Significance
It remains unclear why astrocytes are affected to a lesser extent than neurons in a variety of neurodegenerative diseases. We report the higher activity of C terminus of Hsp70-interacting protein (CHIP), cochaperone of Hsp70, in astrocytes than in neurons, which not only promotes the degradation of misfolded proteins, but also upregulates levels of basal and stress-induced Hsp70 in astrocytes. Furthermore, the low activity of CHIP in neurons is caused by the abundant expression of HspBP1, an inhibitor of CHIP. Knocking down HspBP1 in neurons prevents the accumulation and aggregation of the Huntington’s disease (HD) protein and ameliorates neuropathology in a HD knockin mouse model. These findings suggest that HspBP1 accounts for differential vulnerabilities of neurons and glia to misfolded proteins.
Keywords: polyglutamine, Huntington, chaperone, misfolding, neurodegeneration
Abstract
Although it is well known that astrocytes are less vulnerable than neurons in neurodegenerative diseases, the mechanism behind this differential vulnerability is unclear. Here we report that neurons and astrocytes show markedly different activities in C terminus of Hsp70-interacting protein (CHIP), a cochaperone of Hsp70. In astrocytes, CHIP is more actively monoubiquitinated and binds to mutant huntingtin (mHtt), the Huntington’s disease protein, more avidly, facilitating its K48-linked polyubiquitination and degradation. Astrocytes also show the higher level and heat-shock induction of Hsp70 and faster CHIP-mediated degradation of various misfolded proteins than neurons. In contrast to astrocytes, neurons express abundant HspBP1, a CHIP inhibitory protein, resulting in the low activity of CHIP. Silencing HspBP1 expression via CRISPR-Cas9 in neurons ameliorated mHtt aggregation and neuropathology in HD knockin mouse brains. Our findings indicate a critical role of HspBP1 in differential CHIP/Hsp70 activities in neuronal and glial cells and the greater neuronal vulnerability to misfolded proteins in neurodegenerative diseases.
Protein misfolding causes a wide range of neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and polyglutamine diseases, such as Huntington’s disease (HD) (1). Although all these disease proteins are ubiquitously expressed in different types of cells, they preferentially accumulate in neuronal cells and cause neurotoxicity (2). In the brain, more than 90% of cells are glial cells, which are affected to a much lesser extent than neurons in neurodegenerative diseases (3). A challenging issue in the pathogenesis of neurodegenerative diseases is why misfolded proteins preferentially kill neurons rather than glial cells in the brain. Understanding the mechanism for the selective neurodegeneration is important for developing effective treatments of neurodegenerative diseases.
It is established that the accumulation of misfolded proteins in neuronal cells is a prerequisite for their toxicity (4, 5). Preventing the accumulation of misfolded proteins in cells relies highly on molecular chaperones, which refold misfolded proteins, and the ubiquitin–proteasome system (UPS) and autophagy, which degrade misfolded proteins. C terminus of Hsp70-interacting protein (CHIP), a cochaperone of Hsp70 with ubiquitin E3 ligase, links chaperones to the UPS and plays a critical role in protein triage decisions. Mice with CHIP deficiency show increased levels of toxic oligomer proteins and decreased proteasomal activity (6). CHIP is recruited to chaperone–substrate complexes and consequently polyubiquitinates the chaperone-bound substrates, which are then degraded by the proteasome (7–9). Hsp70-binding protein 1 (HspBP1), a cochaperone of Hsp70 that inhibits the activity of Hsp70 ATPase (10), also associates with CHIP and prevents CHIP-mediated ubiquitination on substrates (11). However, it remains unclear whether CHIP/Hsp70 activities are differentially regulated in neuronal and glial cells and whether differential CHIP/Hsp70 activities account for the preferential neuronal vulnerability to misfolded proteins in neurodegenerative diseases.
In the current study, we investigated the mechanism underlying the differential vulnerability of neurons and glia to misfolded proteins. We found that the higher CHIP activity in astrocytes than neurons accounts for the higher Hsp70 activity and faster degradation of misfolded proteins in astrocytes. Neurons express much more abundant HspBP1, which inhibits CHIP activity, and knocking down HspBP1 in neurons rescued neuropathology in a HD knockin (KI) mouse model. Our findings provide mechanistic insight into the differential vulnerabilities of neuronal and glial cells to misfolded proteins and also offer a therapeutic target in neurodegenerative diseases.
Results
Increased Monoubiquitinated CHIP Indicates Active CHIP in Astrocytes.
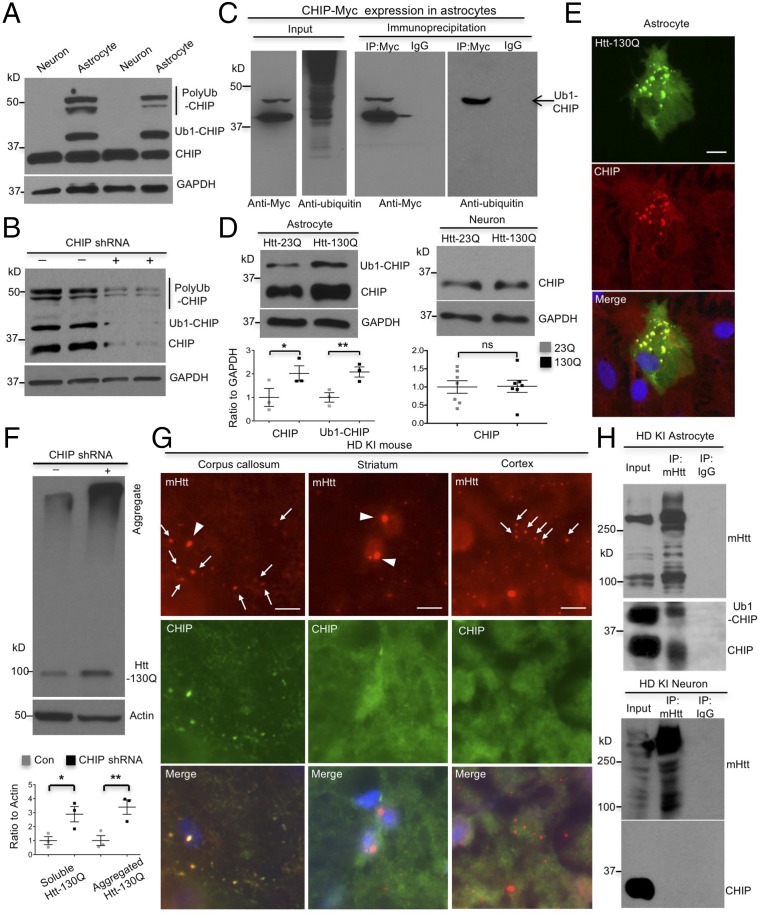
Our recent study showed that astrocytes degraded mutant huntingtin (mHtt), the HD protein, faster than neurons (12). Since CHIP is a cochaperone of Hsp70 with ubiquitin E3 ligase activity for degradation of misfolded proteins (7–9), we examined its expression in cultured astrocytes and neurons and found more abundant CHIP with a high molecular weight (MW) in astrocytes than neurons (Fig. 1A). This high-MW CHIP is reminiscent of monoubiquitinated CHIP (Ub1-CHIP) as reported previously (9) and consistently, is decreased by CHIP knockdown (KD) (Fig. 1B). To provide further evidence for the ubiquitination of the high-MW CHIP, we transfected CHIP-myc in cultured astrocytes and immunoprecipitated CHIP with anti-myc antibody. Immunoblotting with anti-ubiquitin to detect endogenous ubiquitin in immunoprecipitates clearly showed that the high-MW band represents monoubiquitinated CHIP in astrocytes (Fig. 1C).
Fig. 1.
CHIP is more actively involved in mHtt degradation in astrocytes. (A) More abundant monoubiquitinated CHIP (Ub1-CHIP) is present in cultured astrocytes than in neurons. (B) CHIP knockdown decreased Ub1-CHIP in astrocytes (n = 3 independent experiments). (C) Transfected CHIP-myc in cultured astrocytes was immunoprecipitated by anti-myc. Immunoblotting of the immunoprecipitates with anti-ubiqutitin indicated the presence of monoubiquitinated CHIP in astrocytes. (D) Expression of transfected mHtt (Htt-130Q) selectively increases the levels of CHIP and Ub1-CHIP in astrocytes but not in neurons [n = 3 independent experiments (astrocytes) and n = 5 independent experiments (neurons)]. (E) Immunofluorescent staining shows the colocalization of transfected mHtt (Htt-130Q) with endogenous CHIP in cultured astrocytes. (Scale bar, 5 μm.) (F) CHIP knockdown increases both soluble and aggregated mHtt (Htt-130Q) in astrocytes (n = 3 independent experiments). (G) Colocalization of CHIP with mHtt aggregates was detected in glial cells in the corpus callosum, but not in neuronal cells in the striatum and cortex, in HD140Q KI mice at the age of 12 mo. Arrowheads indicate nuclear mHtt aggregates; arrows indicate cytoplasmic aggregates in glial cells or neuropil mHtt aggregates in neurons. (Scale bar, 10 μm.) (H) Association of mHtt with CHIP and Ub1-CHIP was detected in HD140Q KI astrocytes but not in KI neurons. Quantitative data beneath the blots are represented as mean ± SEM (error bar). *P < 0.05, **P < 0.01 (unpaired two-tailed Student’s t test); ns, not significant.
