Significance
An in vivo chemical screen in zebrafish identified glucocorticoids (GCs) as activators of hypoxia-inducible factor transcriptional responses in the liver. This cross-talk is conserved in human liver and requires glucocorticoid receptor signaling but not DNA binding. In human liver cells, GCs down-regulate Von Hippel Lindau expression at a posttranscriptional level most likely through c-src–mediated proteasomal degradation. Since the liver is an important regulator of blood glucose and hypoxia-inducible factors regulate gluconeogenesis/glycogen synthesis, cross-talk between these transcriptional regulators may be essential to control glucose metabolism in the liver. This identified, conserved, noncanonical pathway may have wider physiological significance in health and disease.
Keywords: hypoxia-inducible factor, glucocorticoid signaling, Von Hippel Lindau, metabolism, liver
Abstract
Glucocorticoid (GC) and hypoxic transcriptional responses play a central role in tissue homeostasis and regulate the cellular response to stress and inflammation, highlighting the potential for cross-talk between these two signaling pathways. We present results from an unbiased in vivo chemical screen in zebrafish that identifies GCs as activators of hypoxia-inducible factors (HIFs) in the liver. GCs activated consensus hypoxia response element (HRE) reporters in a glucocorticoid receptor (GR)-dependent manner. Importantly, GCs activated HIF transcriptional responses in a zebrafish mutant line harboring a point mutation in the GR DNA-binding domain, suggesting a nontranscriptional route for GR to activate HIF signaling. We noted that GCs increase the transcription of several key regulators of glucose metabolism that contain HREs, suggesting a role for GC/HIF cross-talk in regulating glucose homeostasis. Importantly, we show that GCs stabilize HIF protein in intact human liver tissue and isolated hepatocytes. We find that GCs limit the expression of Von Hippel Lindau protein (pVHL), a negative regulator of HIF, and that treatment with the c-src inhibitor PP2 rescued this effect, suggesting a role for GCs in promoting c-src–mediated proteosomal degradation of pVHL. Our data support a model for GCs to stabilize HIF through activation of c-src and subsequent destabilization of pVHL.
Glucocorticoids (GCs) are steroid hormones secreted from the adrenal glands that regulate carbohydrate, lipid, and protein metabolism. GCs are widely used as anti-inflammatory agents for treating pathological conditions where hypoxia plays a role in disease progression such as rheumatoid arthritis and chronic obstructive pulmonary disease. GCs and hypoxia pathways have a close interplay in physiology and disease (1–3); however, recent studies report conflicting results on the cross-talk between GC action and hypoxia (4, 5). Hypoxia-inducible factors (HIFs) are oxygen-sensitive transcriptional complexes constituted by α- and β-subunits that activate diverse pathways regulating cellular glucose and lipid metabolism and proliferation (6, 7). Under normoxic conditions, the HIF-1α transcriptional subunit is recognized by prolyl hydroxylases and targeted for degradation via the Von Hippel Lindau (VHL)-mediated ubiquitin proteasome pathway; however, under hypoxic conditions HIF-1α is stabilized and translocates to the nucleus to exert its transcriptional activity. HIFs play a central role in many disease processes and provide a therapeutic target for treating pathological conditions including cancer, ischemia, stroke, inflammation, and chronic anemia (8–11). Screens to identify agents that stabilize HIFs have identified numerous agents, with the majority acting either via iron chelation or as 2-oxyglutarate analogs (12). In vitro HIF-reporter screening methods, although extremely valuable, do not provide physiological information and may overlook tissue-specific activators that require a physiological context.
To identify regulators of the HIF pathway, we developed several HIF-reporter zebrafish lines (13) and completed an unbiased chemical screen. GCs activated HIF-associated transcriptional responses most prominently in the liver. Importantly, we translate these observations to human tissue and show that GCs stabilize HIF in primary human hepatocytes and intact liver slices. Our data support a model where GCs act via a transcriptional independent mechanism by activating c-src to repress Von Hippel Lindau (pVHL) expression and stabilize HIF protein under normoxic conditions. Our study identifies a role for GCs to stabilize HIF and to regulate liver glucose metabolism.
Results
GCs Activate Hypoxic Signaling.
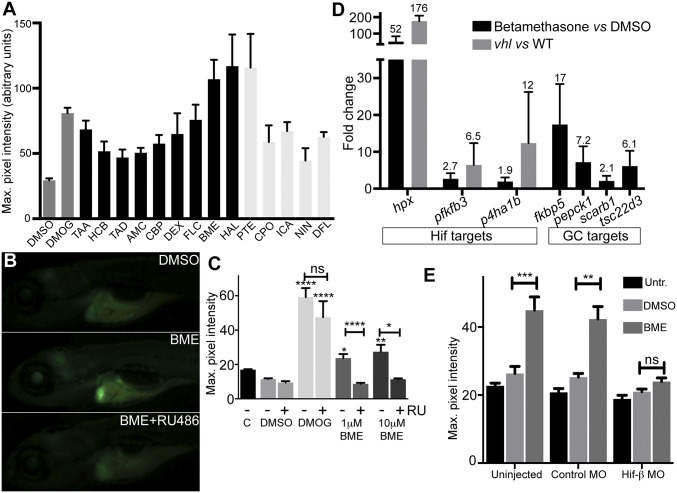
Prolyl hydroxylase 3 (PHD3) transcription is regulated by HIFs, and we used our zebrafish phd3:eGFP HIF-reporter line to screen a chemical library (Dataset S1) for HIF activators. Dimethyloxaloylglycine (DMOG), a well-established activator of HIF signaling, was used as a positive control (14). Of the 41 initial hits, several GCs activated the reporter to comparable levels as DMOG (60 µM) (Fig. 1A). We confirmed that two GR agonists, betamethasone 17,21-dipropanoate (BME) and dexamethasone (DEX), activated the reporter (Fig. 1 B and C). We noted increased phd3-GFP expression mainly in the liver (Fig. 1B), suggesting that activation may be due to nonspecific stress as result of drug metabolism. To address this potential issue, reporter embryos were treated with GCs in the presence or absence of the GR antagonist RU-486 that reduced GFP intensity of BME-treated embryos (Fig. 1 B and C), while having minimal effect on DMOG-dependent activation (Fig. 1C), demonstrating that GC-augmented phd3:eGFP activity is GR-dependent.
Fig. 1.
Identification of glucocorticoids as HIF activators. (A) Retest of hits from the Spectrum screen for activators of the HIF response in phd3:eGFP zebrafish, where compounds showing an average GFP level of >44 are shown. Averaged maximum pixel intensity and SEM are shown for between five and nine embryos. Dark gray: controls; black: GC-like compounds; light gray: non-GC hits. AMC, amcinonide; BME, betamethasone; CBP, clobetasol propionate; CPO, ciclopirox olamine; DEX, dexamethasone; DFL, 8,2-dimethoxyflavone; FLC, fluocinonide; HAL, halcinonide; HCB, hydrocortisone butyrate; ICA, icariin; NIN, 7-nitroindazole; PTE, ptaeroxylin; TAA, triamcinolone acetonide; TAD, triamcinolone diacetate. (B) BME activates phd3:eGFP in an RU-486–dependent manner; representative images show the activation specific to the liver in BME-treated embryos and reduced GFP expression following BME+RU-486 cotreatment. (Magnification: 16×.) (C) Quantitation of phd3:eGFP response. DMSO was added to 1% vol/vol in all experiments except Control (C), DMOG to 60 μM, and RU-486 to 5 μM as indicated (n ≥ 9). (D) BME induction of hypoxia-regulated genes and GC target genes, where values above bars indicate average fold change (FC) and triplicate samples of 20 embryos were used. For the hypoxia-induced genes, FC induction in vhl mutants vs. wild type is given as a comparison, where FC is calculated using ΔΔCT ± SEM. (E) HIF1β-MO abrogates BME activation of phd3:eGFP. Maximum pixel intensity in embryos as grouped by injection showing mean ± SEM (n = 14). Significance was calculated using Kruskal–Wallis one-way ANOVA followed by Dunn’s multiple comparison test for treatments against DMSO (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
To characterize the effects of BME on endogenous hypoxia-associated transcriptional responses, we quantified selected HIF-responsive genes (pfkfb3, p4ha1b, and hpx) and GR-responsive genes (tsc22d3, scarb1, fkbp5, and pepck1) (15–17) by qPCR. phd3:eGFP embryos [5 days postfertilization (dpf)] were treated with BME (10 μM) for 8 h before isolating RNA, the optimal time for phd3 induction. (Fig. S1). BME induced the transcription of HIF-target genes to variable extents, albeit less than in a vhl mutant background, with hpx being the most responsive gene (Fig. 1D). To determine whether BME activation of phd3:eGFP reporter is HIF dependent, we silenced Hif-1β expression, an essential binding partner of both HIF-1α and HIF-2α (18). Hif-1β morphants were incubated with BME from 56 h postfertilization (hpf) until 72 hpf, and HIF-1β knockdown significantly reduced GFP intensity in the treated embryos compared with control morpholino (MO)-injected embryos (Fig. 1E), demonstrating that GC activation of phd3:eGFP is HIF-dependent.
