Significance
Oxidative damage is frequently associated with aging and aging-related disease, but, paradoxically, several recent studies have shown that artificial boosts of reactive oxygen species (ROS) can also extend life span in young individuals. Here, we show that physiological levels of ROS promote diapause, thereby extending life span in pupae of the moth Helicoverpa armigera. Insect diapause, like the dauer stage of nematodes, is a period of developmental rest that results in a profound extension of life span. ROS appears to contribute to this life span extension by acting through components of the insulin-signaling pathway. Our results thus suggest a new molecular mechanism regulating life span and help to explain the dual nature of ROS action in animals.
Keywords: insulin signaling, Akt, PRMT1, insects, diapause
Abstract
Reactive oxygen species (ROS) are well-known accelerants of aging, but, paradoxically, we show that physiological levels of ROS extend life span in pupae of the moth Helicoverpa armigera, resulting in the dormant state of diapause. This developmental switch appears to operate through a variant of the conventional insulin-signaling pathway, as evidenced by the facts that Akt, p-Akt, and PRMT1 are elevated by ROS, but not insulin, and that high levels of p-Akt fail to phosphorylate FoxO through PRMT1-mediated methylation. These results suggest a distinct signaling pathway culminating in the elevation of FoxO, which in turn promotes the extension of life span characteristic of diapause.
Diapause in insects is akin to dauer, the slowed developmental phase in Caenorhabditis elegans (1) and hibernation in vertebrates (2). These dormant stages share a similar phenotype, characterized by suppressed metabolic activity and arrested development. The brain functions as both a receptor and programmable center to perceive environmental signals to induce diapause. When reared under long daylength and low temperature (20 °C) the cotton bollworm Helicoverpa armigera quickly progresses through the pupal stage and develops immediately into an adult, but when reared under short daylengths at the same temperature the bollworm enters pupal diapause (3). The life span of this “nonaging” diapause pupa is extended many months (4), making H. armigera an excellent model for life span research.
Numerous reports indicate that reactive oxygen species (ROS) promote aging processes (5) and are associated with diverse medical disorders, including Alzheimer’s disease, Parkinson’s disease, cancer, diabetes, and others (6, 7). However, pioneering work in yeast, C. elegans, and Drosophila melanogaster has shown that increased ROS from chemical inhibition or mutations that affect mitochondrial function or allotopic expression can also lengthen life span (8–11). What is unclear is whether naturally occurring, physiological levels of ROS can also regulate life span. It is also unclear how ROS play a dual function in these species and what molecular pathway is evoked by ROS to extend life span. ROS appear to elicit distinct responses at different developmental stages: inducing aging in older individuals while promoting life span extension in younger individuals. To evaluate this dichotomy of function, we focused on ROS in diapausing pupae, a nonaging stage that is locked into a developmental arrest.
The insulin-signaling pathway plays a critical role in regulating the dauer state in C. elegans (1), life span extension in adults of D. melanogaster (12), and adult diapause (reproductive arrest) in the mosquito Culex pipiens (13). Insulin activates Akt, which in turn phosphorylates FoxO, promoting FoxO degradation (14) and elevation of metabolic activity (13, 15). Low insulin signaling results in down-regulation of Akt, leading to activation of FoxO, which in turn promotes life span extension by regulating transcription in a number of critical downstream genes (16). Modulation of the insulin-signaling pathway by ROS through PI3K/Akt/FoxO (17) implicates ROS as a potential regulator of life span through components of the insulin-signaling pathway.
Numerous genes, proteins, and metabolites that are differentially expressed in diapause-destined individuals are involved in carbohydrate metabolism (18–21). Diapause-destined pupae of H. armigera are characterized by low glucose levels in the blood but high glucose levels in the brain, and we know that restriction of glucose by 2-deoxy-d-glucose (DOG) injection can delay development of nondiapausing individuals (22). In C. elegans, reduced glucose metabolism increases life span in an ROS-dependent manner through an impaired insulin pathway or glucose restriction (23). These results suggest that insulin signaling and ROS may play important roles in diapause regulation.
