 2016]. [Google Scholar]
- 4.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach – Second edition, 2016. http://www.who.int/hiv/pub/arv/arv-2016/en/ [Accessed 18 October 2016]. [PubMed] [Google Scholar]
- 5.Arias E. United States life tables, 2011. National vital statistics reports; vol 64 no 11 Hyattsville, MD: National Center for Health Statistics, 2015. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_11.pdf [Accessed 19 October 2016]. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. HIV among people aged 50 and over, 2016. http://www.cdc.gov/hiv/group/age/olderamericans/ [Accessed 20 October 2016]. [Google Scholar]
- 7.Boccara F. Cardiovascular health in an aging HIV population. AIDS 2017; 31 suppl 2:S157–S163. [DOI] [PubMed] [Google Scholar]
- 8.Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr 2003; 33:506–512. [DOI] [PubMed] [Google Scholar]
- 9.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sorensen HT, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis 2007; 44:1625–1631. [DOI] [PubMed] [Google Scholar]
- 11.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec's public health insurance database. J Acquir Immune Defic Syndr 2011; 57:245–253. [DOI] [PubMed] [Google Scholar]
- 12.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Womack JA, Chang CC, So-Armah KA, Alcorn C, Baker JV, Brown ST, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc 2014; 3:e001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr 2015; 68:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanna DB, Ramaswamy C, Kaplan RC, Kizer JR, Anastos K, Daskalakis D, et al. Trends in cardiovascular disease mortality among persons with HIV in New York City, 2001–2012. Clin Infect Dis 2016; 63:1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162:335–344. [DOI] [PubMed] [Google Scholar]
- 17.Post WS, Budoff M, Kingsley L, Palella FJ, Jr, Witt MD, Li X, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014; 160:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh NI, Gerschenson M, Bennett K, Gangcuangco LM, Lopez MS, Mehta NN, et al. Lipoprotein concentration, particle number, size and cholesterol efflux capacity are associated with mitochondrial oxidative stress and function in an HIV positive cohort. Atherosclerosis 2015; 239:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 2005; 352:48–62. [DOI] [PubMed] [Google Scholar]
- 20.Ballocca F, Gili S, D’Ascenzo F, Marra WG, Cannillo M, Calcagno A, et al. HIV infection and primary prevention of cardiovascular disease: lights and shadows in the HAART era. Prog Cardiovasc Dis 2016; 58:565–576. [DOI] [PubMed] [Google Scholar]
- 21.Stein JH, Hsue PY. Inflammation, immune activation, and CVD risk in individuals with HIV infection. JAMA 2012; 308:405–406. [DOI] [PubMed] [Google Scholar]
- 22.Beltran LM, Rubio-Navarro A, Amaro-Villalobos JM, Egido J, Garcia-Puig J, Moreno JA. Influence of immune activation and inflammatory response on cardiovascular risk associated with the human immunodeficiency virus. Vasc Health Risk Manag 2015; 11:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vos AG, Idris NS, Barth RE, Klipstein-Grobusch K, Grobbee DE. Pro-inflammatory markers in relation to cardiovascular disease in HIV infection. A systematic review. PLoS One 2016; 11:e0147484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borges AH, O’Connor JL, Phillips AN, Ronsholt FF, Pett S, Vjecha MJ, et al. Factors associated with plasma IL-6 levels during HIV infection. J Infect Dis 2015; 212:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westhorpe CL, Schneider HG, Dunne M, Middleton T, Sundararajan V, Spelman T, et al. C-reactive protein as a predictor of cardiovascular risk in HIV-infected individuals. Sex Health 2014; 11:580–582. [DOI] [PubMed] [Google Scholar]
- 27.Phillips AN, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther 2008; 13:177–187. [DOI] [PubMed] [Google Scholar]
- 28.Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis 2010; 201:318–330. [DOI] [PubMed] [Google Scholar]
