Abstract
Glaciers cover ∼10% of the Earth’s land surface, but they are shrinking rapidly across most parts of the world, leading to cascading impacts on downstream systems. Glaciers impart unique footprints on river flow at times when other water sources are low. Changes in river hydrology and morphology caused by climate-induced glacier loss are projected to be the greatest of any hydrological system, with major implications for riverine and near-shore marine environments. Here, we synthesize current evidence of how glacier shrinkage will alter hydrological regimes, sediment transport, and biogeochemical and contaminant fluxes from rivers to oceans. This will profoundly influence the natural environment, including many facets of biodiversity, and the ecosystem services that glacier-fed rivers provide to humans, particularly provision of water for agriculture, hydropower, and consumption. We conclude that human society must plan adaptation and mitigation measures for the full breadth of impacts in all affected regions caused by glacier shrinkage.
Keywords: glacier, runoff, biogeochemistry, biodiversity, ecosystem services
Global-scale understanding of glacier mass loss has improved significantly in the past decade, principally via advances in satellite remote-sensing instrumentation and processing. Previous estimates of mass loss were extrapolated from sparsely distributed in situ observations (1), whereas field measurements are now combined routinely with globally complete glacier inventories (2) and direct measurements of volume and mass change from satellite altimetry and gravimetry to provide accurate estimates of global glacier mass change (3). The most recent estimate of global glacier change indicates a mass loss rate of 259 ± 28 Gt y−1 between 2003 and 2009 (3), with global runoff from glaciers exceeding 1,350 km3 y−1 (4). Research to date on the associated downstream impacts of this glacier mass loss has focused largely on sea-level rise (3, 5) and to a lesser extent, on water availability and runoff (6). The scale of ice loss worldwide creates an urgent need to evaluate comprehensively the broader spectrum of downstream effects on freshwater and near-shore marine systems. This increased understanding is vital to aid planning for adaptation and mitigation measures in the most significantly affected regions. The largest individual contributions to global glacier mass loss come from the glaciers of the Gulf of Alaska, the Canadian Arctic, and the ice sheet peripheries of Greenland and Antarctica. However, the glaciers with the most negative mass balances are located in the European Alps and at low latitudes in the South American Andes (3). In the European Alps, atmospheric warming has been pronounced in the last 30 years, especially during the summer months, which when combined with decreased snowfall, has led to a 54% loss of ice area since 1850; current projections suggest that just 4–13% of the 2003 European Alps ice area will remain by 2100 (7, 8). Similarly, in South America, Bolivian glaciers have lost nearly 50% of their mass in the last 50 years (9), and in western Canada, glacier ice volume relative to 2005 is projected to decrease 70 ± 10% by 2100 (10). Glaciers in the Ganges, Indus, and Brahmaputra Rivers basins of the Himalaya are currently losing 24 ± 2 Gt y−1 of ice (11), equivalent to around 10% of the global glacier mass loss between 2003 and 2009 (3). Climate-driven changes in glacier volume will alter the timing, magnitude, and frequency of downstream discharge, sediment transport, and the speciation of nutrients, with far-reaching consequences for downstream ecosystems, including rivers, lakes, and the coastal zones of major oceans, and the human populations that depend on them (12).
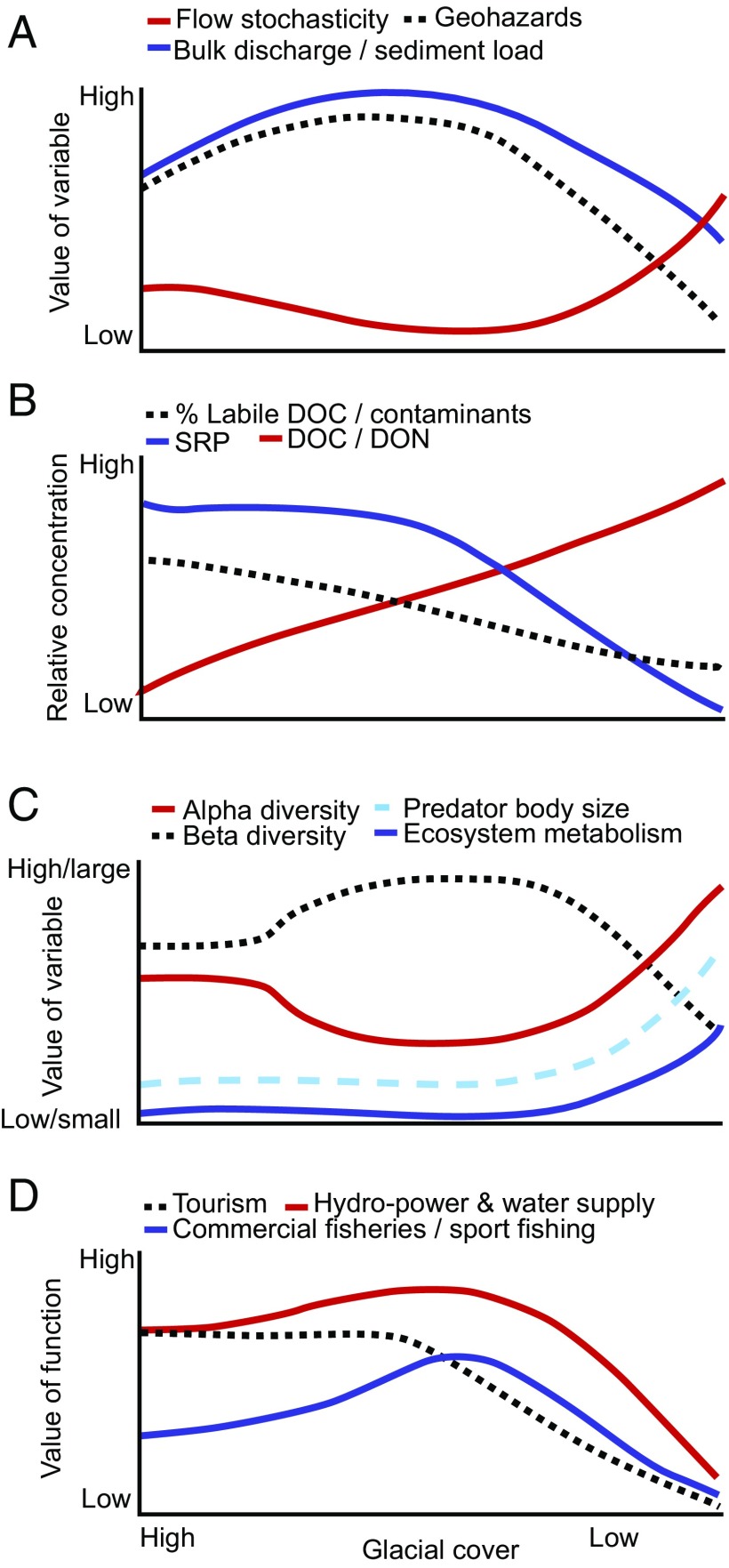
The downstream impacts along the continuum from glaciers to rivers to the ocean affect various ecosystem levels ranging from shifts in biogeochemical and sediment fluxes (13, 14) to loss of biodiversity (15, 16) and perturbed food web dynamics (17). Glacier shrinkage also will have important implications for the ecosystem services that glacier-fed rivers provide, particularly in regions where glacier runoff is integral to multiple human activities, such as farming, energy production, water supply/quality, and tourism. The scale of potential changes faced by society is immense. For example, Himalayan glaciers alone provide water to 1.4 billion people (18). Here, we examine multiple lines of evidence from glacier-fed systems globally to develop a framework to synthesize existing information and expert understanding about how glacier shrinkage and changes in runoff regimes will impact downstream ecosystems and human society. We have developed integrated conceptual models of how discharge, nutrients, ecological communities, and ecosystem services respond as glacial cover decreases over time with ongoing climate warming (Fig. 1). Building on this framework, we present a globally integrated perspective into the complex adaptation decisions required with respect to changing ecosystem services and associated societal adaptations within glacierized regions.
Fig. 1.
Conceptual response curves for (A) river flow, geohazards, and sediment load; (B) nutrients and contaminants; (C) ecological community attributes; and (D) ecosystem services for large and small glaciers with decreasing glacial cover. Note that, for each panel, the y axis is relative to the system type (i.e., low discharge for a large valley glacier in A will be greater than low discharge for a small alpine glacier). A–C are based on literature findings as referenced in the text, and D is hypothetical based on understanding.
Hydro- and Geomorphological Implications of Glacier Melt
Estimating the quantity and timing of melt water runoff in relation to glacier mass balance is a major challenge because of complex hydroclimatological interactions across different spatial scales and climatic zones (6). A significant amount of “glacial” runoff is sourced from the melt of annual snowfall that passes over or on the surface of the glacial ice, which will still occur in the absence of a glacier. However, snowmelt-dominated systems have a more variable and temporally constrained hydrological regime compared with glacially-dominated systems. Glacier runoff continues as long as there is energy for melt because of the ice reservoir. Hence, the loss or substantial reduction of ice will lead to distinct changes in the timing and magnitude of runoff peaks (19). Glacial ice melt runoff typically peaks during summer in mid- to high-latitude regions, when runoff from other sources is typically low, thereby buffering dry season stream discharge from precipitation and snowmelt variability (Fig. 2A, high glacier cover) (20) and preventing drought in some regions (12). Over longer timescales, regions with extensive glacier cover, such as the northeastern Canadian Arctic and Svalbard, are predicted to see an initial increase in annual glacier runoff (4) because of the earlier disappearance of the reflective snow cover and the exposure of ice with lower surface albedo. However, this initial bulk discharge increase (Fig. 1A) will be followed by reduced annual runoff caused by the ensuing decrease in glacier volume (21), such as is presently observed with smaller glaciers. Paired long-term glacier mass balance and streamflow records in Alaska suggest that the overall loss of glaciers will decrease total catchment runoff by 10–20% (22). During the coming century, as total runoff from glaciers decreases in most regions (Fig. 1A), river flow in summer will become more sensitive to precipitation events, particularly if these become more extreme with climate change.