Here, we monitored insulin-like peptide (ILP) levels in pupal blood and found that high ILP levels led to insect development, whereas low levels were associated with diapause. However, surprisingly, Akt, p-Akt, and FoxO levels were higher in brains of diapause-destined pupae of H. armigera, compared with their nondiapausing counterparts. This result was not consistent with the observations of (i) low ILP levels and (ii) negative regulation of FoxO by p-Akt. Further experiments showed that ROS, but not ILPs, elicit high expression of Akt and abundant p-Akt in diapause-destined pupal brains. We conclude that high p-Akt levels activate the target protein glucose transporter (Glut) to sense low glucose in the blood and enhance its uptake by the brain as an energy resource. Elevation of protein arginine methyltransferase 1 (PRMT1), a predominant member of the PRMT family (24), blocks FoxO phosphorylation to reduce FoxO protein degradation, thus promoting accumulation of FoxO in brains of diapause-destined pupae, leading to life span extension (diapause). The results suggest a mechanism by which the brain naturally controls life span extension through a distinct ROS-mediated insulin-signaling pathway, indicating that physiological levels of ROS are exploited by insects to extend life span in young individuals.
Results
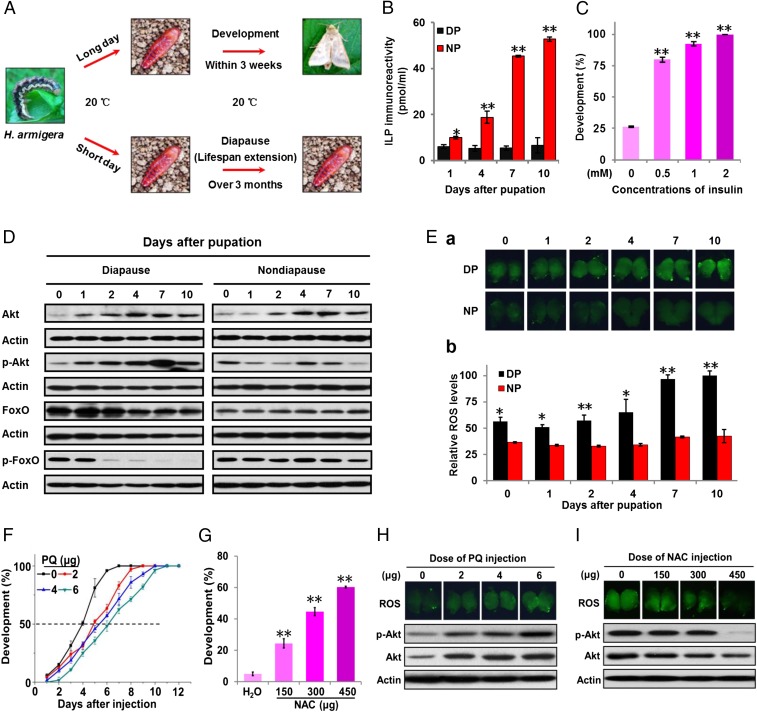
Nondiapause- and diapause-destined pupae of H. armigera were cultured at 20 °C, differing only in the photoperiod they received. Reared under long daylengths, pupae failed to enter diapause and emerged as adults 21–23 d after pupation. By contrast, pupae reared under short daylengths entered diapause 8–10 d after pupation, and their pupal life span was extended beyond 3 mo (Fig. 1A). We focused on the diapause initiation phase from day 0 to day 10 after pupation. To elucidate the relationship between insulin signaling and diapause, we measured ILP titers in blood from nondiapause- and diapause-destined pupae using competitive ELISA; ILP titers in nondiapause-destined pupae gradually increased from days 0 to 10 after pupation, whereas titers in diapause-destined pupae remained low through day 10 (the onset of diapause) (Fig. 1B). Injection of exogenous insulin into day-1 diapause-destined pupae averted diapause and prompted development (Fig. 1C), suggesting that high insulin elicits development, while low insulin levels lead to diapause.
Fig. 1.
