Significance
In cereal crops, the number of florets in a spikelet is an important factor affecting the grain number per panicle and then the grain yield. In wild-type rice, one spikelet produces one fertile floret. This study characterized a gain-of-function mutant lateral florets 1 (lf1) in rice. In lf1, the spikelet developed lateral florets with proper floral organ identities in the axil of the sterile lemma, showing that the rice spikelet has the potential to restore the “three-florets spikelet” which may have existed in ancestors. Therefore, it provides strong evidence supporting the three-florets spikelet hypothesis and presents a prospect for increasing grain number per panicle by breeding rice with three-floret spikelets.
Keywords: lateral floret, three-florets spikelet, evolution, yield, rice
Abstract
The spikelet is a unique inflorescence structure in grass. The molecular mechanisms behind the development and evolution of the spikelet are far from clear. In this study, a dominant rice mutant, lateral florets 1 (lf1), was characterized. In the lf1 spikelet, lateral floral meristems were promoted unexpectedly and could generally blossom into relatively normal florets. LF1 encoded a class III homeodomain-leucine zipper (HD-ZIP III) protein, and the site of mutation in lf1 was located in a putative miRNA165/166 target sequence. Ectopic expression of both LF1 and the meristem maintenance gene OSH1 was detected in the axil of the sterile lemma primordia of the lf1 spikelet. Furthermore, the promoter of OSH1 could be bound directly by LF1 protein. Collectively, these results indicate that the mutation of LF1 induces ectopic expression of OSH1, which results in the initiation of lateral meristems to generate lateral florets in the axil of the sterile lemma. This study thus offers strong evidence in support of the “three-florets spikelet” hypothesis in rice.
Flower development is a key process in the reproduction of angiosperms. Under suitable conditions, flowering signals are transmitted to shoot apical meristems (SAMs), which are transformed first into inflorescence meristems (IMs). Floral meristems (FMs) are then initiated on the top and/or lateral domains of the IMs and subsequently transformed into the four whorls of floral organs. The spikelet is a unique unit of inflorescence architecture in grasses and consists of a pair of glumes and a fixed or variable number of florets. Some grass-specific genes are involved in regulating spikelet development. For example, FRIZZY PANICLE (FZP) functions in regulating spikelet meristem (SM) identity in rice. In the fzp mutant, axillary meristems (AMs) are formed instead of FMs, and these then develop into higher-order branches (1). Three genes that encode members of the AP2/ERF superfamily, SUPERNUMERARY BRACT (SNB), INDETERMINATE SPIKELET 1 (OsIDS1), and MULTI-FLORETS SPIKELET 1 (MFS1), are involved in regulating spikelet determinacy in rice (2, 3). In these mutants, the transition from SM to FM is delayed, and extra organs or florets are produced. However, our knowledge about the details of spikelet development in rice remains limited.
In most members of Oryzeae, the spikelet is composed of one pair of rudimentary glumes, one pair of sterile lemmas, and one terminal fertile floret, which consists of one pair of hulls (lemma and palea) and inner floral organs (4). Regarding the origin of the sterile lemmas, the “three-florets spikelet” hypothesis proposes that the putative ancestor of the rice spikelet contained two lateral florets in addition to a terminal fertile floret. Subsequently, the lemmas of the two lateral florets degenerated into sterile lemmas, and the inner floral organs and palea degenerated markedly and disappeared during evolution (5). In recent years, several reports have supported this hypothesis. The genes LONG STERILE LEMMA (G1), EXTRA GLUME 1 (EG1), PANICLE PHYTOMER 2 (PAP2)/OsMADS34, NONSTOP GLUMES (NSG), and ABERRANT SPIKELET AND PANICLE 1 (ASP1) are involved in the regulation of sterile lemma identity. Loss of function of these genes results in lemma-like sterile lemmas (6–10). In addition, ectopic expression of OsMADS1 causes lemma-like sterile lemmas (11). All these results suggest that the sterile lemma is homologous to the lemma. However, there is no direct evidence that these lemma-like organs are derived from the lemmas of two lateral florets, because no lateral florets with inner floral organs have been observed in these mutants. Given the lack of further evidence, the three-florets spikelet hypothesis remains widely debated.
In this study, we characterized a gain-of-function mutant, lateral florets 1 (lf1), in which the lateral florets were surprisingly formed in the axil of the sterile lemmas. By map-based cloning, we isolated the LF1 gene, which encodes a homeodomain-leucine zipper class III (HD-ZIP III) transcriptional activator. In lf1, LF1 was expressed ectopically because miRNA165/166 could not act on the mutated LF1 mRNA. LF1 then directly activated the expression of the meristem maintenance gene OSH1 to induce the initiation of lateral FMs. Interestingly, the results of the study provide strong evidence in support of the three-florets spikelet hypothesis in rice.
Results
The lf1 Spikelet Developed Lateral Florets.
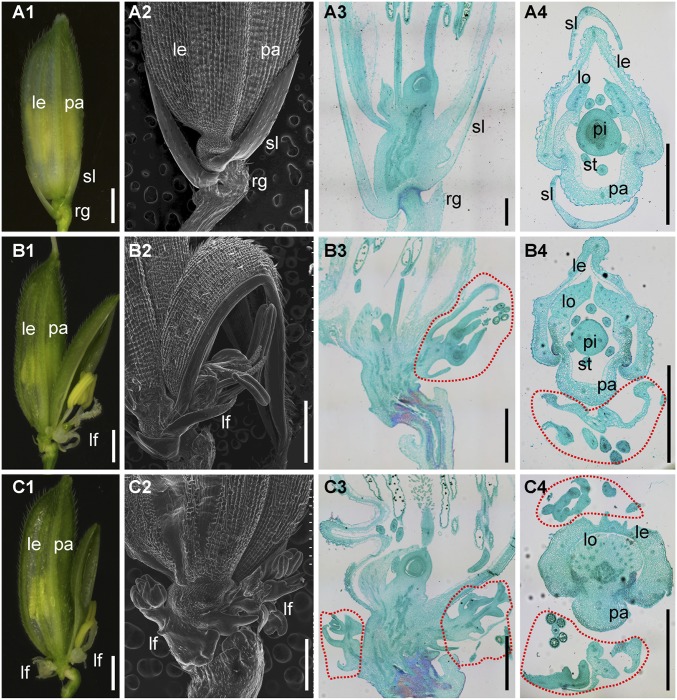
The WT rice spikelet consisted of one pair of rudimentary glumes, one pair of sterile lemmas, and one terminal fertile floret (Fig. 1 A, 1). The rudimentary glumes and sterile lemmas were inserted on the rachilla in an alternate phyllotaxy, whereas the terminal floret was produced on the top of the rachilla (Fig. 1 A, 1). The terminal floret consisted of four whorls of floral organs: one pistil in whorl 4; six stamens in whorl 3; two lodicules at the lemma side in whorl 2, and a lemma and palea that surround the inner floral organs in whorl 1 (Fig. 1A and Fig. S1C). The sterile lemmas were two small white glume-like organs with a smooth surface on the abaxial side (Fig. 1 A, 2–4). The two rudimentary glumes subtended below the sterile lemmas and were degenerate with abundant trichomes on the abaxial side (Fig. 1 A, 2 and 3).
Fig. 1.
Phenotypes of spikelets in the WT and lf1. (A) WT spikelets. (B and C) lf1 spikelets. (A1, B1, and C1) Whole spikelets. (A2, B2, and C2) Scanning electron micrographs of the lower part of spikelets. (A3, B3, and C3) Longitudinal sections of spikelets. (A4, B4, and C4) Transverse sections of spikelets. Red dotted lines represent lateral florets; le, lemma; lf, lateral floret; lo, lodicule; pa, palea; pi, pistil; rg, rudimentary glume; sl, sterile lemma; st, stamen. (Scale bars, 500 µm.)
Fig. S1.
Phenotypes of spikelets in the WT and lf1. (A) Spikelets of WT (A, 1) and lf1 (A, 2 and 3) plants from which the lemma and palea had been removed. (B) Percentage of each type of spikelet among 100 spikelets in lf1. (C and D) Type of floral organs in the terminal florets of WT (C) and lateral florets of lf1 (D). (C, 4 and 5 and D, 4–6) Transverse sections of spikelets in WT and lf1, respectively. Abbreviations are as in Fig. 1. (Scale bars, 500 µm.) (E) Relative expression levels of genes for floral organ identity in the sterile lemma and terminal floret of the WT and lateral florets of lf1. Error bars indicate the SD of three biological repeats.
