Significance
Climate warming is having wide-ranging effects on aquatic ecosystems. Fish are believed to adapt their feeding behavior as temperatures change, but empirical evidence of this behavior in nature and its impacts on individual fitness are lacking. We monitored the feeding behavior and growth of a temperature-sensitive fish population in a pristine lake for 11 y. Fish adjusted their feeding behavior annually in response to differences in temperature. In cooler years, fish ate more large prey from shallow nearshore regions, resulting in higher growth and condition than in warmer years, when fish ate more small prey from deep offshore regions. This suggests that the impacts of warming on aquatic ecosystems can scale from the individual to the food web level.
Keywords: food web, climate change, habitat coupling, lake trout, north-temperate lake
Abstract
There is a pressing need to understand how ecosystems will respond to climate change. To date, no long-term empirical studies have confirmed that fish populations exhibit adaptive foraging behavior in response to temperature variation and the potential implications this has on fitness. Here, we use an unparalleled 11-y acoustic telemetry, stable isotope, and mark–recapture dataset to test if a population of lake trout (Salvelinus namaycush), a cold-water stenotherm, adjusted its use of habitat and energy sources in response to annual variations in lake temperatures during the open-water season and how these changes translated to the growth and condition of individual fish. We found that climate influenced access to littoral regions in spring (data from telemetry), which in turn influenced energy acquisition (data from isotopes), and growth (mark–recapture data). In more stressful years, those with shorter springs and longer summers, lake trout had reduced access to littoral habitat and assimilated less littoral energy, resulting in reduced growth and condition. Annual variation in prey abundance influenced lake trout foraging tactics (i.e., the balance of the number and duration of forays) but not the overall time spent in littoral regions. Lake trout greatly reduced their use of littoral habitat and occupied deep pelagic waters during the summer. Together, our results provide clear evidence that climate-mediated behavior can influence the dominant energy pathways of top predators, with implications ranging from individual fitness to food web stability.
There is growing urgency to understand how ecosystems are responding to climate change (1, 2). Recent work, using latitudinal gradients as proxies to warming, has argued that the behavioral responses of mobile top predators to changing temperatures can drive fundamental shifts in aquatic food webs by altering the coupling of major energy pathways (3, 4). Although this work is intriguing, no one has yet examined long-term empirical data that have explicitly tested if populations of top predators can shift their foraging behavior in response to annual changes in temperature or has evaluated what implications this might have for individual fitness. Temporal studies are critically important in this context because they control for the ecosystem-specific adaptations that can confound latitudinal studies and instead focus on the active responses to changing conditions that are highly relevant to understanding the impacts of climate change.
Mobile top predators display adaptive foraging behavior by moving across spatially disparate habitats in response to changing conditions, most notably prey densities. For example, birds feed on both terrestrial and aquatic prey, effectively coupling these ecosystems (5). Habitat coupling can also occur within ecosystems and has been well described in freshwater lakes, where predatory fish feed upon prey supported by dissimilar energy sources, such as offshore pelagic phytoplankton and nearshore littoral benthic algae (6). These adaptive foraging shifts between littoral and pelagic food chains (i.e., littoral–pelagic coupling) in response to changes in prey densities can be a stabilizing force in aquatic food webs (7–9).
As ectotherms, the body temperatures of fish closely follow that of their ambient environment, and they must occupy species-specific temperature ranges to optimize physiological performance (10–12). Adaptive foraging behavior therefore should be particularly important in north-temperate lakes, because these systems undergo annual cycles in water temperatures and stratify thermally in summer. During thermal stratification, surface waters often exceed the temperature preferences of cold-water fish, substantially increasing the metabolic costs associated with occupying littoral habitats (10–12). In response, cold-water predators exhibit seasonality in their foraging, feeding in the littoral zone in the spring and fall when surface waters are cool and relying on pelagic energy when surface waters are warm in summer (13, 14). Therefore, variations in both prey density and seasonality should be important factors in directing the foraging behavior of fish in north-temperate lakes.
Recent studies have shown that lake-surface temperatures have risen globally over the past 30 y (15), with north-temperate lakes also having longer open-water seasons and undergoing shifts in the phenology of seasonal water temperatures (16). These observed changes in lake temperatures suggest that future warming may alter littoral–pelagic coupling by mobile predatory fish. In fact, multilake studies of temperate food webs have shown that cold-water predatory fish alter their littoral–pelagic coupling across gradients of abiotic factors that regulate the physiological costs of foraging in the littoral zone. For example, littoral energy use by lake trout (Salvelinus namaycush), a cold-water stenotherm, has been shown to increase with latitude, because lakes at higher latitudes have littoral zones that are either thermally favorable for longer periods or cooler in summer (3). Lake trout acquisition of littoral energy also has been shown to decrease with increasing littoral zone size due to the greater expanse of warm water to be traversed to access nearshore prey during summer (17). In both cases, the physiological constraint imposed by temperature was suggested as the key factor in controlling littoral energy use by lake trout, and together these studies suggest that the expected warmer conditions also could alter littoral–pelagic coupling by cold-water fish populations within single lakes.
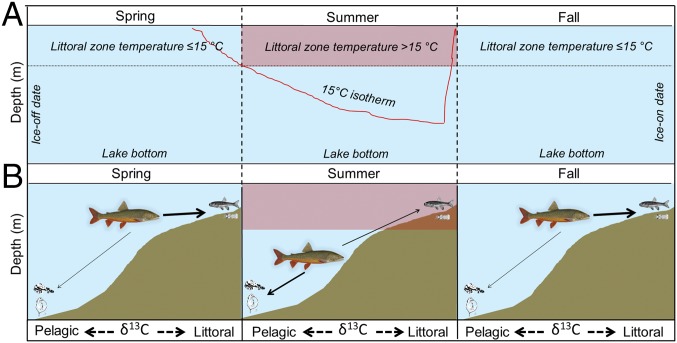
Here, we sought to understand if and how annual variations in water temperatures altered littoral–pelagic coupling by a cold-water predatory fish population and what implications these dietary shifts had on individual fitness as inferred from growth and condition. Our study system was a small, oligotrophic north-temperate lake that did not contain pelagic prey fish. In such lakes, lake trout obtain the majority of their energy from prey fish and benthic invertebrates located in the littoral zone (18), presumably during the spring and fall, when water temperatures are cool. As the lake warms, water temperatures within the littoral zone exceed the thermal preference of lake trout (>15 °C), and they move offshore to deeper water within the pelagic zone and begin to rely increasingly on smaller pelagic prey, including Mysis diluviana (i.e., freshwater shrimp) and zooplankton (Fig. 1) (13, 14, 18, 19). We hypothesized that because of the direct influence of temperature on fish physiology, annual changes in the phenology of littoral zone water temperatures, which are closely linked to air temperature variations (16), would influence littoral–pelagic coupling by lake trout (Fig. 1). We also expected that climate-driven year-to-year differences in access to prey-rich littoral regions would be manifested in the growth and condition of lake trout. To test these hypotheses, we used 11 consecutive years of acoustic telemetry and stable isotope data to quantify annual littoral habitat use and energy sources of our study population and related these findings to annual variations in water temperatures, prey fish abundance, and the growth and condition of individual lake trout from annual mark–recapture sampling.
Fig. 1.
Theoretical illustration of how seasonality in water temperatures during the open-water season impacts foraging behavior of lake trout in small Boreal Shield lakes. (A and B) Cold water temperatures immediately after ice-out in the spring and before ice-on in the fall allow lake trout to access the littoral zone (<6 m depth) without thermal consequence. However, during summer warm littoral temperatures impose an energetic cost to lake trout accessing the littoral zone. Therefore, (B) lake trout should exhibit greater use of littoral habitat and prey (prey fish and benthic invertebrates/aquatic insects) when springs and falls are longer, and conversely, should use more pelagic habitat and prey (Mysis and zooplankton) when summers are longer. In B, increasing arrow thickness denotes expected increased use of energy pathways based on littoral water temperatures.
Results
Lake Temperatures.
The length of spring, when lake trout can access the littoral zone without thermal consequence (≤15 °C), averaged 43 d and varied in duration by nearly a month (31–59 d) over the study. The summer period, when lake trout are putatively thermally restricted from accessing the littoral zone (>15 °C), was on average 2.7 times longer than the spring and averaged 109 d with a difference of 1 mo (36 d) between the shortest (85 d) and longest (121 d) summers. Longer summers typically had warmer littoral zone temperatures (Pearson correlation: n = 11, r = 0.79, P < 0.01). The length of the fall season, when lake trout spawn but can also use the littoral zone for feeding without thermal consequence, averaged 61 d (range: 51–72 d) and was on average 1.5 times longer than the spring and 1.8 times shorter than the summer. In a given year, the length of the spring and summer seasons showed a negative correlation (n = 11, r = −0.59, P = 0.06), spring and fall lengths were not significantly correlated (n = 11, r = 0.42, P = 0.20), and neither were fall and summer lengths (n = 11, r = −0.32, P = 0.34).