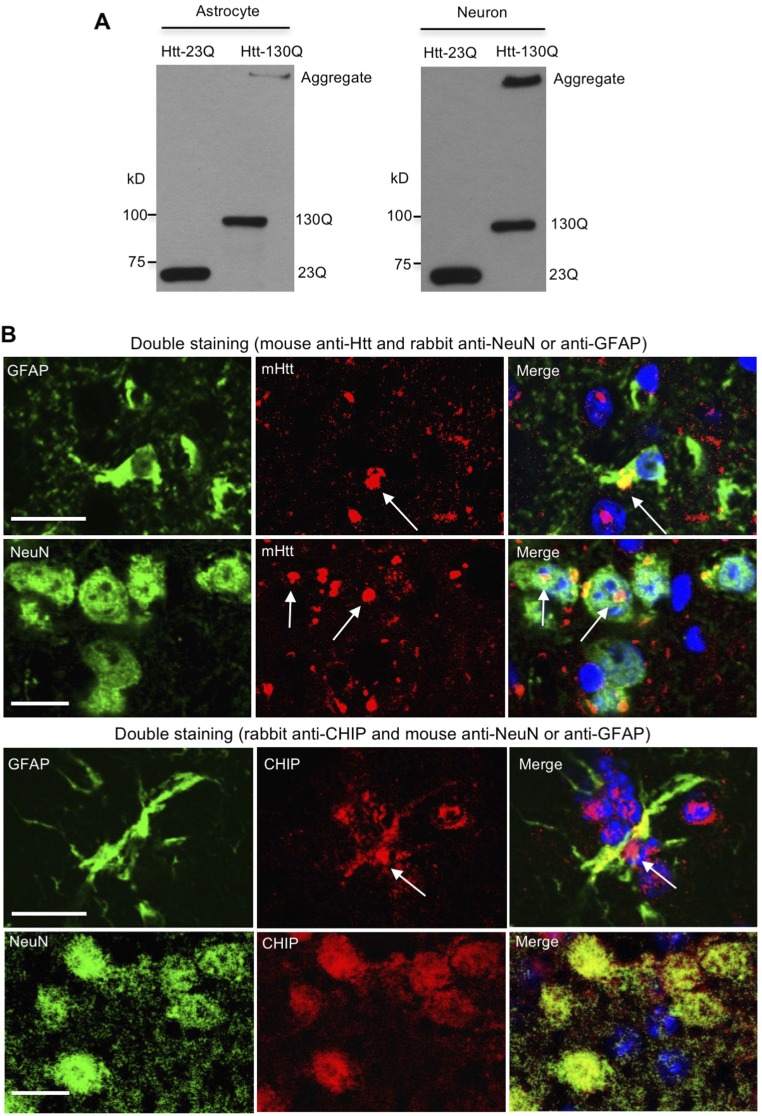
Because CHIP is monoubiquitinated when it polyubiquitinates misfolded proteins (9), increased Ub1-CHIP suggests that CHIP is more active in astrocytes than neurons tackling misfolded proteins. To test this idea, we transfected N-terminal Htt (1–230 amino acids), which contained normal 23 (Htt-23Q) or expanded 130 (Htt-130Q) glutamine repeats conjugated with the photoswitchable fluorescent protein Dendra2 (12, 13) into astrocytes and neurons. Compared with Htt-23Q, Htt-130Q increased the expression of CHIP and Ub1-CHIP in astrocytes but not in neurons (Fig. 1D and Fig. S1A), suggesting that CHIP plays a specific role in coping with mHtt in astrocytes. Indeed, we found that CHIP colocalizes with transfected Htt-130Q (Fig. 1E), and CHIP knockdown stabilized Htt-130Q in astrocytes (Fig. 1F). Furthermore, we found that in HD 140Q KI mouse model in which full-length mHtt is expressed at endogenous levels, CHIP is colocalized with mHtt aggregates in the corpus callosum (CC) where astrocytes are enriched (Fig. 1G). It has been reported that in the brains of HD patients and KI mice, large and round aggregates are localized in neuronal nuclei, and small, bead-like aggregates are localized in neuropils (14, 15). However, there is absence of CHIP in neuronal aggregates (Fig. 1G), suggesting that CHIP does not associate with mHtt in neurons. Double immunofluorescent staining was also unable to reveal CHIP aggregates in neuronal cells in KI mice (Fig. S1B). Furthermore, by immunoprecipitating endogenous mHtt from cultured neurons and astrocytes of KI mice, we also saw an association of mHtt with both CHIP and Ub1-CHIP in astrocytes, but not in neurons (Fig. 1H). Collectively, these data suggest that CHIP is more active to clear mHtt in astrocytes.
Fig. S1.
Expression of huntingtin in primary cultures and the brain of HD knockin mouse. (A) Wild-type (23Q) or mutant (130Q) huntingtin was transfected in astrocytes and neurons, and their expression was confirmed by Western blotting. (B) mHtt aggregates are formed in both NeuN-positive neurons and GFAP-positive astrocytes of 12-mo-old HD 140Q KI mice. However, CHIP puncta, which are indicative of localization of CHIP in mHtt aggregates, are found in astrocytes of the corpus callosum, but not in neurons of the striatum. (Scale bar, 10 μm.)
Active CHIP Promotes Ubiquitination of mHtt in Astrocytes.
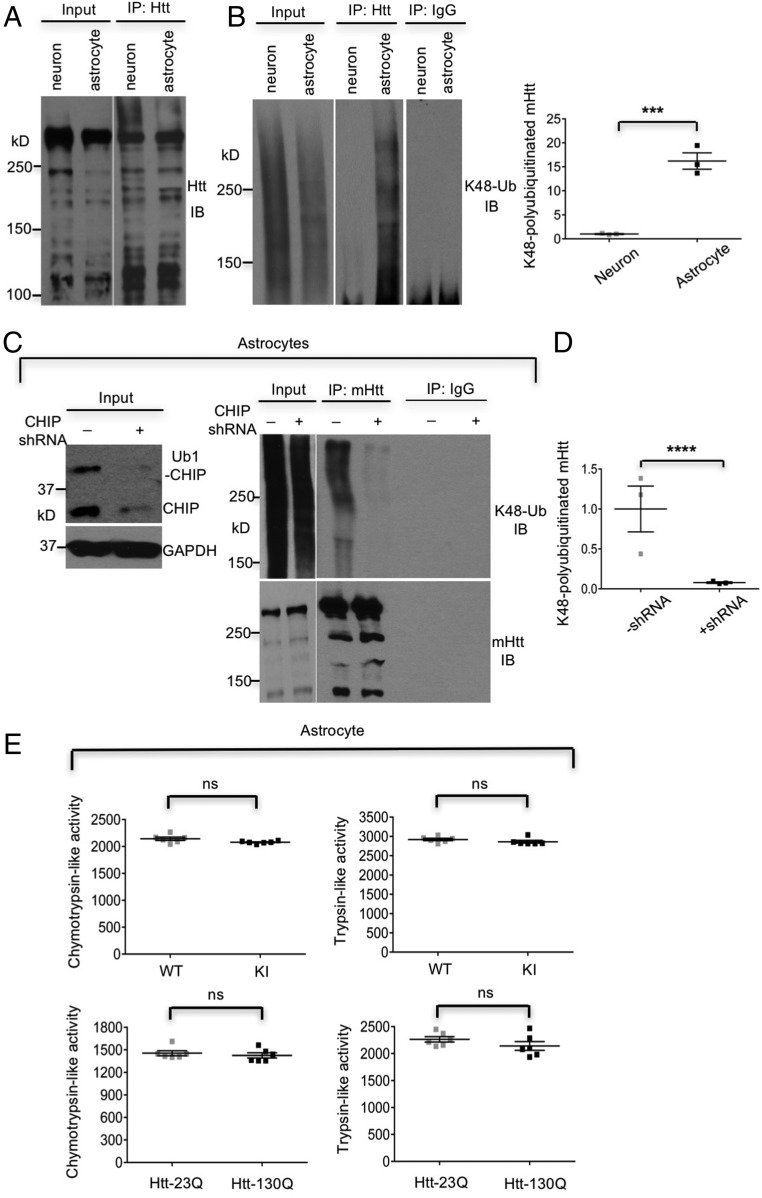
CHIP has ubiquitin E3 ligase activity and polyubiquitinates substrates for proteasomal degradation (7, 8). Indeed, our results indicated that astrocyte-specific association between CHIP and mHtt increased K48-linked polyubiquitinated mHtt in HD KI astrocytes (Fig. 2 A and B), which is corroborated by elimination of the enhanced K48-polyubiquitinated mHtt via CHIP knockdown (Fig. 2 C and D). Using in vitro proteasome activity assay, we ruled out the possibility that the increased K48-linked polyubiquitination of mHtt in astrocytes is due to mHtt-causing proteasomal dysfunction (Fig. 2E). Given that K48 polyubiquitination targets substrates for proteasomal degradation, increased K48-linked polyubiquitinated mHtt explains the faster clearance of mHtt in astrocytes (12).
Fig. 2.
CHIP enhances K48-linked polyubiquitination on mHtt in astrocytes. (A and B) There are more K48-polyubiquitinated mHtt in KI cortical astrocytes than KI neurons. Ratios of K48-polyubiquitinated mHtt to total immunoprecipitated mHtt are from three independent experiments. (C and D) CHIP knockdown suppressed K48-linked polyubiquitination on endogenous mHtt in HD140Q KI cortical astrocytes. Ratios of K48-polyubiquitinated mHtt to total immunoprecipitated mHtt are from three independent experiments. (E) Expression of mHtt in Htt-130Q transfected astrocytes and HD140Q KI astrocytes did not affect proteasomal function compared with Htt-23Q transfected or wild-type (WT) astrocytes (n = 3 independent experiments). In A–D, cultured cells were treated with lactacystin (10 μM for 6 h) to inhibit proteasomal degradation. ***P < 0.001, ****P < 0.0001. ns, not significant. Data are represented as mean ± SEM. Error bars indicate SEM.
Active CHIP Increases Hsp70 and Facilitates Degradation of Various Misfolded Proteins in Astrocytes.
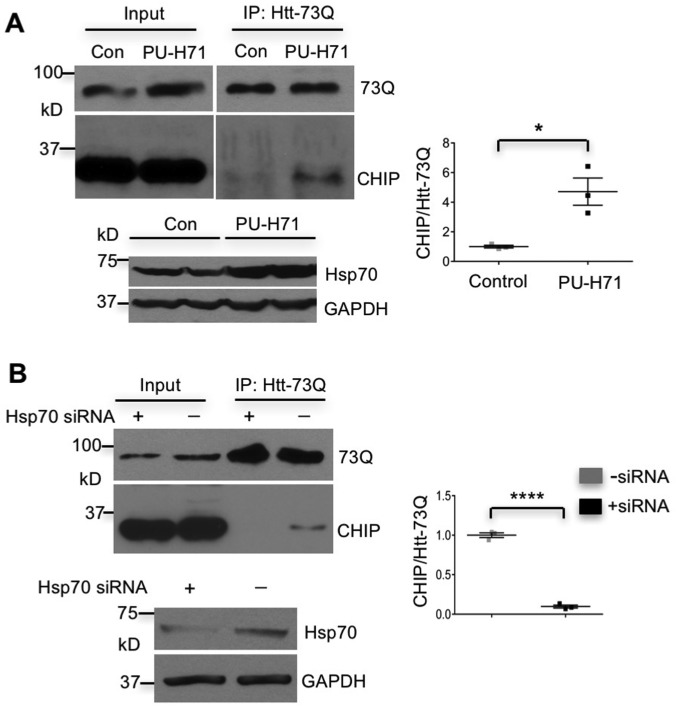
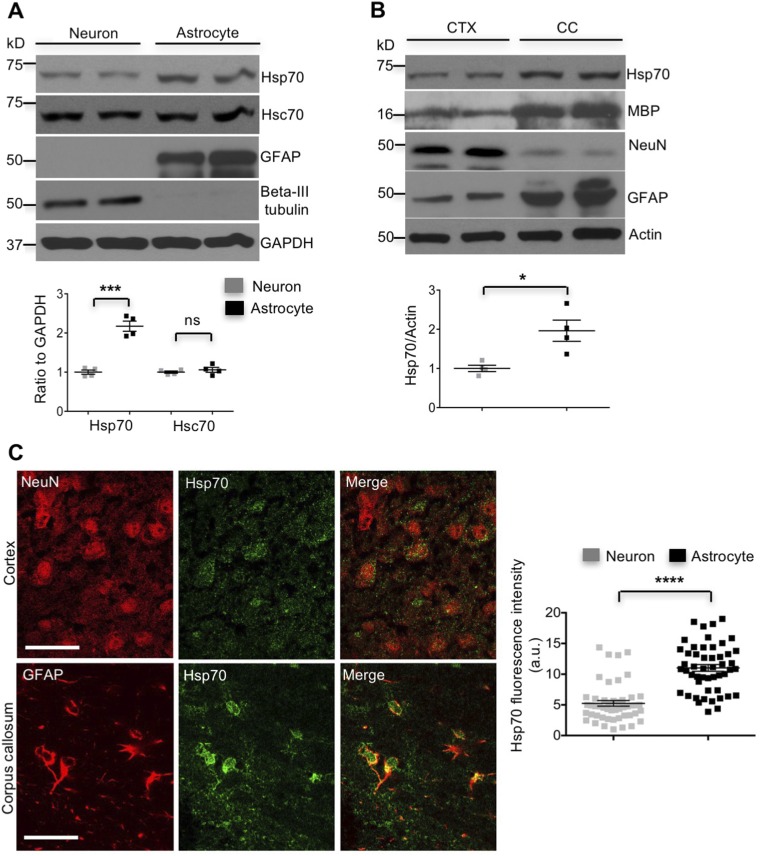
Although the binding of CHIP to substrates depends on Hsp70 (16, 17) and CHIP is able to enhance Hsp70 expression in cultured fibroblast cells in response to heat shock (18), its relation with Hsp70 in neuronal and glial cells remained elusive. By inducing Hsp70 in HEK293 cells with PU-H71, a compound that up-regulates Hsp70 transcription (19), we found that increased Hsp70 promoted the association of CHIP with transfected mHtt (Htt-73Q) and that this association was suppressed by Hsp70 knockdown (Fig. S2). Importantly, we also found that basal Hsp70 was more abundant in cultured astrocytes than neurons (Fig. S3A). To validate this difference in the brain, we isolated the CC, a brain region enriched in astrocytes, and the cerebral cortex (CTX) with less abundant astrocytes to examine Hsp70 levels. Western blotting also showed a higher level of basal Hsp70 in the astrocyte-enriched corpus callosum (Fig. S3B). Consistently, double immunostaining confirmed the higher level of Hsp70 in astrocytes than neurons in the mouse brain (Fig. S3C).