Roles of insulin-like peptides (ILPs) and ROS in diapause and associated changes in Akt and FoxO. (A) A schematic representation of pupal diapause regulation in the moth, Helicoverpa armigera. (B) ILP titers in pupal hemolymph detected by competitive ELISA. Hemolymph from 10 pupae was collected and mixed as a sample for competitive ELISA using an insulin antibody. DP, diapause-destined pupae; NP, nondiapause-destined pupae. (C) Developmental fate of diapause-destined pupae following an insulin injection. Diapause-programmed pupae were injected with insulin on day 1 following pupation, and the onset of development was determined by observing timing of the disappearance of the pupal stemmata. (D) Western blot showing patterns of abundance for Akt, p-Akt, FoxO, and p-FoxO. Proteins from brains were extracted and detected with corresponding antibodies. (E) ROS levels in the brain. (a) Brains were incubated with the ROS detector CM-H2CFDA for 1 h. (b) Quantification of ROS levels in the brains. Relative ROS levels indicate the highest value as 100. (F) Developmental delay in nondiapause-destined pupae caused by injection of the ROS generator, paraquat (PQ). PQ was injected into day-1 pupae, and developmental delay was determined by examining location of the pupal stemmata on different days after injection. (G) Developmental fate of diapause-destined pupae following injection of the ROS scavenger, N-acetyl-l-cysteine (NAC). Day-1 pupae were injected with NAC or H2O as a control, and the onset of development was determined by observing disappearance of the pupal stemmata. Each point represents the mean ± SD of three independent replicates. *P < 0.05; **P < 0.01 (determined by an independent t test). Effects of (H) PQ and (I) NAC on levels of ROS, Akt, and p-Akt in the brain. Nondiapause-destined pupae were injected on day 1 with PQ for 48 h, and diapause-destined pupae were injected on day 7 with NAC for 48 h. ROS were detected as above, and proteins from brains were extracted and detected with corresponding antibodies.
To clarify how insulin signaling regulates this developmental switch we monitored brain abundance of two downstream gene products, Akt and FoxO, in pupae by Western blots (Fig. 1D and SI Appendix, Fig. S1). Akt levels in nondiapause- and diapause-destined pupae were similar, but the active form of Akt, p-Akt, was significantly higher in diapause-destined pupae. FoxO abundance in diapause-destined pupae was significantly higher, but p-FoxO levels were lower. These results are inconsistent with the predicted and anticipated low ILP levels, high Akt and p-Akt levels, and low p-FoxO levels observed in diapause-destined individuals, thus indicating an unexpected but distinct use of components of the insulin-signaling pathway in regulating diapause in this moth.
The known role of ROS in modulating insulin signaling to extend life span (23) prompted our examination of ROS activity in the two types of pupae (Fig. 1E). ROS levels were significantly higher in diapause-destined pupal brains than in brains from nondiapause-destined pupae. To further test this relationship, we injected the mitochondrial superoxide generator paraquat (PQ) into day-1 nondiapause-destined pupae to elevate ROS levels. In control pupae, 50% completed stemmata migration (a marker for development) in 4 d, whereas individuals injected with PQ showed delayed development and required 5–6 d to complete stemmata migration (Fig. 1F). We also injected the ROS scavenger N-acetyl-l-cysteine (NAC) into day-1 diapause-destined pupae to decrease ROS levels: significantly more pupae were channeled into nondiapause than in the controls that did not receive NAC (Fig. 1G). Injection of PQ into day-1 nondiapause-destined pupae elevated Akt and p-Akt levels in the brain, accompanied by increased ROS activity (Fig. 1H and SI Appendix, Fig. S2A). When NAC was injected into day-7 diapause-destined pupae, ROS were significantly lower, accompanied by decreased brain levels of Akt and p-Akt (Fig. 1I and SI Appendix, Fig. S2B). Collectively, these results indicate that ROS can elevate Akt and p-Akt levels, suggesting that high Akt and p-Akt in diapause-destined individuals are dependent on ROS, but not ILPs, and that ROS are important regulators of insect diapause.