Habitat Use.
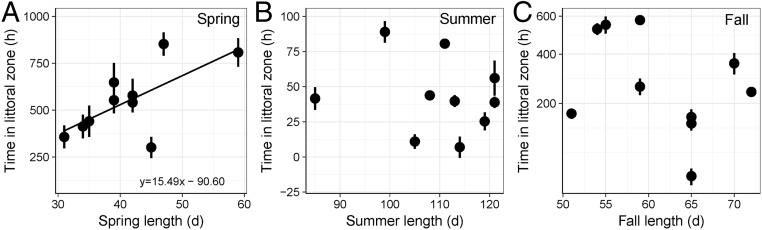
Lake trout displayed clear seasonal shifts in habitat use and behavior that followed changes in mean littoral zone temperatures (Fig. 2). Immediately following ice-out, lake trout often spent several hours or entire days within the littoral zone (Fig. 2). As mean littoral zone water temperatures exceeded 15 °C (summer), lake trout greatly reduced their forays into the littoral zone until water temperatures cooled to 15 °C in the fall, when lake trout quickly reoccupied the littoral zone (Fig. 2). The total time spent by lake trout in the littoral zone during the spring of each year averaged 550 h and increased with spring length (log10; F1,8 = 6.75, P = 0.03, r2 = 0.46) (Fig. 3A). In contrast, the number (F1,8 = 0.28, P = 0.61) or average duration (F1,8 = 1.13, P = 0.32) of littoral forays made in the spring was not predicted by spring length. Rather, lake trout made a greater number (F1,8 = 12.22, P < 0.01, r2 = 0.60) of shorter (F1,8 = 5.24, P = 0.05, r2 = 0.40) forays in springs with higher prey fish densities (Fig. S1 A and B). The contrasting effect of number and duration of forays meant that prey fish abundance (measured as catch per unit effort, CPUE) did not alter the total time lake trout spent in the littoral zone during the spring but only how they used that time (F1,8 = 0.00, P = 0.98) (Fig. S1C).
Fig. 2.
Daily estimates of littoral zone use by individual lake trout implanted with acoustic transmitters during each annual open-water period (n = 420–1,906 per year), including (A) the number of littoral forays, (B) the average foray duration in hours, and (C) the total time in hours. Red vertical dashed lines indicated the start and end dates of the summer period (i.e., when the mean littoral zone temperature exceeds 15 °C) each year. Each data point is a daily estimate for an individual fish. Note there were no telemetry data for 2005 (Materials and Methods).
Fig. 3.
Relationships between the length of (A) spring, (B) summer, and (C) fall and time spent in the littoral zone by acoustically tagged lake trout. Note differences in y-axis scales. Least squares mean (±SD) estimates of time in the littoral zone are shown. Spring data were log10 transformed for analysis.
Fig. S1.
Relationships between prey fish CPUE and least squares mean (±SD) spring littoral habitat use (A–C) and between mean summer littoral zone water temperatures and least square mean estimates (±SD) of summer littoral habitat use (D–F). Habitat use measures include the number of littoral forays (n = 10) (A and D), average littoral foray duration (n = 10) (B and E), and total time spent within the littoral zone (n = 10) (C and F). Note that error bars are very small in A and D.
The amount of time that lake trout spent in the littoral zone each summer averaged 43 h and was not predicted by summer length (F1,8 = 0.09, P = 0.77) (Fig. 3B) or prey fish CPUE (F1,8 = 0.01, P = 0.93). The number or average duration of littoral forays made by lake trout in the summer also was not predicted by summer length (log10; number of forays: F1,8 = 0.55, P = 0.48; average foray duration: F1,8 = 2.31, P = 0.17) or prey fish CPUE (log10; number of forays: F1,8 = 0.69, P = 0.43; average foray duration: F1,8 = 0.09, P = 0.78). The mean summer water temperature in the littoral zone also did not predict the time spent by lake trout within the littoral zone during the summer (F1,8 = 0.01, P = 0.92) Fig. S1D or the number of littoral forays (log10; F1,8 = 1.76, P = 0.22). However, lake trout made shorter forays as mean summer littoral zone water temperatures increased (F1,8 = 3.79, P = 0.08, r2 = 0.32) (Fig. S1 E and F). The time spent within the littoral zone the during summer was not related to the length of the preceding spring (F1,8 = 0.01, P = 0.92).
The amount of time that lake trout spent in the littoral zone each fall averaged 301 h but, unlike spring, was not predicted by fall length (square-root; F1,8 = 1.13, P = 0.32) (Fig. 3C) or prey fish CPUE (square-root; F1,8 = 0.04, P = 0.85). The number or average duration of littoral forays made by lake trout in fall also was not predicted by fall length (number of forays: F1,8 = 3.14, P = 0.12; average foray duration: F1,8 = 0.02, P = 0.90) or prey fish CPUE (number of forays: F1,8 = 0.58, P = 0.47; average foray duration: F1,8 = 0.24, P = 0.64). The time spent within the littoral zone during the fall also was not related to the length of the preceding spring (square-root; F1,8 = 0.20, P = 0.67) or summer (square-root; F1,8 = 0.14, P = 0.72).
Energy Sources.
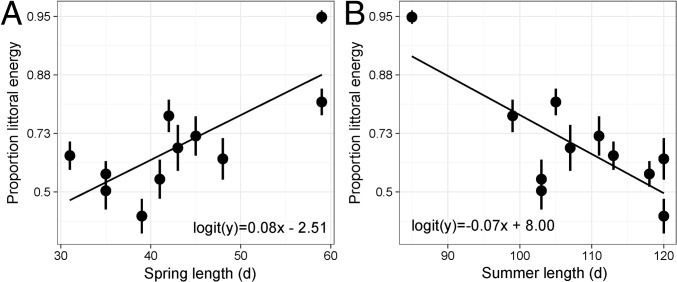
The proportion of littoral energy assimilated by lake trout, estimated using δ13C values (logit-transformed for analyses), averaged 0.66 (range 0.40–0.95) over the study and was positively related to spring length (F1,9 = 12.66, P < 0.01, r2 = 0.59) (Fig. 4A) and negatively related to summer length (F1,9 = 12.58, P < 0.01, r2 = 0.58) (Fig. 4B). The proportion of littoral energy assimilated by lake trout was not predicted by prey fish CPUE (F1,9 = 1.53, P = 0.25) but was negatively related to mean summer littoral zone temperature (F1,9 = 7.49, P = 0.02, r2 = 0.45).
Fig. 4.
Relationships of mean estimates (±SD) of littoral energy use (logit-transformed; n = 11) to the number of (A) spring and (B) summer days.
Lake trout stomachs contained more prey in spring (6.33 ± 6.15 g, mean ± SD) than in summer (1.73 ± 1.45 g) and fall (3.45 ± 2.75 g), and the composition of these prey items varied seasonally. Stomach contents collected in spring contained 60% benthic invertebrates and insects, 37% littoral prey fish, 3% Mysis, and no zooplankton. Stomachs collected in summer contained 45% littoral prey fish, 2% benthic invertebrates and insects, 27% Mysis, and 26% zooplankton. Fall stomach contents contained 22% littoral prey fish, no benthic invertebrates or insects, 72% Mysis, and 6% zooplankton.
Growth and Condition.