Fig. S2.
Hsp70-dependent association of CHIP with mHtt. (A) PU-H71 induction promoted the association of transfected Htt-73Q with CHIP (Upper) and also increased the expression of Hsp70 (Lower). The ratios of immunoprecipitated CHIP to Htt-73Q in transfected HEK293 cells untreated (control) or treated with PU-H71 from three independent experiments are also shown. (B) Knocking down Hsp70 via siRNA abolished the coimmunoprecipitation of Htt-73Q with CHIP (Upper) and diminished the level of Hsp70 in transfected HEK293 cells (Lower). Right shows the ratios of CHIP to Htt-73Q in coimmunoprecipitation from three independent experiments. *P < 0.05, ****P < 0.0001 (unpaired two-tailed Student’s t test). Data are represented as mean ± SEM. Error bars indicate SEM.
Fig. S3.
More abundant Hsp70 in astrocytes than in neurons. (A) Hsp70 is expressed at a higher level in wild-type cultured cortical astrocytes than neurons (n = 4 independent experiments). (B) The higher level of Hsp70 in the corpus callosum (CC) than in the cortex (CTX) of 3-mo-old wild-type mice (n = 4 independent experiments). (C) Levels of Hsp70 are higher in astrocytes than in neurons in the mouse brain (n = 3 mice) (Scale bar, 20 μm.) *P < 0.05, ***P < 0.001, ****P < 0.0001 (unpaired two-tailed Student’s t test); ns, not significant. Data are represented as mean ± SEM (error bar).
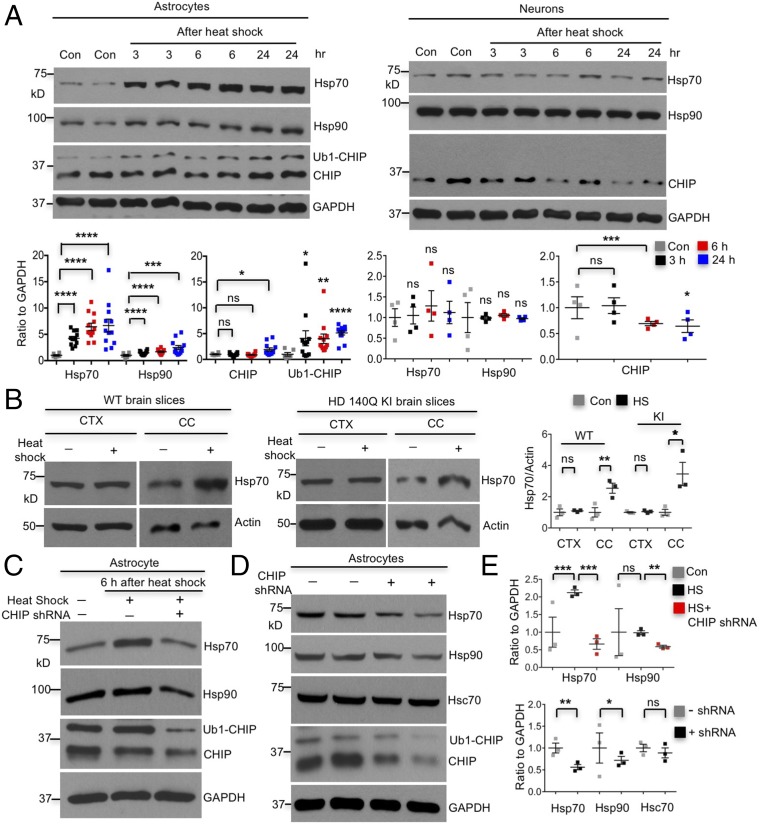
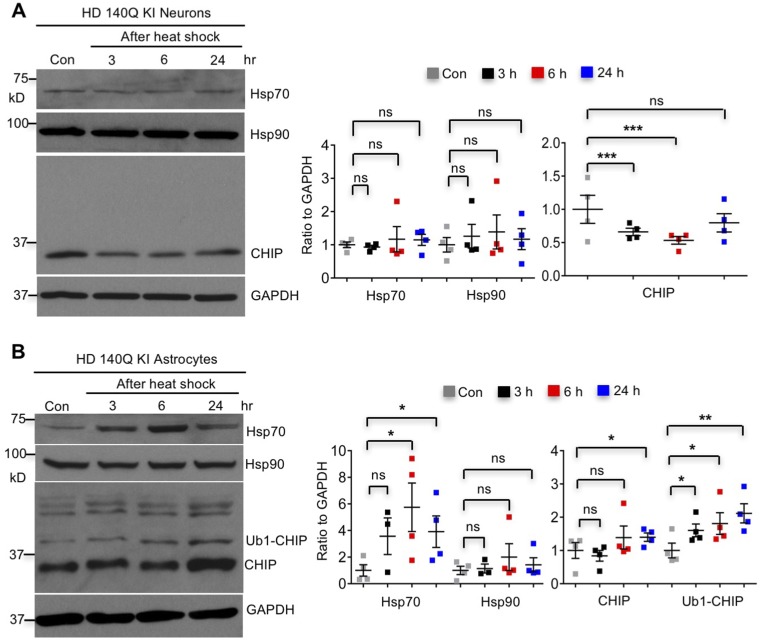
We then compared heat-shock responses in wild-type neurons and astrocytes under hyperthermia conditions and found robustly increased Hsp70 in astrocytes but not in neurons (Fig. 3A). In addition, expression of mHtt did not have an impact on these differential responses (Fig. S4). Strikingly, both CHIP and Ub1-CHIP were increased in wild-type and HD 140Q KI astrocytes, whereas CHIP declined in neurons after heat shock (Fig. 3A and Fig. S4). To verify that the different Hsp70 responses in cultured neurons and astrocytes also occur in brain tissues, we isolated brain slices from wild-type and HD140Q KI mice. The brain slices consisting of the cerebral cortex and corpus callosum were incubated under normal physiological or heat-shock stress conditions. The cortex and corpus callosum were then dissected for Western blotting. Consistently, hyperthermia induced Hsp70 in glia- but not neuron-enriched regions in both wild-type and HD140Q KI mouse brains (Fig. 3B). The differential heat-shock response found here is consistent with previous findings that heat-shock response is absent in neurons, but robust in astrocytes (20–22).
Fig. 3.
Differential CHIP activity causes distinct heat-shock responses in astrocytes and neurons. (A) Typical heat shock (42 °C for 1 h) induced Hsp70, Hsp90, CHIP, and Ub1-CHIP specifically in wild-type cortical astrocytes around 30 days in vitro (DIV 30) (Left), but not in wild-type cortical neurons at DIV 10–14 (Right) (at least three independent experiments). (B) Heat-shock stress increased Hsp70 in the corpus callosum, but not in the cortex, of brain slices from wild-type (WT) and HD140Q KI mice at the age of 3 mo (n = 3 independent experiments). CC, corpus callosum; CTX, cortex; HS, heat shock. (C) Induction of Hsp70 by heat shock (42 °C, 1 h) was completely inhibited by CHIP knockdown, and no Hsp90 was induced in wild-type astrocytes at DIV 45–60. (D) CHIP knockdown decreased the expression of Hsp70/Hsp90, but did not affect levels of Hsc70 in DIV 45–60 wild-type astrocytes. (E) Ratios of indicated proteins to GAPDH are from three independent experiments (Upper for C and Lower for D). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (unpaired two-tailed Student’s t test). ns, not significant. Data are represented as mean ± SEM (error bar).
Fig. S4.
Increased CHIP, monoubiquitinated CHIP, and Hsp70 after heat shock specifically in HD140Q KI astrocytes. (A) Hsp70 and Hsp90 were not inducible in cultured KI neurons at DIV 10–14 by 30 min of heat-shock stress, and CHIP decreased after heat shock (n = 4 independent experiments). (B) Heat shock for 30 min was able to induce Hsp70, CHIP, and Ub1-CHIP, but not Hsp90, in cultured KI astrocytes at DIV 21–30 (n = 4 independent experiments). *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired two-tailed Student’s t test); ns, not significant. Data are represented as mean ± SEM. Error bars indicate SEM.
The exact mechanism underlying distinct heat-shock responses from astrocytes and neurons is unknown. We postulated that differential activities of CHIP in astrocytes and neurons cause different heat-shock responses. Indeed, CHIP knockdown abolished heat-shock induction of Hsp70 in astrocytes (Fig. 3 C and E), confirming that the specific induction of Hsp70 by heat-shock stress in astrocytes is CHIP mediated. Furthermore, CHIP knockdown also decreases the basal levels of Hsp70/Hsp90 but not Hsc70 in astrocytes (Fig. 3 D and E), which is the opposite of previous findings that CHIP knockdown enhanced the basal Hsp70 in immortal cell lines (18). The basal levels of Hsp70 may be various in different types of cells. The finding that CHIP is more active in astrocytes also explains why astrocytes have the higher basal levels of Hsp70 than neurons. Together, our results suggest that CHIP-dependent up-regulation of Hsp70 in astrocytes contributes to the preferential and Hsp70-dependent binding of CHIP to mHtt in astrocytes.