- 29.Ryom L, Lundgren JD, El-Sadr W, Reiss P, Phillips A, Kirk O, et al. Association between cardiovascular disease & contemporarily used protease inhibitors [Abstract 128LB]. Conference on Retroviruses and Opportunistic Infections (CROI), February 13-16, 2017, Seattle, WA, USA. http://www.croiconference.org/sessions/association-between-cardiovascular-disease-contemporarily-used-protease-inhibitors (abstract) and http://www.croiwebcasts.org/p/2017croi/croi33599 (webcast) [Accessed 2 March 2017]. [Google Scholar]
- 30.Berk PD, Wolkoff AW. Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL. Bilirubin metabolism and the hyperbilirubinemias. Harrison's principles of internal medicine 15th ed.New York: McGraw-Hill; 2001. 1715–1720. [Google Scholar]
- 31.McDonald C, Uy J, Hu W, Wirtz V, Juethner S, Butcher D, et al. Clinical significance of hyperbilirubinemia among HIV-1-infected patients treated with atazanavir/ritonavir through 96 weeks in the CASTLE study. AIDS Patient Care STDS 2012; 26:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang SJ, Lee C, Kruzliak P. Effects of serum bilirubin on atherosclerotic processes. Ann Med 2014; 46:138–147. [DOI] [PubMed] [Google Scholar]
- 33.Horsfall LJ, Nazareth I, Pereira SP, Petersen I. Gilbert's syndrome and the risk of death: a population-based cohort study. J Gastroenterol Hepatol 2013; 28:1643–1647. [DOI] [PubMed] [Google Scholar]
- 34.de Saint-Martin L, Bressollette L, Perfezou P, Bellein V, Ansart S, Vallet S, et al. Impact of atazanavir-based HAART regimen on the carotid intima–media thickness of HIV-infected persons: a comparative prospective cohort. AIDS 2010; 24:2797–2801. [DOI] [PubMed] [Google Scholar]
- 35.Stein JH, Ribaudo HJ, Hodis HN, Brown TT, Tran TT, Yan M, et al. A prospective, randomized clinical trial of antiretroviral therapies on carotid wall thickness. AIDS 2015; 29:1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow D, Kohorn L, Souza S, Ndhlovu L, Ando A, Kallianpur KJ, et al. Atazanavir use and carotid intima media thickness progression in HIV: potential influence of bilirubin. AIDS 2016; 30:672–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chow D, Shikuma C, Ritchings C, Guo M, Rosenblatt L. Atazanavir and cardiovascular risk among human immunodeficiency virus-infected patients: a systematic review. Infect Dis Ther 2016; 5:473–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Estrada V, Monge S, Gomez-Garre MD, Sobrino P, Masia M, Berenguer J, et al. Relationship between plasma bilirubin level and oxidative stress markers in HIV-infected patients on atazanavir- vs. efavirenz-based antiretroviral therapy. HIV Med 2016; 17:653–661. [DOI] [PubMed] [Google Scholar]
- 39.Kelesidis T, Tran TT, Stein JH, Brown TT, Moser C, Ribaudo HJ, et al. Changes in inflammation and immune activation with atazanavir-, raltegravir-, darunavir-based initial antiviral therapy: ACTG 5260s. Clin Infect Dis 2015; 61:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moser CB, Currier JS, Stein JH, Ribaudo HJ, Dube MP, Brown TT, et al. Hyperbilirubinemia during ATV/r initiation may slow the progression of carotid wall thickening through its effects on glucose and lipid metabolism: a structural equation modeling approach from ACTG A5260s [Abstract O15]. Antivir Ther 2016; 21 suppl 1:A17. [Google Scholar]
- 41.Monforte A, Reiss P, Ryom L, El-Sadr W, Dabis F, De Wit S, et al. Atazanavir is not associated with an increased risk of cardio- or cerebrovascular disease events. AIDS 2013; 27:407–415. [DOI] [PubMed] [Google Scholar]
- 42.Braithwaite RS, Kozal MJ, Chang CC, Roberts MS, Fultz SL, Goetz MB, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS 2007; 21:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gandhi NR, Tate JP, Rodriguez-Barradas MC, Rimland D, Goetz MB, Gibert C, et al. Validation of an algorithm to identify antiretroviral-naive status at time of entry into a large, observational cohort of HIV-infected patients. Pharmacoepidemiol Drug Saf 2013; 22:1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.CMS Chronic Conditions Data Warehouse (CCW). CCW Condition Algorithms, 2016. https://www.ccwdata.org/web/guest/condition-categories [Accessed 15 February 2017]. [Google Scholar]