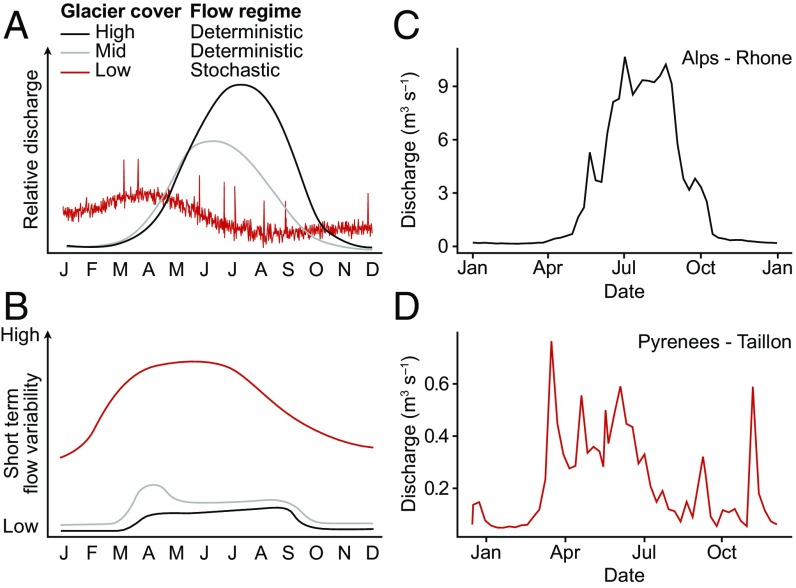
Fig. 2.
(A) Conceptual representation of the changing hydrograph for alpine glacier-fed rivers. For the low-glacier cover scenario, a change in climate is also anticipated: in particular, an increase in the magnitude and frequency of extreme events and a decrease in the fraction of precipitation falling as snow. (B) Hypothesized sensitivity of river flow to extreme events (droughts and high-intensity storms) and short-term flow variability (i.e., variability in the weekly mean discharge); (C) discharge for the Rhone River, Italy with high glacial cover; and (D) discharge of the Taillon River, French Pyrenees with low glacial cover, indicating stochastic discharge events caused by rainfall or rain on snow events.
On annual timescales, glacier runoff produces a deterministic, predictable hydrograph in temperate and Arctic glacier-fed rivers. Future decreases in glacier runoff will result in a shift toward a more stochastic hydrological regime in these watersheds (Fig. 2A). In particular, the replacement of predictable summer glacier melt by less predictable rainfall events and snowmelt runoff regimes will increase the relative magnitude and seasonal duration of short-term flow variability (Fig. 2B). These shifts are already evident for glacierized catchments in the Pyrenees and Alps, showing that, in watersheds with low glacial influence, river flows in spring and fall will be higher with more variability (Fig. 2D) than when glacier cover is intermediate or high (Fig. 2C). This is caused by a combination of the earlier onset of snow melt and an increased fraction of winter precipitation falling as rain with climate warming (Fig. 2A) (23). Clearly, glacier-driven changes in hydrology will have major implications for downstream ecosystem processes and ecological communities given the known importance of predictable annual flow pulses for river biodiversity (24). However, this can vary according to region; for example, in the Himalaya, precipitation from the monsoon system provides a deterministic element to the annual flow cycle, although the year-to-year variability will increase without the buffering capacity of glacial runoff (25). Equatorial systems typically have no seasonality but high diurnal variability (26). These systems are ablation-driven with no snow accumulation; therefore, the quantity of meltwater will decrease over time, and the magnitude of this diurnal variability will gradually decrease.
In Nepal and Greenland, basins have formed between the glacier and the lateral or end moraines with rapid ice recession in recent times. Stagnant ice in these basins may melt to form glacial lakes, which can grow rapidly in size (27) and catastrophically discharge as a glacial lake outburst floods. Such geohazards are predicted to increase with ongoing glacier recession in heavily glacierized regions (28) (Fig. 1A), leading to significant societal impacts via loss of life and damage to land, property, and infrastructure (29).
In the long term, reduced glacial runoff will increase summer water temperature downstream because of (i) an increase in atmospheric energy receipt with warmer air temperature (although radiation could be reduced by increased cloudiness), (ii) a decline in the relative contribution of cold water from glacial melt, and (iii) the reduced heat capacity of rivers with lower flow (30). Concomitantly, bedload and suspended sediment load are expected to decrease in the long term as glacial runoff becomes reduced and erosion decreases (31) (Fig. 1A). The resultant decrease in turbidity will increase light penetration to the bed of streams and rivers, with implications for primary production and the photochemical transformation of organic matter enhanced by increased channel stability where braiding of river channels is less (31).
Glacier retreat is likely to expose new areas of unconsolidated glacial sediments and to destabilize slopes in many areas (32), creating the potential for hazardous landslides (Fig. 1A) and short-term increases in sediment yields from glacial basins. On steeper slopes, where vegetation cannot be established, sediment yields will occur for a longer time period (33). An additional consequence of a reduction in glacier volume in polar areas may be a decrease in sediment yield from glaciers undergoing changes in their thermal regime (34). Ice overburden pressure and heat release by deformation and strain heating will be reduced by glacier thinning, potentially reducing the energy available for subglacial erosion, especially in cases where the energy loss causes a reduction in basal sliding. Ultimately, thinning glaciers may even freeze onto the bed. However, this decrease in sediment yield may be compensated for by mobilization of paraglacial sedimentary materials from thermoerosion of ice-cored moraines and increased prevalence of ice-marginal landslides and debris flows (35). The net result is a shift from glacial- to paraglacial-dominated mountain catchments, which in the short term (coming decades), will increase sediment supply and associated geohazards within mountain ranges (Fig. 1A).
Biogeochemical Fluxes and Cycling
Recent discoveries highlight glacier perturbations of global and regional biogeochemical cycles (14, 36). Glaciers receive and accumulate dissolved organic carbon (DOC) and inorganic nutrients from both wet and dry deposition. Red-colored green algae growing on the glacier surface can reduce the albedo, thereby increasing melting—a phenomenon known as the “bioalbedo” effect (37). These species are frequently derived from remote sources (38, 39). A wide variety of microorganisms (including algae) assimilate inorganic nutrients within the snow and ice and in meltwater flowing down crevasses and entering sedimentary habitats at the glacier bed (40). Microorganisms transform labile inorganic nutrients, such as ammonium and phosphate, into organic forms (41) that ultimately contribute to the pool of ice-locked organic matter. At a global scale, ice-locked organic matter is substantial and represents a hitherto poorly understood reservoir of carbon (36). The biogeochemical diversity of this organic matter is also unexpectedly high, with many constituents containing proteinaceous molecules, lignins, and tannins (39).
Supraglacial microbial communities are important drivers of the production and export of DOC from glacial ecosystems (42, 43). Studies on Alaskan (38) and alpine (39) glaciers have shown that ice-locked DOC is highly bioavailable (up to 80%) to microbial heterotrophs and that, on release, this DOC can stimulate riverine carbon cycling (39) and sustain aquatic food webs (44) in glacier-fed rivers. Bioreactive proteinaceous molecules have a near-global distribution within glacial meltwaters (45), and changes to the export of this labile substrate to downstream ecosystems will occur with glacial recession. DOC, dissolved inorganic nitrogen (DIN), and dissolved organic nitrogen (DON) concentrations in runoff increase as glacier coverage in the catchment decreases (Fig. 1B and Fig. S1 A–C), although overall DOC bioavailability decreases (Fig. 1B) (38). Although DOC concentrations in glacier runoff are low, fluxes of labile DOC to downstream ecosystems can be substantial because of the relatively high specific discharge in glacierized watersheds. Glacier runoff may also act as a source of N. For example, a significant flux of reactive N, equivalent to the sum of the dissolved nitrogen, may be associated with NH4 adsorption onto suspended sediments (14). Glacial sediments also seem to promote nitrification, thereby making glacier basins net exporters of nitrate to downstream ecosystems (46).
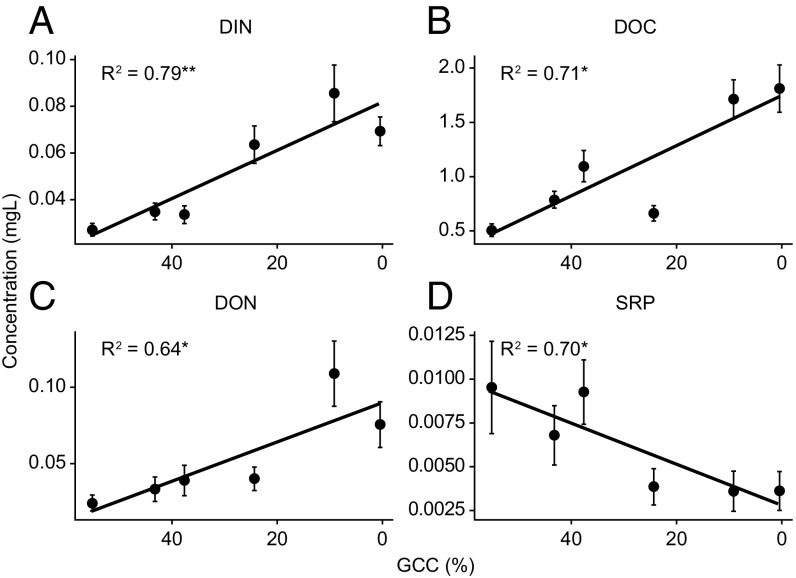
Fig. S1.