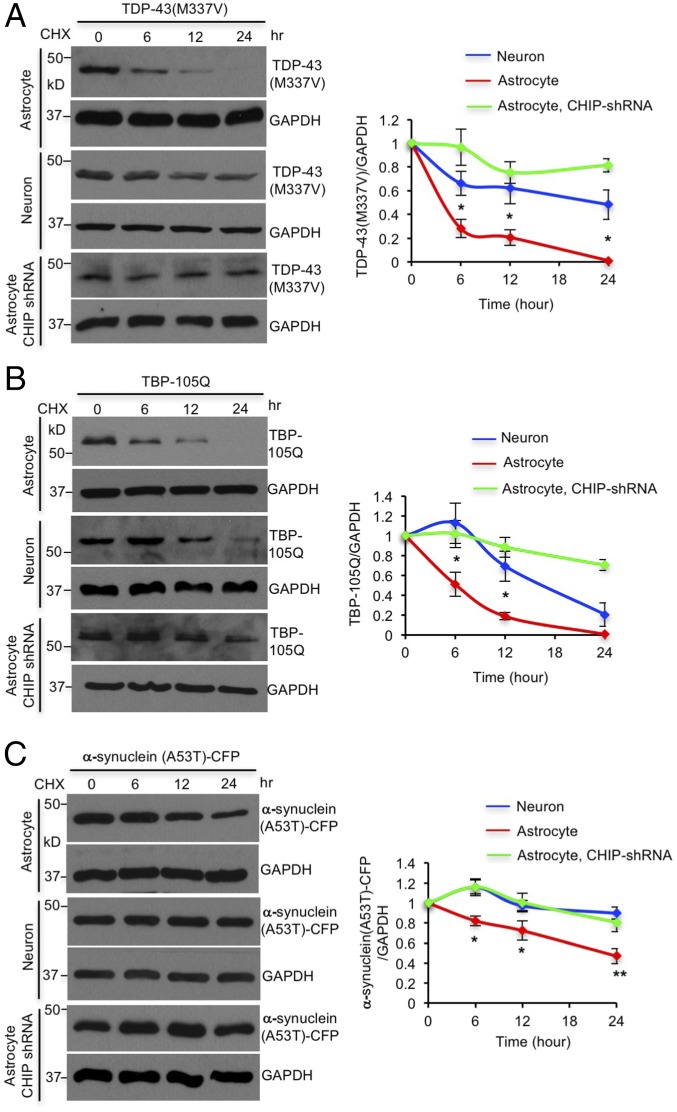
To investigate whether CHIP also promotes the clearance of other misfolded proteins in astrocytes, we transfected mutant TDP-43 (M337V), polyQ expanded TATA box binding protein (TBP-105Q), or mutant α-synuclein (A53T), in astrocytes and neurons, as these mutant proteins cause neurodegenerative diseases (23, 24). Cycloheximide (CHX) half-life assay demonstrated that all these mutant proteins were removed faster by astrocytes than neurons, which was abolished by CHIP knockdown (Fig. 4). These results suggest that astrocytes use CHIP to accelerate the degradation of misfolded proteins, which protects astrocytes from misfolded proteins in neurodegenerative diseases.
Fig. 4.
CHIP mediates faster degradation of misfolded proteins in astrocytes. (A–C) Mutant TDP-43 (M337V), TBP-105Q, and α-synuclein (A53T)-cyan fluorescent protein (CFP) were degraded faster by astrocytes than neurons, which was eliminated by CHIP knockdown in astrocytes. *P < 0.05, **P < 0.01 (unpaired two-tailed Student’s t test). ns, not significant. Data are represented as mean ± SEM (error bar).
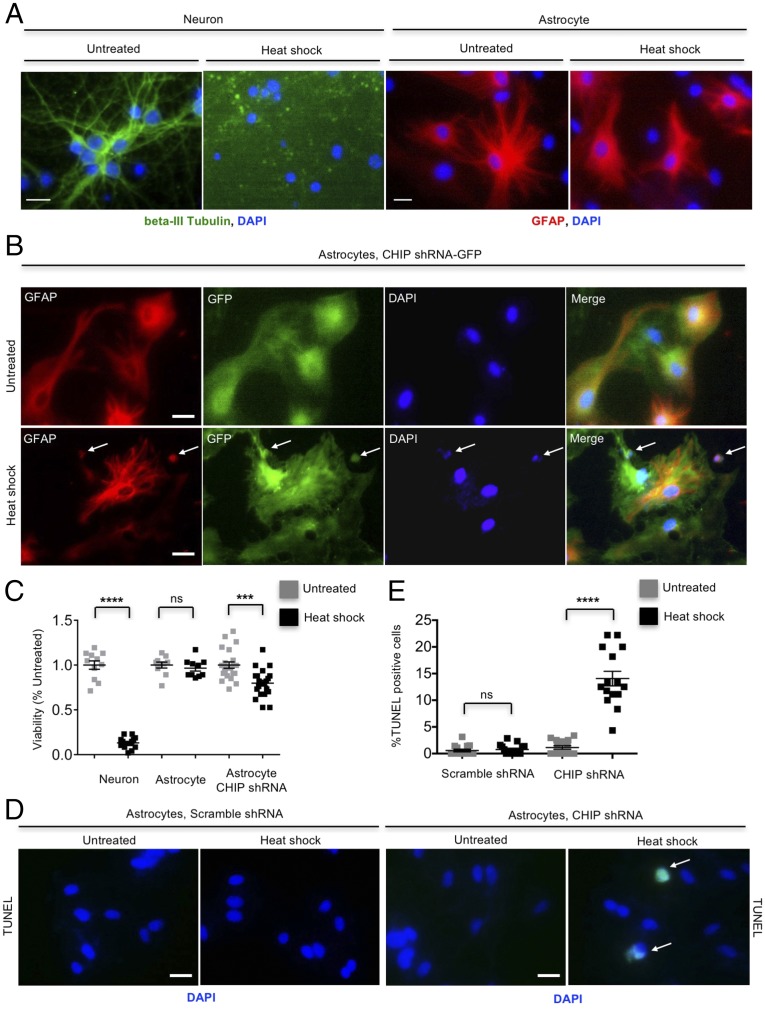
We further investigated the role of CHIP in viability of astrocytes under severe heat-shock stress (45 °C for 30 min). Astrocytes were resistant to heat-shock stress, which was indicated by no difference in viability and morphology between untreated and hyperthermia-treated astrocytes (Fig. 5 A and C). In contrast, ∼86% neurons were killed by the same hyperthermia condition (Fig. 5 A and C). However, the lethality of astrocytes after hyperthermia was increased when CHIP was knocked down (Fig. 5 B–E), indicating that the high activity of CHIP is involved not only in the prompt clearance of mHtt, but also in protection of cell viability.
Fig. 5.
High activity of CHIP contributes to astrocytic resistance to hyperthermia. (A) Apparent demise of cultured cortical neurons at DIV 10–12 after heat-shock stress (45 °C, 30 min). The same heat-shock stress (45 °C, 30 min) did not affect viability of cultured cortical astrocytes at DIV 40–45. Neurons and astrocytes were fixed and immunostained at 24 h after heat shock. (B) CHIP knockdown caused death of a portion of CHIP shRNA-transfected astrocytes, which was characterized by shrunken nucleus, at 24 h after heat shock (45 °C, 30 min). Arrows indicate corpse of astrocytes. (C) Quantitative results of Fig. 5 A and B. At least 10 visual fields were randomly picked up for each group, and the live cells in each visual field were quantified. (D and E) TUNEL assay showing increased susceptibility of CHIP knockdown astrocytes to heat-shock stress (45 °C, 30 min). Arrows indicate apoptotic astrocytes (TUNEL-positive cells indicated by green fluorescence). ***P < 0.001, ****P < 0.0001 (unpaired two-tailed Student’s t test); ns, not significant. Data are represented as mean ± SEM. Error bars indicate SEM. (Scale bar, 10 μm.)
Differential HspBP1 Expression Results in Distinct CHIP Activity Between Astrocytes and Neurons.
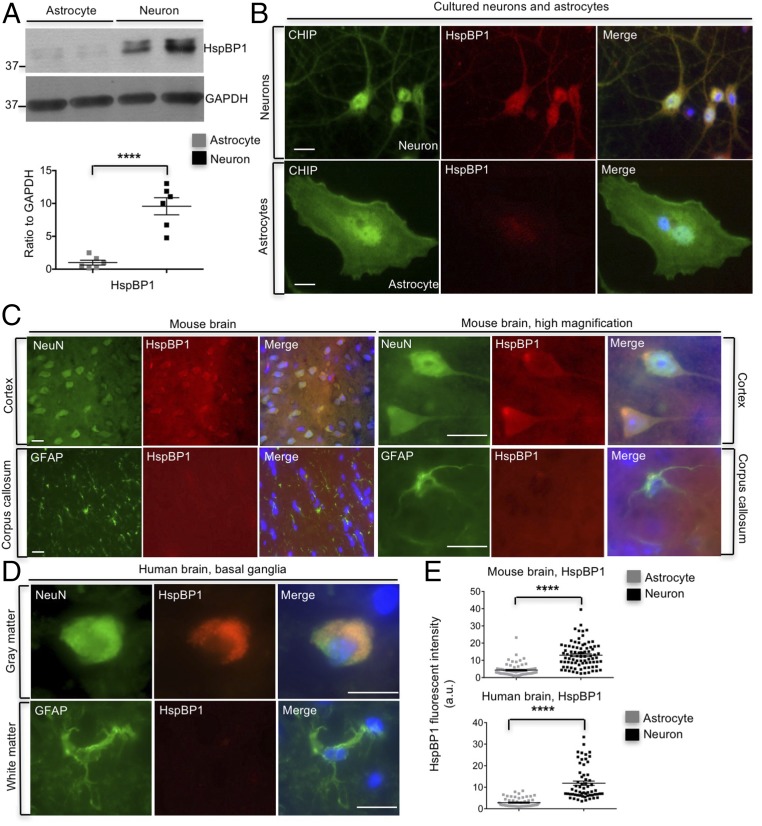
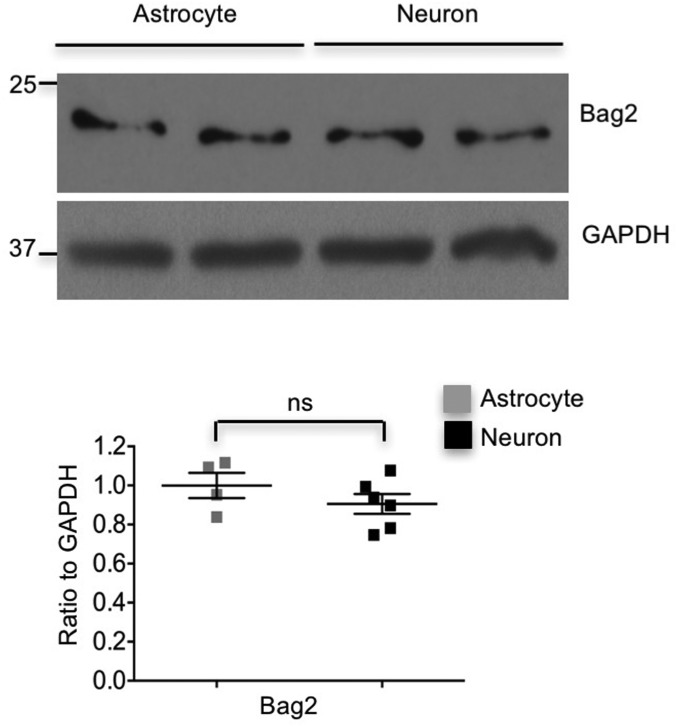
Our findings raise a key question of why CHIP is more active in astrocytes. Using RNA-seq database to compare the profiles of gene expression between neurons and astrocytes (25), we found that HspBP1, a cochaperone of Hsp70 that inhibits CHIP E3 ligase activity (11), shows approximately fivefold increase at the transcriptional level in neurons compared with astrocytes. The different expression level of HspBP1 was first confirmed with Western blotting, showing the abundant level of HspBP1 in cultured neurons but the almost undetectable level in cultured astrocytes (Fig. 6A). In addition, HspBP1 is distributed throughout the neurons, and its cellular localization is quite consistent with that of CHIP (Fig. 6B). In contrast, only faint or background staining of HspBP1 was seen in astrocytes (Fig. 6B). Since Bag2 has been reported to be another CHIP inhibitor, we also examined the level of Bag2 and found there was no difference between neurons and astrocytes, suggesting that Bag2 is not the determinant of the high activity of CHIP in astrocytes (Fig. S5). Furthermore, we performed HspBP1 immunostaining of mouse brains, in which neurons and astrocytes were labeled respectively with antibodies to cell-specific markers, NeuN and glial fibrillary acidic protein (GFAP). Indeed, there was more HspBP1 staining in neurons, whereas HspBP1 was almost undetectable in astrocytes (Fig. 6 C and E). Consistently, in the brain section of human basal ganglia, human neurons exhibited a considerable amount of HspBP1, whereas human astrocytes lacked HspBP1 staining (Fig. 6 D and E). Together, our results indicate intrinsic and significant difference of HspBP1 expression in neurons and astrocytes, which may account for differential CHIP activities in astrocytes and neurons.