In the lf1 spikelet, no significant abnormalities were detected in the terminal floret and rudimentary glumes (Fig. 1 B, 1 and 2 and C, 1 and 2 and Fig. S1 A, 1–3). However, the lateral florets contained inner floral organs that grew obliquely in the axil of the upper and/or lower sterile lemma in an alternate phyllotaxy in the lf1 spikelet (Fig. 1 B and C). The statistical analysis showed that 78% of lf1 spikelets developed one lateral floret only in the axil of the upper sterile lemma, and 4% developed two lateral florets in the axils of both the upper and lower sterile lemmas (Fig. S1B). In these lateral florets, all types of inner floral organs were discovered. The pistil with an ovary and bifid stigmas was same as that in the WT. The transparent and oval-shaped lodicules and the yellow stamens with anthers growing in the slender filaments were very similar to those in the WT, although their numbers were decreased in some lateral florets. The palea, which had three vascular bundles that consisted of the body of the palea (bop) and two marginal regions (mrps), was similar to those in the WT, even though some of them showed abnormal shapes (Fig. S1 C and D). Furthermore, known genes for floral organ identity were expressed at high levels in the WT terminal floret and lf1 lateral floret, whereas almost no expression was detected in the WT sterile lemma by qRT-PCR (Fig. S1E). These genes included the lemma and palea identity gene OsMADS1 (12), lodicule and stamen identity genes OsMADS4 and OsMADS16 (13, 14), stamen and carpel identity genes OsMADS3 and OsMADS58 (15), pistil identity gene DROOPING LEAF (DL) (16), and the ovule identity gene OsMADS13 (17). Therefore, these results indicated that the identities of the floral organs were specified correctly in lf1 lateral florets, although defects in the shape and number of floral organs were observed in some lateral florets. They also suggested that the reversion to lateral florets occurred in the lf1 spikelet. Unfortunately, no sterile lemmas were transformed into lemmas, and in fact some even degenerated further in some spikelets (Fig. 1 B and C).
The lf1 Mutation Induced Lateral FMs During Early Spikelet Development.
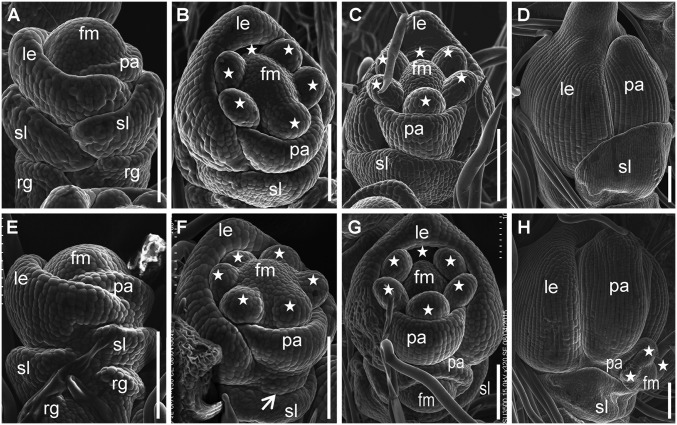
In the WT, the primordia of the rudimentary glumes, sterile lemmas, lemma, and palea had formed or initiated in an alternate phyllotaxis during spikelet stage 4 (Sp4) (Fig. 2A). During stages Sp5–Sp8 the inner floral organs developed in the following order: two lodicules developed first, followed by six stamens and a pistil (Fig. 2 B–D). In lf1, no significant difference was observed during stage Sp4 compared with the WT (Fig. 2E). However, during stage Sp5, when lodicule and stamen primordia were forming in the terminal floret, the lateral FM became visible as a protrusion at the axil of the sterile lemma primordia in lf1 (Fig. 2F). During approximately stages Sp6/Sp7, when the pistil primordium was forming in the terminal florets, the formation of the palea primordium was completed in the lateral florets (Fig. 2G). The initiation of the inner floral organ primordia, e.g., the stamen and pistil, was not finally finished in the lateral florets until stage Sp8, after the lemma and palea had closed in the terminal floret (Fig. 2H). Therefore, the developmental stage of the lateral florets was delayed by approximately two spikelet stages compared with the terminal floret (Fig. 2). This delay might be caused by the florets in the three-florets spikelet developing in acropetal succession (18). In addition, degradation of the lf1 sterile lemma was observed during stages Sp4–Sp8 (Fig. S2).
Fig. 2.
Scanning electron micrographs of spikelets at early developmental stages in the WT and lf1. (A–D) WT spikelets. (E–H) lf1 spikelets. (A and E) Stage Sp4. (B and F) Stage Sp5. (C and G) Stage Sp6–Sp7. (D and H) Stage Sp8. Stars indicate stamens. fm, floral meristem; other abbreviations are as in Fig. 1. (Scale bars, 100 µm.)
Fig. S2.
Scanning electron micrographs of florets at early developmental stages in lf1 showing degradation of the sterile lemma in lf1 plants during stages Sp4 (A), Sp5–6 (B), Sp7 (C), and Sp8 (D). rg, rudimentary glume; sl, sterile lemma. (Scale bars, 100 µm.)
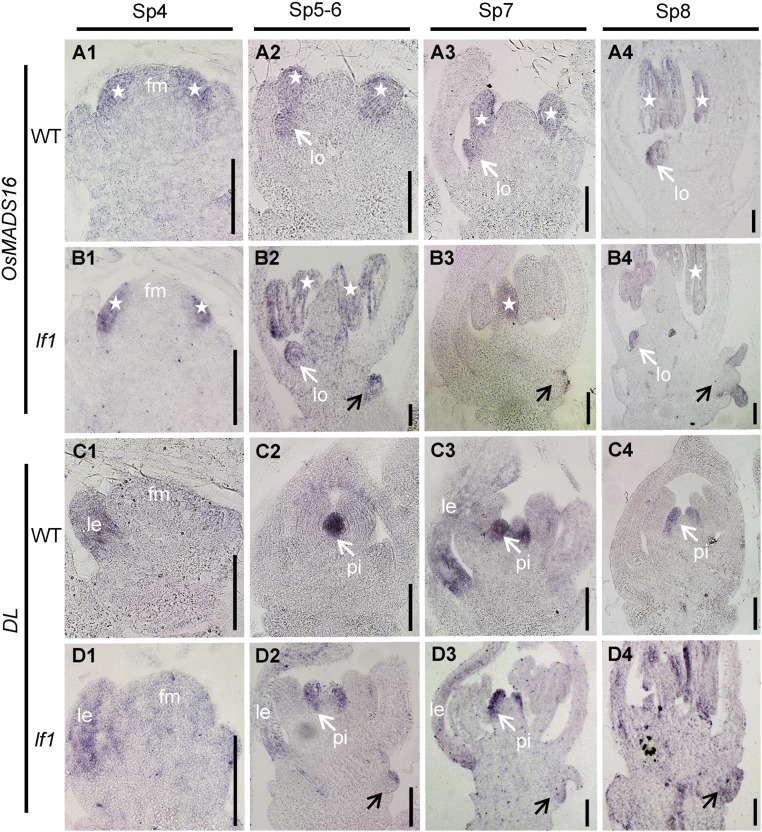
To confirm the identity of the organs in lf1 lateral florets during the early developmental stages of the spikelet, the expression of OsMADS16 and DL was investigated by in situ hybridization. Expression of OsMADS16 was detected in the lodicule and stamen of terminal florets in both the WT and lf1 during stages Sp4–Sp8 (Fig. S3 A and B). High levels of expression were also detected in the lodicule and stamen of lf1 lateral florets during stages Sp5–Sp8 (Fig. S3 B, 2–4). In the terminal florets of the WT and lf1, DL was expressed first in lemma primordia during stages Sp4–Sp6 (Fig. S3 C, 1 and D, 1) and then was expressed in pistil primordia during stages Sp7–Sp8 (Fig. S3 C, 2–4 and D, 2–4). Ectopic expression of DL was also detected in the lateral florets of lf1 during stages Sp6–Sp8 (Fig. S3 D, 2–4). In conclusion, the initiation of FMs and subsequent formation of the primordia of the floral organs in lateral florets occurred during the early developmental stages of spikelets in lf1.
Fig. S3.
OsMADS16 and DL gene expression in WT and lf1 flowers at early developmental stages. Expression of OsMADS16 and DL is shown in WT flowers (A and C, respectively) and in lf1 flowers (B and D, respectively). White arrows indicate gene expression in the lodicules or pistil. Stars indicate gene expression in the stamens. Black arrows indicate gene expression in the lateral florets of lf1. fm, floral meristem; other abbreviations are as in Fig. 1. (Scale bars, 50 µm.)
Map-Based Cloning of the LF1 Gene.
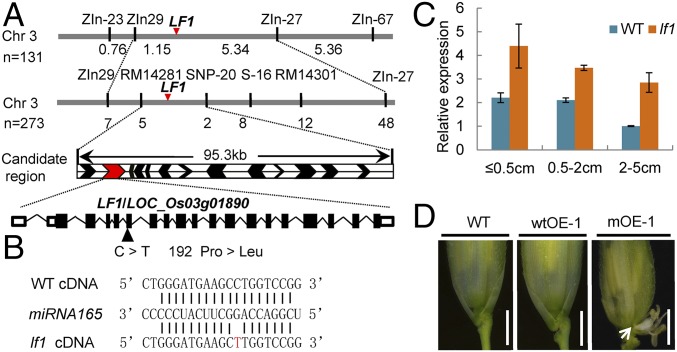
A map-based cloning strategy was used to isolate the LF1 gene. Genetic analysis of the F2 progeny showed that the segregation ratio of mutant individuals and WT plants was a good fit to 3:1 (1,194 of 1,598 were mutant individuals; χ2 = 0.053 < χ20.05 = 3.84), which demonstrated that the lf1 trait was controlled by a single dominant gene. By using the 404 WT plants in the F2 population, we narrowed the location of the LF1 gene to a 95.3-kb region between markers RM14281 and SNP-20 on the short arm of chromosome 3 in which there were 13 Michigan State University (MSU) Rice Genome Annotation Project-annotated genes (Fig. 3A).