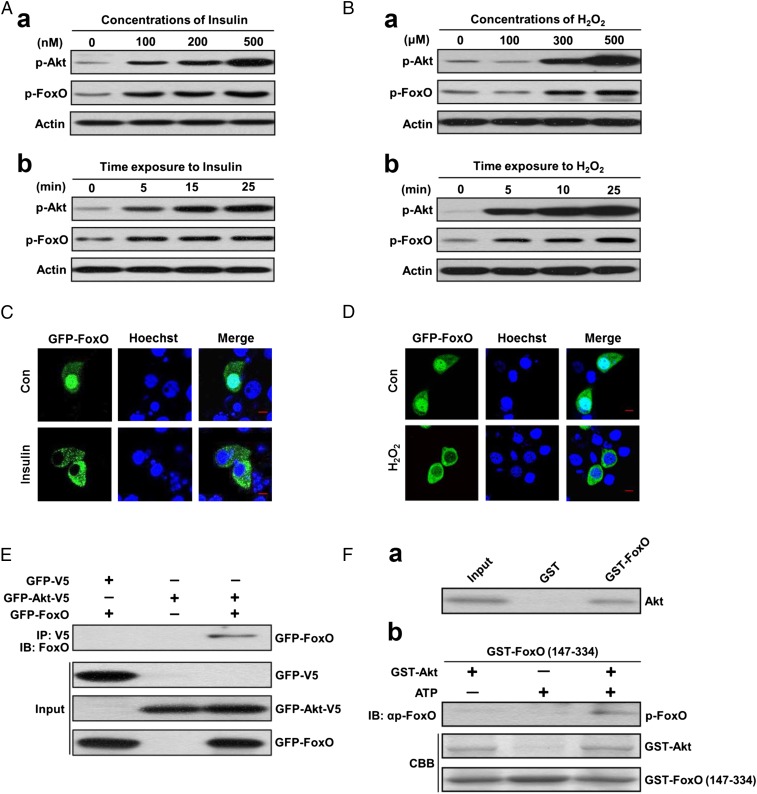
The high p-Akt and low p-FoxO levels in diapause-destined individuals imply that Akt does not phosphorylate FoxO. To clarify the role of Akt in regulating FoxO phosphorylation in response to insulin and ROS, we performed cell experiments with an HzAm1 cell line from Helicoverpa zea, a close relative of H. armigera. Cell culture experiments clearly showed that both p-Akt and p-FoxO respond respectively to insulin (Fig. 2A and SI Appendix, Fig. S3A) or oxidative stress (Fig. 2B and SI Appendix, Fig. S3B); increased p-FoxO was accompanied by elevated p-Akt. In addition, insulin or oxidative stress increased nuclear export of FoxO (Figs. 2C and 2D). These results suggest that Akt phosphorylates FoxO as previously reported (25). The structure of FoxO in H. armigera revealed two consensus Akt phosphorylation motifs (RXRXXS) at amino acids 186–191 and 250–255, corresponding to Akt phosphorylation motifs of FoxOs in other taxa, including mammals, C. elegans, D. melanogaster, and Bombyx mori (SI Appendix, Fig. S4). The RXRXXS motif of FoxO undergoes Akt-mediated phosphorylation at Ser, but this can be prevented by prior methylation at Arg from the PRMT1 (26). Using coimmunoprecipitation, we found that Akt and FoxO specifically bind to each other in vitro (Fig. 2E). We then performed in vitro phosphorylation assays using GST-fused Akt and FoxO fragment containing the RXRXXS motif, and the result showed that Akt specifically interacts with FoxO to phosphorylate FoxO (Fig. 2F), implying that Akt-mediated phosphorylation of FoxO in diapause-destined individuals may be abolished by prior PRMT1 methylation.
Fig. 2.
P-Akt and p-FoxO levels in response to insulin and oxidative stress, and Akt binding to and phosphorylation of FoxO. P-Akt, and p-FoxO levels in response to (A) insulin and (B) H2O2. (a) Dose-related response to insulin or H2O2. (b) Time-related response to insulin or H2O2. HzAm1 cells were cultured with various doses of insulin or H2O2 for 25 min and with 500 nM insulin for 0, 5, 15, or 25 min or 500 μM H2O2 for 0, 5, 10, or 25 min. (C and D) Nuclear FoxO localization in response to insulin or H2O2. HzAm1 cells were transfected with a GFP-FoxO plasmid for 48 h and then treated with distilled water as a control (Con) or with 500 nM insulin or with 300 μM H2O2 for 30 min. Hoechst 33342 labels the nuclei. GFP-FoxO, recombinant GFP-FoxO protein. (Scale bar, 10 μm.) (E) Akt physically associates with FoxO as shown by coimmunoprecipitation. (F) Akt binds to and phosphorylates FoxO. (a) Akt interacts with FoxO as shown in a pull-down assay. (b) In vitro phosphorylation assay. CBB, Coomassie brilliant blue.