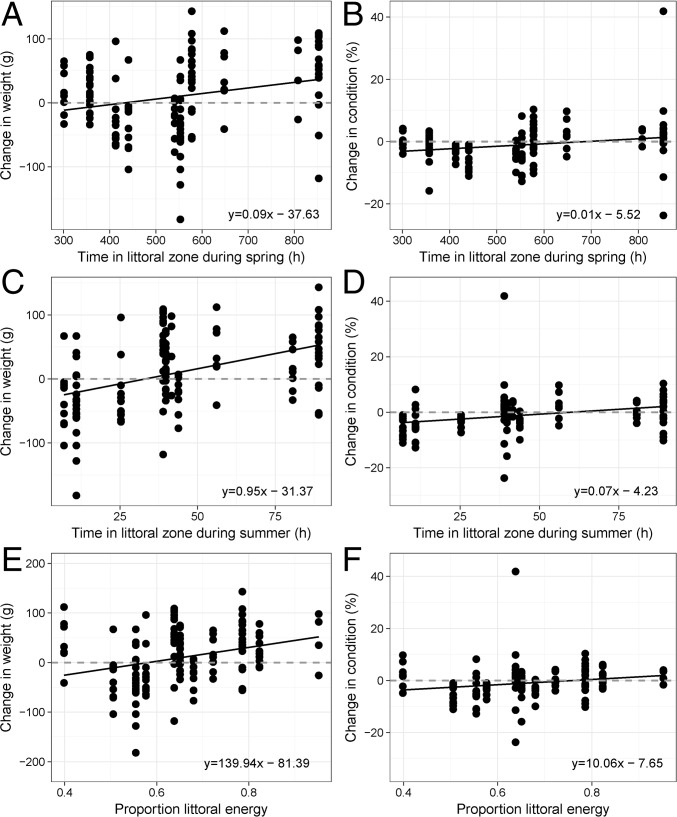
The growth (i.e., change in weight) and condition of individual lake trout were positively related to the time spent within the littoral zone during the spring (weight: F[1,127] = 8.69, P < 0.01, r2 = 0.07; condition: F[1,127] = 5.82, P = 0.02, r2 = 0.04) (Fig. 5 A and B) and summer (weight: F[1,126] = 30.39, P < 0.01, r2 = 0.19; condition: F[1,126] = 11.95, P < 0.01, r2 = 0.09) (Fig. 5 C and D). Growth and condition were also positively related to the proportion of littoral energy assimilated (weight: F[1,139] = 13.96, P < 0.01, r2 = 0.09; condition: F[1,138] = 5.44, P < 0.01, r2 = 0.04) (Fig. 5 E and F). Consistent with behavioral data, growth and condition of individual lake trout were also positively correlated to spring length (weight: F[1,139] = 8.56, P < 0.01, r2 = 0.06; condition: F[1,138] = 6.74, P = 0.01, r2 = 0.05); however, neither was related to summer length (weight: F[1,139] = 0.17, P = 0.68; condition: F[1,138] = 0.56, P = 0.46).
Fig. 5.
Annual changes in the weight and condition of individual lake trout captured in consecutive years as a function of spring littoral habitat use (A and B) (n = 129), summer littoral habitat (C and D) (n = 129), and littoral energy use (E and F) (n = 141). The gray dashed lines indicate zero.
Discussion
We found that lake trout, a mobile cold-water predator, made consistent and predictable seasonal shifts in habitat use that were triggered by changes in lake-water temperatures. As the phenology of littoral zone temperatures shifted from year to year, so did lake trout use of nearshore habitat and acquisition of energy from this prey-rich habitat. In warmer years, when littoral energy acquisition was lower, individual lake trout had reduced growth and condition. This ability of a single population of aquatic top predators to adjust its foraging behavior rapidly to interannual changes in climatic conditions and the corresponding impacts on measures of fitness highlight the adaptability of this glacial relict species to persist under adverse conditions. Indeed, recent evidence that many fish species from various thermal guilds and trophic levels may be capable of flexible foraging behavior (20) suggest that our results may be broadly applicable for understanding the impact of climate change on aquatic ecosystems.
Our results suggest that water temperature phenology (i.e., seasonality) supersedes prey density as the main controller of littoral–pelagic coupling by lake trout. Although prey fish abundance and summer littoral zone temperature influenced foraging strategy (i.e., the balance of the number and duration of littoral forays), these factors did not affect the overall time spent within the littoral zone. Rather, longer springs resulted in greater occupation of littoral habitat and acquisition of littoral energy. Conversely, littoral energy use declined with increasing summer length, which was negatively correlated to spring length in a given year. These results suggest that changes in temperature can fundamentally rewire energy pathways, a result that has the potential to impact food web stability (7–9, 21), but of course the direction and magnitude of these impacts would vary depending on the how future warming alters the phenology of water temperatures.
Reductions in the growth and condition of individual lake trout observed in years with reduced littoral habitat use and littoral energy use could result from a combination of factors. First, increased reliance on Mysis and zooplankton would be less energetically efficient than foraging on larger prey fish or benthic invertebrates because predatory fish are more active when forced to feed on numerous, smaller prey (22). This increased energetic cost of feeding on small prey has been illustrated by studies that found predatory fish in lakes without pelagic prey fish had increased muscle activity and greater activity rates than those same species in lakes containing pelagic prey fish (23–25) and by studies showing that more active fish generally grow more slowly (26). Additionally, in oligotrophic lakes, the littoral zone is often more productive and smaller in volume than the pelagic zone (6, 17), and therefore the probability of a lake trout’s encountering prey would be higher in the littoral zone than in the pelagic zone, increasing foraging success but also reducing the time required to find prey.
Despite thermally suboptimal temperatures in littoral regions during summer, lake trout used this habitat to access preferred prey in all years. Regular, although limited, foraging into waters with temperatures above their thermal preference indicates the energetic importance of acquiring large, energy-dense prey (27). This suggests that capturing prey fish in warm water could be a more efficient strategy than foraging on small prey within the pelagic zone—at least for some parts of summer. These rapid forays into warm, shallow water have been documented previously for lake trout (28, 29). However, during the peak of summer, when littoral temperatures were highest, lake trout seemed to reduce greatly and even stop use of the littoral zone. This behavior also has been exhibited by other temperature-sensitive fish, including brook trout (Salvelinus fontinalis) and rainbow trout (Oncorhynchus mykiss), which have been documented to stop foraging and seek cool-water refuge when temperatures exceed their thermal preferences (30, 31). Therefore, continued warming of surface-water temperatures during the summer (15, 16) could extend periods of limited littoral access in salmonids, increasing their reliance on pelagic-derived energy.
The fall period also provides thermal conditions in which lake trout could exploit littoral prey without consequence and presumably could offset the constraints imposed by the stressful conditions during the preceding seasons. Although the fall periods generally were longer than the spring periods, lake trout on average spent less time in the littoral zone during the fall than in the spring, even though the littoral zone is also the region where spawning occurs. Moreover, lake trout did not increase their use of this habitat in fall periods that were preceded by stressful conditions (i.e., shorter spring and/or longer summers), and their fall diet reflected a reliance on pelagic prey, mainly Mysis, as earlier studies have noted (13, 14). At least in our study system, the fall does not seem to be a period when lake trout make extensive use of littoral resources. Based on the apparent limited acquisition of littoral energy in summer and fall periods, it appears that the spring period, which is strongly influenced by climate (16), is critical for the annual growth and condition of lake trout.
Observed reductions in individual growth and condition associated with reduced access to littoral energy also have important implications for population persistence through impacts on reproduction and recruitment. Further reductions in access to littoral regions with future warming could prevent lake trout from accumulating sufficient energy to spawn in the fall (27), potentially increasing the frequency of skip-spawning (32, 33). The prospective smaller postadult body size with warming could also lead to reductions in fecundity, which is positively correlated with body size (34). Furthermore, increased reliance on pelagic energy by adult lake trout during longer and/or warmer summers would increase competition with juvenile lake trout that rely almost exclusively on Mysis and zooplankton (35, 36). Together, the potential for less frequent spawning, production of fewer eggs, and lower recruitment to adulthood posed by warming could have severe implications for the ability of lake trout populations within small lakes to persist through future climate change.
It is worth noting that our study lake did not contain pelagic prey fish (e.g., cisco, Coregonus artedi), and this type of food web represents only a subset of lake trout lakes (18). In lakes with cisco, lake trout would be able to access prey fish throughout periods of thermal limitation (13), and so the impacts of seasonal phenology on foraging behavior and fitness in cisco-containing lakes may differ from our findings. However, a study by King, et al. (37) found that lake trout inhabiting a cisco-containing lake had reduced growth in years with earlier onset of thermal stratification, likely because of reduced access to littoral prey in the spring. This suggests that the impacts of seasonal phenology on the foraging behavior and fitness of lake trout may be similar across lake types; however, the magnitude of these changes may vary. Finally, the adaptive behavior of lake trout illustrated here also has implications for predicting how future warming may alter the geographic distribution of cold-water fish species, particularly around southern range borders, where surface temperatures may exceed thermal limits. At these southern edges, adaptive foraging behavior and use of refuge may allow cold-water populations to persist for extended periods when simple surface models would predict range contractions (2).
Materials and Methods
Study Site.