Fig. 3.
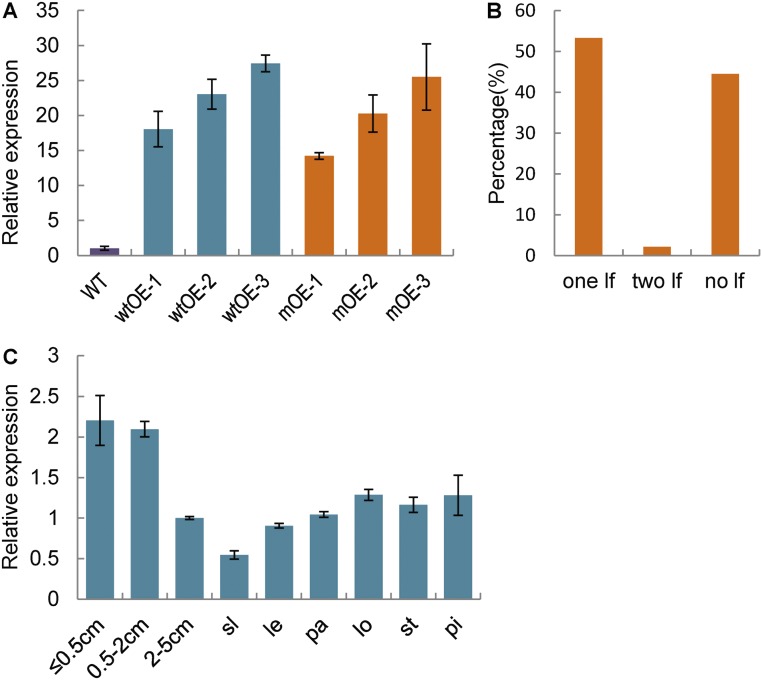
Map-based cloning of LF1. (A) Map position of the LF1 locus. Arrowheads indicate the sites of predicted genes in the RM14281–SNP-20 interval. The genomic structure of LF1 and the site of the mutation in the lf1 mutant are shown. (B) Base pairing between cDNA from WT and LF1 with miRNA165. Vertical lines indicate the complementary mRNA sequence to miRNA165. The mutated nucleotide is noted in red. (C) qRT-PCR analysis of LF1 in developing WT and lf1 panicles at different stages: ≤0.5 cm, young panicles ≤0.5 cm; 0.5–2 cm, young panicles 0.5–2 cm; 2–5 cm, young panicles 2–5 cm. Error bars indicate the SD of three biological repeats. (D) Phenotypes of WT, WT overexpressing (wtOE), and mutant overexpressing (mOE) transgenic plants. The arrow indicates lateral florets. (Scale bars, 500 µm.)
A single-nucleotide substitution from C to T (proline 192 to leucine 192) was found in annotated gene LOC_Os03g01890 in the lf1 mutant (Fig. 3A). Further analysis showed that the mutation site was located in a putative target sequence for miRNA165/166 (Fig. 3B). In general, miRNAs repress gene expression in plants by binding to complementary sites in their target mRNAs and then guiding mRNA cleavage (19). Consequently, it was possible that the expression of WT LOC_Os03g01890 was repressed by miRNA165/166, whereas the mutated LOC_Os03g01890 could not be degraded. Next, the expression of LOC_Os03g01890 was shown by qRT-PCR to be higher in the lf1 mutant than in the WT in all stages of the young panicles (Fig. 3C). In addition, we expressed the WT LOC_Os03g01890CDS-GFP and mutated LOC_Os03g01890CDS-GFP fusion genes under the control of the ubiquitin promoter in WT plants. In the transgenic plants overexpressing mutated LOC_Os03g01890, 53.3% of spikelets developed one lateral floret, and 2.2% developed two lateral florets, similar to the findings in lf1 (Fig. 3D and Fig. S4 A and B). These results suggested that miRNA165/166 could not act on the mutated LOC_Os03g01890 mRNA, which then led to ectopic expression of LOC_Os03g0189 to induce lateral florets in both the lf1 mutant and the transgenic plants overexpressing mutated LOC_Os03g01890. Although the transcription level of LOC_Os03g01890 was greatly increased in the transgenic plants overexpressing WT LOC_Os03g01890 (Fig. S4A), the spikelets displayed normal morphology (Fig. 3D). This might be due to miRNA165/166 functioning normally to repress LOC_Os03g01890 in the appropriate tissues. Therefore, these results suggested that the dominant phenotype in lf1 was probably caused by the ectopic expression of LOC_Os03g01890 in corresponding tissues due to a mutation in the complementary site for miRNA165/166 rather than by the single amino acid change in the LOC_Os03g01890 protein. In summary, LOC_Os03g01890 is the LF1 gene.
Fig. S4.
Expression pattern of LF1 and the percentage of each type of spikelet among 100 spikelets in transgenic plants that overexpressed mutated LF1CDS-GFP. (A) LF1 expression in the young spikelets of transgenic plants overexpressing mutated LF1CDS-GFP (mOE1–3) and WT LF1CDS-GFP (wtOE1–3), respectively. (B) Percentage of each type of spikelet among 100 spikelets in transgenic plants that overexpressed mutated LF1CDS-GFP. (C) LF1 expression in young panicles at different stages and different floral organs was detected by qRT-PCR: ≤0.5 cm, young panicles ≤0.5 cm; 0.5–2 cm, young panicles 0.5–2 cm; 2–5 cm, young panicles 2–5 cm. Error bars indicate the SD of three biological repeats. Abbreviations are as in Fig. 1.
Expression Pattern of the LF1 Gene and miRNA165/166.
By qRT-PCR analysis, LF1 was expressed generally in young panicles, with high levels in panicles <2 cm in size and low levels in those measuring 2–5 cm, and its expression was still detected in the sterile lemma and all floral organs during the heading stage (Fig. S4C).
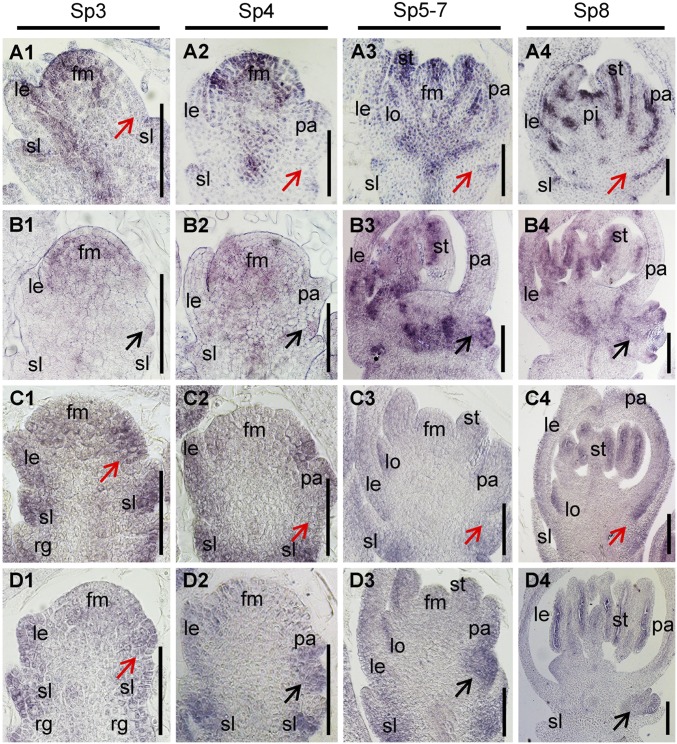
The expression pattern of LF1 in spikelets was investigated by in situ hybridization. In WT spikelets, LF1 was expressed strongly in the FM domain and stamen, lodicule, and pistil primordia, and weak signals were detected in the primordia of the sterile lemma, lemma, and palea during stages Sp3–Sp7, whereas no obvious signal was detected at the axil of the sterile lemma (Fig. 4 A, 1–3). During Sp8, after the primordia of the floral organs had entered the differentiation phase, strong transcript signals were found in the adaxial domain of all the lateral organs, including the sterile lemma, lemma/palea, lodicule, and stamen (Fig. 4 A, 4). The expression pattern suggested that LF1 functioned both in the maintenance of the meristem of the terminal floret and the polarity development of organs in the WT spikelet. Likewise, although the expression of the LF1 signal was enhanced in the spikelets of transgenic plants overexpressing WT LF1, it was not expressed at the axil of the sterile lemma during stages Sp4–Sp8 (Fig. S5 A, 1–3). In the lf1 spikelet, the expression domain of LF1 was obviously expanded (Fig. 4B). First, the expression of LF1 was detected in cells located between the sterile lemma and palea, which might be precursors of the lateral FM during stages Sp3–Sp4 (Fig. 4 B, 1 and 2). Next, LF1 was expressed in the same domain where the FM or floral organs of the lateral floret were formed during stages Sp5–Sp8 (Fig. 4 B, 3 and 4).