Variations in (A) dissolved inorganic nitrogen, (B) dissolved organic carbon, (C) dissolved organic nitrogen, and (D) soluble reactive phosphorus as a function of glacier coverage in the catchment (GCC) *P < 0.05 and **P < 0.01 (47).
The concentration of soluble reactive phosphorus (SRP) is predicted to decrease with declining glacier coverage in the watershed (Fig. 1B and Fig. S1D) (47), and a large proportion of that reactive P flux can also be bound loosely to suspended sediment (14). However, with increasing glacier retreat, DOC, DIN, and DON concentrations are likely to increase, while SRP concentrations will decrease (Fig. 1B). In the short term, dependent on factors such as the flux of glacier meltwater and the concentration of glacial suspended sediment, elevated fluxes of potentially bioavailable NH4+ and P may mitigate declines in dissolved N and P.
An unexpected impact of glacier shrinkage is the liberation of contaminants including emission products from industrial activity (48), such as black carbon and associated compounds, mercury, pesticides, and other persistent organic pollutants (49). Uncertainty exists in how climate change is releasing these contaminants from glacier ice, leading to their transport to downstream systems by meltwater, which should reduce in concentration as glacial ice shrinks (Fig. 1B) (50). Importantly, glaciers now represent the most unstable stores of the so-called legacy pollutants, such as dichlorodiphenyltrichloroethane, in European and other mountain areas flanking large urban centers. Their accumulation in lake ecosystems downstream from glacierized mountains has been modeled using climate-driven mass balance change to alpine glaciers in Europe (48). However, their impacts as they cascade through downstream ecosystems remain largely unexplored, suggesting that the additional study of contaminant dynamics should be extended to a wide range of mountain environments, such as the Himalaya, where meltwater supplies drinking and irrigation water to billions of people.
Biodiversity
Diverse ecological communities have adapted over evolutionary timescales to the harsh environmental conditions in glacier-fed streams and rivers. Benthic invertebrate communities play important roles in the flux of biomass and nutrients (C, N, and P) from lower (algal, microbial, detrital resources) to upper trophic levels (fish, birds, mammals, amphibians). Shifts in the hydrological regime will alter the physical and chemical template to which these communities have adapted, ultimately leading to overall loss of biodiversity, ranging from algae to fish (51). The scale of these effects on biodiversity remains poorly quantified (16), with studies to date focusing primarily on community-level changes. Here, we focus on differences among biological groups regarding their ability to withstand changing conditions and the role of phenotypic plasticity and genetic adaptations in response to climatic change.
Microbial and Algal Communities.
Microbial life in glacier-fed streams is dominated by biofilms that extensively colonize the sediment surface (52). Encapsulated in a polymeric matrix, species from all three domains of life (Archaea, Bacteria, and Eukarya) coexist in these biofilms, where they are protected against harsh environmental conditions, including pronounced variability in flow, high UV radiation, and low availability of organic carbon (52). Benthic biofilms in mid- to high-latitude glacier-fed streams can flourish during optimal environmental windows in spring, when channels unfreeze, flow is low, and the water is clear, and in fall, when similar conditions exist (53). Similarly, benthic biofilms can develop markedly where channels remain open during winter because of upwelling groundwater (54).
Biofilms drive most of the aquatic ecosystem respiration (ER) and gross primary production (GPP) and are, therefore, critical to the carbon and nutrient cycles in glacier-fed rivers. The extension of the temporal period of clear water may cause sites of increased primary production (55) dominated by diatoms and the gold filamentous alga (Hydrurus foetidus) (56). Channels become more stable as glacial inputs diminish, analogous to nonglacial Arctic rivers (57), and bryophytes may become more abundant and compete with benthic algae. Overall, these changes would increase the annual GPP, likely followed by higher ER in these streams as glacial influence becomes markedly reduced (Fig. 1C).
Molecular analyses have recently highlighted surprisingly high bacterial richness in biofilms in glacier-fed rivers (58) caused by turnover between putatively active and dormant taxa continuously recruited from upstream sources in the catchment (e.g., from soils, rocks, groundwater), which maintains high bacterial richness (59). This is advantageous for biofilms to react rapidly to an environment that is continuously subject to physical disturbance and environmental fluctuations. Previous studies suggest that community assembly of microbial biofilms is largely deterministic, involving a series of environmental filters (58, 60). However, with ongoing glacier shrinkage, increasingly unpredictable flows during the spring window when glacier cover is low (Fig. 1A) will likely reduce the optimal period for biofilm growth in glacier-fed rivers. In addition, as glacial runoff further decreases, bacterial communities in biofilms will become more similar to those in rivers fed predominantly by groundwater, snowmelt, and rain, thereby leading to a homogenization of microbial diversity at the landscape level (i.e., loss of β diversity) and decreasing regional diversity (γ diversity) (Fig. 1C) (58, 61).
Macroinvertebrates.
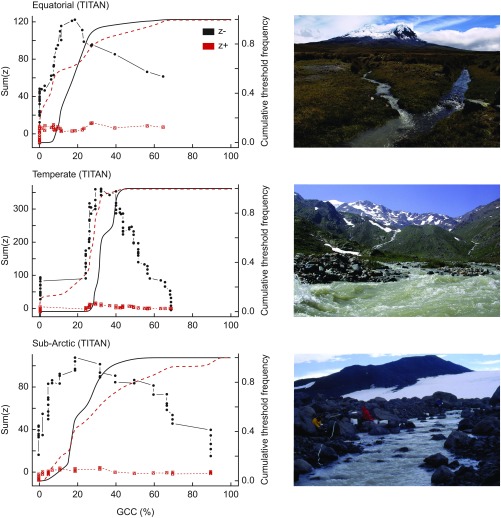
This group has been well-studied in freshwaters globally and therefore, is typically considered a “model” group for tracking environmental change. Glacially-dominated rivers are characterized typically by the deterministic nature of their benthic macroinvertebrate communities caused by the overriding conditions of low water temperature and low channel stability (62, 63). Substantial changes in macroinvertebrate local diversity (α diversity) and distribution patterns are likely as glacial cover and meltwater runoff decline in catchments and the hydrological regime in glacier-fed rivers become more stochastic (15, 24, 64) (Fig. 1C). Threshold analyses from glacierized river systems in three regions suggests cold stenothermic organisms become extinct when glacial cover falls below 19% for the sub-Arctic, 20.4% for temperate, and 31.8% for equatorial (SI Thresholds and Tipping Points; see Fig. S2 and Table S1). In general, α diversity is expected to increase at the reach level as environmental conditions ameliorate with lower summer discharge and turbidity and higher channel stability and water temperature, with communities tracking optimal water temperature by migrating upstream (65).
Fig. S2.
Community change points identified across glacier influence gradients [glacier cover in catchment (GCC)] for equatorial (Ecuador), temperate (Italian Alps), and sub-Arctic (Iceland) systems. Change points were estimated using Threshold indicator taxa analysis (TITAN) (113), a nonparametric technique that orders and partitions observations along the gradient using IndVal scores (114) to define groupings. Multiple candidate change points are identified, and IndVal scores are calculated for each taxon (250 permutations) and then standardized (z scores). Declining (z−) and increasing (z+) taxa are used to identify community-level change points, and uncertainty is estimated via bootstrapping (500 replicates). Photographs represent the individual sites.
Table S1.
Threshold indicator taxa analysis results for the three regions
| Method | Iceland | Ecuador | Italy | |||||||||
| Obs. | 0.05 | 0.5 | 0.95 | Obs. | 0.05 | 0.5 | 0.95 | Obs. | 0.05 | 0.5 | 0.95 | |
| Sum z− | 25.5 | 11.0 | 19.0 | 45.0 | 17.7 | 8.2 | 15.9 | 27.2 | 31.8 | 26.6 | 31.8 | 41.6 |
| Sum z+ | 36.0 | 5.0 | 32.0 | 90.0 | 33.4 | 0.0 | 7.3 | 68.5 | 11.9 | 0.0 | 23.8 | 31.8 |
| nCPA.bc | 25.5 | 11.0 | 32.0 | 68.5 | 17.7 | 7.7 | 17.7 | 33.4 | 30.3 | 27.8 | 31.8 | 41.9 |
Observed change points (Obs) and 5th, 50th,and 95th quantiles of bootstrapped change points correspond to the values resulting in the largest sum of IndVal z scores for z− and z+ taxa. Threshold indicator taxa analysis (TITAN) or nonparametric change point analysis (nCPA) thresholds correspond to the maximum deviance reduction calculated using Bray–Curtis distance (bc).
A longitudinal displacement of species composition with changes in runoff is intriguing, because during the initial phase of loss of larger glacial masses, increased glacial runoff may cause stream communities to migrate downstream, thereby reducing α diversity (65). However, although in the longer term, α diversity will increase, β and γ diversity are expected to decrease at the stream and catchment level (15, 24, 64, 66) (Fig. 1C), and genetic diversity is expected to decrease at the regional level (66). River reaches will become gradually more homogenous, and unique glacial river specialist species will become extinct because of increase in water temperature and competition for food and space with newly colonizing species and predator range shifts (67, 68). This is similar to the response for biofilm microbial communities described above. Nevertheless, even as riverine conditions become more favorable to colonization by new, often more ubiquitous species, other factors may restrict establishment (for example, weak dispersal ability, mountain barriers, lack of suitable microhabitats, scarcity of food, or other possible environmental conditions) (51, 69).