The study occurred over 11 y (2003–2013) within the International Institute for Sustainable Development (IISD)-Experimental Lakes Area, Canada (49°40′N, 93°44′W) (38). Lake 373 (L373) is an unmanipulated long-term reference lake used to monitor natural variation. It is a small (surface area 27.3 ha, maximum depth 20.5 m), single-basin lake that thermally stratifies during summer, when mean littoral zone temperatures typically range from 17–21 °C. The lake supports a native, naturally reproducing lake trout population that consisted of ≈285 adults during our study. The lake does not contain pelagic prey fish, and the main prey items for adult lake trout are littoral prey fish (Phoxinus eos, Phoxinus neogaeus, Margariscus margarita, and Cottus cognatus), benthic invertebrates, insects, Mysis, and zooplankton. The lake also contains white sucker (Catostomus commersonii). We considered the littoral zone to be water depths <6 m (39). Because L373 is bowl-shaped, with no islands or shoals, littoral regions were located only along the perimeter of the lake.
Lake Temperatures.
Annual ice-on and ice-off dates were monitored at nearby (≈12 km) Rawson Lake (54.3 ha) and were assumed to be the same for L373 because the lakes are similar in size and depth. Water temperatures in L373 were measured every 30 min using a string of data loggers (HOBO Temp Pro H20-001; Onset) deployed over the deepest point of the lake (19). Logger data from 29 April to 20 June 2003 were not available, and biweekly temperature profile data were supplemented.
We estimated the mean daily temperature of the littoral zone during each open-water season using data from temperature loggers at 1–6 m. The period between ice-off and the date that the mean littoral zone temperature exceeded 15 °C was denoted “spring.” The period when the mean littoral zone temperature exceeded 15 °C was denoted “summer.” “Fall” was the period between the date that the lake cooled to ≤15 °C and ice-on. “Winter” was considered the ice-covered period. Because lake trout spawn inshore in the fall (40), we were unable to distinguish between foraging and spawning behavior. By using the date when littoral water temperatures exceeded 15 °C, we provide a measure of how access to littoral energy is controlled by temperature (19, 41). We did not use the existence of a planar thermocline to define summer, because this can occur when littoral temperatures are ≤15 °C and would not represent a physiological barrier for lake trout (41).
Habitat Use.
We monitored the depths of lake trout using acoustic telemetry. Telemetry data were collected from 41 individual fish (fork length 380–501 mm) implanted with depth-sensing transmitters (V16P-4L or V13P-1L; VEMCO Ltd.) that randomly transmitted a coded signal every 16–64 s (V16) or every 120–300 s (V13) (Tables S1 and S2 for transmitter and biological details). The depths of transmitter-implanted fish were monitored year-round using four or five omnidirectional hydrophone receivers (VR2; VEMCO Ltd.) with overlapping detection ranges distributed throughout the lake (19, 42).
Table S1.
Comparison of transmitter type specifications of transmitter types used in the study
| Transmitter type | Weight, g | Length, mm | Mean ping frequency, s | Ping frequency range, s | Mean burst duration, s | Burst duration range, s | Minimum ping frequency, s |
| V16P-1L | 12.6 | 80.0 | 40.5 | 15–64 | 2.80 | 2.00–4.60 | 17.00 |
| V13P-1L | 6.00 | 33.0 | 210.0 | 120–300 | 3.54 | 2.22–4.02 | 122.22 |
Ping frequency is how often a transmitter emits a signal with the transmitter identification number and the associated depth of the fish encoded for receivers to detect. Burst duration is how long the encoded pings take to emit. Minimum ping frequency therefore is the shortest possible ping frequency plus the minimum burst frequency.
Table S2.
Biological characteristics and data collection details of the 41 lake trout implanted with acoustic transmitters and monitored during the study period (2003–2013)
| Tag ID | Fork length, mm | Weight, g | Date implanted | Date end | Tag type |
| 11.0001 | 444 | 1,000 | 2002-05-14 | 2005-04-29 | V16P-4L |
| 20.0001 | 439 | 916 | 2002-05-14 | 2004-08-23 | V16P-4L |
| 18.0001 | 427 | 825 | 2002-05-16 | 2005-07-07 | V16P-4L |
| 19.0001 | 480 | 1,331 | 2002-05-16 | 2005-05-23 | V16P-4L |
| 12.0001 | 440 | 970 | 2002-05-21 | 2004-06-14 | V16P-4L |
| 13.0001 | 440 | 883 | 2002-05-21 | 2003-07-24 | V16P-4L |
| 14.0001 | 446 | 1,056 | 2002-05-22 | 2005-07-02 | V16P-4L |
| 15.0001 | 443 | 894 | 2002-05-22 | 2003-07-18 | V16P-4L |
| 16.0001 | 462 | 1,137 | 2002-05-22 | 2005-05-04 | V16P-4L |
| 129.001 | 394 | 700 | 2005-05-14 | 2005-09-27 | V13P-1L |
| 130.001 | 427 | 898 | 2005-05-14 | 2009-06-28 | V13P-1L |
| 131.001 | 427 | 759 | 2005-05-14 | 2010-05-31 | V13P-1L |
| 132.001 | 380 | 653 | 2005-05-14 | 2006-07-13 | V13P-1L |
| 133.001 | 417 | 822 | 2005-05-14 | 2009-11-14 | V13P-1L |
| 136.001 | 430 | 871 | 2005-05-14 | 2005-09-20 | V13P-1L |
| 157.001 | 418 | 816 | 2006-05-10 | 2008-06-25 | V13P-1L |
| 158.001 | 448 | 905 | 2006-05-10 | 2009-02-16 | V13P-1L |
| 159.001 | 408 | 858 | 2006-05-10 | Alive | V13P-1L |
| 160.001 | 420 | 767 | 2006-05-10 | 2008-10-22 | V13P-1L |
| 161.001 | 399 | 789 | 2006-05-11 | Alive | V13P-1L |
| 202.001 | 388 | 625 | 2007-05-10 | 2010-11-27 | V13P-1L |
| 203.001 | 448 | 998 | 2007-05-10 | 2010-11-28 | V13P-1L |
| 204.001 | 450 | 809 | 2007-05-10 | 2010-11-27 | V13P-1L |
| 219.001 | 419 | 746 | 2008-05-13 | 2012-01-13 | V13P-1L |
| 223.001 | 435 | 829 | 2008-05-13 | 2008-10-30 | V13P-1L |
| 224.001 | 429 | 829 | 2008-05-13 | 2012-01-12 | V13P-1L |
| 225.001 | 421 | 794 | 2008-05-13 | 2010-10-23 | V13P-1L |
| 226.001 | 465 | 1,029 | 2008-05-13 | 2012-01-13 | V13P-1L |
| 227.001 | 459 | 952 | 2008-05-13 | 2011–05-11 | V13P-1L |
| 228.001 | 445 | 925 | 2008-05-13 | 2009-05-26 | V13P-1L |
| 244.001 | 400 | 701 | 2009-05-11 | 2013-01-10 | V13P-1L |
| 250.001 | 501 | 1,018 | 2009-05-11 | 2009-08-17 | V13P-1L |
| 1.003 | 430 | 811 | 2011-05-20 | Alive | V13P-1L |
| 2.003 | 412 | 773 | 2011-05-20 | Alive | V13P-1L |
| 3.003 | 422 | 884 | 2011-05-20 | 2011-11-03 | V13P-1L |
| 4.003 | 401 | 733 | 2011-05-20 | Alive | V13P-1L |
| 5.002 | 396 | 727 | 2011-05-20 | Alive | V13P-1L |
| 227.002 | 426 | 879 | 2011-05-24 | 2012-01-26 | V13P-1L |
| 14.003 | 430 | 806 | 2012-05-15 | Alive | V13P-1L |
| 15.003 | 444 | 959 | 2012-05-15 | Alive | V13P-1L |
| 16.003 | 419 | 785 | 2012-05-15 | 2012-09-30 | V13P-1L |
Alive indicates the fish was alive at the end of the final study year (fall 2013). Date end is the date that the transmitter or fish was deemed dead, and data on or after this date were not used.
Raw telemetry data were filtered before analyses (details are given in Supporting Information and Figs. S2 and S3). The final telemetry dataset for estimation of spring, summer, and fall habitat use consisted of 1,979,775 detections (range 63,016–368,755 per year) from 29 individual fish (range two to nine fish per year). No telemetry data from 2005 were available, because all new transmitters were implanted that spring, and we did not use data from the year in which transmitters were implanted to avoid potential effects of tagging on behavior. Using data for each fish, we calculated daily estimates of (i) the number of forays into the littoral zone; (ii) the average duration of each littoral foray; and (iii) the total time spent in the littoral zone during the spring, summer, and fall of each open-water season. A littoral foray was denoted by a fish’s depth changing from ≥6 m to <6 m between consecutive detections. The total number of forays performed by an individual fish on a given day was then summed. Daily estimates of the total time spent in the littoral zone by each fish were calculated as the ratio of littoral zone detections to total detections scaled to a 24-h period. Daily estimates of average foray duration for each fish were then calculated as the total time spent in the littoral zone divided by the number of littoral forays on that day. We note that our method would categorize a lake trout moving into depths <6 m in offshore regions as a littoral foray, but we assumed all forays occurred in the nearshore region.