Fig. 6.
Deficient expression of HspBP1 in astrocytes. (A) Higher levels of HspBP1 in cultured cortical neurons than astrocytes were shown by Western blotting (n = 3 independent experiments). (B) Representative images of immunostaining showed differential expression levels of HspBP1 between cultured neurons and astrocytes. (Scale bar, 5 μm.) (C) Fluorescent immunostaining revealing greater abundance of HspBP1 in neurons than astrocytes in mouse brain. (Scale bar, 20 μm.) (D) Immunostaining demonstrated more HspBP1 expression in neurons in the gray matter of human basal ganglia than that in astrocytes in the white matter of basal ganglia. (Scale bar, 20 μm.) (E) Statistical results for C and D [mice n = 3, human n = 2 (57 y)]. ****P < 0.0001 (unpaired two-tailed Student’s t test). Data are represented as mean ± SEM (error bar).
Fig. S5.
Expression of Bag2 in neurons and astrocytes. The levels of Bag2 do not show difference between cultured neurons and astrocytes. Data are represented as mean ± SEM (error bar). ns, not signficant.
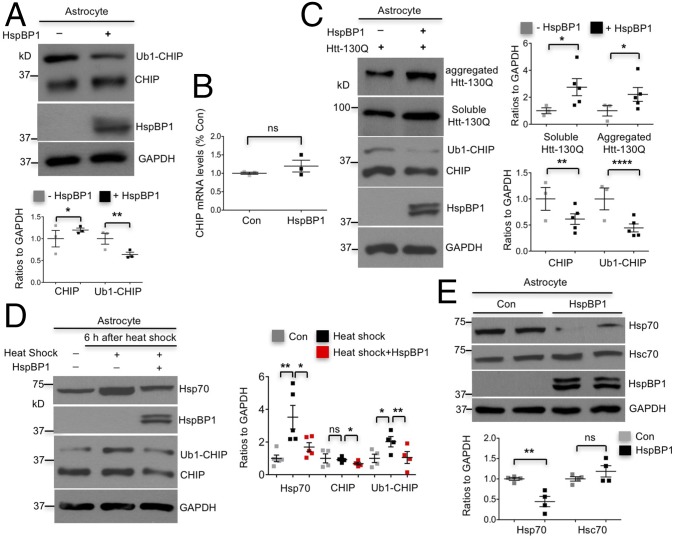
To investigate whether expression of HspBP1 could inhibit CHIP activation and CHIP-mediated degradation of mHtt in astrocytes, we transfected HspBP1 into cultured astrocytes. Overexpression of HspBP1 reduced Ub1-CHIP and increased the unmodified form of CHIP correspondingly (Fig. 7A). This blockade of conversion of CHIP to Ub1-CHIP suggests an inhibition of CHIP activity by HspBP1 in astrocytes. In addition, increased unmodified CHIP was not caused by alteration in CHIP expression since HspBP1 overexpression did not affect the transcription of CHIP in astrocytes (Fig. 7B). Furthermore, cotransfection of HspBP1 and mHtt (Htt-130Q) into cultured astrocytes stabilized mHtt, suggesting that CHIP-mediated degradation of mHtt was inhibited by HspBP1. In addition, HspBP1 suppressed mHtt-induced increases in CHIP and Ub1-CHIP, which is evidenced by decreased Ub1-CHIP and CHIP in cotransfected astrocytes (Fig. 7C).
Fig. 7.
Deficient expression of HspBP1 in astrocytes contributes to high activity of CHIP in astrocytes. (A) Expression of HspBP1 in cultured astrocytes reduced the level of Ub1-CHIP, and major form of CHIP increased correspondingly (n = 3 independent experiments). (B) qRT-PCR showed that overexpression of HspBP1 did not influence transcription of CHIP in cultured astrocytes (n = 3 independent experiments). (C) HspBP1 expression in cultured astrocytes stabilized aggregated and soluble mHtt and suppressed induction of CHIP and Ub1-CHIP by mHtt, which was seen in Fig. 1D (n = 3 independent experiments). (D) Induction of Hsp70, CHIP, and Ub1-CHIP by heat-shock stress at 42 °C for 1 h was significantly inhibited by HspBP1 expression in DIV 30–50 astrocytes (n = 4 independent experiments). (E) Expression of HspBP1 decreased basal levels of Hsp70 in astrocytes (n = 3 independent experiments). *P < 0.05, **P < 0.01, ****P < 0.0001 (unpaired two-tailed Student’s t test). Data are represented as mean ± SEM (error bar).
Given that CHIP is required for heat-shock response in astrocytes (Fig. 3C), we also investigated whether expression of HspBP1 could inhibit heat-shock response via suppressing CHIP activity. Indeed, HspBP1 suppressed heat-shock induction of Hsp70, CHIP, and Ub1-CHIP in astrocytes (Fig. 7D). Moreover, HspBP1 reduced Hsp70, but not Hsc70, under physiological conditions (Fig. 7E). Taken together, the inhibitory effect of HspBP1 on CHIP-mediated protective cellular processes in astrocytes supports our conclusion that lack of HspBP1 accounts for the high activity of CHIP in astrocytes.
Silencing HspBP1 Expression Activated CHIP in Neurons and Ameliorated Neuropathology.
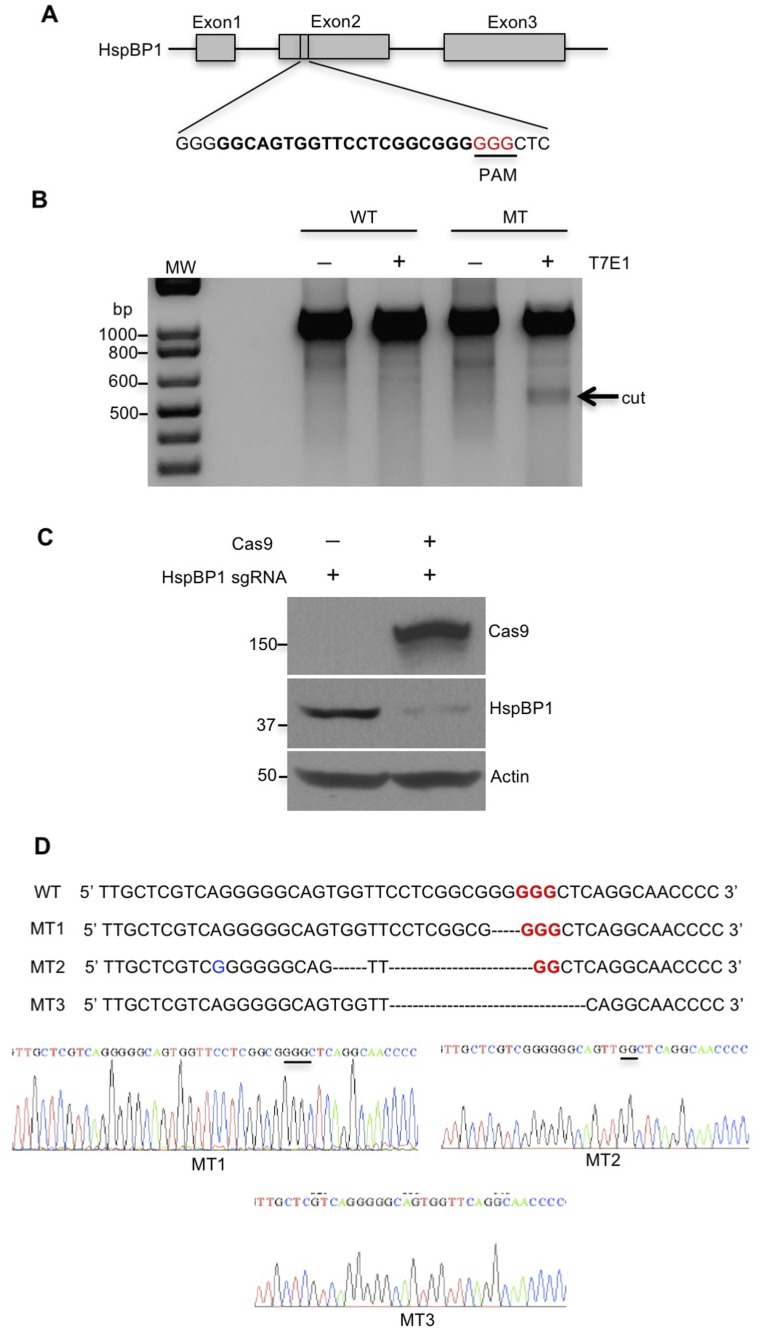
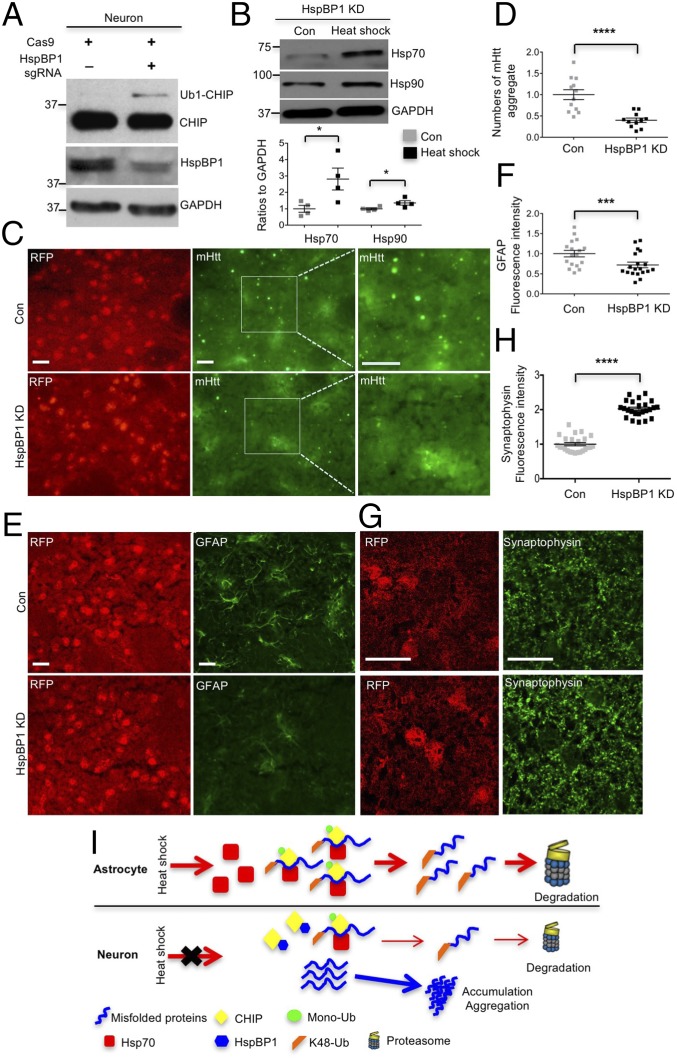
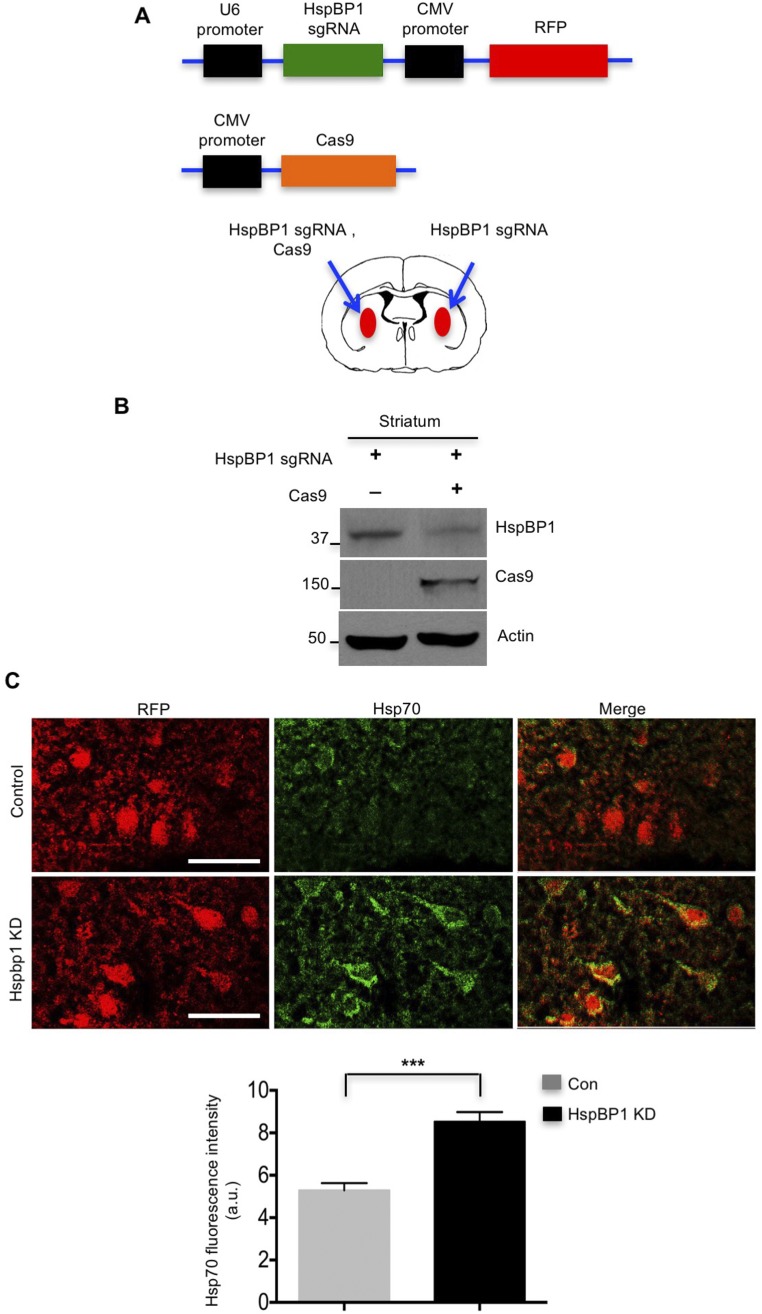
If the expression of HspBP1 in neuronal cells is responsible for the increased accumulation of misfolded proteins, knocking down HspBP1 in neurons should alleviate misfolded proteins causing neuropathology. Thus, we used CRISPR-Cas9 to target the HspBP1 gene (Fig. S6A) and silence the expression of HspBP1 in the striatum of HD 140Q KI mice via stereotaxic injection of adeno-associated viruses (AAVs) expressing Cas9 and HspBP1 sgRNA. T7E1 assay and Western blotting validated the genome editing of HspBP1 sgRNA/Cas9 and efficient reduction of HspBP1 at the protein level in N2a cells, respectively (Fig. S6 B and C). The targeted HspBP1 DNA was also subcloned for sequencing, which verified Cas9/gRNA-mediated mutations in the HspBP1 gene (Fig. S6D). Furthermore, HspBP1 KD induced the