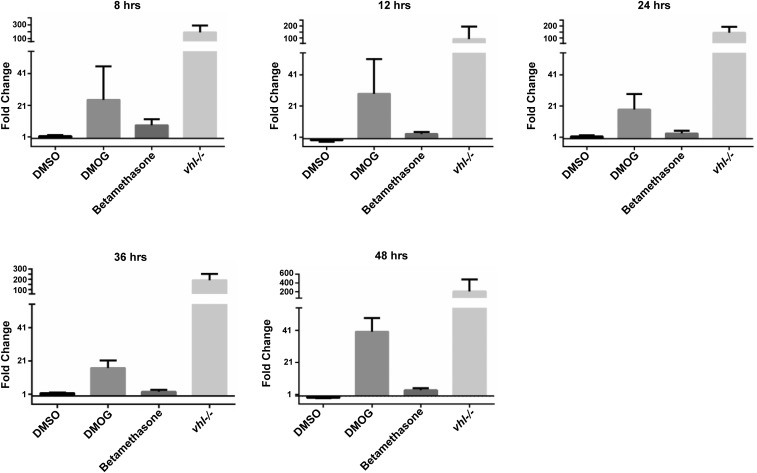
Fig. S1.
Time course of phd3 induction by BME: BME induction of phd3 is short-lived. We sought to establish whether BME activation of phd3 was a time-dependent phenomenon and whether or not the effects might be an artifact of the phd3:GFP transgene (for example, due to external enhancers present near the transgene insertion site). Therefore, qPCR for endogenous phd3 was performed using cDNA created from RNA isolated after 8, 12, 24, 36, and 48 h of incubation from 3-dpf onward. This showed a rather transient induction of phd3 by glucocorticoids, which peaked at the first sampled time point. Fold change was calculated using the ΔΔCT method for each treatment with DMOG and BME compared against the DMSO control and vhl compared against the wild type. Mean fold change values at +8 h were the following: DMSO vs. untreated: 1.5; DMOG vs. DMSO: 24.4; BME vs. DMSO: 8.4; vhl vs. WT: 190.8. Mean fold change values at +12 h were the following: DMSO vs. untreated: −1.0; DMOG vs. DMSO: 28.8; BME vs. DMSO: 3.1; vhl vs. WT: 92.4. Mean fold change values at +24 h were the following: DMSO vs. Untreated: 1.5; DMOG vs. DMSO: 18.7; BME vs. DMSO: 3.5; vhl vs. WT: 145.0. Mean fold change values at +36 h were the following: DMSO vs. untreated: 1.7; DMOG vs. DMSO: 16.8; BME vs. DMSO: 2.5; vhl vs. WT: 191.8. Mean fold change values at +48 h were the following: DMSO vs. untreated: −1.0; DMOG vs. DMSO: 40.2; BME vs. DMSO: 3.6; vhl vs. WT: 205.4. No significant difference was observed when Kruskal–Wallis one-way ANOVA was performed on β-actin CT values with Dunn’s multiple comparison between DMSO vs. WT, DMOG vs. DMSO, BME vs. DMSO, and vhl vs. wild type. Error bars represent upper and lower limits calculated from the SEM using ΔΔCT.
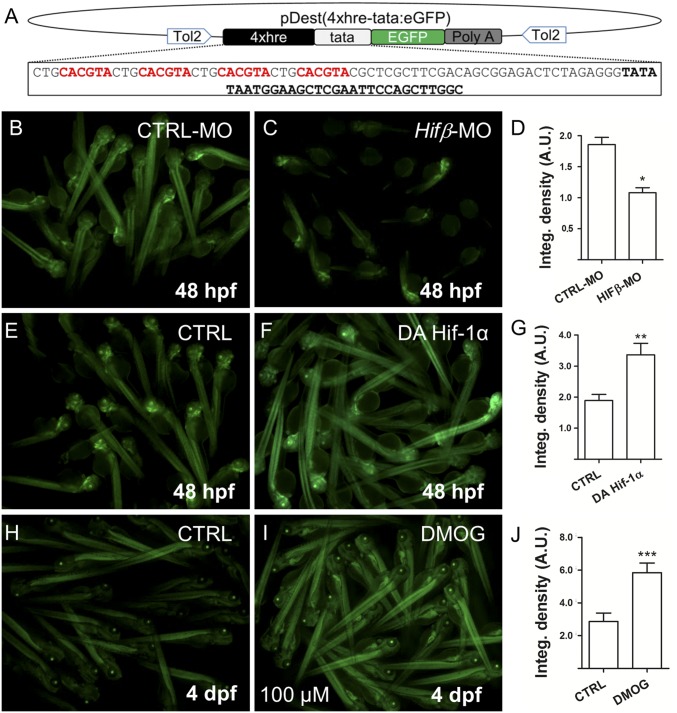
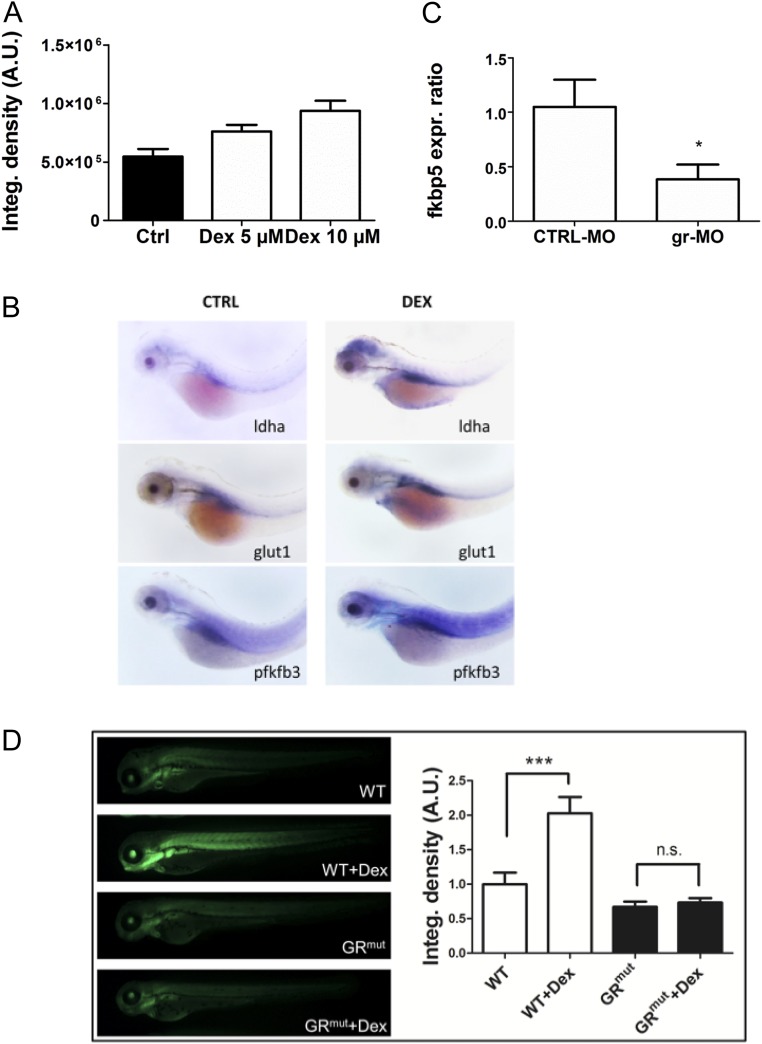
To confirm that GCs directly activate HIF-transcriptional activity, we created a HIF reporter zebrafish expressing tandem copies of a hypoxia response element (HRE) driving eGFP expression, named Tg(4xhre-tata:eGFP)ia21. Reporter activity is decreased in Hif-1β morphants and increased by expression of a dominant active form of HIF-1α mRNA or DMOG treatment, confirming that the transgene responds to modulators of the HIF-1–signaling pathway (Fig. 2 and Fig. S2). To examine the influence of GCs, the offspring obtained from a Tg(4xhre-tata:eGFP)ia21 carrier were exposed to increasing concentrations of DEX for 24 h. Treated embryos showed a dose-dependent activation of the 4xhre-tata transgene, confirming that GCs activate HIF transcriptional responses under normoxic conditions (Fig. 3A and Fig. S3A). To independently assess whether GCs affect HIF-1 signaling and the glycolytic pathway, we analyzed the expression of three known hypoxia-dependent regulators of glucose metabolism in zebrafish (19) using whole-mount in situ hybridization (WISH); as expected, pfkfb3, ldha, and glut1 were up-regulated after DEX treatment as a consequence of HIF-1 activation (Fig. S3B).