Fig. 4.
Expression pattern of LF1 and miRNA165/166. (A and B) In situ hybridization in the spikelets of the WT (A) and lf1 (B) during stages Sp3–Sp8 using an LF1 antisense probe. (C and D) In situ hybridization in the spikelets of the WT (C) and lf1 (D) during stages Sp3–Sp8 using a miRNA166 complementary locked nucleic acid (LNA)-modified DNA probe. Black arrows in B and D indicate the lateral floral meristem and lateral floret. Red arrows in A and C indicate the axil of the sterile lemma. fm, floral meristem; other abbreviations are as in Fig. 1. (Scale bars, 50 µm.)
Fig. S5.
Expression pattern of LF1 and miRNA165/166. (A) In situ hybridizations of LF1 (1–3) and miRNA165/166 (4–6) in the spikelets of the transgenic plants overexpressing WT LF1 during stages Sp4–Sp8 using an LF1 antisense probe and a miRNA166 complementary LNA-modified DNA probe, respectively. (B) Serial sections for the in situ hybridization of miRNA165/166 in Fig. 4C, 2. Red arrows indicate the axil of the sterile lemma. Abbreviations are as in Fig. 1. (Scale bars, 50 µm.)
The expression pattern of miRNA165/166 was also investigated. In the WT spikelet, miRNA165/166 was expressed mainly in the precursor cells or primordia of organs, such as the sterile lemma, lemma/palea, lodicule, and stamen, during stages Sp3–Sp7, whereas no obvious signal was detected in the central domain of the FM (Fig. 4 C, 1–3). During stage Sp8, the expression of miRNA165/166 was observed mainly in the abaxial sides of all organs in the spikelet except the pistil (Fig. 4 C, 4). In the spikelets of transgenic plants overexpressing WT LF1, the expression pattern of miRNA165/166 was similar to that of WT during stages Sp4–Sp8 (Fig. S5 A, 4–6). In the lf1 spikelet, the expression pattern of miRNA165/166 was also similar to that of the WT (Fig. 4D), except that obvious expression of miRNA165/166 was detected in the lateral FM domain at the axil of the sterile lemma (Fig. 4 D, 2–4). Thus, the data indicated that expression of miRNA165/166 and LF1 is mutually exclusive in the WT spikelet but not in the lf1 mutant, which suggests that miRNA165/166 is responsible for the restriction of WT LF1 expression.
In particular, during stage Sp3, before the palea primordium was initiated, strong expression of miRNA165/166 was observed in the margin domain of the FM located next to the primordium of the upper sterile lemma in the spikelet of WT and lf1 (Fig. 4 C, 1 and D, 1). Therefore, it is possible that the precursor cells of the lateral FM in the WT were repressed due to degradation of the LF1 mRNA by miRNA165/166 in this domain at stage Sp3. The fact that no signal for miRNA165/166 was detected at the axil of the sterile lemma in the WT might be due to the absence of target cells here after stage Sp3 (Fig. 4 C, 2–4), and the serial sections of the picture in Fig. 4 C, 2 further demonstrate that there was no expression of the miRNA165/166 signal (Fig. S5B). However, it was expressed in the lf1 mutant due to the presence of target cells after stage Sp3 (Fig. 4 D, 2–4). Taken together with all the above data, these results suggest that the accumulation of miRNA165/166 in the presumptive lateral FM region suppresses the expression of LF1 in the WT, whereas once a mutation had occurred in the miRNA165/166 target sequence of LF1, the latter was expressed ectopically to induce initiation of the lateral FM at the axil of the sterile lemma in lf1.
LF1 Encodes a HD-ZIP III Transcriptional Activator.
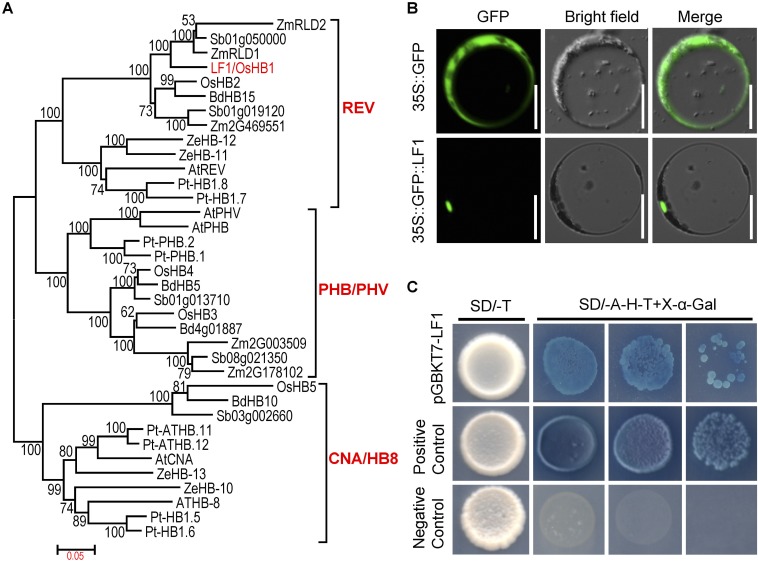
LF1 was found to encode the rice HD-ZIP III transcription factor OsHB1 and shared high sequence similarity with other HD-ZIP III genes in monocots and dicots (Fig. S6). The results of phylogenetic analysis revealed that the angiosperm HD-ZIP III genes formed three strongly supported clades, referred to as the “REV,” “PHB/PHV,” and “CNA/HB8” clades, and that LF1/OsHB1 belonged to the REV clade (Fig. S7A). Previous studies showed that this family of genes regulates embryo patterning, meristem function, vascular development, lateral organ polarity, and interfascicular fiber differentiation in angiosperms (20–23). In this study, we identified a function of LF1/OsHB1 that is associated with lateral FM fate in rice.
Fig. S6.
Protein sequence alignment of OsHB1 and REV. The homeodomain is marked by a blue box. The START domain is marked by red bars. The bZIP domain is marked by gray bars. The MEKHLA domain is marked by black bars.
Fig. S7.
LF1 encodes a HD-ZIP III protein. (A) Phylogenetic tree of the LF1 protein. The phylogenetic tree was constructed using the maximum likelihood method based on the Jones–Taylor–Thornton matrix-based model. Bootstrap values were calculated from 500 replicates and are given at the branch nodes. At, Arabidopsis; Bd, Brachypodium distachyon; Os, rice; Pt, poplar; Sb, Sorghum bicolor; Ze, Zinnia elegans; Zm, maize. (B) Analysis of the subcellular localization of the LF1 protein in rice protoplasts. (Scale bars, 50 µm.) (C) Analysis of the transcriptional activation of LF1. Transcriptional activation of LF1 was tested by yeast two-hybrid analysis. The positive control was pGBKT7-53+pGADT7-T, and the negative control was pGBKT7-Lam+pGADT7-T.
Next, constructs that encoded GFP and the GFP-LF1ORF fusion protein were expressed transiently in rice protoplasts. For GFP, green fluorescence was detected consistently throughout the cell, apart from in the vacuole, whereas the GFP-LF1ORF fusion protein was localized in the nucleus (Fig. S7B). Further, a GAL4 DNA-binding domain and LF1 fusion protein (pGBKT7-LF1) was expressed in Y2HGold yeast cells. The yeast cells that contained pGBKT7-LF1 and the positive control yeast cells were able to grow in the absence of adenine, histidine, and tryptophan and showed X-α-Gal activity, whereas yeast cells that contained the negative controls did not (Fig. S7C). These results suggested that LF1 is a transcriptional activator.
LF1 Regulates (Floral) Meristem-Related Genes to Initiate the Lateral FM.