expression of Ub1-CHIP in cultured neurons, suggesting increased activity of CHIP (Fig. 8A). In addition, Hsp70/Hsp90 were induced by heat shock in HspBP1 knockdown neurons (Fig. 8B). To knock down HspBP1 in the HD KI mouse brain, both HspBP1 sgRNA and Cas9 were transduced into the mouse brain striatal region via stereotaxic injection (Fig. S7A). Four to 6 wk after injection of AAV-HspBP1 sgRNA and AAV-Cas9, HspBP1 was markedly decreased in the striatum of HD KI mice compared with the control injected only with AAV-HspBP1 sgRNA (Fig. S7B). In this experiment, cells expressing AAV-HspBP1 sgRNA could be identified as red fluorescent protein (RFP) cells. Consequently, Cas9-mediated HspBP1 knockdown markedly increased Hsp70 in RFP-positive cells (Fig. S7C) and reduced mHtt aggregates in the striatum (Fig. 8 C and D). Astrogliosis is characterized by increased GFAP staining and is an early neuropathological event caused by neuronal injury in HD KI mouse brains (26). Reducing mHtt accumulation in neuronal cells via knocking down HspBP1 attenuated the extent of reactive astrocytes (Fig. 8 E and F). Synaptophysin is a presynaptic marker protein whose loss is also found in HD KI mice (27). HspBP1 knockdown restored the intensity of immunostaining of synaptophysin in the striatum of HD KI mice (Fig. 8 G and H). All these results suggest that knocking down HspBP1 in neuronal cells could increase CHIP activity, enhance the clearance of mHtt, and subsequently reduce mHtt-mediated neuropathology. Based on our findings, we propose that the high-level expression of HspBP1 in neurons accounts for the preferential accumulation of misfolded proteins in neurons and their greater vulnerability than astrocytes to misfolded proteins (Fig. 8I).
Fig. S6.
Genome-editing activity of HspBP1 sgRNA. (A) For increasing specificity and decreasing off-target binding, we used Target Finder, which is generated by Feng Zhang’s laboratory, Massachusetts Institute of Technology, Cambridge, MA to design gRNA. The chosen target sites of sgRNA are shown in bold font, and the PAM sequence is highlighted in red. (B) Genome-editing activity of HspBP1 sgRNA was tested using T7E1 assay. N2a cells were cotransfected with HspBP1 sgRNA and Cas9 plasmids or transfected only with HspBP1 sgRNA plasmid as control. The T7E1 assay was carried out using PCR products of genome DNA from transfected N2a cells. The arrow indicates cleaved PCR products by T7E1 enzyme. MT, mutant; WT, wild type. (C) Western blotting assay showed knockdown of HspBP1 at the protein level in N2a cells that were cotransfected with HspBP1 sgRNA and Cas9 plasmids. (D) Mutations in target HspBP1 gene were identified by TA cloning and subsequent sequencing.
Fig. 8.
Knocking down HspBP1 is neuroprotective in HD 140Q KI mice. (A) HspBP1 knockdown (KD) in cultured neurons induced Ub1-CHIP expression. (B) Hsp70/90 were induced 6 h after heat shock (42 °C for 1 h) in HspBP1 knockdown neurons (n = 3 independent experiment). (C–H) HspBP1 KD decreased mHtt aggregates and reactive astrocytes, rescued the loss of synaptophysin in the striatum of HD140Q KI mice at the age of 13–14 mo, compared with the injection with HspBP1 sgRNA alone [control (Con)]. *P < 0.05, ***P < 0.001, ****P < 0.0001 (unpaired two-tailed Student’s t test). (Scale bar, 20 μm.) Data are represented as mean ± SEM (error bar). (I) A proposed model for the differential accumulation and toxicity of misfolded proteins in neurons and astrocytes: CHIP is more active and monoubiquitinated in astrocytes due to deficient HspBP1 expression. Activated CHIP preferentially binds to and polyubiquitinates misfolded proteins and confers higher Hsp70, resulting in the prompt proteasomal degradation of misfolded proteins in astrocytes.
Fig. S7.
Knocking down HspBP1 in the mouse striatum using CRISPR-Cas9. (A) Schematic representation of DNA constructs of HspBP1 sgRNA and Cas9. RFP is expressed under the cytomegalovirus (CMV) promoter in the same AAV vector for expressing HspBP1 sgRNA. Viral injection of HspBP1 sgRNA and Cas9 into one striatum of HD 140Q KI mice was performed to knock down endogenous HspBP1, and injection of HspBP1 sgRNA alone into the striatum of the other hemisphere served as a control. (B) In the striatum of HD140Q KI mice, HspBP1 knockdown by CRISPR-Cas9 was validated by Western blotting. (C) Knocking down HspBP1 enhances the level of Hsp70 in the striatum of HD 140Q KI mice (n = 3 mice). RFP labeling indicates cells expressing HspBP1 sgRNA. (Scale bar, 20 μm.) ***P < 0.001 (unpaired two-tailed Student’s t test). Data are represented as mean ± SEM (error bar).