Fig. 2.
Generation of 4xhre-tata Hif-reporter lines. (A) Schematic representation of the Tol-2 vector used to generate the Tg(4xhre-tata:eGFP)ia21 line. The construct consists of a 98-bp fragment encoding four HREs (in red) from the murine lactate dehydrogenase followed by a TATA minimal promoter (in boldface), EGFP (in green), and the poly(A) signal. (B–D) Representative image of Tg(4xhre-tata:eGFP)ia21 embryos at 48 hpf injected with a control MO (B) or the HIFβ-MO MO (C). (Magnification: 5×.) Down-regulation of Hifβ significantly decreases transgene activity as reported by the integrated density analysis of fluorescence (D). (E–G) HIf-1α dominant active (DA) mRNAs injected in Tg(4xhre-tata:eGFP)ia21 embryos increase transgene expression (F) compared with control embryos (E). (Magnification: 5×.) (H–J) Treatment from 72 hpf to 96 hpf with 100 μM DMOG significantly increases 4xhre-tata transgene activity (I) with respect to the control embryos (H). (Magnification: 4×.) (D, G, and J) Average values of fluorescence integrated density calculated for treated embryos and controls. Values represent the mean ± SEM (***P < 0.001; **P < 0.01; *P < 0.05). A.U., arbitrary units.
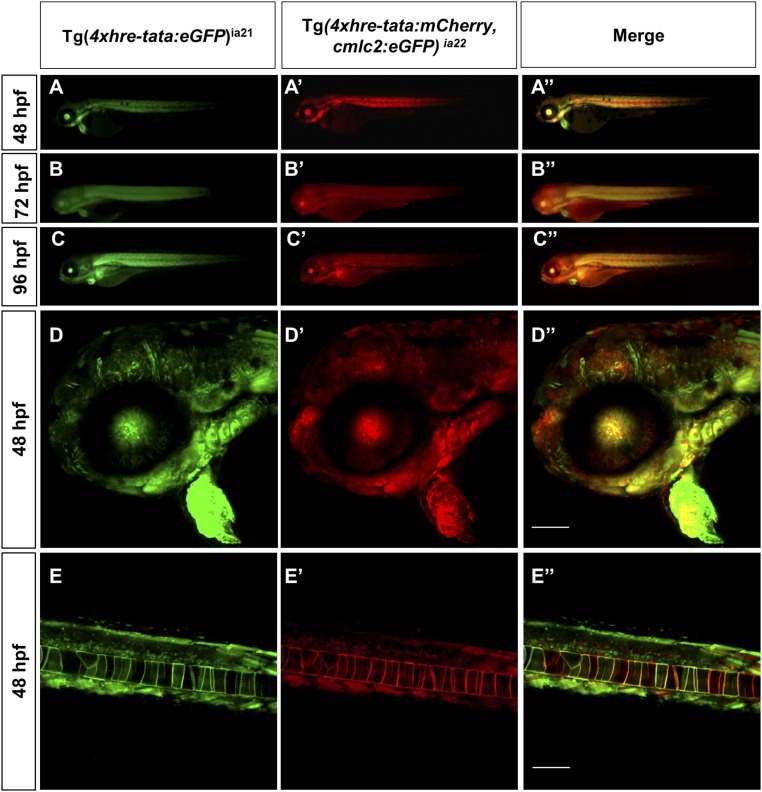
Fig. S2.
HRE transgene expression: Tg(4xhre-tata) lines have overlapping expression patterns. Conventional (A, A′, A′′, B, B′, B′′, C, C′, and C′′) and confocal (D, D′, D′′, E, E′, and E′′) lateral views of double transgenic Tg(4xhre-tata:eGFP)ia21/Tg(4xhre-tata:mCherry,cmlc2:eGFP)ia22 embryos. (D, D′, D′′, E, E′, and E′′) The 48-hpf embryos, with anterior to the left. (D–D′′) Head region of double transgenic embryo: D, green channel; D′, red channel; D′′, merge. (E–E′′) Tail region of double transgenic embryo: E, green channel; E′, red channel; E′′, merge. (Scale bar: 100 μm.) Comparisons between the two reporter lines revealed significant overlap in the expression profile of the 4xhre-tata transgene, suggesting the independence of the transgene expression from the genomic site of insertion. (Magnification: A–C, 8×.)
Fig. 3.
Cross-talk between the HIF-1 and glucocorticoid-signaling pathways is mediated by the glucocorticoid receptor and HREs. (A) The 72-hpf Tg(4xhre-tata:eGFP)ia21 embryos were incubated with different concentrations of DEX. DEX activates the 4xhre-tata transgene. (Magnification: 8×.) (B) Images of 72-hpf Tg(4xhre-tata:eGFP)ia21 larvae injected with a gr-MO and a CTRL-MO alone or combined with DEX (Bottom). DEX activation of 4xhre-tata transgene expression is maintained in CTRL-MO–injected larvae and ablated in GR morphants. (Magnification: 8×.) (C) Histograms showing the average values (±SEM) of the fluorescence-integrated density in 72-hpf Tg(4xhre-tata:eGFP)ia21 embryos injected with gr-MO and CTRL-MO with or without DEX. A.U., Arbitrary units. (D) Fold changes (±SEM) in phd3 gene expression in 72-hpf embryos injected with gr-MO and CTRL-MO with or without DEX (24 h) compared with nontreated/noninjected controls (set at 1). Target gene mRNA levels were normalized to β-actin. *P < 0.05; ***P < 0.001.
Fig. S3.
DEX induces Hif-1 pathway activation and expression of glycolytic regulators and is GR-dependent. (A) Histograms showing the average values of the integrated density of fluorescence in 72-hpf Tg(4xhre-tata:eGFP)ia21 embryos treated with different doses of DEX. DEX incubation induces a dose-dependent activation of the Hif-1 pathway as shown by the increase of the 4xhre-tata transgene expression. A.U., arbitrary units. (B) In situ hybridization for glycolytic regulators ldha, glut1, and pfkfb3 showing increased expression after DEX treatment. (Magnification: 17.5×.) (C) Fold changes in gene expression of fkbp5 in 72-hpf embryos injected with gr-MO and CTRL-MO compared with nontreated/noninjected control (set at 1). Real-time PCR analysis revealed that the expression of fkbp5 was significantly reduced in gr-MO–injected embryos, confirming the ability of gr-MO to impair the expression of the glucocorticoid receptor. (D, Left) Images of 72-hpf Tg(4xhre-tata:eGFP)ia21 in wild-type and GR CRISPR mutant background treated with 10 μM DEX in DMSO for 24 h or with vehicle control. (Magnification: 10×.) (Right) Quantification of GFP reporter fluorescence, showing that the HRE reporter failed to respond in the GR mutant background. Generation of this GR mutant is described in SI Text.
GC Activation of HIF Is Independent of GR DNA Binding.