Fig. S2.
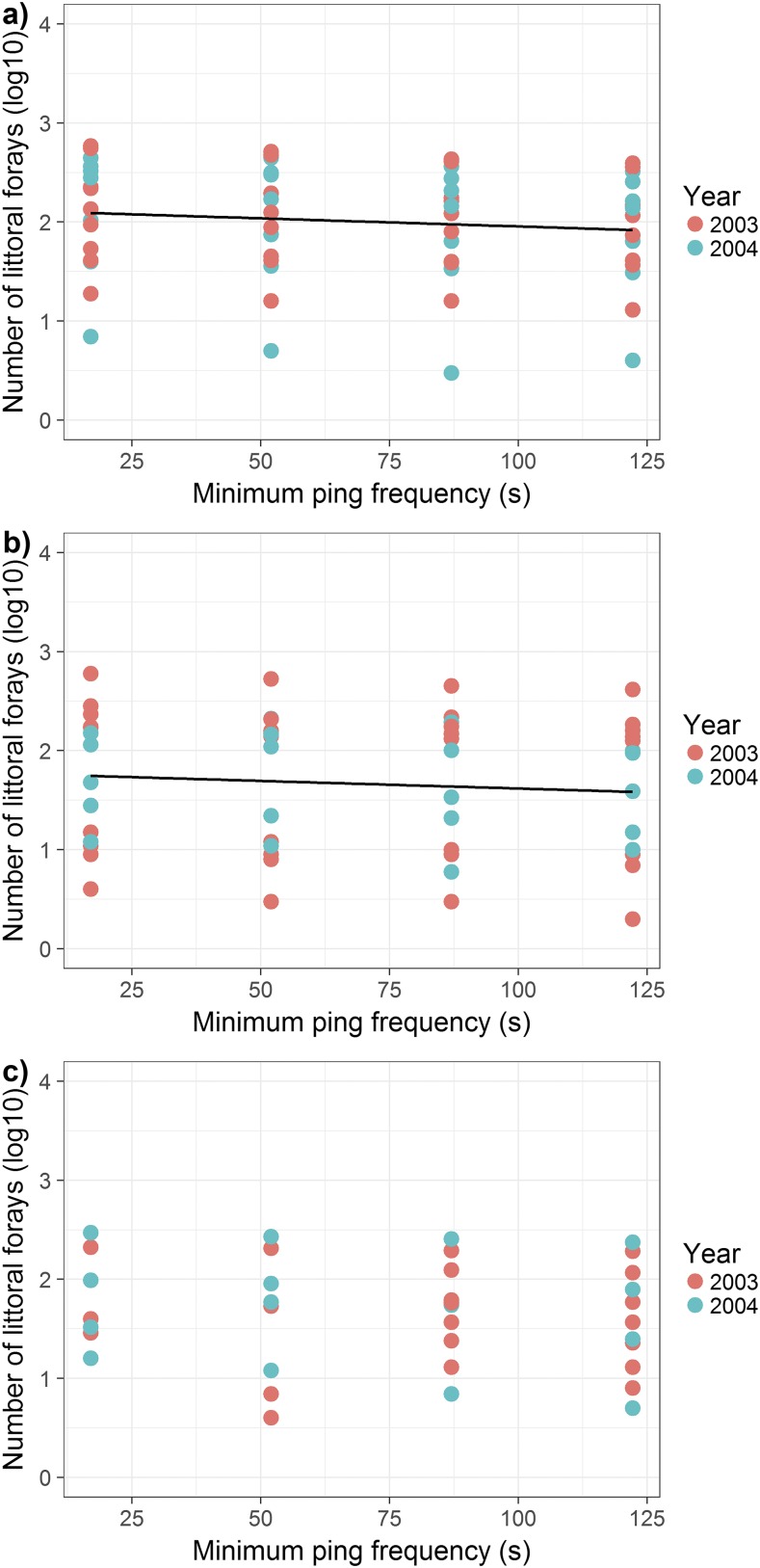
Relationship between minimum ping frequency and estimates of the number of forays by individual lake trout into the littoral zone of L373 during the (A) spring (n = 54), (B) summer (n = 46), and (C) fall (n = 36) periods of 2003 and 2004. Points represent estimates for individual fish in a given year at each minimum ping frequency. The slope of the relationship between minimum ping frequency and corresponding estimates of the number of littoral forays did not differ by year for either spring or summer, and estimates of the mean number of littoral forays also did not differ by year for either season. The best-fit equation in A was log(y) = −0.0016x + 2.13 and in B was log(y) = −0.0027x + 1.87, where x is the minimum ping frequency and y is the estimate of the number of littoral forays. For fall, the number of littoral forays estimated was not influenced by minimum ping frequency, year, or their interaction.
Fig. S3.
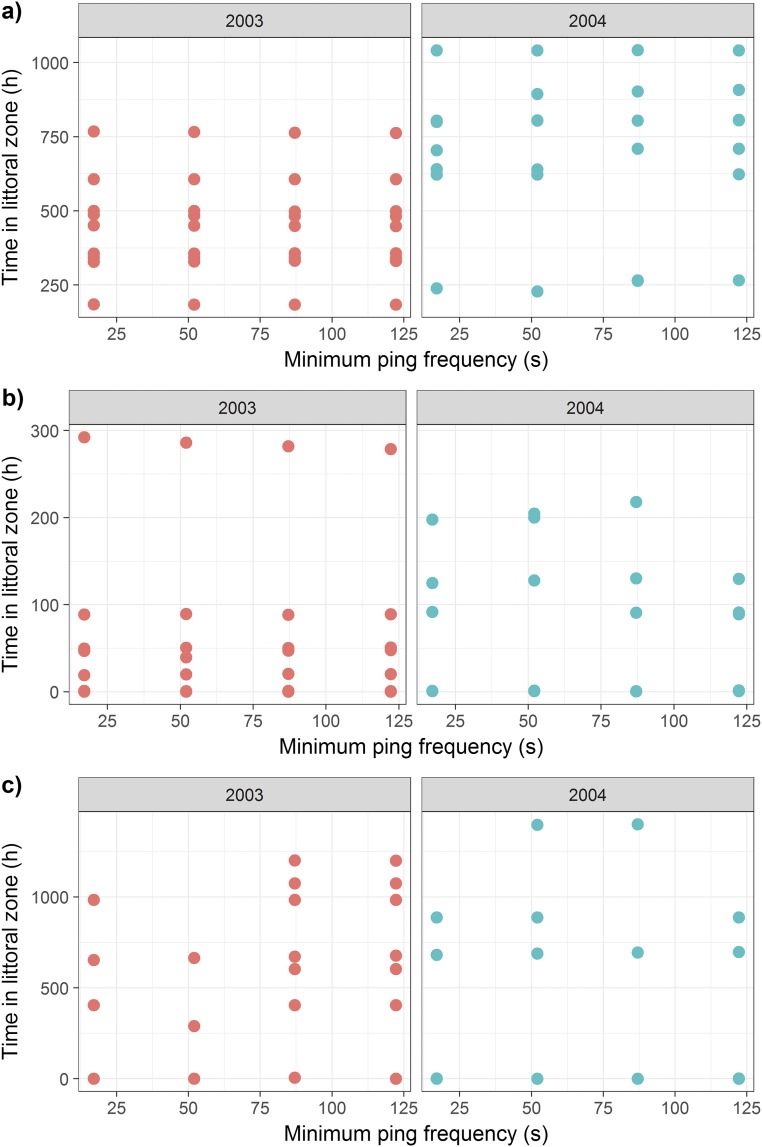
The amount of time (in hours) spent in the littoral zone by individual lake trout implanted with acoustic transmitters in L373 during the (A) spring, (B) summer, and (C) fall periods of 2003 (n = 20) and 2004 (n = 16) under various minimum ping frequencies. Estimates of the time spent in the littoral zone were not influenced by the minimum ping frequency of transmitters for any season but did differ by year for spring and summer, but not for fall.
Energy Sources.