Discussion
Although misfolded proteins are ubiquitously expressed in neuronal and glial cells in the brain, they are more readily to accumulate in neurons and kill neuronal cells (2). This differential vulnerability to misfolded proteins is likely due to distinct capacities of maintaining protein homeostasis between neuronal and glial cells. However, whether and how neuronal and glial cells have differential capacities to maintain protein folding and to prevent protein misfolding have not been rigorously investigated. Our findings demonstrate for the first time that the higher activity of CHIP in astrocytes than neurons is related to monoubiquitinated CHIP. Additional new finding is that the differential HspBP1 expression accounts for different CHIP/Hsp70 activities in neurons and astrocytes.
Our results also revealed two major functional consequences of increased CHIP activity in astrocytes, both of which account for their faster degradation of misfolded proteins. First, CHIP associates with mHtt specifically in astrocytes, resulting in polyubiquitination and subsequent proteasomal degradation of mHtt. The increased mHtt ubiquitination in astrocytes explains why astrocytes clear mHtt more efficiently than neurons (12). In support of this idea, clearance of several other neurodegeneration-related misfolded proteins is also CHIP dependent in astrocytes. Second, active CHIP enhances both basal and inducible levels of Hsp70 in astrocytes, and increased Hsp70 prevents aggregation of misfolded proteins and facilitates the binding of CHIP to misfolded proteins.
Heat-shock response plays a vital role in maintaining cellular homeostasis. Chaperone network is extremely sophisticated and well coordinated. For example, CHIP activates HSF1 (28) but is inhibited by HspBP1 (11). Our findings provide compelling evidence that HspBP1 is differentially expressed in neurons and astrocytes, which accounts for cell-type-dependent regulation of CHIP and Hsp70 activities. Consistent with this idea, expression of HspBP1 attenuates CHIP-dependent protective effects in astrocytes. Conversely, silencing HspBP1 expression with CRISPR-Cas9 in neurons activated CHIP and alleviated neuropathology in the HD knockin mouse model.
Our findings offer a mechanistic insight into the phenomenon that astrocytes, the largest cell population in the brains, are able to clear misfolded protein more efficiently so that they are affected to a much lesser extent than neurons in a variety of neurodegenerative diseases (3). Since CHIP is an ubiquitin E3 ligase that bridges the chaperone system and the UPS (7, 8), the increased CHIP activity may also contribute to the higher proteasomal activity in astrocytes than neurons (29). The finding that inhibition of HspBP1 in HD140Q KI mouse brain can diminish mHtt aggregation and neuropathology also suggests the therapeutic benefits by removing endogenous inhibition of CHIP activity in neuronal cells, which can be an alternative approach to treat a wide range of neurodegenerative diseases that are caused by the accumulation of misfolded proteins in neuronal cells.
Materials and Methods
Animals.
Full-length mHtt CAG140Q (HD KI) mice were kindly provided by Michael Levine, University of Caifornia Los Angeles (30) and maintained at the Emory University animal facility. This study was carried out in strict accordance with the recommendations in the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of Emory University (permit number: 2003631).
Stereotaxic Injection of Viral Vectors.
Mice were anesthetized with an i.p. injection of Avertin (0.5 mg/g). Their heads were placed and fixed in a Kopf stereotaxic frame (model 1900) equipped with a digital manipulator, and a UMP3-1 Ultra pump. Mice were kept deeply anesthetized as assessed by monitoring pinch withdrawal and respiration rate. Viral vector injections were given in the striatum (0.6 mm anterior to bregma, 2.0 mm lateral to the midline, and 3.5 mm ventral to dura). The injections were performed at a rate of 0.2 μL/min. The needle was left in place for 10 min after each injection to minimize upward flow of viral solution after raising the needle.
Additional information is provided in SI Materials and Methods.
SI Materials and Methods
Plasmids, Antibodies, and Reagents.
Htt-23Q, -73Q, and-130Q were generated by subcloning N-terminal fragments of huntingtin (1–230 amino acids) containing 23Q, 73Q, or 130Q into pDendra2-N (Clontech) using SalI and ApaI cloning sites. Murine HspBP1 was cloned into a pRK5 vector using BamH1 and EcoR1 cloning sites. sgRNA backbone vector and Cas9 plasmids were purchased from Addgene. Synthetic HspBP1 target site sequence (20 bp) was cloned into the sgRNA backbone vector using Sap1 sites to generate HspBP1 sgRNA plasmid. TDP-43 (M334V), TBP-105Q, and α-synuclein (A53T)-CFP plasmids were generated in our previous studies (23, 31). Mouse CHIP-myc vector was obtained from Origene (MR204258). Antibodies used were anti-CHIP (PA5-32046, Thermo), anti-expanded polyQ (1C2) (MAB1574, Millipore), anti-ubiquitin (7780, Abcam; z0458, Dako), anti-ubiquitin, K48-specific (05–1307, Millipore), anti-huntingtin (mEM48), anti-Hsp70 (4872S, Cell Signaling; sc-24, Santa Cruz), anti-Hsc70 (sc-7298, Santa Cruz), anti-Hsp90 (4874S, Cell Signaling), anti-TDP-43 (H00023435-M01, Abnova), anti-TBP (70102, QED Bioscience), anti-CFP (632381, Clontech), anti-HspBP1 (sc-390467, Santa Cruz), anti-Cas9 (MAC133, Millipore), anti-GFAP (RB-087-A1, MA5-12023, Thermo), anti-NeuN (ABN78, MAB377, Millipore), anti-synaptophysin (04–1019, Millipore), anti-MBP (AB980, Millipore), anti-Bag2 (29-709, ProSci), anti-beta III tubulin (AB9354, Millipore), anti-GAPDH (MA1-10036, Thermo), anti-Myc (2276S, 2272S, Cell Signaling), and anti–β-actin (A5060, Sigma). Secondary antibodies were HRP-labeled donkey anti-mouse, donkey anti-rabbit, donkey anti-mouse Alexa Fluor 488 or 594, and donkey anti-rabbit Alexa Fluor 488 or 594 from Jackson ImmunoResearch. Lactacystin, PU-H71, PYR-41, and cycloheximide (CHX) were purchased from Sigma, as were proteinase inhibitor mixtures.
Knockdown Assay.
The Hsp70 siRNA duplexes and negative control siRNA were purchased from Origene (SR418598). The following combination of oligonucleotides was used to target the Hsp70 gene: rGrUrCrUrUrArArArCrArArArCrGrUrCrUrUrGrGrCrArCTG, rGrGrCrArCrCrGrArUrUrArCrUrGrUrCrArArGrGrUrUrATT, and rUrGrGrGrAr
ArGrArCrArUrArUrArGrUrCrUrArGrCrUrGCC. The shRNA of CHIP and negative control shRNA were products of Origene (TL503190). The combination of plasmids carrying the following oligonucleotides were transfected into cells: GCCTGCTACGGCCGCGCCATCACTCGGAA, GCTAAGAAGAAGCGCTGGA
ACAGTATCGA, ATCCACCAGGAGAGTGAGCTGCATTCATA, and CATTGA
CGCTTTCATCTCTGAGAACGGCT.
Cell Cultures.
Brains of postnatal (days 1–3) murine pups were used for culturing cortical astrocytes. Following dissection, cortex was subjected to 0.3 mg/mL papain digestion. Cell suspension flew through 70-μm nylon cell strainers (Fisher). Cells were plated onto Petri dishes, and culture medium was replaced 24 h later and once every 3 d thereafter. Microglia and oligodendrocytes were removed from cultures by shaking at DIV 14. Remaining cells were detached with 0.25% trypsin and plated for the experiments that follow below. For cortical neuron cultures, cortical neurons were prepared from postnatal day 0 murine pups. Cortex was digested with 0.3 mg/mL papain. Cell suspension was filtered through 40-μm nylon cell strainers (Fisher) to remove debris. Neurons were plated at 1 ×106 on poly-d-lysine-coated six-well plates, and cultured in Neurobasal-A medium supplemented with B27 and glutamine (Invitrogen). Half the culture medium was changed with fresh medium every 3 d. To reduce glial proliferation, cytosine was added to the cultures 3 d after plating. N2a cells were cultured in DMEM, containing 10% FBS (Invitrogen) and penicillin/streptomycin at 37 °C and 5% CO2.
Cycloheximide Chase Assay.
DIV 2–3 cortical neurons and DIV 21–30 cortical astrocytes were transfected with plasmids encoding misfolded proteins. Transfected cells were treated with 100 μM cycloheximide at various time points from 0 to 24 h. After CHX treatment, cells were harvested and lysed in ice-cold 0.5% Triton X-100 in PBS with protease inhibitor mixture and PMSF on ice. Following this, the lysates were sonicated, and protein concentrations were determined by BCA assay (Thermo Scientific). Samples were loaded into SDS/PAGE gels and subjected to Western blotting.
Heat Shock.
Heat shock was performed on cortical astrocytes and neurons, which were seeded on six-well plates. To study effects of CHIP knockdown on heat-shock response, CHIP shRNA was transfected into cortical astrocytes. Before heat shock, the medium was replaced with fresh medium prewarmed to 42 °C. Primary cultures were incubated at 42 °C for 1 h in a water bath. For HD140Q KI neurons that were less tolerable to heat shock, they were treated with heat shock for 30 min. The plates were sealed with parafilm. Control cells were maintained in a 37 °C incubator. After heat shock, cells were allowed to recover in the same medium at 37 °C for various time periods. For heat shock on brain slices, we cut the brains of 3-mo-old mice into 1-mm coronal slices with a vibratome (Leica) in chilled artificial cerebral spinal fluid (ACSF). Before heat shock, slices were plated on Transwells (Costar), maintained with culture medium containing 50% MEM/Hepes (Gibco), 25% heat-inactivated horse serum (Gibco), 25% Hanks’ solution (Gibco), 6.5 mg/mL glucose (Sigma), pH 7.2, and allowed to recover in a 37 °C, 5% CO2 incubator for 1 h. Heat shock was performed on slices from one hemisphere, and those from the other hemisphere were used as controls. For heat shock, slices on Transwells were incubated at 42 °C for 1.5 h in the water bath. Following heat shock, slices recovered for 6 h in a 37 °C, 5% CO2 incubator, and then the cortex and corpus callosum were dissected and prepared for Western blotting.
Western Blot Analysis.
Cells or brain tissues were lysed in ice-cold RIPA buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1 mM EDTA, pH 8.0, 1 mM EGTA, pH 8.0, 0.1% SDS, 0.5% DOC, and 1% Triton X-100) containing protease inhibitor mixture and phosphatase inhibitors. The lysates were sonicated and subjected to SDS/PAGE. The proteins on the gel were transferred to a nitrocellulose membrane, which was then blocked with 5% milk/TBST for 1 h at room temperature. The blot was incubated with primary antibodies in 5% milk/TBST overnight at 4 °C. After three washes in TBST, the blot was incubated with HRP-conjugated secondary antibodies in 5% milk/TBST for 1 h at room temperature. After three washes in TBST, ECL Prime (GE Healthcare) was used to detect immunoreactive bands on the blot.