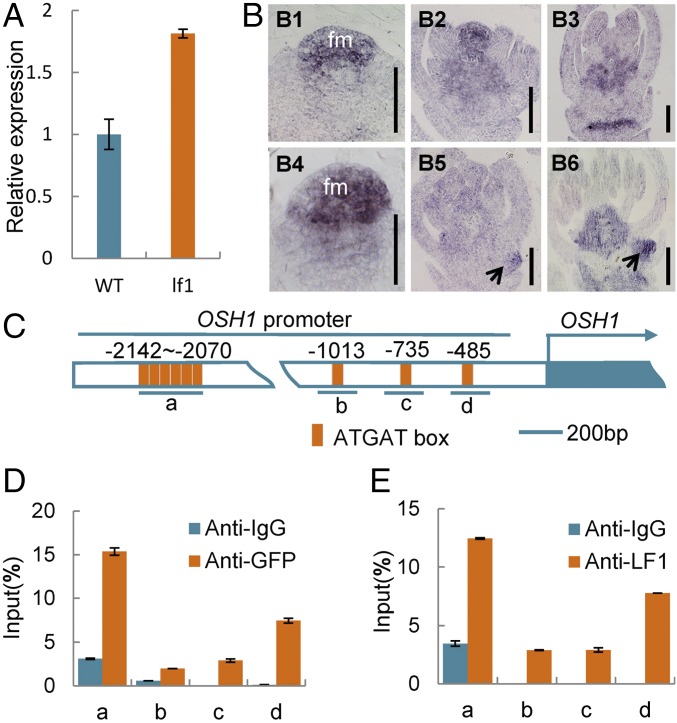
Previous studies have shown that REV (the ortholog of LF1 in rice) up-regulates the expression of STM (the ortholog of OSH1 in rice) in leaf axil meristem cells directly to ensure the initiation of axillary meristems in Arabidopsis (24). In the present study, the expression level of OSH1 was increased significantly in the panicles of lf1 compared with the WT by qRT-PCR analysis (Fig. 5A). Next, in situ hybridization was used to compare the expression pattern of OSH1 in WT and lf1. In the WT, OSH1 was expressed specifically in the FM and receptacle at a high level during stages Sp4–Sp6 (Fig. 5 B, 1 and 2). After stage Sp7, when the carpel primordium formed, only a slight signal was detected in the rachilla (Fig. 5 B, 3). In the terminal floret of lf1, the expression pattern of OSH1 was generally similar to that in the WT during stages Sp4–Sp8 (Fig. 5 B, 4–6). As expected, a strong expression signal for OSH1 was observed in the lf1 lateral FM during stages Sp5–Sp8 (Fig. 5 B, 5 and 6). Subsequently, ChIP assays were used to examine whether LF1 protein can bind directly to the OSH1 promoter. Chromatin isolated from young panicles of the mutated LF1-GFP (mLF1-GFP) transgenic and WT plants was immunoprecipitated with an anti-GFP antibody and an anti-LF1 antibody, respectively, and then was subjected to qRT-PCR analysis. The results showed that both mLF1-GFP and the WT LF1 protein cloud bound to four regions of the OSH1 promoter, which contained one to six conserved binding motifs (ATGAT) for the HD-ZIP III protein (Fig. 5 C–E). These findings suggested that LF1 might regulate the OSH1 gene directly in both the lf1 and WT spikelet.
Fig. 5.
LF1 regulates the meristem maintenance gene OSH1. (A) qRT-PCR analysis of OSH1 in WT and lf1 panicles. (B) In situ hybridization of OsH1 in WT (B1–B3) and lf1 (B4–B6) panicles. Black arrows indicate gene expression in the lateral FM of lf1 panicles. fm, floral meristem. (C) Distribution of the conserved binding site ATGAT in the promoter regions of OsH1. Underlining indicates the DNA fragments amplified in the ChIP assays. (D and E) ChIP enrichment compared with the input sample was tested by qPCR. (D) Chromatin isolated from fresh young WT panicles and immunoprecipitated with anti-LF1 antibody. (E) Chromatin isolated from fresh young panicles of mLF1CDS-GFP transgenic plants and immunoprecipitated with anti-GFP antibody. Error bars indicate the SD of three biological repeats. (Scale bars, 50 µm.)
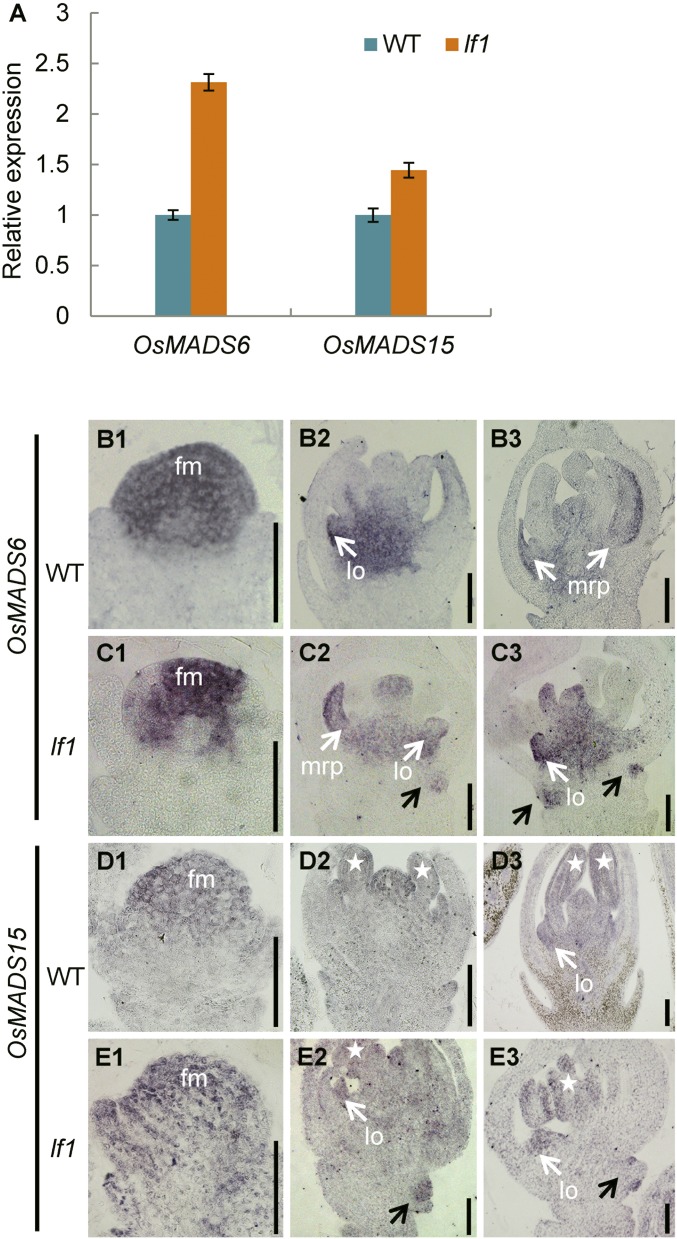
Analysis by qRT-PCR also showed that expression of the FM identity genes OsMADS6 and OsMADS15 was increased significantly in the panicles of lf1 compared with that in the WT (Fig. S8A). Next, the expression patterns of OsMADS6 and OsMADS15 were determined in detail by in situ hybridization. During stage Sp4, strong expression of OsMADS6 and OsMADS15 was detected in the terminal FM in both the WT and lf1 (Fig. S8 B, 1; C, 1; D, 1; and E, 1). From stages Sp5–Sp8, in the terminal florets of both the WT and lf1, OsMADS6 was expressed mainly in the mrp, lodicule, and carpel (Fig. S8 B, 2 and 3 and C, 2 and 3), whereas OsMADS15 was expressed in all the floral organs (Fig. S8 D, 2 and 3 and E, 2 and 3). Therefore, no differences in expression pattern were found between the WT and lf1 terminal florets. However, clear expression signals for OsMADS6 and OsMADS15 were detected in the lf1 lateral FM from stages Sp5–Sp8 (Fig. S8 C, 2 and 3 and E, 2 and 3). Thus, it is suspected that the FM identity genes OsMADS6 and OsMADS15 might also be involved in initiation of the lateral FM in lf1.
Fig. S8.
LF1 regulates genes for floral meristem identity. (A) qRT-PCR analysis of OsMADS6 and OsMADS15 in WT and lf1 panicles. Error bars indicate the SD of three biological repeats. (B–E) In situ hybridization in WT and lf1 panicles using OsMADS6 and OsMADS15 antisense probes. (B and C) Expression of OsMADS6 in the spikelets of the WT and lf1. (D and E) Expression of OsMADS15 in the spikelets of the WT and lf1. Black arrows indicate gene expression in the lateral floral meristem of lf1. White arrows indicate gene expression in the lodicules or mrp. Stars indicate gene expression in the stamens. (Scale bars, 50 µm.)