We used stable carbon (δ13C) isotopes to estimate use of littoral- vs. pelagic-derived energy by lake trout (35). Pectoral fin-ray tips (lake trout) and dorsal muscle (littoral prey fish) were collected each fall. Mysis and bulk zooplankton were collected monthly during each open-water season. Samples were dried, ground, loaded into tin capsules, and analyzed using standard methods at the University New Brunswick or the University of Waterloo. Stable isotope values were conveyed in δ notation (‰): δ13C = [(13C/12C sample/13C/12C standard) − 1] × 1,000.
The δ13C values of lake trout fins were first corrected to equivalent muscle values using the equation δ13Cmuscle = 0.73 × δ13CFin − 8.11 (43). We mathematically lipid-corrected the δ13C values of prey fish and Mysis using δ13Cmuscle (normalized) = δ13Cmuscle + [−3.32 + (0.99 × C:N)] (44). The correction was not applied to lake trout because lipids should not be an issue for fin tissue. From 2003–2005, stable isotope data for prey fish were not available; in these years we estimated the δ13C values of prey fish by adding the mean difference in δ13C values of prey fish and littoral mayflies during years when both these items were analyzed to the mean values of littoral mayflies. We note that lake trout fin-ray tips have been found to turn over at similar rates to white muscle (43). We determined the proportion of littoral energy assimilated by each lake trout using a two-source mixing model that treated Mysis and littoral prey fish as the pelagic and littoral end members, respectively (45): proportion littoral energy = (δ13Clake trout − δ13CMysis)/(δ13Cprey fish − δ13CMysis). The lake trout δ13C dataset contained 194 samples (n = 15–20 per year) with fork lengths ranging from 199–502 mm.
A small number of lake trout stomach contents obtained from nonlethal gastric lavages were collected in the spring (23 and 28 May, n = 14) and summer (12 August, n = 4) of 2014 and from fall mark–recapture sampling mortalities (various dates between 1–21 October 1986–2012, n = 24) to support stable isotopes. Prey items from each stomach were identified and grouped into the following groups: benthic invertebrates and insects, prey fish, Mysis, and zooplankton. We then calculated the proportion of the total stomach content weight for each diet item.
Growth and Condition.
Lake trout growth was examined using data from annual mark–recapture sampling. Fish were captured each fall using trap nets and short (<30 min) evening gill net sets on spawning shoals (46, 47). Following capture, the weights, fork lengths (in millimeters), and tag numbers from previously captured fish were recorded. Newly captured fish received a tag for future identification. Condition was estimated as a percentage of standard weight specific for lake trout (48). We identified 141 instances in which an individual fish was captured in consecutive years during the study period.
Prey Fish Abundance.
The relative abundance of littoral prey fish were calculated as CPUE based on annual collections (46, 49). Each spring, two or three small-diameter mesh trap nets were set for 27–39 consecutive days to target the nearshore-littoral zone. Annual CPUE estimates were calculated by dividing the total number of prey fish by the number of net days (i.e., number of trap nets × number of fishing days). We included all cyprinid species and slimy sculpin in putative littoral prey fish CPUE values.
Statistical Analysis.
Analyses were carried out in R, v. 3.3.2 (50). Assumptions of linear mixed effect models (LMMs) and linear regression were evaluated (51, 52). Where necessary, data were transformed to meet assumptions as reported in Results. LMMs were fit using the nlme package (53), and fits were evaluated (51, 52). We calculated least squares means for each factor level in LMMs using the lsmeans package (54). Because of the small sample size (11 y), we considered P < 0.1 as statistically significant.
We analyzed habitat use data from spring, summer, and fall seasons separately. LMMs were applied to each habitat measure (number of littoral forays, average foray duration, and total time in littoral zone) over all years for each season, for a total of nine LMMs, which treated year as a fixed factor and individual fish as random intercepts. Mean estimates (±SD) of habitat use for each year/season were obtained using the least squares means from each LMM and were subsequently used as response variables in linear regression to test if each seasonal habitat use measure was predicted by the corresponding season length, prey fish CPUE, length of the preceding season (summer and fall only), and mean summer littoral zone temperatures (summer only).
Mean estimates of the proportion of littoral energy assimilated for each study year were obtained by calculating means (±SD) over all individual lake trout analyzed for δ13C within each year. The linear regression procedure described above for habitat use then was used to test if the mean proportion of littoral energy was predicted by season length (spring or summer), prey fish CPUE, and summer littoral zone water temperatures. Isotope data were not related to fall length, because samples were collected near the start of the fall in each year.
We determine how variations in littoral energy and habitat use translated to growth by treating annual changes in individual lake trout weight and condition as response variables in separate linear regressions with spring–summer littoral energy use, spring and summer littoral habitat use, and the length of the spring, summer, and winter periods used a predictor variables. Fall habitat use or fall length was not used as a predictor for annual growth because fish collections occurred throughout this period.
SI Preparation of Telemetry Data
Before analysis we filtered raw telemetry data to remove dead fish, malfunctioning tags, detections outside the depth range of the lake, and multiple/false detections. Because transmitters were implanted during the spring, data from the year a fish was tagged were excluded to avoid potential effects of tagging on behavior (55). Only fish that had data for an entire year from their date of tagging were included in the final analyses. The two types of telemetry transmitters used over the course of this 11-y study differed in how frequently they transmitted coded signals (i.e., “pings”) (V16: 15–64 s, V13: 120–300 s) (Table S1). Both transmitter types recorded pressure, which was converted to an instantaneous depth, and emitted this information, along with individual fish identification, at random intervals within a prescribed time period. When a transmission was detected by a receiver, it was given a time stamp. The V16 transmitters were implanted in spring 2002 and were active for 1–4 y (Table S2). In spring 2005 we began using V13 transmitters and continued to do so for the duration of the study (Table S2). Because V16 transmitters emitted pings about five times more frequently than V13 transmitters, on average, the probability that a V16 transmitter would ping and be detected during a quick foray by a lake trout into the littoral zone was, by chance, greater than that of the slower-pinging V13 transmitters. A difference in the probability of detecting a fish in the littoral zone because of transmitter type would bias among-year comparisons of telemetry data in this 11-y record. Therefore, to remove any potential bias, we needed to “thin” detection data of fish implanted with V16 transmitters so that their detection frequencies were comparable to those of the V13 transmitters. To do so, we removed detections from V16 data with intervals (i.e., time difference between subsequent detections of that transmitter) that were less than the minimum ping frequency of V13 transmitters (122.22 s) (Table S1). To thin V16 data, we used a function written in the R statistical computing package that calculated the transmission intervals for all detections for each fish in a given year and then removed the first (chronologically) detection that had a transmission interval less than a set time of 122.22 s. This process was repeated until the new dataset contained only detections with intervals less than 122.22 s.
To test if increasing minimum ping frequencies resulted in underestimates of littoral habitat use, we used data collected from lake trout implanted with V16 transmitters in 2003 and 2004, previously reported as a “warm” and “cool” years, respectively (19), for comparison of a range of littoral use. First, all dead fish/transmitters and multiple detections were removed (when the same ping was detected by multiple receivers, only the first detection was kept). Next, we created four separate V16 detection datasets with varying minimum ping frequencies for each year of data for each fish (i.e., a fish with data for both 2003 and 2004 would have eight separate detection datasets). The four datasets for each year for each fish included (i) detection data that were not thinned (minimum ping frequency of the V16 transmitters: 17 s) and detection data that were thinned to minimum ping frequencies of (ii) 52 s, (iii) 87 s, and (iv) 122.22 s, in which 52 s and 87 s represent midpoints of the minimum ping frequencies of raw V16 (17 s) and V13 (122.22 s) transmitters. Having multiple detection datasets with differing minimum ping frequencies allowed us to determine if our estimates of the number of forays into and total time spent in the littoral zone by lake trout each year were altered by the minimum ping frequency of the transmitters used and to apply corrections as needed. Datasets then were divided into spring and summer, based on the criteria described in the main text. Hypotheses were tested separately for each season using LMMs with individual fish treated as random intercepts to account for repeated measures and minimum ping frequency treated as a continuous fixed factor (52). Assumptions of LMM were tested using q-q plots, boxplots, and scatter plots of normalized residuals (51, 52). Where necessary, data transformations were used to meet assumptions and are provided in Results.
SI Results
Number of Littoral Forays.