GCs regulate gene transcription through binding and activating cytoplasmic GR, through nuclear translocation, and through direct binding to GC response elements (GRE) (20). To analyze the mechanism underlying GC/HIF cross-talk, we assessed whether DEX-induced effects on HIF-reporter activity were dependent on GR and its DNA binding activity. Single-cell stage Tg(4xhre-tata:eGFP)ia21 embryos were microinjected with a splice-blocking MO against full-length GR (gr-MO) (21). Real-time PCR analysis of fkbp5 showed significantly reduced expression to confirm gr-MO activity (Fig. S3C). Morphants and control Tg(4xhre-tata:eGFP)ia21 embryos (48 hpf) were incubated with or without DEX (10 μM) for 24 h, and reporter activity was measured. DEX increased 4xhre-tata activity in controls but had minimal effect on the gr morphants (Fig. 3 B and C). Moreover, HIF activation was independently confirmed by measuring endogenous phd3 mRNA expression in 72-hpf gr morphants and control embryos (Fig. 3D). Finally, GR dependency of HIF transcriptional activation was observed in a CRISPR-induced null mutant of the GR gene (Fig. S3D).
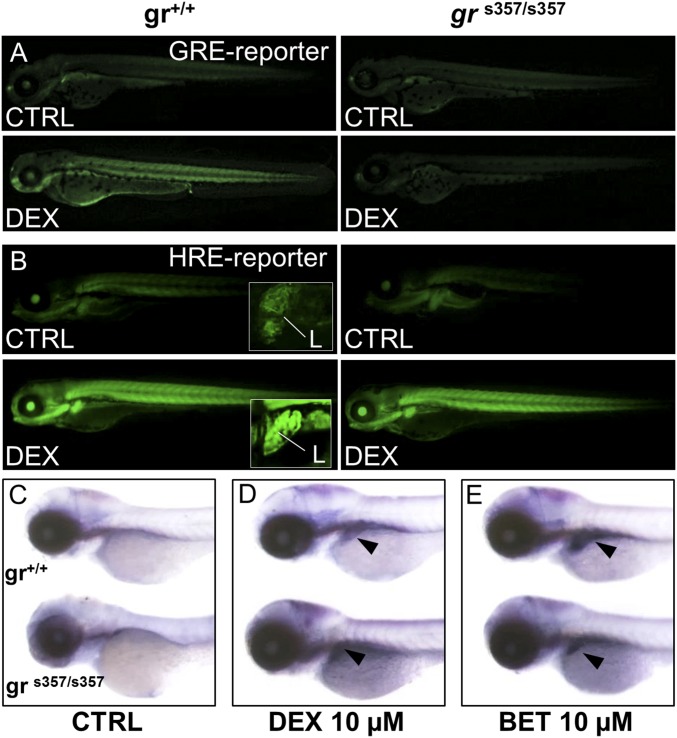
We also studied the zebrafish grs357mutant (22) that lacks DNA-binding function and abrogates GR transcriptional activity (Fig. 4A). Interestingly, DEX activated the Tg(4xhre-tata:eGFP)ia21 reporter in a grs357 mutant background (Fig. 4B), suggesting that a nontranscriptional mechanism underlies GC activation of HIF signaling. To independently assess the role of the GR DNA-binding domain in GC/HIF cross-talk, offspring from two grs357 homozygous carriers (72 hpf) were incubated with DEX or BME, and phd3 mRNA levels were analyzed by WISH (Fig. 4 C–E). DEX and BME activated phd3 transcription in both wild-type and grs357 mutant embryos, confirming that HIF transcriptional activity was preserved.
Fig. 4.
Glucocorticoid induction of HIF-1 activity is independent of glucocorticoid receptor DNA binding. (A) Treatment with 10 μM DEX for 24 h activates the wild-type Tg(9xGCRE-HSV.Ul23:eGFP)ia20 (23) glucocorticoid reporter (GRE-reporter) line, while in grs357 mutant background (Right) DEX is unable to activate GRE reporter. (Magnification: 14.5×.) (B) Images of wild-type and grs357 embryos in the Tg(4xhre-tata:eGFP)ia21 background (HRE-reporter), treated for 24 h with DEX 10 µM. DEX activates the 4xhre-tata transgene in both wild-type and grs357 mutant larvae and induces a significant and generalized increase of fluorescence that is particularly evident in the liver (L in Insets). (Magnification: 14.5×; Insets, 72.5×.) (C–E) In situ hybridization of phd3 mRNA antisense probe in wild-type and grs357 mutants at 80 hpf. (Magnification: 17.5×.) DEX (D) or BME (E) activated phd3 gene expression in the liver (arrowheads).
GCs Activate HIF Signaling in Human Hepatocytes.
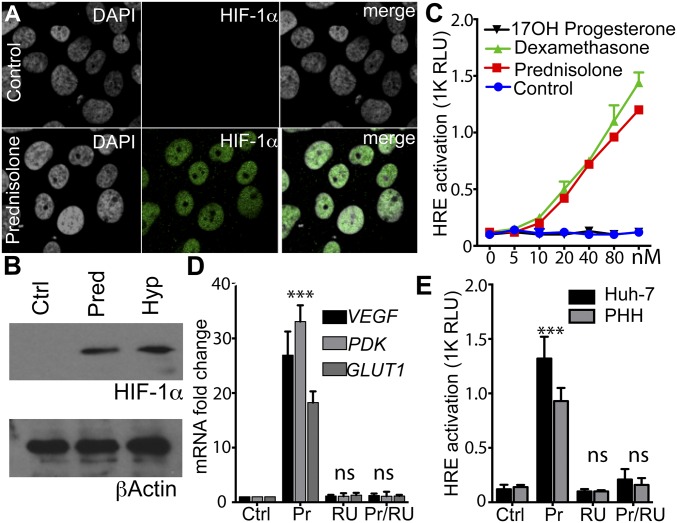
To verify whether the GC/HIF cross-talk observed in zebrafish is relevant to humans, we focused our efforts on human liver-derived cells. Culturing hepatocyte-derived Huh-7 cells under low oxygen stabilized HIF-1α at comparable levels to prednisolone treatment (Fig. 5 A and B). To assess transcriptional responses, Huh-7 cells were transfected with a hre-luciferase (hre-luc) reporter and treated with prednisolone and dexamethasone that activated the reporter (Fig. 5C). Furthermore, prednisolone induced mRNA levels of three endogenous HIF target genes (VEGF, PDK, and GLUT1) to a similar degree as low-oxygen treatment, thus paralleling the in vivo results observed in zebrafish embryos (Fig. 5D). Furthermore, GC activation of HRE activity was sensitive to the GR antagonist RU-486 (Fig. 5D), demonstrating GR dependency. Next we screened a panel of hypoxia-regulated target genes in Huh-7 cells and found that 29/38 HIF target genes were induced by prednisolone, whereas only 3 of 38 were induced by cortisone (Dataset S2).
Fig. 5.
Glucocorticoids stabilize HIF in human cell lines. (A) Mock or prednisolone-treated (100 nM) Huh-7 cells were stained for HIF-1α expression (green) and counterstained with DAPI (gray); images show nuclear localization of HIF-1α. (Magnification: 260×.) (B) HIF-1α expression in hypoxic (3% O2) and prednisolone (Pred)-treated Huh-7 cells. (C) Huh-7 cells were transfected with a HRE luciferase reporter and 24 h later treated with increasing doses of various steroids (100 nM) for16 h. GC agonists activated the HRE reporter while 17OH did not. (D) Huh-7 cells treated with Pred/RU-486 (100 nM) for 24 h, lysed, and RNA-purified to quantify HIF transcriptional targets. Data represents fold change over control sample (Dataset S2). (E) Huh-7 cells and primary human hepatocytes (PHH) were transfected with a HRE luciferase reporter and 24 h later treated with Pred (100 nM) and/or RU-486 (100 nM) for 16 h, and HRE luciferase activity was measured. ***P < 0.001; ns, not significant (ANOVA). Data are represented as mean ± SD.
To confirm that the response is not simply a result of using immortalized Huh-7 cells, we showed that prednisolone activated the HRE reporter in primary human hepatocytes (Fig. 5E). To ascertain whether GCs increase HIF expression and associated transcriptional activity in an authentic liver microenvironment, we treated human liver slices with prednisolone or culture under low oxygen (3%) for 16 h and imaged nuclear HIF-1α (Fig. 6A) and quantified HIF target gene mRNA levels (Fig. 6 B and C). Both prednisolone and low-oxygen treatments stabilized HIF-1α (Fig. 6A) and increased HIF target gene expression (Fig. 6 B and C). Together, these data demonstrate that, as in the zebrafish, GCs can activate HIF signaling in the human liver.