Discussion
Our data suggested that the miRNA165/166–LF1–OSH1 pathway plays a key role in regulating the induction of lateral florets. First, a single-nucleotide substitution in the complementary site for miRNA165/166 in the LF1 gene and the ectopic expression of the latter in the lateral FM was observed in lf1. The expression domains of miRNA165/166 and LF1 were mutually exclusive in the WT spikelet. These results fit well with the assumption that the LF1 mRNA was degraded by miRNA165/166 in the precursor cells of the lateral FM at the axil of the sterile lemma in the WT, whereas miRNA165/166 could not act on the mutated LF1 mRNA, resulting in its ectopic expression at the axil of the sterile lemma to induce the formation of lateral florets in lf1. Second, in transgenic plants overexpressing the mutated LF1 gene, the spikelet developed lateral florets, similarly to lf1, whereas the spikelet showed a normal morphology in the transgenic plants overexpressing WT LF1. This indicated that the gain-of-function phenotypes that were observed in the lf1 mutant were probably due to ectopic expression rather than to overexpression of LF1. In addition, the LF1 gene belongs to the REV clade of the HD-ZIP III gene family. Previous studies have shown that most HD-ZIP III genes share conserved complementary sites for miRNA165/166 and similar regulatory mechanisms (25). In several gain-of-function mutants of HD-ZIP III genes, such as phb-d, phv-d, and rev in Arabidopsis and Rld1 in maize, the mutation sites were all located in the complementary site for miRNA165/166, and the gain of function was shown to be caused by the disruption of miRNA165/166 binding rather than by the amino acid substitution in the ligand-binding domain (20, 21, 26). Furthermore, the function of miRNA165/166 in maintaining the correct expression domain of these HD-ZIP III genes is conserved between dicots and monocots. In the present study, OSH1 was expressed ectopically in the axil of the lf1 sterile lemma where LF1 was expressed ectopically, and its promoter could be bound directly by the LF1 protein, which suggested that OSH1 might be activated by LF1 to promote initiation of the lateral FM in the lf1 spikelet. Previous studies have demonstrated that REV is required for initiation of the lateral meristem by promoting the expression of the meristem maintenance gene STM in the axil in Arabidopsis (24). Therefore, the miRNA165/166–LF1–OSH1 pathway in rice is similar to the miRNA165/166–REV–STM pathway in Arabidopsis. In WT rice, miRNA165/166 represses the expression of LF1 in the axil of the sterile lemma, which prevents the activation of OSH1 so that a single-floret WT spikelet is produced. In the lf1 mutant, the point mutation in the complementary site for miRNA165/166 resulted in the ectopic expression of LF1, which in turn induced the ectopic expression of OSH1 and finally resulted in initiation of the lateral FM.
From the findings of morphological studies, it has been hypothesized that a putative ancestor of the Oryzeae spikelet had three florets (two lateral and one terminal); the two lateral florets degenerated during the course of evolution, leaving only one fertile terminal floret (5). This is referred to as the “early three-florets spikelet” hypothesis. Previous studies have demonstrated that the sterile lemmas in extant Oryza species probably evolved from the lemmas in the two lateral florets. Mutation of G1, EG1, OsMADS34, NSG, or ASP1 or ectopic expression of OsMADS1 causes homeotic transformation of the sterile lemma into a lemma to various degrees (6–11), which suggests that sterile lemmas are homologous to lemmas and partly supports the above hypothesis. This paper reports the observation of lateral florets with normal floral organs in rice and other Oryzeae (Fig. 6), which constitutes much stronger evidence in support of the three-florets spikelet hypothesis than the transformation of the sterile lemma into a lemma. It is notable that the sterile lemma was not restored to a lemma-like organ in the lf1 mutant, in contrast to mutants such as g1, eg1, and pap2. In contrast, the lf1 mutant developed inner floral organs in lateral florets, whereas those other mutants did not. This interesting finding has two implications. First, in the process of evolution from the three-florets to one-floret spikelet, the degradation of lemmas was controlled by the G1, EG1, and OsMADS34 genes, among others, whereas the degradation of other floral organs as a whole was controlled by the miRNA165/166–LF1–OSH1 pathway (Fig. 6), although it is not clear which degradation occurred earlier in the evolutionary process. The other is that the lemma (or sterile lemma) is not a real floral organ but is a bract-like organ. In fact, the identities of the lemma and palea have long been debated (4). In one opinion, their positions on the axis suggest distinct identities of the lemma and palea as a bract (formed on a spikelet axis) and a prophyll (formed on a floret axis), respectively (5, 27). In the other opinion, the lemma and palea are perceived as modified outer tepals (sepals) (28, 29). Our results obviously support the first point of view. Therefore, both the lemma and sterile lemma might be bract-like organs, with the real floret consisting of the palea and other inner floral organs that form in the axil of the lemma or sterile lemma.
Fig. 6.
Model of hypothesized changes in spikelet architecture during Oryza evolution and putative function of LF1. During the evolutionary process the G1, EG1, OsMADS34, NSG, ASP1, and OsMADS1 genes determined the degeneration from the lemma to sterile lemma in lateral florets, whereas the miRNA165/166–LF1–OSH1 pathway controlled the degeneration of floral organs in lateral florets. Red arrows indicate positive regulation, the red “T” shape patterning indicates negative regulation, and green arrows show the two evolutionary lines of the WT spikelet. The blue arrows and “X” shape patterning suggest a possible method to breed a rice hybrid with a three-florets spikelet by combining the lf1 mutant and other materials with a lemma-like sterile lemma.
Finally, the three-florets spikelet has potential value in rice breeding. Increasing the grain number per panicle is one of the key factors to increase yield. In rice, the grain number per panicle is correlated mainly with the first and secondary branches. Some previously reported genes, such as OsCKX2/Gn1a, Ghd7, DENSE AND ERECT PANICLE 1 (DEP1), DEP2, and OsSPL14/IPA1, which have been used to make major contributions to increasing yield, are all involved in regulating panicle branching in rice (30–34). According to the present study, it should be possible to breed a three-florets spikelet in rice, for example by combining the lf1 mutant and other lemma-like sterile lemma mutants or transgenic plants (Fig. 6), and this presents the prospect for increasing grain number per panicle.
Materials and Methods
The rice mutant lf1 was identified from an ethylmethane-sulfonate–treated population of Oryza sativa subsp. indica cultivar Jinhui 10. All plant materials were grown in experimental fields of the Rice Research Institute of Southwest University in Chongqing and Hainan, China. A map-based cloning strategy was used to isolate the LF1 gene. Expression of LF1 was investigated in different organs at different growth stages by real-time RT-PCR and in situ hybridization. See SI Materials and Methods and Table S1 for full details. The fold enrichment of the mLF1-GFP and WT LF1 target promoter regions pulled down by the anti-GFP antibody and anti-LF1antibody, respectively, was compared with the input sample, and an IgG antibody was included as a negative control. Three biological repeats with three technical repeats each were used to produce data for the statistical analysis. Experimental procedures for ChIP-qPCR were performed as described previously (35). DNA fragments of the OSH1 promoter regions, which were positively enriched in ChIP-qPCR, were amplified using a SYBR Premix Ex Taq II Kit (TaKaRa).
Table S1.
Primers used in the study
| Purpose | Primer name | Sequence | Remarks | |
| Mapping | ZIn-23F | CCCAGGACAGAGTAGGCACAAT | Indel | |
| ZIn-23R | CGCTGTGCCTATGGAAACATACT | Indel | ||
| ZIn29F | CATTACCAACCTCTACGCGC | Indel | ||