Rivers with high glacial influence typically support communities with low taxonomic diversity dominated by highly adapted taxa, which display a convergence of functional traits. Analysis of a unique 27-year record of community assembly after a reduction in glacier cover in southeast Alaska showed that niche-filtering processes were dominant with high glacier cover (63). However, significant shifts in the trait composition of stream invertebrate communities occur as catchment glacial cover decreases (63, 70), with stochastic and deterministic assembly processes co-occurring. Specialist taxa are replaced by more generalist taxa with a greater diversity and width of biological traits (SI Thresholds and Tipping Points). The size spectra of aquatic biota typically increase with warmer thermal regimes and higher productivity. Thus, as glaciers retreat, larger-sized predators (e.g., fish, amphibians, mammals, large invertebrates) will colonize rivers or move upstream in greater abundance (Fig. 1C) (65). Functional diversity and functional redundancy increase rapidly (63, 70), as new colonizers possess many similar traits to taxa already present, thereby contributing to the functional resilience to additional ecosystem change.
The adaptive potential (evolutionary and phenotypic) of aquatic biota to projected changes in glacial runoff is not well-known (71), but dispersion and phenotypical plasticity provide a possibility for some threatened taxa to respond to climate and environmental changes in the short term (72). Dispersal propensity and the level of specialization and capacity to exploit refugia are important considerations. Traits, such as semivoltinism, light bodies and good adult fliers, eurythermy, and generalist feeding, typically associated with high dispersal capacity might allow threatened invertebrates to survive environmental changes. Some species may avoid extinction as glacial relicts in groundwater systems as shown by the cold stenothermal stygobiotic crustacean Niphargus strouhali alpinus found trapped for at least 150 years in 30-cm sediments in the Italian Alps (73).
Salmonids.
Both Atlantic and Pacific species of salmon and trout provide important sources of proteins to millions of people worldwide and sustain major commercial and sport fisheries. Rising water temperature has been linked to increased mortality of juvenile Chinook salmon, raising concerns over the future viability of this important species (74). Population models indicate a 17% chance of catastrophic loss in the Chinook salmon population by 2100 (74). However, where glacier cover is both intermediate and high, increased glacial melt with climate change will have a cooling effect on monthly mean stream temperature during the summer. For each 10% increase in glacier cover in a catchment, mean stream summer temperature can decrease by >1 °C (75), potentially reducing fish growth. Conversely, future reductions in glacier runoff may actually improve the thermal suitability of glacially-dominated rivers for salmon. An extensive amount of salmon habitat has been created because of glacial recession in Alaska, with >500 systems documented along the southeast coast (76). More work is needed to understand the implications of interacting phenomena, as more extreme weather events, like floods, can conversely significantly reduce salmonid population sizes (77).
Glacier-fed rivers conserve a greater proportion of their annual flow budget for late summer and through winter than streams and rivers fed by snowmelt (78). Increased summer flows in glacier-fed rivers facilitate the migration of anadromous salmon through river corridors to spawning grounds at a time when other sources to flow are typically low (78). Some of the largest runs of anadromous salmon in southcentral Alaska that support commercial and sport fishing occur in glacially influenced rivers (79), but glacier shrinkage will diminish summer discharge, thereby potentially restricting adult salmon spawners migrating upstream. In addition, a more stochastic flow regime in summer (Fig. 1A) will have a negative effect on stream juvenile-rearing salmonids and other fish species. Sockeye salmon have also been shown to prefer the turbid glacial meltwaters for spawning (80), and turbidity can provide cover for rearing fish from predators (81). However, potentially higher flows in winter and early spring may be of benefit to reduce mortality, particularly for overwintering salmonids, a time period when mortality is typically at its highest. Climatic change will likely have widely divergent impacts on the timing of salmon migration, but watershed glacier cover does not influence long-term shifts in the phenology of migration timing (82).
SI Thresholds and Tipping Points
The concept of ecological thresholds is particularly relevant for glacier-fed streams, with their environment changing at a rapid pace because of glacier shrinkage (111). Thus, it is increasingly important to identify transition or tipping points between alternate ecosystem states to aid conservation and mitigation efforts (112). Here, we present a threshold analysis conducted on three comprehensive datasets of macroinvertebrate density from equatorial (E; Ecuador), temperate (T; Italian Alps), and sub-Arctic (sA; Iceland) glacierized river systems. For each region, quantitative benthic macroinvertebrate samples were collected along a gradient of glacier influence (quantified as percentage of glacier cover in the catchment). We used Threshold Indicator Taxa Analysis (113), a nonparametric technique that orders and partitions observations along environmental gradients using indicator value (IndVal) scores (114), to define ecological thresholds. Multiple candidate change points were identified (250 permutations), and IndVal scores were calculated for each taxon and then standardized (z scores). Declining (z−) and increasing (z+) taxa were used to identify community-level change points and uncertainty estimated via bootstrapping (500 replicates). For all systems, a distinct peak in the z− taxa, taxa that decrease in density across an increasing glacial gradient, was observed (T: 31.8%; E: 20.4%; sA: 19%). This suggests nonlinear responses of alpine aquatic assemblages to changes in glacier influence (Fig. S2), consistent with global patterns of macroinvertebrate α diversity in glacier-fed rivers (64), and illustrates a phenomenon that is increasingly recognized across differing levels of biological organization in glacier-fed rivers (64, 65, 115). It should be noted, however, that the change point confidence intervals were relatively broad (Table S1), indicative of a gradual decrease/increase in taxa along the gradient. This type of response is often associated with invasive or weedy species (115) and in the case of glacier-fed rivers, represents the replacement of specialist stress-tolerant species (e.g., the nonbiting midge Diamesa spp.) by generalist species, such as the mayfly Baetis alpinus (66). Interestingly, few taxa displayed increases across the gradient, and no distinct peak in z+ was apparent (Fig. S2), possibly because of the patchy distribution of stress tolerant, specialists in glacier-fed streams (64, 66).
Food web complexity and ecosystem functioning
The consequences of species extinction on trophic interactions and related matter fluxes in glacier-fed rivers after glacier shrinkage currently remain elusive (51). We know that food webs in glacier-fed rivers can be partly sustained by ancient DOC released from glaciers, which can be transferred to higher trophic levels, including invertebrates and fish after incorporation into bacterial biomass in biofilms (44). The decrease over the long term of a glacial source of organic carbon with ongoing glacier shrinkage will likely be replaced by instream and terrestrial primary production. Species turnover in glacier-fed rivers is linked strongly to glacier shrinkage, where colonizing species likely compete for resources with native species. These changes can be “bottom up” (e.g., from biofilm) (83) or “top down” (e.g., colonization by larger predatory invertebrates) (Fig. 1C) (68). Both mechanisms will potentially influence food web structure and trophic interactions as glaciers vanish (84). This is supported by a four-year experimental flow manipulation in the Ecuadorian Andes, where meltwater reduction led to increased biomass of benthic algal and invertebrate herbivores and furthermore, altered invertebrate community composition within a few weeks (55).
Ecosystem Services
The seasonal predictability of glacial melt facilitates a range of important socioeconomic ecosystem services, spanning provisioning, regulating, and cultural services (Fig. 3). Provisioning ecosystem services associated with glacier and snowpack storage of water is perhaps the most prominent of the three ecosystem services. The seasonally predictable flow regimes of glacier-fed rivers enable navigation on larger rivers; provide water for hydropower, human consumption, and irrigation for agriculture; and in some regions, support significant freshwater and inshore fisheries. This is particularly important in semiarid and arid regions, where other water source contributions are limited (12, 85). These ecosystem services will be perturbed markedly by the expected shifts of the hydrological regime owing to glacier shrinkage, causing significant economic ramifications.
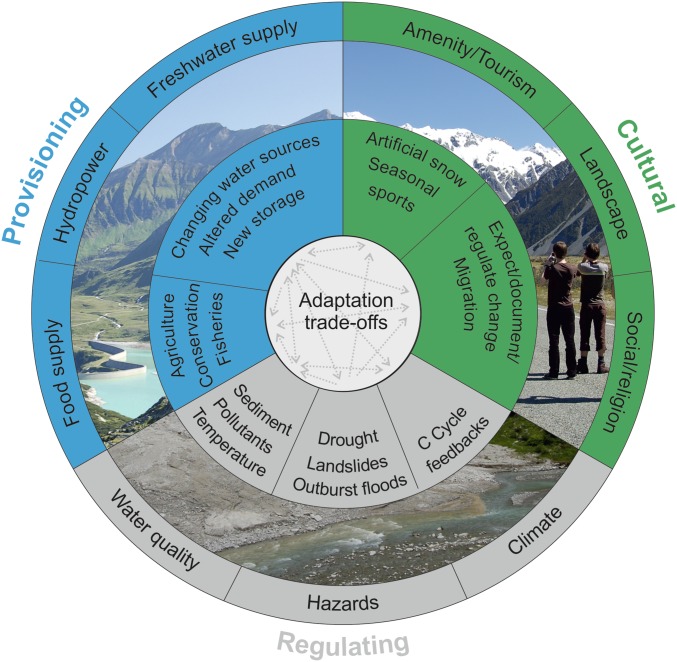
Fig. 3.
Conceptual framework integrating the effects of glacier shrinkage on provisioning, regulating, and cultural ecosystem services. The outer ring highlights broad groups of ecosystem services provided by glacier-fed watersheds. The inner ring highlights specific services potentially altered by glacier retreat and disappearance. The center highlights the complexity of interactions among the various services, which will necessitate tradeoffs as society adapts to cryospheric change (Table S2).