Fig. 6.
Glucocorticoids stabilize HIF in human liver slices. (A) HIF-1α expression in human liver slices treated with prednisolone or cultured under low oxygen (3%) for 24 h and counterstained with DAPI. Both prednisolone and hypoxic treatments induce nuclear HIF-1α expression. Images are representative of five donor livers. (Magnification: 55×.) (B and C) HIF target gene PDK1 and VEGF mRNA levels in human liver slices from three donors treated with Pred/RU-486 for 24 h. (D) VHL expression in Huh-7 cells treated with prednisolone (Pred) or dexamethasone (Dex) in presence or absence of proteasome inhibitor MG-132 at 10 μM. (E and F) Huh-7 cells treated with prednisolone (Pred) or dexamethasone (Dex) in presence or absence of Src inhibitor PP2 at 5 μM and probed for VHL (E) or HIF-1α (F). All samples were stained for actin as a loading control. ***P < 0.001; ns, not significant (ANOVA). Data are represented as mean ± SD.
To determine how GCs activate HIF signaling, we investigated the effect of GCs on pVHL expression, a crucial negative regulator of HIFα protein. We showed that GCs reduce pVHL expression in Huh-7 cells (Fig. 6D). This is unlikely to be mediated by a transcriptional mechanism since VHL mRNA levels were not affected (Fig. S4A). pVHL is degraded by the proteasome (Fig. 6D) following posttranslational modification by kinases such as c-src and CKII (24). Indeed, we found that inhibiting c-src activity with PP2 (25) rescued the inhibitory effects of GC on VHL expression (Fig. 6E), blocked HIF-1α induction (Fig. 6F), and normalized HIF target gene expression (Fig. S4B), suggesting a mechanism for GCs to stabilize HIFs via degrading VHL.
Fig. S4.
Effects of GC agonists on VHL protein and mRNA expression and PP2 effect on GC-induced HIF target gene expression. (A) Huh-7 and HEK293 cells treated with prednisolone and RU-486 (100 nM) for 24 h were lysed and RNA purified to detect VHL mRNA. Data represent fold change over HEK293 control sample. (B) Huh-7 cells were treated with prednisolone and dexamethasone, a c-src inhibitor PP2 (10 μM), for 24 h, lysed, and RNA-purified to detect HIF-1α transcriptional targets VEGF, PDK, and Glut1. Data represent fold change over control sample.
Discussion
The Spectrum Collection (Microsource Discovery Systems) includes compounds that modulate a wide variety of pathways; however, only GCs showed a consistent and robust HIF activation. It is surprising that GCs were not previously identified as HIF modifiers in cell-based chemical screens. We can only speculate what the apparent liver specificity of the GC effect may have contributed. Thus, in vivo chemical screens, providing a variety of cell types in a physiological context, are a useful expansion compared with more classical screening approaches, even with “well-trodden” pathways like HIF.
We confirmed that GCs promote HRE-dependent HIF transcriptional activity in fish and human liver cells. Surprisingly, fish expressing a GR mutant lacking a functional DNA-binding domain (DBD) still activated the phd3 promoter and 4xhre reporter models after treatment with DEX. However, complete knockdown of GR and a newly generated truncated GR mutant abrogated the ability of DEX to stabilize HIF. The GR is essential for survival as demonstrated by the report that 90% of GR KO mice die soon after birth (26) and only 10% of GR KO zebrafish survive, with a reduced fitness. Of note, both mouse and zebrafish homozygous mutants of the GR-DBD survive with minor defects (22, 27), suggesting essential roles for GRE-independent GR activities, as exemplified by our study.
Since GCs are reported to activate c-src kinase (28, 29), which is known to phosphorylate and target pVHL for proteosomal degradation (24), we hypothesized a role for GCs in stabilizing HIF via c-src degradation of VHL. Consistent with our results, RU-486 inhibited such nongenomic effects of GR (30). Our in vitro studies confirm that GCs destabilize VHL protein expression, and this was rescued by cotreating cells with the c-src inhibitor PP2 (Fig. 6E). As VHL is a negative regulator of HIF, this results in HIF expression under normoxic conditions.
Although we see activation of phd3 and 4xhre promoters and other hypoxia response genes by GCs, GCs do not fully replicate a hypoxic response. We find increased expression of many HIF target genes (Dataset S2), but some exceptions were noted such as Enolase1 and Carbonic anhydrase 9, which were down-regulated by prednisolone. A direct interaction between ligand-activated GR and HIF proteins cannot be excluded, as Kodama et al. (5) have suggested that GR binds the HIF dimer. However, a simpler explanation is that, in addition to stabilizing HIF protein, GR binds a subset of promoters to regulate transcription. A complex interplay is suggested by other reports: for example, while HIF up-regulates VEGF expression and promotes angiogenesis, GCs are generally angiostatic (31) and negatively regulate VEGF expression (32–34). Similarly, during high altitude sickness, GC and HIF may have apparently opposing effects (35, 36). Therefore, we propose that the interplay that we have identified is likely to be tissue- and possibly context-specific.
We found that GC agonists increased mRNA levels of the classical HIF target erythropoietin both in cell culture (Dataset S2) and in zebrafish embryos and adult zebrafish livers (Fig. S5). Our data are consistent with reports showing a synergistic effect of GCs and HIFs in hematopoiesis (37, 38), and it will be interesting to investigate whether this cooperativity is GR-DBD–dependent. GCs received their name because they promote blood glucose levels as well as gluconeogenesis and glycogen storage in the liver and provide an acute response to stress (39). HIF signaling has a profound impact on cellular metabolism and induces gluconeogenesis and glycogen storage (40, 41). We suggest that GCs modulation of VHL and HIF may contribute to their ability to modulate blood glucose and glycogen storage. In addition, HIF has been shown to impact lipid metabolism in the liver, where its (in)appropriate activation reduces beta oxidation and increases lipid storage capacity leading to steatosis (42, 43). It is worth noting that long-term treatment with GCs was reported to increase steatosis (44). Activation of the HIF pathway via cross-talk with the GR may explain how GC excess regulates hepatic fat metabolism and the steatotic phenotype. Our experiments show that GCs predominantly stabilize HIF in the liver and may activate only a subset of HIF targets, which could be addressed by studying tissue-specific knockout models. Based on the elegant experiments of Rankin et al. (43), we would predict that liver-specific deletion of HIF-2a should protect mice from GC-induced steatosis and may reduce the effect of GCs on blood glucose levels.
Fig. S5.
Effect of GC agonist BME or DEX on epo expression. (A) BME weakly increases epo expression in zebrafish larvae. The 6-dpf larvae were exposed to 20 μM BME or vehicle (DMSO) for 8 h, and groups of 10 embryos were used to isolate RNA for qPCR (n = 8). Target-gene mRNA levels were normalized to β-actin. *P < 0.05 (t test). Graph shows mean and SEM average expression after DMSO treatment was set to 1. (B) Dexamethasone increases epo expression in the liver of adult zebrafish. Three adults were exposed to either 5 μM dexamethasone or vehicle (DMSO) for 48 h. At the end of treatment, fish were euthanized and their liver was used to isolate RNA for qPCR. The epo gene mRNA levels were normalized to acidic ribosomal protein (arp) mRNA. **P < 0.01 (t test). Graph shows mean and SEM average expression after DMSO treatment was set to 1.
It is becoming clear that HIF signaling is regulated not only by low oxygen, but also by several other inflammatory mediators (45–47). Our data support GC as a further key modulator of HIF signaling to add to this growing list. It is interesting to note that many common GC and HIF gene targets are implicated in regulating hepatic metabolism. This is potentially important, as GCs are secreted in a rhythmic circadian fashion, where circulating GC levels reach a maximum to coincide with the onset of the active phase—morning in humans, evening in mice. Since metabolic processes in the liver are coupled to the circadian clock, our data provide an additional level of control, where GC activation of the HIF pathway helps to ensure that the changing metabolic demands throughout the day are met. Our data suggest a role for GC-driven circadian components in other aspects of HIF signaling.