The numbers of littoral forays in both spring and summer were log10 transformed to meet assumptions of LMM. For spring data, we found that the estimated number of littoral forays decreased linearly as minimum ping frequencies increased (minimum ping frequency: F1,54 = 5.34, P = 0.02). The slopes (minimum ping frequency × year: F1,52 = 0.28, P = 0.60) and intercepts (year: F1,53 = 0.52, P = 0.47) of the relationship between the number of littoral forays and minimum ping frequency did not differ among years (Fig. S2A). Similarly, for summer data, we also found that the estimated number of forays decreased linearly as minimum ping frequencies lengthened (minimum ping frequency: F1,46 = 4.48, P = 0.03). The slopes (minimum ping frequency × year: F1,44 = 1.89, P = 0.18) and intercepts (year: F145 = 0.01, P = 0.96) of the relationship between the number of littoral forays and minimum ping frequency did not differ among years (Fig. S2B). The spring and summer analyses supported our hypothesis that when transmitters pinged more often, they had a higher probability of transmitting while a fish was in the littoral zone. However, for fall, we did not find any effect of minimum ping frequency (minimum ping frequency: F1,26 = 1.53, P = 0.23), year (year: F1,28 = 0.02, P = 0.90), or their interaction (minimum ping frequency × year: F1,28 = 0.01, P = 0.93) on the estimated number of littoral forays.
To determine the degree to which the number of forays was underestimated by increasing minimum ping frequencies during the spring and summer, we used the models described in the paragraph above to predict the average number of littoral forays that would be detected at minimum ping frequencies of 17, 52, 87, and 122.22 s. Assuming that a minimum ping frequency of 0 (model intercept) meant that a transmitter was pinging continuously, we calculated the percent difference between the modeled number of littoral forays of minimum ping frequencies of 17 s, 52 s, 87 s, and 122.22 s with that of the intercept (Table S3). Most important to our study, we found that a minimum ping frequency of 122.22 s (that of V13 transmitters) underestimated the number of forays by ≈63% in spring and by 86% in summer. Therefore, we adjusted our estimates of the number of spring and summer littoral forays based on tags with minimum ping frequencies of 122.22 s by 63% and 86%, respectively.
Table S3.
The extent to which the number littoral forays by acoustically tagged lake trout during spring and summer were underestimated because of transmitter minimum ping frequency
| Underestimate of littoral forays, % | |||
| Data type | Minimum ping frequency, s | Spring | Summer |
| Intercept | 0.00 | 0 | 0 |
| Raw V16 | 17.00 | 12 | 22 |
| Thinned | 52.00 | 33 | 54 |
| Thinned | 87.00 | 50 | 74 |
| Thinned | 122.22 | 63 | 86 |
Differences were calculated using the slope estimate of the model in Fig. S1, and the percent underestimated was calculated using the estimated change in the number of forays at each minimum ping frequency relative to the intercept. For raw V16 data, only multiple detections were removed (i.e., successive detections with timestamp differences <17 s). For thinned data, all detections with timestamps less than 52 s, 87 s, or 122.22 s were removed. Underestimates for fall are not provided because minimum ping frequency did not influence the number of forays estimated during this season.
Time Spent in the Littoral Zone.
The total time spent within the littoral zone each spring and summer was square root transformed before analysis to meet assumptions of LMM. For spring, we found that neither the interaction between minimum ping frequency and year (minimum ping frequency × year: F1,52 = 0.10, P = 0.75) nor minimum ping frequency alone (minimum ping frequency: F1,53 = 0.06, P = 0.80) had an effect on our estimates of time spent in the littoral zone. We did, however, find that estimates of littoral zone use differed among years (year: F1,54 = 58.84, P < 0.001) (Fig. S3). Similarly, for summer, we found that neither the interaction between minimum ping frequency and year (minimum ping frequency × year: F1,44 = 2.36, P = 0.13) nor minimum ping frequency alone (minimum ping frequency: F1,45 = 1.02, P = 0.32) had an effect on our estimates of time spent in the littoral zone. We did, however, find that estimates of littoral zone use differed among years (year: F1,46 = 17.76, P < 0.001) (Fig. S3). For fall, we found that the interaction between minimum ping frequency and year (minimum ping frequency × year: F1,27 = 1.28, P = 0.27), the year (year: F1,29 = 0.01, P = 0.94), and the minimum ping frequency (minimum ping frequency: F1,29 = 1.37, P = 0.25) all had no effect on our estimates of time spent in the littoral zone (Fig. S3). The fact that estimates of time spent in the littoral zone with a minimum ping frequency of 17 s (which is quite rapid) did not differ from those at 122.22 s provides confidence in our using minimum ping frequencies of 122.22 s in our estimates.
Acknowledgments
We thank M. Paterson, S. Schiff, and K. Kidd for contributing stable isotope data; L. Hrenchuk and L. Tate for maintaining telemetry data; A. Chapelsky and C. Rogers for help preparing stable isotope samples; K. Mills and S. Chalanchuk for collecting and maintaining the fish population data; staff and students over the years for field support; and two anonymous reviewers and M. Paterson for comments that improved this paper. Funding was provided by the Natural Sciences and Engineering Research Council of Canada, the W. Garfield Weston Foundation, Manitoba Fish Futures, the University of Manitoba, DeBeers Canada, Fisheries and Oceans Canada, and the IISD-Experimental Lakes Area.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the open source database Zenodo at https://doi.org/10.5281/zenodo.832921. Additional data are available upon request from the IISD-Experimental Lakes Area (https://www.iisd.org/ela/science-data/our-data/data-requests/).
See Commentary on page 9764.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702584114/-/DCSupplemental.
References
- 1.International Panel on Climate Change 2014 Fifth assessment report: Synthesis report. Available at www.ipcc.ch/report/ar5/syr/. Accessed November 5, 2015.
- 2.Schmitz OJ, Post E, Burns CE, Johnston KM. Ecosystem responses to global climate change : Moving beyond color mapping. Bioscience. 2003;53:1199–1205. [Google Scholar]
- 3.Tunney TD, McCann KS, Lester NP, Shuter BJ. Effects of differential habitat warming on complex communities. Proc Natl Acad Sci USA. 2014;111:8077–8082. doi: 10.1073/pnas.1319618111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMeans B, et al. The adaptive capacity of lake food webs: From individuals to ecosystems. Ecol Monogr. 2016;86:4–19. [Google Scholar]
- 5.Murakami M, Nakano S. Indirect effect of aquatic insect emergence on a terrestrial insect population through predation by birds. Ecol Lett. 2002;5:333–337. [Google Scholar]
- 6.Schindler DE, Scheuerell MD. Habitat coupling in lake ecosystems. Oikos. 2002;98:177–189. [Google Scholar]
- 7.Kondoh M. Foraging adaptation and the relationship between food-web complexity and stability. Science. 2003;299:1388–1391. doi: 10.1126/science.1079154. [DOI] [PubMed] [Google Scholar]
- 8.Post DM, Conners ME, Goldberg DS. Prey preference by a top preator and the stability of linked food chains. Ecology. 2000;81:8–14. [Google Scholar]
- 9.Rooney N, McCann K, Gellner G, Moore JC. Structural asymmetry and the stability of diverse food webs. Nature. 2006;442:265–269. doi: 10.1038/nature04887. [DOI] [PubMed] [Google Scholar]
- 10.Pörtner HO, Knust R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science. 2007;315:95–97. doi: 10.1126/science.1135471. [DOI] [PubMed] [Google Scholar]
- 11.Fry F. Effects of the environment on animal activity. Publ Ontario Fish Res Lab. 1947;55:1–62. [Google Scholar]
- 12.Magnuson JJ, Crowder LB, Medvick PA. Temperature as an ecological resource. Integr Comp Biol. 1979;19:331–343. [Google Scholar]
- 13.Fry FEJ. A comparative study of lake trout fisheries in Algonquin Park, Ontario. Publ Ontario Fish Res Lab. 1939;46:1–69. [Google Scholar]