Irrigation and Water Supply.
In Wyoming, crop production to support a United States dollar (USD) $800 million cattle industry is dependent on glacial meltwater to supply a stable source of water during the growing season. However, from 1985 to 2005, glacier surface area decreased on average by 32%, resulting in 10% less stream flow during this period (86). Agriculture in Alberta is also dependent on meltwater from the Peyto Glacier and proximal glaciers in the Canadian Rockies, which are estimated to lose 90% of their present volume by 2100 (87). As an example of potential future changes in some regions of the world, the Tien Shan region of China is relatively arid but was once known as the “Green Labyrinth” because of glacial runoff being a significant source of water for agriculture. However, a 20% reduction in volume of 446 glaciers within the region over the period 1964–2004 is already impacting the sustainability of the region’s water sources (88).
In the Andean region of South America, many large populations depend heavily on glacial sources during this season, not only for agriculture but also, for drinking water. For example, 27% of La Paz’s water consumption during the dry season is glacial meltwater from the Cordillera Real (89), which is projected to decrease annually by 12% (90). During the dry season, 40% of the discharge of the Rio Santa, which drains the Cordillera Blanca in Peru, is from glacial runoff (91), providing extensive irrigated areas along the dry Atacama coast as well as 5% of Peru’s electricity (89). Estimated glacial retreat within the upper Rio Santa could lead to a decrease in the dry season average flow by 30% (92), further impacting the provisioning of water, with significant implications for associated provision of agricultural crops.
Hydropower.
In countries where this is the main source of electricity, glacial runoff contributes significantly to its generation (Iceland: 91% and Norway: 15–20%) (93). Austria generates 70% of its electricity from hydropower, with many large facilities being glacier-fed, while the glacier-fed Rhone River has 19 hydropower plants, supplying 25% of France’s hydropower. In Switzerland, 25% of its electricity is sourced from glacial runoff. The effects of glacier retreat on hydropower revenue are uncertain as a consequence both of predictions in meltwater runoff in future as well as market fluctuations (94). For glacier-fed rivers, ecosystem service valuations are best developed for this sector. For example, the Mauvoisin power plant in Switzerland gathers runoff from nine glaciers, and revenue is expected to increase initially from between USD $87.5–104.3 million (1981–2010) to between USD $87.5–108.6 million in 2021–2050 as a result of increasing rates of glacier retreat and thus, extra runoff generation potential. However, revenue is estimated to fall to USD $74.0–91.3 million by 2071–2100 as glacier runoff declines (94). Estimates of revenue shifts are needed from other regions to develop a more coherent picture of the economic issues associated with glacier retreat.
Current reservoir capacities may be unable to store the additional water from increasing summer melt from larger alpine glaciers, leading to only marginal electricity generation gains but increased numbers of overspill events and associated hazards (95). On the other hand, in the short term, hydropower may benefit from higher hydrological yields in summer (96) but not in the long term as glaciers shrink further or disappear (Fig. 1D). Glacier shrinkage also may open up new terrain for the construction of new dams and reservoirs in valleys not previously suitable for hydropower and now fed from other water sources (96). Shifting of the timing of peak melt compared with peak electricity demand may require society to invest more in storage reservoirs. Although typically holding less ice, rock glaciers may prove to be an important alternative source of water (97). Research into the importance rock glaciers as water sources is relatively new, and a wider inventory and understanding are critical to ensure their future protection.
Fisheries and Water Quality.
River fisheries provide important sources of food in many regions, and the potential for significant local–regional scale economic perturbations is high if glacier-fed river fisheries change with reductions in glacial melt (Fig. 1D). For example, in the glacial Kenai River in Alaska, sport and commercial fisheries generate as much as USD $70 million to the state’s economy, but increased flow variability and flooding caused by enhanced glacier melting and glacial outburst floods can significantly affect these important fisheries (78). Associated large-scale changes in basin geometry and rerouting of meltwater may significantly affect salmonid habitat (98). However, the creation of new salmon habitat as documented along the Pacific Northwest coast of North America (76) can potentially benefit fisheries.
Glaciers and their downstream river systems provide important regulating services, most notably related to water quality, hazard generation/mitigation, and climate feedbacks via C cycling. Glacial meltwater can dilute pollutants and mitigate water quality modifications associated with river regulation (24). Glaciers can also regulate the influence of harmful contaminant release from the snowpack through storage, which prolongs the opportunities for volatilization and other transformations (99). However, the liberation of historically deposited pollutants could significantly reduce water quality downstream. Reduced suspended sediment load caused by glacier retreat can lead to decreases in erosional processes. Glacier shrinkage is, furthermore, creating thousands of new lakes worldwide, where outburst floods are increasingly threatening hydropower facilities and downstream settlements (28, 29). There are only a handful of detailed studies available at present to underpin our understanding of current and future regulating services of glacier-fed rivers and a pressing need for detailed studies integrating a range of scenarios.
Cultural Services.
Among those provided by glacierized mountain landscapes, tourism makes a particularly important contribution to the economy of many regions. For example, the glaciers of Banff National Park, Canada attract >3 million visitors each year (100); in Austria, the Pasterze Glacier attracts an estimated 800,000 people annually, while Glacier Bay National Park, Alaska typically attracts in excess of 400,000 people per year. In New Zealand, glacier tourism is estimated to contribute USD $84 million annually to the economy. Tourism numbers are likely to be affected by glacier retreat and loss in the long term, although counterintuitively, there may be increases in visitor numbers in some areas if access improves or there is a desire to learn about climate change and see glaciers before they are lost (101) (Fig. 1D). This is true in South America, where glacier tourism represents an important economic factor; in the late 2007, the Pastoruri Glacier became the first tourism destination in Peru to be closed because of the adverse effect of too many visitors degrading the glacial surface (102). Similar problems have been reported in China (101).
In addition to sightseeing, glaciers can provide important tourism and recreation opportunities for year-round skiing. However, in parts of the Austrian and Swiss Alps, rising numbers of visitors during the 1980s and 1990s are stagnating (103). In Italy, at the Vedretta Piana, where the glacier area has shrunk by ≈2/3 since 1965, the threat of glacier retreat to the ski industry has prompted the redistribution of snow from accumulation zone to fill in crevasses and cover debris (104). Other adaptation measures include extension of ski areas to higher elevations and artificial snow-making machinery, which is expensive [e.g., more than USD $400 million in 2006–2007 (105)]. In contrast, the closure of the world’s highest ski resort in Boliva (Chacaltaya) occurred in 2009, while in parts of the Alps, summer glacier skiing has been abandoned, because conditions are too dangerous (103).
Cultural services associated with glaciers incorporate religious beliefs and/or landscape character. Glacierized mountain peaks are considered as spiritual to some indigenous peoples and thus, accorded high cultural significance (106). For example, thousands of pilgrims annually traverse the Gangotri Glacier in India, considering it a sacred site (107), and in Peru and the Yukon Territory of Canada, indigenous people consider glaciers as gods. In Peru, the loss of ice and snow from mountain peaks is thus associated with the god’s departure and the end of the world (108). On the Tibetan Plateau, residents consider the glacierized Mount Yulong Snow their spiritual home, but already >65% have recognized the necessity to potentially migrate to adapt to climate change and achieve a sustainable livelihood (107). These social upheavals would clearly lead to implications across the wider array of services that human populations use from glacier-fed rivers. In addition, conflicts may occur in the future between neighboring countries as water resources become more scarce because of lower glacial runoff as highlighted for certain regions in Asia (12).
Our conceptual synthesis of the multiple ecosystem services associated with glacial rivers, which will be altered by the retreat or loss of ice masses, reveals the potential for several interlinked effects (Fig. 3 and Table S2). For example, some links may be considered as unidirectional effects, such as new water storage reservoirs (provisioning) altering landscape character (cultural). Alternatively, more complex bidirectional effects occur with meltwater reductions leading to more extreme droughts, with a counterresponse being the development of more storage facilities to mitigate these effects, thereby further altering river flows. However, it is more likely that society will be faced with more complex tradeoffs (109), and suitable management strategies must be developed and adopted to mitigate these societal impacts of profound changes in glacial runoff. In the Andes, for example, glacier retreat may necessitate a shift to a less water-intensive agriculture to reduce demand, but there is still likely to be a need to create new highland reservoirs to supply water during the dry season, leading to knock on changes among regulating services currently provided by downstream river systems. This could necessitate a shift from hydropower electricity generation to other sources (90) to retain water for food. Other solutions could be behavioral (for example, reductions in water use and waste) and managerial in terms of water source management (9). The development of new storage facilities would, in turn, lead to alteration to landscape character, which for some groups, may be seen as a negative for cultural tourism-related services. In 2010, Argentina became the first country to adopt a multifaceted Glacier Protection Act that aims to preserve glaciers as “strategic reserves of water for human consumption, for agriculture and as suppliers of water to recharge basins, for the protection of biodiversity; and as a source of scientific and tourist attraction” (110). This kind of solution may become more common to prevent modifications within glacierized watersheds but is unlikely to be useful in the face of global climate change unless adopted internationally as an additional driver of emissions mitigation policies.
Table S2.