| ZIn29R | CGTACTCACGGTTGTAATACTCTCC | Indel | ||
| ZIn27F | GCAGCTAGTGCAAGACGGAG | Indel | ||
| ZIn27R | GATGAGCCGTATGATCCGC | Indel | ||
| ZIn67F | CATGTTCCAAGTATTCCTGGG | Indel | ||
| ZIn67R | AGCTAGGGCACTAGCATTCG | Indel | ||
| RM14281F | CATGCATGCAACTCTGCTAAACG | SSR | ||
| RM14281R | ATCAGGATCCAGGAAATCGAACC | SSR | ||
| SNP-20F | CCTCTTTCTATATCATGACTCGATC | SNP | ||
| SNP-20R | CATGGAAGGTGGGAGGACAG | SNP | ||
| S-16F | GCTCTCTCTCTTGTTCCCCTCTCA | SSR | ||
| S-16R | CATGGATCGGCGATGGGT | SSR | ||
| RM14301F | TCATCATCATCAGTCCAGCATCG | SSR | ||
| RM14301R | GCGGCGATTGAATTGTTTCTTAGG | SSR | ||
| Overexpression | LF1OE-F | GCCGGTACCATGGCTGCGGCAGTGGCAAT | KpnI | |
| LF1OE-R | GCCACTAGTCACAAAGGACCAGTTGACGAAACAGAAG | SpeI | ||
| GFP-F | GCCACTAGTATGGTGAGCAAGGGCGAGGAG | SpeI | ||
| GFP-R | GCCGAGCTCTTACTTGTACAGCTCGTCCATGCCGT | SacI | ||
| RNA interference | LF1Ri-F1 | GCCGGATCCGTGATCCATGGCCGTGGCT | BamHI | |
| LF1Ri-R1 | GCCGGTACCGAGCCAAGCAAGCACAACCACT | KpnI | ||
| LF1Ri-F2 | GCCGAGCTCGTGATCCATGGCCGTGGCT | SacI | ||
| LF1Ri-R2 | GCCACTAGTGAGCCAAGCAAGCACAACCACT | SpeI | ||
| Subcellular localization | GFP-PA7-F | TCACCATTTACGAACGATACTCGAGATGGTGAGCAAGGGCGAGGA | Homologous recombination | |
| GFP-LF1-R | ATTGCCACTGCCGCAGCCATCTTGTACAGCTCGTCCATGCCGT | |||
| LF1-GFP-F | ACGGCATGGACGAGCTGTACAAGATGGCTGCGGCAGTGGCAAT | |||
| LF1-PA7-R | TGATGCATTCCCGGGTCTAGATCACACAAAGGACCAGTTGACGAAAC | |||
| qRT-PCR | Actin-F | TGCTATGTACGTCGCCATCCAG | ||
| Actin-R | AATGAGTAACCACGCTCCGTCA | |||
| qLF1-F | CTGTGAATCAAATGTGACTACCCCT | |||
| qLF1-R | GGACGATCTTTCAATATCTCTACCAC | |||
| qOsMADS1-F | GCTGCAACTACAACTCACAGG | |||
| qOsMADS1-R | TGATGGTGAGCATGAGGGTG | |||
| qOsMADS4-F | GATGGACCACTGGAGGATGC | |||
| qOsMADS4-R | CTGGAGGTTGGGGTGGCT | |||
| qOsMADS16-F | CCGCTACCAGCAAGCCAT | |||
| qOsMADS16-R | CTTGTAGGTTTCAGTCTGTGTGG | |||
| qOsMADS3-F | AGAGAACAGGCTGGAGAAAGGC | |||
| qOsMADS3-R | GGCTGCTGTCCCCTCTCATTC | |||
| qOsMADS58-F | AACAGTGACACCTCCAACGC | |||
| qOsMADS58-R | TTGGTGATCTGTTGCTTCAGC | |||
| qOsMADS13-F | TGGCGATAATGTGAGCAACCT | |||
| qOsMADS13-R | TCCATGTTGTCGTTCTGAAGC | |||
| qDL-F | CCCATCTGCTTACAACCGCTT | |||
| qDL-R | GTTGGAGGTGGAAACCGTCG | |||
| qOSH1-F | CAGGACCTGGAGCTTCGCCAG | |||
| qOSH1-R | GGAGAGCGTGTTGAGCTGCGTCT | |||
| qOsMADS6-F | AGAGAAAGACGCAACTGATGATGG | |||
| qOsMADS6-R | AGGCTTGCTGCATGGCTCTG | |||
| qOsMADS15-F | GTCACAAACACCTCATGGGAGAGGAT | |||
| qOsMADS15-R | CACATTCTTCTGCCTCTCCACCAGT | |||
| In situ | LF1-F | CGGATTCGGTTGGTATTGTGGC | ||
| LF1SP6-R | AGATTTAGGTGACACTATAGAACGTTAAAGCCTCTGCTCAGCCTT | |||
| M16-F | TACGGCGGCAACCACGA | |||
| M16SP6-R | AGATTTAGGTGACACTATAGAATCCAAGCACAGTTGCACACC | |||
| DL-F | ATGGATCTCGTGTCGCCGT | |||
| DLSP6-R | AGATTTAGGTGACACTATAGAATCACAACGAAGGGTGCTCT | |||
| OsH1-F | ATGGAGGAGATCTCCCACCACTT | |||
| OsH1SP6-R | AGATTTAGGTGACACTATAGAATCAGAAGAGCCGGAGGAAAGGAT | |||
| M6-F | GACGCAACTGATGATGGAACAAG | |||
| M6SP6-R | AGATTTAGGTGACACTATAGAAGACGCAGAAGGTGCAAACAGC | |||
| OsMADS15-F | TGAAGCGGATAGAGAACAAGATCAACAG | |||
| OsMADS15SP6-R | AGATTTAGGTGACACTATAGAACACATTCTTCTGCCTCTCCACCAGT | |||
| Transactivation activity assay | LF1-pGBKT7-F | GCCGAATTCATGGCTGCGGCAGTGGCAAT | EcoRI | |
| LF1-pGBKT7-R | GCCGTCGACCACAAAGGACCAGTTGACGAAACAGAAG | SalI | ||
| ChIP-qPCR | OsH1-aF | GTGATTTATGATATATGATTTGGAGATGATT | ||
| OsH1-aR | GATTTCACTCCATTCCCAAATCAT | |||
| OsH1-bF | CCTTGACATACAACCAATGATACCAT | |||
| OsH1-bR | TATTAGTTCAGGCCATCACCCGT | |||
| OsH1-cF | CATCGTCTAAAGTACACGCAAGATTC | |||
| OsH1-cR | GAGAAACACGATACGCTTTACTACTACT | |||
| OsH1-dF | CTCTAATGATAGCTTGGAGCAAGTTC | |||
| OsH1-dR | AGTGTGTATGATAGATGGGACCAGAT |
SI Materials and Methods
Map-Based Cloning of LF1.
The lf1 mutant was crossed with cultivar Xinong 1A. A total of 404 F2 progeny that displayed the WT phenotype were used for mapping LF1. Simple sequence repeat (SSR) markers that had been created from publicly available rice databases, including Gramene (www.gramene.org) and the Rice Genomic Research Program (rgp.dna.affrc.go.jp/index.html.en). and insertion/deletion and SNP markers that had been created from a comparison between genomic sequences from cultivar Xinong 1A and Jinhui 10 in our laboratory were used for mapping. The information on annotations and candidate genes came from the MSU website (rice.plantbiology.msu.edu/cgi-bin/gbrowse/rice/). The primer sequences for these markers that were used for gene mapping and candidate gene analysis are listed in Table S1.
RNA Isolation and qRT-PCR Analysis.
Total RNA was isolated from young panicles, sterile lemmas, pistils, stamens, lodicules, paleas, and lemmas of lf1, WT, and transgenic plants using the RNAprep Pure Plant RNA Purification Kit (Tiangen). The first-strand cDNA was synthesized from 2 µg of total RNA using oligo(dT)18 primers in a 25-µL reaction volume using the SuperScript III Reverse Transcriptase Kit (Invitrogen). Half a microliter of the reverse-transcribed RNA was used as a PCR template with gene-specific primers (Table S1). Real-time qPCR analysis was performed with a 7500 Real-Time PCR System (Applied Biosystems) and a SYBR Premix Ex Taq II Kit (TaKaRa), and actin was used as an endogenous control. At least three replicates were performed, from which the mean value was used to represent the expression level.
In Situ Hybridization.
For the LF1 probe, a 636-bp gene-specific cDNA was amplified. Probes for the known floral organ genes were prepared using the same method. All the probes were labeled using a DIG RNA Labeling Kit (Roche). Detection of miR165/166 was performed using a miR166 complementary LNA-modified DNA probe, 5′-GgGGaATgAAgCCtGGtCCgA-3′ (the lowercase letters represent LNAs). Pretreatment of sections, hybridization, and immunological detection were performed as described previously (36). Hybridization conditions were similar for all probes, with the exception of miR166, which was carried out at 60 °C overnight; then the slides were washed four times at 62 °C. The primer sequences are listed in Table S1.
Vector Construction.
To construct the LF1 overexpression plasmids, we amplified the WT LOC_Os03g01890CDS, mutated LOC_Os03g01890CDS [the lf1 mutant coding sequence (CDS)], and GFPCDS and inserted them into the expression cassette pTCK303-ubiquitin-NOS to construct the WT LOC_Os03g01890CDS-GFP and mutated LOC_Os03g01890CDS-GFP fusion vectors. The recombinant plasmids were transferred into calli of indica rice cultivar Jinhui 10 by an Agrobacterium tumefaciens-mediated transformation method. The primer sequences are listed in Table S1.
Microscopy Analysis.
Panicles at different developmental stages from lf1 and WT plants were collected and fixed in FAA solution (50% ethanol, 0.9 M glacial acetic acid, and 3.7% formaldehyde) overnight at 4 °C. They were then dehydrated in a series of ethanol solutions, substituted with xylene, and embedded in paraffin (Sigma). Samples were sectioned at 8 mm and transferred onto poly-l-lysine–coated glass slides, deparaffinized with xylene, and dehydrated through a series of ethanol solutions. The sections were stained with 1% safranin (AMRESCO) and 1% Fast Green (AMRESCO), dehydrated through a series of ethanol solutions, infiltrated with xylene, and mounted beneath a coverslip. Light microscopy was performed using a Nikon Eclipse E600 microscope. For scanning electron microscopy, fresh samples were examined using a Hitachi SU3500 scanning electron microscope with a −40 °C cool stage.
Phylogenetic Analysis.
Protein sequences were obtained by BLAST in PHYTOZOME, using a 10−5 EXPECT value threshold (https://phytozome.jgi.doe.gov/pz/portal.html). A phylogenetic tree was constructed using MEGA 5.0 (37). The tree was constructed using the maximum likelihood method based on the Jones–Taylor–Thornton matrix-based model with the lowest Bayesian information criterion scores (37). Bootstrap support values for each node from 500 replicates are shown next to the branches.
Subcellular Localization of LF1 Protein.
The full-length coding region of LF1 was amplified and cloned into the expression cassette 35S-GFP-NOS (pA7) to generate the GFP-LF1 fusion vector. The GFP and GFP-LF1 plasmids were transformed into rice protoplasts. After overnight incubation at 28 °C, GFP fluorescence was observed by Olympus confocal laser-scanning microscopy. The primer sequences are listed in Table S1.