- 45.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J 2004; 148:99–104. [DOI] [PubMed] [Google Scholar]
- 46.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke 2005; 36:1776–1781. [DOI] [PubMed] [Google Scholar]
- 47.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deb S, Austin PC, Tu JV, Ko DT, Mazer CD, Kiss A, et al. A review of propensity-score methods and their use in cardiovascular research. Can J Cardiol 2016; 32:259–265. [DOI] [PubMed] [Google Scholar]
- 49.Bedimo RJ, Westfall AO, Drechsler H, Vidiella G, Tebas P. Abacavir use and risk of acute myocardial infarction and cerebrovascular events in the highly active antiretroviral therapy era. Clin Infect Dis 2011; 53:84–91. [DOI] [PubMed] [Google Scholar]
- 50.Desai M, Joyce V, Bendavid E, Olshen RA, Hlatky M, Chow A, et al. Risk of cardiovascular events associated with current exposure to HIV antiretroviral therapies in a US veteran population. Clin Infect Dis 2015; 61:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryom L, Lundgren JD, Ross M, Kirk O, Law M, Morlat P, et al. Renal impairment and cardiovascular disease in HIV-positive individuals: the D:A:D study. J Infect Dis 2016; 214:1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crane HM, Nance RM, Heckbert SR, Budoff M, Tirschwell D, Ritchings C, et al. Association between initial antiretroviral therapy regimen and cardiovascular disease events among persons living with HIV in the US [Abstract O13]. Antivir Ther 2016; 21 suppl 1:A15. [Google Scholar]
- 53.Marconi VC, So-Armah K, Tate J, Lim J, Lo Re V, Butt AA, et al. Hyperbilirubinemia prevents cardiovascular disease for HIV+ and HIV− individuals [Abstract 127]. Conference on Retroviruses and Opportunistic Infections (CROI), February 13-16, 2017, Seattle, WA, USA. http://www.croiconference.org/sessions/hyperbilirubinemia-prevents-cardiovascular-disease-hiv-and-hiv-individuals (abstract) and http://www.croiwebcasts.org/p/2017croi/croi33598 (webcast) [Accessed 2 March 2017]. [Google Scholar]
- 54.Venuto CS, Hunt PW, McComsey GA, Morse GD, Messing S. inflammation investigated as a source of atazanavir pharmacokinetic variability [Abstract 428]. Conference on Retroviruses and Opportunistic Infections (CROI), February 22-25, 2016, Boston, MA, USA. http://www.croiconference.org/sessions/inflammation-investigated-source-atazanavir-pharmacokinetic-variability (abstract) and www.croiconference.org/sites/default/files/posters-2016/428.pdf (poster) [Accessed 2 March 2017]. [Google Scholar]
- 55.Levy ME, Greenberg AE, Hart R, Powers Happ L, Hadigan C, Castel A. High burden of metabolic comorbidities in a citywide cohort of HIV outpatients: evolving healthcare needs of people aging with HIV in Washington, DC. HIV Med 2017; [Epub ahead of print]. doi:10/1111/hiv.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Zoest RA, van der Valk M, Wit FW, Vaartjes I, Kooji KW, Hovius J, et al. Primary and secondary cardiovascular prevention is suboptimal in HIV-infected patients: results for the AGEhIV Cohort Study. Antivir Ther 2016; 21 suppl 1:A21. [Google Scholar]
- 57.Langebeek N, Kooij KW, Wit FW, Stolte IG, Sprangers MAG, Reiss P, et al. Impact of co-morbidity and aging on health-related quality of life in HIV-positive and HIV-negative individuals. AIDS 2017; 31:1471–1481. [DOI] [PubMed] [Google Scholar]
- 58.Ross JS, Keyhani S, Keenan PS, Bernheim SM, Penrod JD, Boockvar KS, et al. Dual use of Veterans Affairs services and use of recommended ambulatory care. Med Care 2008; 46:309–316. [DOI] [PubMed] [Google Scholar]
- 59.Drozd DR, Kitahata MM, Althoff KN, Zhang J, Gange SJ, Napravnik S, et al. Increased risk of myocardial infarction in HIV-infected individuals in North America compared with the general population. J Acquir Immune Defic Syndr 2017; 75:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