Examples of unidirectional and bidirectional linkages that are likely to arise as a consequence of glacier retreat (link with Fig. 3)
| Ecosystem service linkages | Implications |
| Changing water sources ←→ artificial snow | Retreating glaciers and snow packs lead to a need for artificial snow to maintain the winter sports industry, adding additional pressures on water resources (105) |
| Changing water sources → landscape | Development of new storage facilities further alters landscape character (96) |
| Changing water sources ←→ C cycle | New reservoirs may release more CO2/CH4, adding to water source changes caused by climate change (116) |
| Changing water sources ←→ hazards | Receding glaciers may lead to more extreme droughts, with a response being the development of more storage facilities to mitigate the effects (12) |
| Changing water sources → agriculture/fisheries | Altered river flows lead to changes in water supply for irrigation and fisheries (90) |
| Changing water sources ←→ water quality | Pollutant release from glacier stores may degrade water sources for drinking/irrigation users; reservoirs can alter river thermal and sediment regimes (24) |
| Food supply ←→ cultural | Loss of water for agriculture could mean a need for populations to migrate, leading to a reduced need for food supply services from these rivers (12) |
| Water quality → fisheries | Warmer thermal regimes may be detrimental to some fish species (51, 75) |
| Water quality → C cycle | Elevated nutrients may enhance carbon cycling (36, 42), leading to positive or negative feedbacks that alter thermal regimes or nutrient loads (40) |
| Hazards → water quality | Low flows can lead to excessive water temperature (30); landslides often deposit large amounts of sediments in rivers |
| Hazards → landscape/social | Landslides and/or outburst floods will alter existing landscape character or make some areas unsafe for habitation (28, 29, 32) |
| Hazards → amenity/tourism | Droughts reduce ability for artificial snow generation; landslides may make some areas dangerous for tourism (32) |
These linkages will not be mutually exclusive, leading to the prospect of complex adaptive tradeoffs having to be negotiated among human populations that are affected by glacier retreat. ←→, bidirectional; →, unidirectional.
Summary
The area of land occupied by glaciers will decrease significantly by the end of the present century. Although the impact of melting glaciers on sea levels has received much attention to date, our synthesis clearly outlines other multiple downstream effects that will alter riverine ecosystems with significant societal implications. There will be major changes to flow regimes in glacierized watersheds, with a shift to greater stochasticity as glacial runoff decreases and flow becomes more dependent on unpredictable precipitation events and snow melt. Among the major impacts, there are profound changes to ecosystem functions and services via altered provision of water resources to human society, reorganization of the regulatory processes that shape water quality and geohazards, and cultural changes associated with tourism, landscape character, and religion. Clearly, the breadth and complexity of interactions among the various impacts will underpin calls for a global research agenda involving interdisciplinary research to meet the following research priorities, which need urgent attention to inform effective societal adaptation.
-
i)
High-resolution (space and time) mapping of glacier mass loss based on new imagery and technologies.
-
ii)
Global census and continuous monitoring of key biogeochemical variables, contaminants, and biodiversity in glacier-fed rivers using a network of sites with a wide geographical coverage and adopting standard techniques of sampling so as to make findings comparable. This will allow detection of early warning signals and regime shifts based on temporal and spatial analyses while providing greater understanding of current and future regulating services of glacier-fed rivers.
-
iii)
Valuation of provisioning, regulating, and cultural ecosystem services associated with glacier-fed systems, in particular considering the implications of emerging concerns about contaminant loads and more detailed understanding of salmon habitat linking to commercial and sport fisheries.
-
iv)
Critical adaptive management decisions in the most sensitive areas to mitigate ecosystem service effects that will have major ramifications for billions of people, with international legislation to protect strategic glacier-derived water resources.
Acknowledgments
We thank Chantal Jackson for assistance drawing some of the figures. We also thank the European Science Foundation for sponsoring the exploratory workshop entitled “Glacier-fed rivers, hydroecology and climate change; current knowledge and future network of monitoring sites (GLAC-HYDROECO-NET)” held in Birmingham, United Kingdom in September of 2013 that generated the initial ideas for this perspective. Our underpinning research has been funded, in part, by UK Natural Environment Research Council Grants NE/E003729/1, NE/E004539/1, NE/E004148/1, NE/G523963/1, and NE/L002574/1; European Union Arctic and Alpine Stream Ecosystem Research Project ENV-CT95-0164; and European Union Framework Programme 7 Assessing Climate Impacts on the Quality and Quantity of Water Project 212250.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619807114/-/DCSupplemental.
References
- 1.Kaser G, Gogley JG, Dyurgerov MB, Meier MF, Ohmura A. Mass balance of glaciers and ice caps: Consensus estimates for 1961-2004. Geophys Res Lett. 2006;33:L19501. [Google Scholar]
- 2.Pfeffer WT, et al. The Randolph Glacier Inventory: A globally complete inventory of glaciers. J Glaciol. 2014;60:537–552. [Google Scholar]
- 3.Gardner AS, et al. A reconciled estimate of glacier contributions to sea level rise: 2003 To 2009. Science. 2013;340:852–857. doi: 10.1126/science.1234532. [DOI] [PubMed] [Google Scholar]
- 4.Bliss A, Hock R, Radić V. Global response of glacier runoff to twenty-first century climate change. J Geophys Res Earth Surf. 2014;119:717–730. [Google Scholar]
- 5.Jacob T, Wahr J, Pfeffer WT, Swenson S. Recent contributions of glaciers and ice caps to sea-level rise. Nature. 2012;482:514–518. doi: 10.1038/nature10847. [DOI] [PubMed] [Google Scholar]
- 6.Ragettli S, Immerzeel WW, Pellicciotti F. Contrasting climate change impact on river flows from high-altitude catchments in the Himalayan and Andes Mountains. Proc Natl Acad Sci USA. 2016;113:9222–9227. doi: 10.1073/pnas.1606526113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zemp M, Haeberli W, Hoelzle M, Paul F. Alpine glaciers to disappear within decades? Geophys Res Lett. 2006;33:L13504. [Google Scholar]
- 8.Huss M. Extrapolating glacier mass balance to the mountain-range scale: The European Alps 1900–2100. Cryosphere. 2012;6:713–727. [Google Scholar]
- 9.Rangecroft S, et al. Climate change and water resources in arid mountains: An example from the Bolivian Andes. Ambio. 2013;42:852–863. doi: 10.1007/s13280-013-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke GKC, Jarosch AH, Anslow FS, Radic V, Menounos B. Projected deglaciation of western Canada in the twenty first century. Nat Geosci. 2015;8:372–377. [Google Scholar]
- 11.Kaab A, Treichler D, Nuth C, Berthier E. Brief communication: Contending estimates of 2003-2008 glacier mass balance over the Pamir-Karakoram-Himalaya. Cryosphere. 2015;9:557–564. [Google Scholar]
- 12.Pritchard H. Asia’s glaciers are a regionally important buffer against drought. Nature. 2017;545:169–174. doi: 10.1038/nature22062. [DOI] [PubMed] [Google Scholar]
- 13.O’Neel S, et al. Icefield-to-ocean linkages across the northern Pacific coastal temperate rainforest ecosystem. Bioscience. 2015;65:499–512. [Google Scholar]
- 14.Hawkings J, et al. The Greenland Ice Sheet as a hot spot of phosphorus weathering and export in the Arctic. Global Biogeochem Cycles. 2016;30:191–210. [Google Scholar]
- 15.Brown LE, Milner AM, Hannah DM. Vulnerability of alpine stream biodiversity to shrinking snowpacks and glaciers. Glob Chang Biol. 2007;13:958–966. [Google Scholar]
- 16.Jacobsen D, Milner AM, Brown LE, Dangles O. Biodiversity under threat in glacier-fed river systems. Nat Clim Chang. 2012;2:361–364. [Google Scholar]
- 17.Gutiérrez MH, Galand PE, Moffat C, Pantoja S. Melting glacier impacts community structure of Bacteria, Archaea and Fungi in a Chilean Patagonia fjord. Environ Microbiol. 2015;17:3882–3897. doi: 10.1111/1462-2920.12872. [DOI] [PubMed] [Google Scholar]
- 18.Bolch T, et al. The state and fate of Himalayan glaciers. Science. 2012;336:310–314. doi: 10.1126/science.1215828. [DOI] [PubMed] [Google Scholar]
- 19.Jansson P, Hock R, Schneider T. The concept of glacier storage: A review. J Hydrol (Amst) 2003;282:116–129. [Google Scholar]
- 20.Jost G, Moore RD, Menounos B, Wheate R. Quantifying the contribution of glacier runoff to streamflow in the upper Columbia River Basin, Canada Hydrol. Earth Syst Sci. 2012;16:849–860. [Google Scholar]