Transcriptional Activation Assay.
The full-length coding region of LF1 was amplified and ligated into the yeast expression vector pGBKT7 (Clontech) to produce pGBKT7-LF1. pGBKT7-LF1, pGBKT7-53+pGADT7-T (plasmid combination for the positive control) (Clontech), and pGBKT7-Lam+pGADT7-T (plasmid for the negative control) (Clontech) were transformed separately into the yeast strain Y2HGold. Transformants were selected on synthetic defined (SD)/−Trp or SD/−Ade/−His/−Trp medium (Clontech). The transactivation activity of each protein was evaluated by comparing growth on permissive and selective medium and the activity of X-α-Gal.
Acknowledgments
This work was supported by National Natural Science Foundation of China Grants 31730063 and 31271304, National Key Research and Development Project Grant 2016YFD0101107, Fundamental Research Funds for the Central Universities Grant XDJK2016A013, Project of Chongqing Science & Technology Commission Grants CSTC2016shms-ztzx0017 and CSTC2017jcyjBX0062, and National Natural Science Foundation of China Grant 31501377.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700504114/-/DCSupplemental.
References
- 1.Komatsu M, Chujo A, Nagato Y, Shimamoto K, Kyozuka J. FRIZZY PANICLE is required to prevent the formation of axillary meristems and to establish floral meristem identity in rice spikelets. Development. 2003;130:3841–3850. doi: 10.1242/dev.00564. [DOI] [PubMed] [Google Scholar]
- 2.Lee DY, An G. Two AP2 family genes, supernumerary bract (SNB) and Osindeterminate spikelet 1 (OsIDS1), synergistically control inflorescence architecture and floral meristem establishment in rice. Plant J. 2012;69:445–461. doi: 10.1111/j.1365-313X.2011.04804.x. [DOI] [PubMed] [Google Scholar]
- 3.Ren D, et al. MULTI-FLORET SPIKELET1, which encodes an AP2/ERF protein, determines spikelet meristem fate and sterile lemma identity in rice. Plant Physiol. 2013;162:872–884. doi: 10.1104/pp.113.216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida H, Nagato Y. Flower development in rice. J Exp Bot. 2011;62:4719–4730. doi: 10.1093/jxb/err272. [DOI] [PubMed] [Google Scholar]
- 5.Arber AR. The Gramineae: A Study of Cereal, Bamboo, and Grass. Cambridge Univ Press Archive; Cambridge, UK: 1934. [Google Scholar]
- 6.Yoshida A, Suzaki T, Tanaka W, Hirano HY. The homeotic gene long sterile lemma (G1) specifies sterile lemma identity in the rice spikelet. Proc Natl Acad Sci USA. 2009;106:20103–20108. doi: 10.1073/pnas.0907896106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, et al. A putative lipase gene EXTRA GLUME1 regulates both empty-glume fate and spikelet development in rice. Plant J. 2009;57:593–605. doi: 10.1111/j.1365-313X.2008.03710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin X, et al. The pleiotropic SEPALLATA-like gene OsMADS34 reveals that the ‘empty glumes’ of rice (Oryza sativa) spikelets are in fact rudimentary lemmas. New Phytol. 2014;202:689–702. doi: 10.1111/nph.12657. [DOI] [PubMed] [Google Scholar]
- 9.Wang N, et al. nonstop glumes (nsg), a novel mutant affects spikelet development in rice. Genes Genomics. 2013;35:149–157. [Google Scholar]
- 10.Yoshida A, Ohmori Y, Kitano H, Taguchi-Shiobara F, Hirano HY. Aberrant spikelet and panicle1, encoding a TOPLESS-related transcriptional co-repressor, is involved in the regulation of meristem fate in rice. Plant J. 2012;70:327–339. doi: 10.1111/j.1365-313X.2011.04872.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, et al. Ectopic expression of OsMADS1 caused dwarfism and spikelet alteration in rice. Plant Growth Regul. 2016;81:433–442. [Google Scholar]
- 12.Wang K, et al. DEP and AFO regulate reproductive habit in rice. PLoS Genet. 2010;6:e1000818. doi: 10.1371/journal.pgen.1000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao SG, Ohmori S, Kimizu M, Yoshida H. Unequal genetic redundancy of rice PISTILLATA orthologs, OsMADS2 and OsMADS4, in lodicule and stamen development. Plant Cell Physiol. 2008;49:853–857. doi: 10.1093/pcp/pcn050. [DOI] [PubMed] [Google Scholar]
- 14.Nagasawa N, et al. SUPERWOMAN1 and DROOPING LEAF genes control floral organ identity in rice. Development. 2003;130:705–718. doi: 10.1242/dev.00294. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi T, et al. Functional diversification of the two C-class MADS box genes OSMADS3 and OSMADS58 in Oryza sativa. Plant Cell. 2006;18:15–28. doi: 10.1105/tpc.105.037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi T, et al. The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa. Plant Cell. 2004;16:500–509. doi: 10.1105/tpc.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreni L, et al. The D-lineage MADS-box gene OsMADS13 controls ovule identity in rice. Plant J. 2007;52:690–699. doi: 10.1111/j.1365-313X.2007.03272.x. [DOI] [PubMed] [Google Scholar]
- 18.Sparks DL. Advances in Agronomy. Academic; Cambridge, MA: 2012. [Google Scholar]
- 19.Rhoades MW, et al. Prediction of plant microRNA targets. Cell. 2002;110:513–520. doi: 10.1016/s0092-8674(02)00863-2. [DOI] [PubMed] [Google Scholar]
- 20.Mallory AC, et al. MicroRNA control of PHABULOSA in leaf development: Importance of pairing to the microRNA 5′ region. EMBO J. 2004;23:3356–3364. doi: 10.1038/sj.emboj.7600340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong R, Ye ZH. Amphivasal vascular bundle 1, a gain-of-function mutation of the IFL1/REV gene, is associated with alterations in the polarity of leaves, stems and carpels. Plant Cell Physiol. 2004;45:369–385. doi: 10.1093/pcp/pch051. [DOI] [PubMed] [Google Scholar]
- 22.Zhong R, Ye ZH. IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain-leucine zipper protein. Plant Cell. 1999;11:2139–2152. doi: 10.1105/tpc.11.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otsuga D, DeGuzman B, Prigge MJ, Drews GN, Clark SE. REVOLUTA regulates meristem initiation at lateral positions. Plant J. 2001;25:223–236. doi: 10.1046/j.1365-313x.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- 24.Shi B, et al. Two-step regulation of a meristematic cell population acting in shoot branching in Arabidopsis. PLoS Genet. 2016;12:e1006168. doi: 10.1371/journal.pgen.1006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Floyd SK, Zalewski CS, Bowman JL. Evolution of class III homeodomain-leucine zipper genes in streptophytes. Genetics. 2006;173:373–388. doi: 10.1534/genetics.105.054239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juarez MT, Kui JS, Thomas J, Heller BA, Timmermans MC. microRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature. 2004;428:84–88. doi: 10.1038/nature02363. [DOI] [PubMed] [Google Scholar]
- 27.Kellogg EA. Evolutionary history of the grasses. Plant Physiol. 2001;125:1198–1205. doi: 10.1104/pp.125.3.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stebbins GL. Natural selection and the differentiation of angiosperm families. Evolution. 1951;5:299–324. [Google Scholar]
- 29.Bowman JL. Evolutionary conservation of angiosperm flower development at the molecular and genetic levels. J Biosci. 1997;22:515–527. [Google Scholar]
- 30.Ashikari M, et al. Cytokinin oxidase regulates rice grain production. Science. 2005;309:741–745. doi: 10.1126/science.1113373. [DOI] [PubMed] [Google Scholar]
- 31.Xue W, et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet. 2008;40:761–767. doi: 10.1038/ng.143. [DOI] [PubMed] [Google Scholar]
- 32.Yan CJ, et al. Identification and characterization of a major QTL responsible for erect panicle trait in japonica rice (Oryza sativa L.) Theor Appl Genet. 2007;115:1093–1100. doi: 10.1007/s00122-007-0635-9. [DOI] [PubMed] [Google Scholar]
- 33.Li F, et al. Rice DENSE AND ERECT PANICLE 2 is essential for determining panicle outgrowth and elongation. Cell Res. 2010;20:838–849. doi: 10.1038/cr.2010.69. [DOI] [PubMed] [Google Scholar]
- 34.Miura K, et al. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat Genet. 2010;42:545–549. doi: 10.1038/ng.592. [DOI] [PubMed] [Google Scholar]
- 35.Xu J, et al. The ABORTED MICROSPORES regulatory network is required for postmeiotic male reproductive development in Arabidopsis thaliana. Plant Cell. 2010;22:91–107. doi: 10.1105/tpc.109.071803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sang X, et al. CHIMERIC FLORAL ORGANS1, encoding a monocot-specific MADS box protein, regulates floral organ identity in rice. Plant Physiol. 2012;160:788–807. doi: 10.1104/pp.112.200980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamura K, et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]