Material and Methods
Zebrafish Strains.
vhl mutant and Tg(phd3:eGFP)i144/i144 (13) fish were maintained in a mixed Tupfel long fin/London wild-type (TL/LWT) background. The Tg(9xGCRE-HSV.Ul23:eGFP)ia20 line is a GC pathway reporter (23). The zebrafish GR mutant line grs357 was incrossed to create homozygotes (22). To obtain wild-type embryos, LWTs were incrossed. To obtain homozygous vhl mutants, vhlhu2117/+;phd3:eGFPi144/i144 fish were incrossed. Embryos were incubated at 28 °C in E3 (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgCl2, pH 7.2) containing methylene blue (Sigma-Aldrich) at 0.0001%. The generation of Tg(4xhre-tata:eGFP)ia21 and Tg(4xhre-tata:mCherry, cmlc2:eGFP)ia22 reporters and GR CRISPR mutation is described in SI Text.
Drug Treatment of Embryos.
The Spectrum Collection (Microsource Discovery Systems) of 2,000 compounds was used; tested compounds and detailed methods can be found in SI Text and Dataset S1.
Hypoxic Target Gene Activation in Zebrafish.
Three biological replicates of 10 phd3:EGFPi144/i144 embryos were treated for 8, 12, 24, 36, and 48 h starting from 72 hpf. Embryos were treated with 10 μM betamethasone 17,21 dipropanoate and 60 μM DMOG in 1% DMSO, with untreated and 1% DMSO controls. The vhlhu2117/hu2117; phd3:EGFPi144/i144mutants were used as positive controls. Biological replicates of 48-hpf Tg(4xhre-tata:eGFP)ia21 embryos were incubated with or without 10 μM DEX and/or 3.8 μM 17-AAG (Sigma) for 24 h. RNA was isolated using TRIzol and quantified using the Nanodrop ND-1000 spectrophotometer. cDNA was reverse-transcribed from 0.5 μg RNA using the SuperScript III First-Strand Synthesis System (Invitrogen). For the temporal phd3 expression profile, qPCR was performed with optimized primers, using the iCycler iQ system (Bio-Rad). Cycling conditions were the following: 95 °C − 3 min, [95 °C − 15 s, 60 °C − 30 s] × 40 cycles, 55–95 °C in 0.5-°C increments 30 s, with β-actin2 as internal control. Primers: see Dataset S3. For hypoxia/glucocorticoid target gene detection, qPCR was carried out using the Applied Biosystems SDS Software v2.4.1 in conjunction with the 7900HT Fast Real-Time qPCR System. The cycling conditions were the following: 50 °C − 2 min, 95 °C − 10 min, [95 °C − 15 s, 60 °C − 1 min] × 40 cycles. Primers: see Dataset S3. Cycle threshold (Ct) values were calculated automatically using the software, with ROX reference dye as the passive reference. Fold change was calculated using the ΔΔCT method.
MO and mRNA Injections.
The translation-blocking MO HIFβ-MO (GGATTAGCTGATGTCATGTCCGACA) was used as reported (18) while a splice-site targeting MO (gr-MO) was used to silence GR (21). A standard control MO (CTRL-MO; Genetools) was used as a negative control. The MO stock solution (8 mg/mL) was diluted in Danieau’s solution, and ∼2 nL was injected per embryo as previously described (48). Dominant-active hif-1αa and hif-1αb (kindly provided by P. Elks, University of Sheffield, Sheffield, UK) were synthesized (mMessageMachine; Ambion, Invitrogen) and injected as described in ref. 49.
Image Analysis in Tg(4xhre-tata:eGFP)ia21 Embryos.
For analysis of Tg(4xhre-tata:eGFP)ia21 embryos, fluorescence was collected using a Leica M165FC dissecting microscope and a Nikon C2 H600L confocal microscope. N-phenylthiourea–treated larvae were anesthetized with tricaine and embedded in 1% low-melting agarose on a glass slide. Images were analyzed with Nikon software. For fluorescence quantification of transgenic embryos, a Leica M165FC microscope and DC500 digital camera were used. All images were acquired using identical parameters, and fluorescence-integrated density was calculated using ImageJ.
Cell Culture, Antibodies, and Treatments.
Huh-7 and HEK293 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS, 1% l-glutamine, and 50 units/mL penicillin/streptomycin (Life Technologies). Primary human hepatocytes were isolated as described (50) and maintained in Williams E Medium (Sigma) supplemented with 10% FBS, 5 mM Hepes/insulin/l-glutamine (Life Technologies). Cells were grown in a humidified incubator at 37 °C, 5% CO2, and 20% O2 (normoxia). For hypoxic conditions, cells were grown at 37 °C in a humidified sealed Galaxy 48R incubator (New Brunswick) at 37 °C, 5% CO2, 92% N2, and 3% O2 (hypoxia). The following antibodies used: anti-mouse HIF-1α (Novus Biologicals), anti-VHL (Cell Signaling #2738), Alexa Fluor goat anti-mouse 488 (Molecular Probes), anti–β-actin antibody (Sigma Aldrich), anti-mouse and anti-rabbit HRP-conjugated secondary antibody (GE Healthcare). Prednisolone, dexamethasone, cortisone, RU-486 17OH progesterone, and MG-132 are from Sigma Aldrich. Src inhibitor PP2 was purchased from Merk Millipore.
Ex Vivo Liver Slices.
Liver tissue samples were collected with local National Health Service research ethics committee approval (Walsall LREC 04/Q2708/40) and with written informed consent. Donor liver tissue surplus to transplantation requirements were collected from the Queen Elizabeth Hospital. Cores were cut from the tissue immediately upon receipt using a Krumdieck Tissue Slicer (Alabama R&D) (51). Briefly, the core was placed into the slicer assembly under aseptic conditions, and 240-µm sections were collected and immediately transferred into Williams E media/1% l-glutamine/0.5 µM insulin. An albumin ELISA (Bethyl Laboratories) was used to monitor the viability of the slices. Samples from five donors were serum-starved followed by incubation under hypoxia (3% O2) or normoxia (20% O2) for 24 h. Liver slices were treated with prednisolone (100 nM) for 24 h before fixing for detection of HIF-1α by confocal microscopy.
Confocal Microscopy on Cells.
Cells were grown on 13-mm borosilicate glass coverslips at a density of 4 × 104/well for 24 h and serum-starved for 5 h before treating with prednisolone (100 nM). Cells or liver slices were fixed in 3% paraformaldehyde for 25 min at room temperature (RT) and permeabilized with 0.01% TX-100/PBS for 10 min. Cells were incubated with anti–HIF-1α for 1 h at RT, unbound antibody was removed by washing, and bound antibody was detected with Alexa-488 secondary antibody. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole, dihydrochlorine (DAPI; Life Technologies). Cells were imaged using a Zeiss Meta Head confocal microscope with a 63× water immersion objective.
HRE Reporter Assay.
Cells were transfected with the HRE reporter (a plasmid containing four tandem HRE copies kindly provided by M. Ashcroft, Cambridge University, Cambridge, UK) using Fugene 6 transfection reagent (Promega) per the manufacturer’s guidelines. After 24 h, cells were serum-starved for 5 h before treating with GC or RU-486, (100 nM) for a further 24 h. Cells were lysed, and reporter activity was quantified using a firefly luciferase assay system (Promega) in a luminometer (Berthold Lumat LB 9507).
Western Blotting.
Cells were harvested in lysis buffer (PBS/1% Triton-X100/0.1% Na deoxycholate/0.1% SDS) containing protease and phosphatase inhibitors (Roche). Lysates were clarified by centrifugation, and protein concentration was determined using a Bradford Protein Assay Reagent (Pierce). Protein lysates (20 μg) were added to sample running buffer (30% glycerol/6% SDS/0.02% bromophenol blue/10% 2-β-mercaptoethanol/0.2 M Tris⋅HCl; pH 6.8) and separated by SDS/PAGE. Separated proteins were transferred to PVDF membranes and incubated with primary antibodies before detection with HRP-conjugated secondary antibody and enhanced chemiluminescence (Geneflow) using a PXi imaging system (Syngene).
SI Text
Screening Conditions and Setup DMOG Activation of phd3:gfp.