- 21.Stahl K, Moore RD, Shea JM, Hutchinson D, Cannon AJ. Coupled modeling of glacier and streamflow response to future climate scenarios. Water Resour Res. 2008;44:W02422. [Google Scholar]
- 22.O’Neel S, et al. Assessing stream flow sensitivity to variations in glacier mass balance. Clim Change. 2014;123:329–341. [Google Scholar]
- 23.Kure S, Jang S, Ohara N, Kavvas ML, Chen ZQ. Hydrologic impact of regional climate change for the snow‐fed and glacier‐fed river basins in the Republic of Tajikistan: Statistical downscaling of global climate model projections. Hydrol Process. 2013;27:4071–4090. [Google Scholar]
- 24.Brown LE, Dickson NE, Carrivick JL, Füreder L. Alpine river ecosystem response to natural and anthropogenic flood pulses. Freshw Sci. 2015;34:1201–1215. [Google Scholar]
- 25.Hannah DM, Kansakar SR, Gerrard AJ, Rees G. Flow regimes of Himalayan rivers of Nepal: Their nature and spatial patterns. J Hydrol (Amst) 2005;308:18–32. [Google Scholar]
- 26.Cauvy-Fraunié S, et al. Technical note: Glacial influence in tropical mountain hydrosystems evidenced by the diurnal cycle in water levels. Hydrol Earth Syst Sci. 2013;17:4803. [Google Scholar]
- 27.Carrivick JL, Quincey DJ. Progressive increase in number and volume of ice-marginal lakes on the western margin of the Greenland Ice Sheet. Global Planet Change. 2014;116:156–163. [Google Scholar]
- 28.Quincey DJ, et al. Early recognition of glacial lake hazards in the Himalaya using remote sensing datasets. Global Planet Change. 2007;56:137–152. [Google Scholar]
- 29.Carrivick JL, Tweed FS. A global assessment of the societal impacts of glacier outburst floods. Global Planet Change. 2016;144:1–16. [Google Scholar]
- 30.Brown LE, Hannah DM. Spatial heterogeneity of water temperature across an alpine basin. Hydrol Process. 2008;22:954–967. [Google Scholar]
- 31.Fleming SW, Clarke GKC. Attentuation of high-frequency interannual streamflow variability by watershed glacial cover. J Hydraul Eng. 2005;131:615–618. [Google Scholar]
- 32.Kellerer-Pirklbauer A, Lieb GK, Avian M, Carrivick JL. Climate change and rock fall events in high mountain areas: Numerous and extensive rock falls in 2007 at Mittlerer Burgstall, central Austria. Geogr Ann Ser B. 2012;94:59–78. [Google Scholar]
- 33.Klaar MJ, et al. Vegetation succession in deglaciated landscapes: Implications for sediment and landscape stability. Earth Surf Process Landf. 2015;40:1088–1100. [Google Scholar]
- 34.Hodson AJ, Ferguson RI. Fluvial suspended sediment transport from cold and warm-based glaciers in Svalbard. Earth Surf Process Landf. 1999;24:957–974. [Google Scholar]
- 35.Irvine-Fynn TDL, Barrand NE, Porter PR, Hodson AJ, Murray T. Recent high-Arctic sediment redistribution: A process perspective using airborne lidar. Geomorphology. 2011;125:27–39. [Google Scholar]
- 36.Hood E, Battin TJ, Fellman J, O’Neel S, Spencer RG. Storage and release of organic carbon from glaciers and ice sheets. Nat Geosci. 2015;8:91–96. [Google Scholar]
- 37.Lutz S, et al. The biogeography of red snow microbiomes and their role in melting arctic glaciers. Nat Commun. 2016;7:11968. doi: 10.1038/ncomms11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hood E, et al. Glaciers as a source of ancient and labile organic matter to the marine environment. Nature. 2009;462:1044–1047. doi: 10.1038/nature08580. [DOI] [PubMed] [Google Scholar]
- 39.Singer GA, et al. Biogeochemically diverse organic matter in Alpine glaciers and its downstream fate. Nat Geosci. 2012;10:710–714. [Google Scholar]
- 40.Hodson AJ, Mumford PN, Lister D. Suspended sediment and phosphorus in proglacial rivers: Bioavailability and potential impacts upon the P status of ice-marginal receiving waters. Hydrol Process. 2004;18:2409–2422. [Google Scholar]
- 41.Anesio AM, Hodson AJ, Fritz A, Psenner R, Sattler B. High microbial activity on glaciers: Importance to the global carbon cycle. Glob Chang Biol. 2009;15:955–960. [Google Scholar]
- 42.Musilova M, et al. Microbially driven export of labile organic carbon from the Greenland ice sheet. Nat Geosci. 2017;10:360–365. [Google Scholar]
- 43.Smith HJ, et al. Microbial formation of labile organic carbon in Antarctic glacial environments. Nat Geosci. 2017;10:356–359. [Google Scholar]
- 44.Fellman JB, et al. Evidence for the assimilation of ancient glacier organic carbon in a proglacial stream food web. Limnol Oceanogr. 2015;60:1118–1128. [Google Scholar]
- 45.Dubnick A, et al. Characterization of dissolved organic matter (DOM) from glacial environments using total fluorescence spectroscopy and parallel factor analysis. Ann Glaciol. 2010;51:111–122. [Google Scholar]
- 46.Wadham JL, et al. Sources, cycling and export of nitrogen on the Greenland Ice Sheet. Biogeosciences. 2016;22:6339–6352. [Google Scholar]
- 47.Hood E, Berner L. Effects of changing glacial coverage on the physical and biogeochemical properties of coastal streams in southeastern Alaska. J Geophys Res. 2009;114:G03001. [Google Scholar]
- 48.Bogdal C, et al. Release of legacy pollutants from melting glaciers: Model evidence and conceptual understanding. Environ Sci Technol. 2010;44:4063–4069. doi: 10.1021/es903007h. [DOI] [PubMed] [Google Scholar]
- 49.Grannas AM, et al. The role of the cryosphere in the fate of organic contaminants. Atmos Chem Phys. 2013;13:3271–3305. [Google Scholar]
- 50.Hodson AJ. Understanding the dynamics of black carbon and associated contaminants in glacial systems. Wiley Interdiscip Rev Water. 2014;1:141–149. [Google Scholar]
- 51.Milner AM, Brown LE, Hannah DM. Hydroecological effects of shrinking glaciers. Hydrol Process. 2009;23:62–77. [Google Scholar]
- 52.Battin TJ, Besemer K, Bengtsson MM, Romani AM, Packmann AL. The ecology and biogeochemistry of stream biofilms. Nat Rev Microbiol. 2016;14:251–263. doi: 10.1038/nrmicro.2016.15. [DOI] [PubMed] [Google Scholar]
- 53.Battin TJ, Wille A, Psenner R, Richter A. Large-scale environmental controls on microbial biofilms in high-alpine streams. Biogeosciences. 2004;1:159–171. [Google Scholar]
- 54.Schutz C, Wallinger M, Burger R, Fureder L. Effect of snow cover on the benthic fauna in a glacier-fed stream. Freshw Biol. 2001;46:1691–1704. [Google Scholar]
- 55.Cauvy-Fraunié S, et al. Ecological responses to experimental glacier-runoff reduction in alpine rivers. Nat Commun. 2016;7:12025. doi: 10.1038/ncomms12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uehlinger U, Robinson CT, Hieber M, Zah R. The physico-chemical habitat template for periphyton in alpine glacial streams under a changing climate. Hydrobiologia. 2010;657:107–112. [Google Scholar]
- 57.Arscott DB, Bowden WB, Finlay JC. Effects of desiccation and temperature/irradiance on the metabolism of 2 arctic stream bryophyte taxa. J N Am Benthol Soc. 2000;19:263–273. [Google Scholar]
- 58.Wilhelm L, Singer GA, Fasching C, Battin TJ, Besemer K. Microbial biodiversity in glacier-fed streams. ISME J. 2013;7:1651–1660. doi: 10.1038/ismej.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilhelm L, et al. Rare but active taxa contribute to community dynamics of benthic biofilms in glacier-fed streams. Environ Microbiol. 2014;16:2514–2524. doi: 10.1111/1462-2920.12392. [DOI] [PubMed] [Google Scholar]
- 60.Besemer K, et al. Unraveling assembly of stream biofilm communities. ISME J. 2012;6:1459–1468. doi: 10.1038/ismej.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freimann R, Bürgmann H, Findlay SEG, Robinson CT. Bacterial structures and ecosystem functions in glaciated floodplains: Contemporary states and potential future shifts. ISME J. 2013;7:2361–2373. doi: 10.1038/ismej.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milner AM, Brittain JE, Castella E, Petts GE. Trends of macroinvertebrate community structure in glacier-fed streams in relation to environmental conditions: A synthesis. Freshw Biol. 2001;46:1833–1848. [Google Scholar]
- 63.Brown LE, Milner AM. Rapid loss of glacial ice reveals stream community assembly processes. Glob Chang Biol. 2012;18:2195–2204. [Google Scholar]
- 64.Jacobsen D, Dangles O. Environmental harshness and global richness patterns in glacier-fed streams. Glob Ecol Biogeogr. 2012;21:647–656. [Google Scholar]
- 65.Jacobsen D, et al. Runoff and the longitudinal distribution of macroinvertebrates in a glacier-fed stream: Implications for the effects of global warming. Freshw Biol. 2014;59:2038–2050. [Google Scholar]
- 66.Finn D, Khamis K, Milner AM. Loss of small glaciers will diminish beta diversity in Pyrenean streams at two levels of organization. Glob Ecol Biogeogr. 2013;22:40–51. [Google Scholar]
- 67.Flory EA, Milner AM. The role of competition in invertebrate community development in a recently formed stream in Glacier Bay National Park, Alaska. Aquat Ecol. 1999;33:175–184. [Google Scholar]
- 68.Khamis K, Brown LE, Hannah DM, Milner AM. Experimental evidence that predator range expansion modifies alpine stream community structure. Freshw Sci. 2015;34:66–80. [Google Scholar]