- 14.Martin NV. A study of the lake trout, Salvelinus namaycush, in two Algonquin Park, Ontario, lakes. Trans Am Fish Soc. 1952;81:111–137. [Google Scholar]
- 15.O’Reilly CM, et al. Rapid and highly variable warming of lake surface waters around the globe. Geophys Res Lett. 2015;42:1–9. [Google Scholar]
- 16.Guzzo MM, Blanchfield PJ. Climate change alters the quantity and phenology of habitat for lake trout (Salvelinus alpinus) Can J Fish Aquat Sci. 2017;74:871–884. [Google Scholar]
- 17.Dolson R, McCann KS, Rooney N, Ridgway MS. Lake morphometry predicts the degree of habitat coupling by a mobile predator. Oikos. 2009;118:1230–1238. [Google Scholar]
- 18.Vander Zanden MJ, Rasmussen J. A trophic position model of pelagic food webs: Impact on contaminant bioaccumulation in lake trout. Ecol Monogr. 1996;66:451–477. [Google Scholar]
- 19.Plumb JM, Blanchfield PJ. Performance of temperature and dissolved oxygen criteria to predict habitat use by lake trout (Salvelinus namaycush) Can J Fish Aquat Sci. 2009;66:2011–2023. [Google Scholar]
- 20.Edmunds NB, Laberge F, McCann KS. A role for brain size and cognition in food webs. Ecol Lett. 2016;19:948–955. doi: 10.1111/ele.12633. [DOI] [PubMed] [Google Scholar]
- 21.Vadeboncoeur Y, McCann KS, Vander Zanden MJ, Rasmussen JB. Effects of multi-chain omnivory on the strength of trophic control in lakes. Ecosystems (N Y) 2005;8:682–693. [Google Scholar]
- 22.Pazzia I, Trudel M, Ridgway M, Rasmussen JB. Influence of food web structure on the growth and bioenergetics of lake trout (Salvelinus namaycush) Can J Fish Aquat Sci. 2002;59:1593–1605. [Google Scholar]
- 23.Kaufman SD, Gunn JM, Morgan GE, Couture P. Muscle enzymes reveal walleye (Sander vitreus) are less active when larger prey (cisco, Coregonus artedi) are present. Can J Fish Aquat Sci. 2006;63:970–979. [Google Scholar]
- 24.Morbey YE, Couture P, Busby P, Shuter BJ. Physiological correlates of seasonal growth patterns in lake trout Salvelinus namaycush. J Fish Biol. 2010;77:2298–2314. doi: 10.1111/j.1095-8649.2010.02804.x. [DOI] [PubMed] [Google Scholar]
- 25.Cruz-font L, Shuter BJ, Blanchfield PJ. Energetic costs of activity in wild Lake Trout: A calibration study using acceleration transmitters and positional telemetry. Can J Fish Aquat Sci. 2016;73:1–14. [Google Scholar]
- 26.Rennie MD, Collins NC, Shuter BJ, Rajotte JW, Couture P. A comparison of methods for estimating activity costs of wild fish populations: More active fish observed to grow slower. Can J Fish Aquat Sci. 2005;62:767–780. [Google Scholar]
- 27.Plumb JM, Blanchfield PJ, Abrahams MV. A dynamic-bioenergetics model to assess depth selection and reproductive growth by lake trout (Salvelinus namaycush) Oecologia. 2014;175:549–563. doi: 10.1007/s00442-014-2934-6. [DOI] [PubMed] [Google Scholar]
- 28.Morbey YE, Addison P, Shuter BJ, Vascotto K. Within-population heterogeneity of habitat use by lake trout Salvelinus namaycush. J Fish Biol. 2006;69:1675–1696. [Google Scholar]
- 29.Sellers TJ, Parker BR, Schindler DW, Tonn WM. Pelagic distribution of lake trout (Salvelinus namaycush) in small Canadian shield lakes with respect to temperature, dissolved oxygen, and light. Can J Fish Aquat Sci. 1998;55:170–179. [Google Scholar]
- 30.Breau C, Cunjak RA, Peake SJ. Behaviour during elevated water temperatures: Can physiology explain movement of juvenile Atlantic salmon to cool water? J Anim Ecol. 2011;80:844–853. doi: 10.1111/j.1365-2656.2011.01828.x. [DOI] [PubMed] [Google Scholar]
- 31.Biro PA. Staying cool: Behavioral thermoregulation during summer by young-of-year brook trout in a lake. Trans Am Fish Soc. 1998;127:212–222. [Google Scholar]
- 32.Sitar SP, Jasonowicz AJ, Murphy CA, Goetz FW. Estimates of skipped spawning in lean and Siscowet lake trout in southern Lake Superior: Implications for stock assessment. Trans Am Fish Soc. 2014;143:660–672. [Google Scholar]
- 33.Morbey Y, Shuter B. Intermittent breeding in the absence of a large cost of reproduction: Evidence for a non-migratory, iteroparous salmonid. Ecosphere. 2013;4:1–18. [Google Scholar]
- 34.Trippel EA. Relations of fecundity, maturation, and body size of lake trout, and implications for management in northwestern Ontario lakes. N Am J Fish Manage. 1993;13:64–72. [Google Scholar]
- 35.France RL, Steedman R. Energy provenance for juvenile lake trout in small Canadian shield lakes as shown by stable isotopes. Trans Am Fish Soc. 1996;125:512–518. [Google Scholar]
- 36.Trippel EA, Beamish FWH. Multiple trophic level structuring in Salvelinus-coregonus assemblages in boreal forest lakes. Can J Fish Aquat Sci. 1993;50:1442–1455. [Google Scholar]
- 37.King JR, Shuter BJ, Zimmerman AP. Empirical links between thermal habitat, fish growth, and climate change. Trans Am Fish Soc. 1999;128:656–665. [Google Scholar]
- 38.Blanchfield PJ, Paterson MJ, Shearer JA, Schindler DW. Johnson and Vallentyne’s legacy: 40 years of aquatic research at the Experimental Lakes Area. Can J Fish Aquat Sci. 2009;66:1831–1836. [Google Scholar]
- 39.Sandstrom S, Rawson M, Lester N. 2013. Manual of Instructions for Broad-Scale Fish Community Monitoring Using North American (NA1) and Ontario Small Mesh (ON2) Gillnets (Ontario Ministry of Natural Resources, Peterborough, ON, Canada). Version 2013.2 35 p. + appendices.
- 40.Muir AM, Blackie CT, Marsden JE, Krueger CC. Lake charr Salvelinus namaycush spawning behaviour: New field observations and a review of current knowledge. Rev Fish Biol Fish. 2012;22:575–593. [Google Scholar]
- 41.Evans DO. Effects of hypoxia on scope-for-activity and power capacity of lake trout (Salvelinus namaycush) Can J Fish Aquat Sci. 2007;64:345–361. [Google Scholar]
- 42.Blanchfield PJ, Tate LS, Plumb JM, Acolas M-L, Beaty KG. Seasonal habitat selection by lake trout (Salvelinus namaycush) in a small Canadian shield lake: Constraints imposed by winter conditions. Aquat Ecol. 2009;43:777–787. [Google Scholar]
- 43.Wellman S, Kidd KA, Podemski CL, Blanchfield PJ, Paterson MJ. Incorporation of wastes by native species during and after an experimental aquaculture operation. Freshw Sci. 2017;36:387–401. [Google Scholar]
- 44.Post DM, et al. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia. 2007;152:179–189. doi: 10.1007/s00442-006-0630-x. [DOI] [PubMed] [Google Scholar]
- 45.Vander Zanden MJ, Vadeboncoeur Y. Fishes as integrators of benthic and pelagic food webs in lakes. Ecology. 2002;83:2152–2161. [Google Scholar]
- 46.Mills KH, Chalanchuk SM, Mohr LC, Davies IJ. Responses of fish populations in lake 223 to 8 years of experimental acidification. Can J Fish Aquat Sci. 1987;44:114–125. [Google Scholar]
- 47.Mills KH, Chalanchuk SM, Allan DJ. Abundance, annual survival,and recruitment of unexploited and exploited lake charr, Salvelinus namaycush, populations at the experimental lakes area, northwestern Ontario. Environ Biol Fishes. 2002;64:281–292. [Google Scholar]
- 48.Piccolo JJ, Hubert WA, Whaley RA. Standard weight equation for lake trout. N Am J Fish Manage. 1993;13:401–404. [Google Scholar]
- 49.Guzzo MM, Rennie MD, Blanchfield PJ. Evaluating the relationship between mean catch per unit effort and abundance for littoral cyprinids in small boreal shield lakes. Fish Res. 2014;150:100–108. [Google Scholar]
- 50.R Core Team 2016 R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna). Available at https://www.r-project.org/. Accessed December 17, 2016.
- 51.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol. 2010;1:3–14. [Google Scholar]
- 52.Zuur AF, Leno EN, Walker NJ, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. Springer; New York: 2009. [Google Scholar]
- 53.Pinheiro J, Bates D, DebRoy S, Sarkar D. R Core Team 2017. nlme: Linear and nonlinear mixed effects models (R Foundation for Statistical Computing, Vienna), Version 3.1-131.
- 54.Lenth RV. Least-squares means: The R package lsmeans. J Stat Softw. 2016;69:1–33. [Google Scholar]
- 55.Rogers KB, White GC. Analysis of movement and habitat use from telemetry data. In: Guy CS, Brown ML, editors. Analysis and Interpretation of Freshwater Fisheries Data. American Fisheries Society; Bethesda: 2007. pp. 625–676. [Google Scholar]