To ensure that the phd3:gfp transgenic line was suitable as an hypoxia reporter for a chemical screen and to find the optimal concentration for use as a positive control, 3-dpf phd3:gfpi144/i144 embryos were treated with DMOG at a range of concentrations and imaged at 5 dpf. DMOG is an inhibitor of PHD proteins and able to induce the activation of the Hif pathway by preventing the degradation of the Hif-α subunits even in normoxic conditions. Activation of phd3:gfp was quantified by measuring maximum pixel intensity in the area of activation using ImageJ. Fluorescence increased as DMOG concentration increased, but the highest concentrations caused variable developmental abnormalities and death. Quantities of 10 μM and 60 μM DMOG were taken as positive controls, 10 μM (an approximately twofold increase) being the benchmark for activation and 60 μM (an approximately fourfold increase) considered the optimal activation.
Drug Treatment of Zebrafish Embryos.
The Spectrum Collection (Microsource Discovery Systems) of 2,000 compounds (Dataset S1) was stored at a concentration of 1 mM in DMSO (Sigma) in microtiter plates at −20 °C in the Medical Research Coucil–Centre for Developmental and Biomedical Genetics Screening Unit. Each 96-well plate contained 80 compounds. Three 3-dpf Tg(phd3:eGFP)i144/i144 embryos were aliquoted per well of a black glass-bottomed 96-well plate (Perkin-Elmer) in E3 medium with the library at a final concentration of 10 μM in 250 μL with 1% DMSO. Remaining wells were used for controls: untreated, 1% DMSO, and 10, 40, and 60 μM DMOG (Sigma). Plates were imaged directly after compound administration, at +24 h (4 dpf) and +48 h (5 dpf). Plates were incubated in darkness at 28 °C. To aid imaging on 3 dpf and 4 dpf, assay plates were chilled by placing the plate on an ice block until embryo movement ceased (±10 min). The 5-dpf embryos were anesthetized using 0.168% wt/vol Tricaine (Sigma). Plates were imaged (z-stacks) using an inverted Nikon Eclipse TE2000-U microscope at 2× magnification in conjunction with Volocity image capture software (Perkin-Elmer). Using Volocity Image Analysis software, embryos were manually assessed for activation. The voxel spy tool was used to detect maximum pixel intensity and to compare this against controls. Initial retests of hits were done in a similar manner but used an Ash plate scanner to capture images (Ash Biotech). Concentration-dependent activation of phd3:gfp reporter embryos by DMOG was measured by GFP intensity. Significance was calculated using Kruskal–Wallis one-way ANOVA followed by Dunn’s multiple comparison test against DMSO (P > 0.05; n = 10 per treatment).
Generation and Validation of 4xhre Tata Transgenics.
To generate a zebrafish HIF/HRE reporter, a construct was made containing four copies derived from an optimized HIF-1–responsive element (CACGTA) located upstream from a TATA minimal promoter (based on ref. 52). We annealed and amplified by PCR two phosphorylated primers 4x-opt-attB1r-F and 4x-opt-attB1r-R (Dataset S3) that were then purified with the Wizard kit (Promega) and recombined with pDONR221 (Invitrogen), according to the manufacturer’s instructions. The resulting p5e(4xhre-tata) entry vector was recombined with the EGFP middle entry vector and the SV40polyA-containing vector (p3E-polyA) to create pDest(4xhre-tata:eGFP) as previously described (23). Similarly, the p5e(4xhre-tata) vector was also cloned upstream of mCherry in a pDestTol2CG2 destination vector to generate pDest(4xhre-tata:mCherry,mlc2:eGFP). The purified plasmids with the construct flanked by Tol2 sites were injected into one- or two-cell stage embryos, together with Tol2 transposase mRNA, to generate mosaic F0 fish (23). Fluorescent F0 embryos were raised and outcrossed, and F1 embryos were screened for reporter expression. Two well-expressing founders, named Tg(4xhre-tata:eGFP)ia21 and Tg(4xhre-tata:mCherry,mlc2:eGFP)ia22, were maintained and used for this study. A comparison of these two lines is shown below.
Generation of a GR CRISPR Mutant.
The single-guide RNA (sgRNA) to target the zebrafish glucocorticoid receptor gene was designed using the CRISPR design tool CHOP CHOP (chopchop.cbu.uib.no/). A total of 100 pg of sgRNA and 300 pg of CAS9 protein (NEB # M0386) were coinjected into one-cell-stage embryos. The gr mutant allele (official allele name nr3c1ia30) presents a 5-bp frameshift insertion occurring in the first coding exon upstream to the DNA-binding domain. The resulting putative encoded protein contains the first 311 aa of the wild-type Gr, plus 20 aa generated by the 5-bp alteration. Homozygous gr−/− mutants display reduced gr transcript levels consistent with nonsense-mediated mRNA decay (53).
Supplementary Material
Acknowledgments
We thank the University of Sheffield and University of Padova aquaria teams for excellent care of zebrafish; J. Tomlinson and A. Clarke for critically reading the manuscript; M. Ashcroft (Cambridge University) for the HRE luciferase construct; P. Elks (University of Sheffield) for DA-Hif1 constructs; H. Baier (Max Planck Institute) for grs357 mutants; and L. Dalla Valle and E. Colletti for help in Padova. Work in the J.A.M. laboratory is funded by the National Institute for Health Research Birmingham Liver Biomedical Research Unit; Medical Research Council Programme Grant G1100247, European Union (EU) FP7 PathCo HEALTH Grant 597F3-2012–305578 and EU Horizon 2020 Hep-CAR Grant 667273. Work in the F.J.M.v.E. laboratory was supported by European Commission FP7 Grant HEALTH-F4-2010-242048 and Biology and Biotechnology Research Council Grant BB/M02332X/1 and an A*Star studentship (to D.G.). Work in the F.A. laboratory is funded by EU Project Grant ZF-HEALTH CT-2010-242048 and by Associazione Italiana per la Ricerca sul Cancro Project IG 10274. L.C.M. is supported by a University of Leeds Academic Fellowship. A.V. is supported by the Italian National Institute of Health Grant GR-2011-02346749.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705338114/-/DCSupplemental.