- 69.Cauvy-Fraunié S, Espinosa R, Andino P, Jacobsen D, Dangles O. Invertebrate metacommunity structure and dynamics in an Andean glacial stream network facing climate change. PLoS One. 2015;10:e0136793. doi: 10.1371/journal.pone.0136793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ilg C, Castella E. Patterns of macroinvertebrate traits along three glacial stream continuums. Freshw Biol. 2006;51:840–853. [Google Scholar]
- 71.Lencioni V, Bernabò P, Jousson O, Guella G. Cold adaptive potential of chironomids overwintering in a glacial stream. Physiol Entomol. 2015;40:43–53. [Google Scholar]
- 72.Shama LNS, Robinson CT. Microgeographic life history variation in an alpine caddisfly: Plasticity in response to seasonal time constraints. Freshw Biol. 2009;54:150–164. [Google Scholar]
- 73.Lencioni V, Spitale D. Diversity and distribution of benthic and hyporheic fauna in different stream types on an alpine glacial floodplain. Hydrobiologia. 2015;751:73–87. [Google Scholar]
- 74.Munoz NJ, Farrell AP, Heath JW, Neff BD. Adaptive potential of a Pacific salmon challenged by climate change. Nat Clim Chang. 2015;5:163–166. [Google Scholar]
- 75.Fellman JB, et al. Stream temperature response to variable glacier coverage in coastal watersheds of southeast Alaska. Hydro Proc. 2014;28:2062–2073. [Google Scholar]
- 76.Soiseth CR, Milner AM. Predicting salmonid occurrence from physical characteristics of streams in Glacier Bay National Park and Preserve. In: Engstrom DR, editor. Proceedings of the Third Glacier Bay Science Symposium, 1993. Department of Interior, US National Park Service, Glacier Bay National Park; Anchorage, AK: 1995. pp. 174–183. [Google Scholar]
- 77.Milner AM, Robertson AE, McDermott M, Klaar MJ, Brown LE. Major flood disturbance alters river ecosystem evolution. Nat Clim Chang. 2013;3:137–141. [Google Scholar]
- 78.Dorava JM, Milner AM. Role of lake regulation of glacier-fed rivers in sustaining high salmon productivity in the Cook Inlet watershed, southcentral Alaska. Hydrol Process. 2000;14:3149–3159. [Google Scholar]
- 79.Fleming SW. Comparative analysis of glacial and nival streamflow regimes with implications for lotic habitat quantity and fish species richness. River Res Appl. 2005;21:363–379. [Google Scholar]
- 80.Young DB, Woody CA. Spawning distribution of sockeye salmon in a glacially influenced watershed: The importance of glacial habitats. Trans Am Fish Soc. 2007;136:452–459. [Google Scholar]
- 81.Richardson JC, Milner AM. Rivers of Pacific Canada and Alaska. In: Benke A, Cushing B, editors. Rivers of North America. Elsevier; Burlington, MA: 2005. pp. 735–775. [Google Scholar]
- 82.Kovach RP, Ellison SC, Pyare S, Tallmon DA. Temporal patterns in adult salmon migration timing across southeast Alaska. Glob Change Biol. 2015;21:1821–1833. doi: 10.1111/gcb.12829. [DOI] [PubMed] [Google Scholar]
- 83.Rott E, Cantonati M, Fureder L, Pfister P. Benthic algae in high altitude streams of the Alps – a neglected component of the aquatic biota. Hydrobiologia. 2006;562:195–216. [Google Scholar]
- 84.Clitherow LR, Carrivick JL, Brown LE. Food web structure in a harsh glacier-fed river. PLoS One. 2013;8:e60899. doi: 10.1371/journal.pone.0060899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sorg A, Bolch T, Stoffel M, Solomina O, Beniston M. Climate change impacts on glaciers and runoff in the Tien Shan (central Asia) Nat Clim Chang. 2012;2:725–731. [Google Scholar]
- 86.Cheesbrough K, Edmunds J, Tootie G, Kerr G, Pochop L. Estimated Wind River range (Wyoming, USA) glacier meltwater contributions to agriculture. Remote Sens. 2009;1:818–828. [Google Scholar]
- 87.Kehrl LM, Hawley RL, Osterberg EC, Winski DA, Lee AP. Volume loss from the Peyto Glacier, Alberta, Canada between 1966 and 2010. J Glaciol. 2014;60:51–56. [Google Scholar]
- 88.Wang L, Li Z, Wang F, Edwards R. Glacier shrinkage in the Ebinur lake basin, Tien Shan, China, during the past 40 years. J Glaciol. 2014;60:245–254. [Google Scholar]
- 89.Chevallier P, Pouyaud B, Suarez W, Condom T. Climate change threats to environment in the tropical Andes: Glaciers and water resources. Reg Environ Change. 2011;11:S179–S187. [Google Scholar]
- 90.Soruco A, Vincent C, Rabatel A, Francou B. Contribution of glacial runoff to the water resources of La Paz city, Boliva. Ann Glaciol. 2015;56:147–154. [Google Scholar]
- 91.Bradley RS, Vuille M, Diaz HF, Vergara W. Climate change. Threats to water supplies in the tropical Andes. Science. 2006;312:1755–1756. doi: 10.1126/science.1128087. [DOI] [PubMed] [Google Scholar]
- 92.Baraer M, et al. Glacier recession and water resources in Peru’s Cordillera Blanca. J Glaciol. 2012;58:134–150. [Google Scholar]
- 93.Andreassen LM, Elvehoy H, Kjollmoen B, Engeset RV, Haakensen N. Glacier mass-balance and length variation in Norway. Ann Glaciol. 2005;42:317–325. [Google Scholar]
- 94.Gaudard L, Gabbi J, Bauder A, Romerio F. Long-term uncertainty of hydropower revenue due to climate change and electricity prices. Water Resour Manage. 2016;30:1325–1343. [Google Scholar]
- 95.Schaefli B, Hingray B, Musy A. Climate change and hydropower production in the Swiss Alps: Quantification of potential impacts and related modeling uncertainties. Hydrol Earth Syst Sci. 2007;11:1191–1205. [Google Scholar]
- 96.Farinotti D, Pistocchi A, Huss M. From dwindling ice to headwater lakes: Could dams replace glaciers in the European Alps? Environ Res Lett. 2016;11:054022. [Google Scholar]
- 97.Brenning A. Geomorphological, hydrological and climatic significance of rock glaciers in the Andes of Central Chile (33–35°S) Permafr Periglac Process. 2005;16:231–240. [Google Scholar]
- 98.Shugar DH, et al. River piracy and drainage basin regorganization led by climate-driven glacier retreat. Nat Geosci. 2017;10:370–375. [Google Scholar]
- 99.Bizzotto EC, Villa S, Vaj C, Vighi M. Comparison of glacial and non-glacial-fed streams to evaluate the loading of persistent organic pollutants through seasonal snow/ice melt. Chemosphere. 2009;74:924–930. doi: 10.1016/j.chemosphere.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 100.Scott D, Jones B, Konopek J. Exploring potential visitor response to climate-induced environmental changes in Canada’s Rocky Mountain National Parks. Tourism Rev Int. 2008;12:43–48. [Google Scholar]
- 101.Purdie H. Glacier retreat and tourism; insights from New Zealand. Mt Res Dev. 2013;33:463–472. [Google Scholar]
- 102.Bury JT, et al. Glacier recession and human vulnerability in the Yanamarey watershed. Clim Change. 2011;105:179–206. [Google Scholar]
- 103.Falk M. The stagnation of summer glacier skiing. Tour Anal. 2016;21:117–122. [Google Scholar]
- 104.Smiraglia C, et al. Glacier changes and their impacts on mountain tourism. In: Orlove B, Wiegandt E, Luckman B, editors. Darkening Peaks: Glacier Retreat, Science, and Society. Univ of California Press; Berkeley, CA: 2008. pp. 206–215. [Google Scholar]
- 105.Matasci C. 2012. Swiss tourism in the age of climate change—vulnerability, adaptive capacity, and barriers to adaptation. PhD thesis (EPFL, Lausanne, Switzerland)
- 106.Xiao CD, Wang SJ, Qin D. A preliminary study of cryopshere service function and value evaluation. Adv Clim Chang Res. 2015;6:181–187. [Google Scholar]
- 107.Wang S, Qin D. Mountain inhabitants perspectives on climate change, and its impacts and adaptation based on temporal and spatial characteristics analysis: A case study of Mt. Yulong Snow, Southeastern Tibetan Plateau. Enivron Haz. 2015;14:122–136. [Google Scholar]
- 108.Steinberg J. Intangible ecologies: Sacred mountain landscapes in a changing climate. Mt Forum Bull. 2008;8:3–4. [Google Scholar]
- 109.Bennett EM, Peterson GD, Gordon LJ. Understanding relationships among multiple ecosystem services. Ecol Lett. 2009;12:1394–1404. doi: 10.1111/j.1461-0248.2009.01387.x. [DOI] [PubMed] [Google Scholar]
- 110.Taillant JD. Glaciers: The Politics of Ice. Oxford Univ Press; Oxford, UK: 2015. p. 304. [Google Scholar]
- 111.Finn DS, Rasanen K, Robinson CT. Physical and biological changes to a lengthening stream gradient following a decade of rapid glacial recession. Glob Chang Biol. 2010;16:3314–3326. [Google Scholar]
- 112.Khamis K, et al. Alpine aquatic ecosystem conservation policy in a changing climate. Environ Sci Policy. 2014;43:39–55. [Google Scholar]
- 113.Baker ME, King RS. Of TITAN and straw men: An appeal for greater understanding of community data. Freshw Sci. 2014;32:489–506. [Google Scholar]
- 114.Dufrene M, Legendre P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol Monogr. 1997;67:345–366. [Google Scholar]
- 115.Khamis K, Hannah DM, Brown LE, Milner AM. Glacier-groundwater stress gradients control alpine stream biodiversity. Ecohydrology. 2016;9:1263–1275. [Google Scholar]
- 116.Deemer BR, et al. Greenhouse gas emissions from reservoir water surfaces: A new global synthesis. Bioscience. 2016;66:949–969. doi: 10.1093/biosci/biw117. [DOI] [PMC free article] [PubMed] [Google Scholar]