Immunoprecipitation.
Cells were harvested and lysed in ice-cold 0.5% Triton X-100/PBS solution with protease inhibitor mixture and 100 μM PMSF on ice. The lysates were centrifuged at 16,000 × g for 30 min. Protein concentrations were measured with BCA assay (Thermo Scientific). Total 300 μg samples were precleared with protein A agarose beads (Sigma), and huntingtin proteins were immunoprecipitated by anti-Htt (mEM48) or 1C2 at 4 °C overnight. Protein A agarose beads were added to capture the immunoprecipitates for 1 h at 4 °C. Ice-cold lysis buffer was used to wash beads three times. Proteins from the immunoprecipitates and inputs were subjected to Western blotting. For immunoprecipitation of ubiquitinated CHIP, CHIP-myc was transfected into cultured astrocytes. Anti-myc antibody was used to pull down CHIP-myc, and the immunoprecipitates were probed with anti-ubiquitin antibody to detect the ubiquitinated form of CHIP.
Immunofluorescence Staining.
Mice were anesthetized, perfused with fresh 4% paraformaldehyde in PBS, and postfixed overnight in the same fixative. Fixed brains were switched to 30% sucrose at 4 °C. Mouse brains were sliced at 15-μm thickness with a cryostat at −20 °C, and then mounted onto gelatin-coated slides. The brain slices were blocked with 3% BSA in PBS supplemented with 0.2% Triton X-100 for 30 min at room temperature. For immunostaining cultured cells, cells were fixed with 4% paraformaldehyde for 8–10 min, followed by blocking with 3% BSA/0.2% Triton X-100 for 30 min at room temperature. Following incubation of fixed cells or brain slices with primary antibodies at 4 °C overnight and washes, fluor-conjugated secondary antibodies and nuclear dye Hoechst were added to the samples for staining. Images were taken using a microscope (Axiovert 200 MOT, Carl Zeiss) and a 63× lens (LD-Achroplan 63×/0.75 NA) with a digital camera (ORCA-100, Hamamatsu Photonics). The Openlab software (PerkinElmer) was used for imaging acquisition.
Proteasomal Activity Assay.
For determining proteasome activity, clear whole-cell extracts or cell fractions were adjusted to 0.5 mg/mL total protein by dilution with homogenization buffer. All assays were done in triplicate. Chymotrypsin-like activity of 20S β5 was determined using the substrate Suc-LLVY-aminomethylcoumarin (40 μM; Bilmol), trypsin-like activity of 20S β2 was determined using the substrate Boc-LRR-AMC (100 μM; Bilmol). Equal amounts (10 μg) of the extracts were incubated with corresponding substrates in 100-μL proteasome activity assay buffer (0.05 M Tris⋅HCl, pH 8.0, 0.5 mM EDTA, 1 mM ATP, and 1 mM DTT) for 30–60 min at 37 °C. The reactions were stopped by adding 0.8 mL of cold water and placing the reaction mixtures on ice for at least 10 min. The free 7-amino-4-methylcoumarin (AMC) fluorescence was quantified by using the CytoFluor multiwell plate reader (FLUOstar, BMG Labtech) with excitation and emission wavelengths at 380 and 460 nm, respectively. All readings were standardized using the fluorescence intensity of an equal volume of free AMC solution (40 mM), normalized by the protein concentrations.
qRT-PCR.
Reverse transcription reactions were performed with 1.5 μg of total RNA using the SuperScript III First-Strand Synthesis System (Invitrogen). One microliter of cDNA was combined with 10 μL SYBR Select Master Mix (Applied Biosystems) and 1 μL of each primer in a 20-μL reaction. The reaction was performed in a thermal cycler (Eppendorf, RealPlex Mastercycler).
T7 Endonuclease 1 Assay.
N2a cells precultured in a six-well plate were cotransfected with HspBP1 sgRNA and Cas9 plasmids or transfected only with HspBP1 sgRNA as control using Lipofectamine 2000 (Invitrogen). Seventy-two hours after transfection, the genomic DNA was extracted from transfected N2a cells. The target genomic region was amplified with PCR. The sequences of the PCR primers are as follows: forward, 5′ ATAGTCTATCTTTAGGCGTGGTG 3′ and reverse, 5′ ACCCAACTACGTTGTGTACGAGT 3′. The PCR products were denatured and reannealed and then incubated with T7 Endonuclease I (New England BioLabs) for 20 min at 37 °C. The reaction products were subjected to 2% agarose gel electrophoresis.
Statistical Analyses.
Unpaired two-tailed Student’s t test was performed with GraphPad Prism 6. Results are expressed as the means ± SEM. A P value of <0.05 was considered significant. Statistical significance level was set as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Acknowledgments
We thank Marta Gaertig for maintaining HD140Q KI mice, Cheryl Strauss for critical reading of this manuscript, and the Integrated Cellular Imaging Core at Emory University for the use of imaging facilities. This work was supported by NIH Grants [NS101701 and NS036232 (to X.-J.L.) and NS095279 and NS095181 (to S.L.)] and the National Natural Science Foundation (91332206).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710549114/-/DCSupplemental.
References
- 1.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 2.Saxena S, Caroni P. Selective neuronal vulnerability in neurodegenerative diseases: From stressor thresholds to degeneration. Neuron. 2011;71:35–48. doi: 10.1016/j.neuron.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 3.Maragakis NJ, Rothstein JD. Mechanisms of disease: Astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- 4.Sherman MY, Goldberg AL. Cellular defenses against unfolded proteins: A cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29:15–32. doi: 10.1016/s0896-6273(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 6.Min JN, et al. CHIP deficiency decreases longevity, with accelerated aging phenotypes accompanied by altered protein quality control. Mol Cell Biol. 2008;28:4018–4025. doi: 10.1128/MCB.00296-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonough H, Patterson C. CHIP: A link between the chaperone and proteasome systems. Cell Stress Chaperones. 2003;8:303–308. doi: 10.1379/1466-1268(2003)008<0303:calbtc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClellan AJ, Frydman J. Molecular chaperones and the art of recognizing a lost cause. Nat Cell Biol. 2001;3:E51–E53. doi: 10.1038/35055162. [DOI] [PubMed] [Google Scholar]
- 9.Scaglione KM, et al. Ube2w and ataxin-3 coordinately regulate the ubiquitin ligase CHIP. Mol Cell. 2011;43:599–612. doi: 10.1016/j.molcel.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raynes DA, Guerriero V., Jr Inhibition of Hsp70 ATPase activity and protein renaturation by a novel Hsp70-binding protein. J Biol Chem. 1998;273:32883–32888. doi: 10.1074/jbc.273.49.32883. [DOI] [PubMed] [Google Scholar]
- 11.Alberti S, Böhse K, Arndt V, Schmitz A, Höhfeld J. The cochaperone HspBP1 inhibits the CHIP ubiquitin ligase and stimulates the maturation of the cystic fibrosis transmembrane conductance regulator. Mol Biol Cell. 2004;15:4003–4010. doi: 10.1091/mbc.E04-04-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao T, Hong Y, Li S, Li XJ. Compartment-dependent degradation of mutant huntingtin accounts for its preferential accumulation in neuronal processes. J Neurosci. 2016;36:8317–8328. doi: 10.1523/JNEUROSCI.0806-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsvetkov AS, et al. Proteostasis of polyglutamine varies among neurons and predicts neurodegeneration. Nat Chem Biol. 2013;9:586–592. doi: 10.1038/nchembio.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiFiglia M, et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Li SH, Yu ZX, Shelbourne P, Li XJ. Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington’s disease mice. J Neurosci. 2001;21:8473–8481. doi: 10.1523/JNEUROSCI.21-21-08473.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul I, Ahmed SF, Bhowmik A, Deb S, Ghosh MK. The ubiquitin ligase CHIP regulates c-Myc stability and transcriptional activity. Oncogene. 2013;32:1284–1295. doi: 10.1038/onc.2012.144. [DOI] [PubMed] [Google Scholar]
- 17.Lee JS, Seo TW, Yi JH, Shin KS, Yoo SJ. CHIP has a protective role against oxidative stress-induced cell death through specific regulation of endonuclease G. Cell Death Dis. 2013;4:e666. doi: 10.1038/cddis.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian SB, McDonough H, Boellmann F, Cyr DM, Patterson C. CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature. 2006;440:551–555. doi: 10.1038/nature04600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pimienta G, Herbert KM, Regan L. A compound that inhibits the HOP-Hsp90 complex formation and has unique killing effects in breast cancer cell lines. Mol Pharm. 2011;8:2252–2261. doi: 10.1021/mp200346y. [DOI] [PubMed] [Google Scholar]
- 20.Marcuccilli CJ, Mathur SK, Morimoto RI, Miller RJ. Regulatory differences in the stress response of hippocampal neurons and glial cells after heat shock. J Neurosci. 1996;16:478–485. doi: 10.1523/JNEUROSCI.16-02-00478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batulan Z, et al. High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J Neurosci. 2003;23:5789–5798. doi: 10.1523/JNEUROSCI.23-13-05789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Chopp M, Yoshida Y, Levine SR. Distribution of 72-kDa heat-shock protein in rat brain after hyperthermia. Acta Neuropathol. 1992;84:94–99. doi: 10.1007/BF00427221. [DOI] [PubMed] [Google Scholar]
- 23.Friedman MJ, et al. Polyglutamine domain modulates the TBP-TFIIB interaction: Implications for its normal function and neurodegeneration. Nat Neurosci. 2007;10:1519–1528. doi: 10.1038/nn2011. [DOI] [PubMed] [Google Scholar]
- 24.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu ZX, et al. Mutant huntingtin causes context-dependent neurodegeneration in mice with Huntington’s disease. J Neurosci. 2003;23:2193–2202. doi: 10.1523/JNEUROSCI.23-06-02193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valencia A, et al. Striatal synaptosomes from Hdh140Q/140Q knock-in mice have altered protein levels, novel sites of methionine oxidation, and excess glutamate release after stimulation. J Huntingtons Dis. 2013;2:459–475. doi: 10.3233/JHD-130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai Q, et al. CHIP activates HSF1 and confers protection against apoptosis and cellular stress. EMBO J. 2003;22:5446–5458. doi: 10.1093/emboj/cdg529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tydlacka S, Wang CE, Wang X, Li S, Li XJ. Differential activities of the ubiquitin-proteasome system in neurons versus glia may account for the preferential accumulation of misfolded proteins in neurons. J Neurosci. 2008;28:13285–13295. doi: 10.1523/JNEUROSCI.4393-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hickey MA, et al. Extensive early motor and non-motor behavioral deficits are followed by striatal neuronal loss in knock-in Huntington’s disease mice. Neuroscience. 2008;157:280–295. doi: 10.1016/j.neuroscience.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan S, et al. TDP-43 causes differential pathology in neuronal versus glial cells in the mouse brain. Hum Mol Genet. 2014;23:2678–2693. doi: 10.1093/hmg/ddt662. [DOI] [PMC free article] [PubMed] [Google Scholar]