References
- 1.Wright A, Brearey S, Imray C. High hopes at high altitudes: Pharmacotherapy for acute mountain sickness and high-altitude cerebral and pulmonary oedema. Expert Opin Pharmacother. 2008;9:119–127. doi: 10.1517/14656566.9.1.119. [DOI] [PubMed] [Google Scholar]
- 2.Dardzinski BJ, et al. Increased plasma beta-hydroxybutyrate, preserved cerebral energy metabolism, and amelioration of brain damage during neonatal hypoxia ischemia with dexamethasone pretreatment. Pediatr Res. 2000;48:248–255. doi: 10.1203/00006450-200008000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Tokudome S, et al. Glucocorticoid protects rodent hearts from ischemia/reperfusion injury by activating lipocalin-type prostaglandin D synthase-derived PGD2 biosynthesis. J Clin Invest. 2009;119:1477–1488. doi: 10.1172/JCI37413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner AE, Huck G, Stiehl DP, Jelkmann W, Hellwig-Bürgel T. Dexamethasone impairs hypoxia-inducible factor-1 function. Biochem Biophys Res Commun. 2008;372:336–340. doi: 10.1016/j.bbrc.2008.05.061. [DOI] [PubMed] [Google Scholar]
- 5.Kodama T, et al. Role of the glucocorticoid receptor for regulation of hypoxia-dependent gene expression. J Biol Chem. 2003;278:33384–33391. doi: 10.1074/jbc.M302581200. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb Symp Quant Biol. 2011;76:347–353. doi: 10.1101/sqb.2011.76.010678. [DOI] [PubMed] [Google Scholar]
- 7.Kaelin WG., Jr Cancer and altered metabolism: Potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases. Cold Spring Harb Symp Quant Biol. 2011;76:335–345. doi: 10.1101/sqb.2011.76.010975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernhardt WM, et al. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol. 2006;17:1970–1978. doi: 10.1681/ASN.2005121302. [DOI] [PubMed] [Google Scholar]
- 9.Shen X, et al. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J Orthop Res. 2009;27:1298–1305. doi: 10.1002/jor.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi T, et al. High-throughput screening identifies CHMP4A associated with hypoxia-inducible factor 1. Life Sci. 2010;87:604–608. doi: 10.1016/j.lfs.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Wan C, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci USA. 2008;105:686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholz CC, Taylor CT. Targeting the HIF pathway in inflammation and immunity. Curr Opin Pharmacol. 2013;13:646–653. doi: 10.1016/j.coph.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Santhakumar K, et al. A zebrafish model to study and therapeutically manipulate hypoxia signaling in tumorigenesis. Cancer Res. 2012;72:4017–4027. doi: 10.1158/0008-5472.CAN-11-3148. [DOI] [PubMed] [Google Scholar]
- 14.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 15.van Rooijen E, et al. Zebrafish mutants in the von Hippel-Lindau tumor suppressor display a hypoxic response and recapitulate key aspects of Chuvash polycythemia. Blood. 2009;113:6449–6460. doi: 10.1182/blood-2008-07-167890. [DOI] [PubMed] [Google Scholar]
- 16.Ciesek S, et al. Glucocorticosteroids increase cell entry by hepatitis C virus. Gastroenterology. 2010;138:1875–1884. doi: 10.1053/j.gastro.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Schaaf MJ, Chatzopoulou A, Spaink HP. The zebrafish as a model system for glucocorticoid receptor research. Comp Biochem Physiol A Mol Integr Physiol. 2009;153:75–82. doi: 10.1016/j.cbpa.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Prasch AL, Tanguay RL, Mehta V, Heideman W, Peterson RE. Identification of zebrafish ARNT1 homologs: 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in the developing zebrafish requires ARNT1. Mol Pharmacol. 2006;69:776–787. doi: 10.1124/mol.105.016873. [DOI] [PubMed] [Google Scholar]
- 19.Greenald D, et al. Genome-wide mapping of Hif-1α binding sites in zebrafish. BMC Genomics. 2015;16:923. doi: 10.1186/s12864-015-2169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker PB, Gloss B, Schmid W, Strähle U, Schütz G. In vivo protein-DNA interactions in a glucocorticoid response element require the presence of the hormone. Nature. 1986;324:686–688. doi: 10.1038/324686a0. [DOI] [PubMed] [Google Scholar]
- 21.Pikulkaew S, et al. The knockdown of maternal glucocorticoid receptor mRNA alters embryo development in zebrafish. Dev Dyn. 2011;240:874–889. doi: 10.1002/dvdy.22586. [DOI] [PubMed] [Google Scholar]
- 22.Ziv L, et al. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol Psychiatry. 2013;18:681–691. doi: 10.1038/mp.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benato F, et al. A living biosensor model to dynamically trace glucocorticoid transcriptional activity during development and adult life in zebrafish. Mol Cell Endocrinol. 2014;392:60–72. doi: 10.1016/j.mce.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Chou MT, Anthony J, Bjorge JD, Fujita DJ. The von Hippel-Lindau tumor suppressor protein is destabilized by Src: Implications for tumor angiogenesis and progression. Genes Cancer. 2010;1:225–238. doi: 10.1177/1947601910366719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanke JH, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 26.Cole TJ, et al. GRKO mice express an aberrant dexamethasone-binding glucocorticoid receptor, but are profoundly glucocorticoid resistant. Mol Cell Endocrinol. 2001;173:193–202. doi: 10.1016/s0303-7207(00)00407-x. [DOI] [PubMed] [Google Scholar]
- 27.Reichardt HM, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 28.Kayahara M, et al. MNAR functionally interacts with both NH2- and COOH-terminal GR domains to modulate transactivation. Am J Physiol Endocrinol Metab. 2008;295:E1047–E1055. doi: 10.1152/ajpendo.90429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews L, et al. Caveolin mediates rapid glucocorticoid effects and couples glucocorticoid action to the antiproliferative program. Mol Endocrinol. 2008;22:1320–1330. doi: 10.1210/me.2007-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br J Pharmacol. 2000;130:289–298. doi: 10.1038/sj.bjp.0703272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shikatani EA, et al. Inhibition of proliferation, migration and proteolysis contribute to corticosterone-mediated inhibition of angiogenesis. PLoS One. 2012;7:e46625. doi: 10.1371/journal.pone.0046625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hegeman MA, et al. Dexamethasone attenuates VEGF expression and inflammation but not barrier dysfunction in a murine model of ventilator-induced lung injury. PLoS One. 2013;8:e57374. doi: 10.1371/journal.pone.0057374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenberger S, Boscolo E, Adini I, Mulliken JB, Bischoff J. Corticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cells. N Engl J Med. 2010;362:1005–1013. doi: 10.1056/NEJMoa0903036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shim SH, Hah JH, Hwang SY, Heo DS, Sung MW. Dexamethasone treatment inhibits VEGF production via suppression of STAT3 in a head and neck cancer cell line. Oncol Rep. 2010;23:1139–1143. doi: 10.3892/or_00000743. [DOI] [PubMed] [Google Scholar]
- 35.Bigham AW, Lee FS. Human high-altitude adaptation: Forward genetics meets the HIF pathway. Genes Dev. 2014;28:2189–2204. doi: 10.1101/gad.250167.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer A, et al. The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev. 1999;13:2996–3002. doi: 10.1101/gad.13.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flygare J, Rayon Estrada V, Shin C, Gupta S, Lodish HF. HIF1alpha synergizes with glucocorticoids to promote BFU-E progenitor self-renewal. Blood. 2011;117:3435–3444. doi: 10.1182/blood-2010-07-295550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo T, McQueen A, Chen TC, Wang JC. Regulation of glucose homeostasis by glucocorticoids. Adv Exp Med Biol. 2015;872:99–126. doi: 10.1007/978-1-4939-2895-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park SK, Haase VH, Johnson RS. von Hippel Lindau tumor suppressor regulates hepatic glucose metabolism by controlling expression of glucose transporter 2 and glucose 6-phosphatase. Int J Oncol. 2007;30:341–348. [PubMed] [Google Scholar]
- 41.Choi JH, et al. Molecular mechanism of hypoxia-mediated hepatic gluconeogenesis by transcriptional regulation. FEBS Lett. 2005;579:2795–2801. doi: 10.1016/j.febslet.2005.03.097. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, et al. HIF-1α and HIF-2α are critically involved in hypoxia-induced lipid accumulation in hepatocytes through reducing PGC-1α-mediated fatty acid β-oxidation. Toxicol Lett. 2014;226:117–123. doi: 10.1016/j.toxlet.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 43.Rankin EB, et al. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol. 2009;29:4527–4538. doi: 10.1128/MCB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel R, Williams-Dautovich J, Cummins CL. Minireview: New molecular mediators of glucocorticoid receptor activity in metabolic tissues. Mol Endocrinol. 2014;28:999–1011. doi: 10.1210/me.2014-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bullen JW, et al. Protein kinase A-dependent phosphorylation stimulates the transcriptional activity of hypoxia-inducible factor 1. Sci Signal. 2016;9:ra56. doi: 10.1126/scisignal.aaf0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Ignazio L, Bandarra D, Rocha S. NF-κB and HIF crosstalk in immune responses. FEBS J. 2016;283:413–424. doi: 10.1111/febs.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peyssonnaux C, et al. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vettori A, et al. Developmental defects and neuromuscular alterations due to mitofusin 2 gene (MFN2) silencing in zebrafish: A new model for Charcot-Marie-Tooth type 2A neuropathy. Neuromuscul Disord. 2011;21:58–67. doi: 10.1016/j.nmd.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Elks PM, et al. Activation of hypoxia-inducible factor-1α (Hif-1α) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood. 2011;118:712–722. doi: 10.1182/blood-2010-12-324186. [DOI] [PubMed] [Google Scholar]
- 50.Mitry RR. Isolation of human hepatocytes. Methods Mol Biol. 2009;481:17–23. doi: 10.1007/978-1-59745-201-4_2. [DOI] [PubMed] [Google Scholar]
- 51.Liaskou E, et al. Regulation of mucosal addressin cell adhesion molecule 1 expression in human and mice by vascular adhesion protein 1 amine oxidase activity. Hepatology. 2011;53:661–672. doi: 10.1002/hep.24085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaluz S, Kaluzová M, Stanbridge EJ. Rational design of minimal hypoxia-inducible enhancers. Biochem Biophys Res Commun. 2008;370:613–618. doi: 10.1016/j.bbrc.2008.03.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Facchinello N, et al. nr3c1 null mutant zebrafish are viable and reveal DNA-binding-independent activities of the glucocorticoid receptor. Sci Rep. 2017;7:4371. doi: 10.1038/s41598-017-04535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.