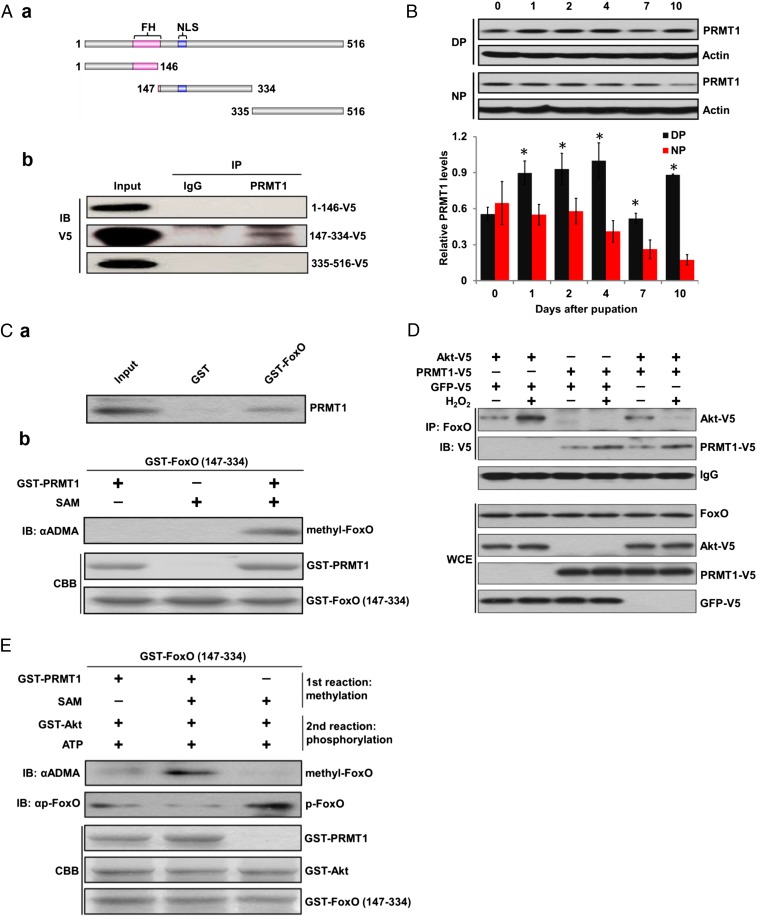
We constructed three FoxO fragments fused with a V5 tag and expressed these fragments in HzAm1 cells; only the 147–334 amino acid fragment containing the RXRXXS motif was able to bind to PRMT1 (Fig. 3A). PRMT1 expression was higher in brains from diapause-destined pupae, a pattern consistent with that of FoxO (Fig. 3B); this suggests that PRMT1 is involved in FoxO methylation. We then performed an in vitro methylation assay using GST-PRMT1 and GST-FoxO fragment. The results showed that PRMT1 specifically interacts with FoxO to methylate FoxO (Fig. 3C).
Fig. 3.
PRMT1 binds to and methylates FoxO and blocks Akt-mediated phosphorylation. (A) FoxO interacts with PRMT1. (a) A schematic representation of FoxO. FH, forkhead box domain; NLS, nuclear localization signal. (b) Coimmunoprecipitation of FoxO fragments and PRMT1. (B) Abundance of PRMT1 in the brain by Western blot. DP, diapausing pupae; NP, nondiapausing pupae. Protein bands were quantified and normalized to the levels of actin, using 1 as the highest value. Each point represents the mean ± SD of three independent replicates. *P < 0.05 (determined by an independent t test). (C) PRMT1 binds to and methylates FoxO. (a) PRMT1 interacts with FoxO as shown in a pull-down assay. (b) In vitro methylation assay. SAM, S-adenosyl-methionine; CBB, Coomassie brilliant blue. (D) PRMT1 diverts Akt to associate with FoxO in HzAm1 cells. WCE, whole-cell extracts. (E) In vitro sequential methylation and phosphorylation assays.
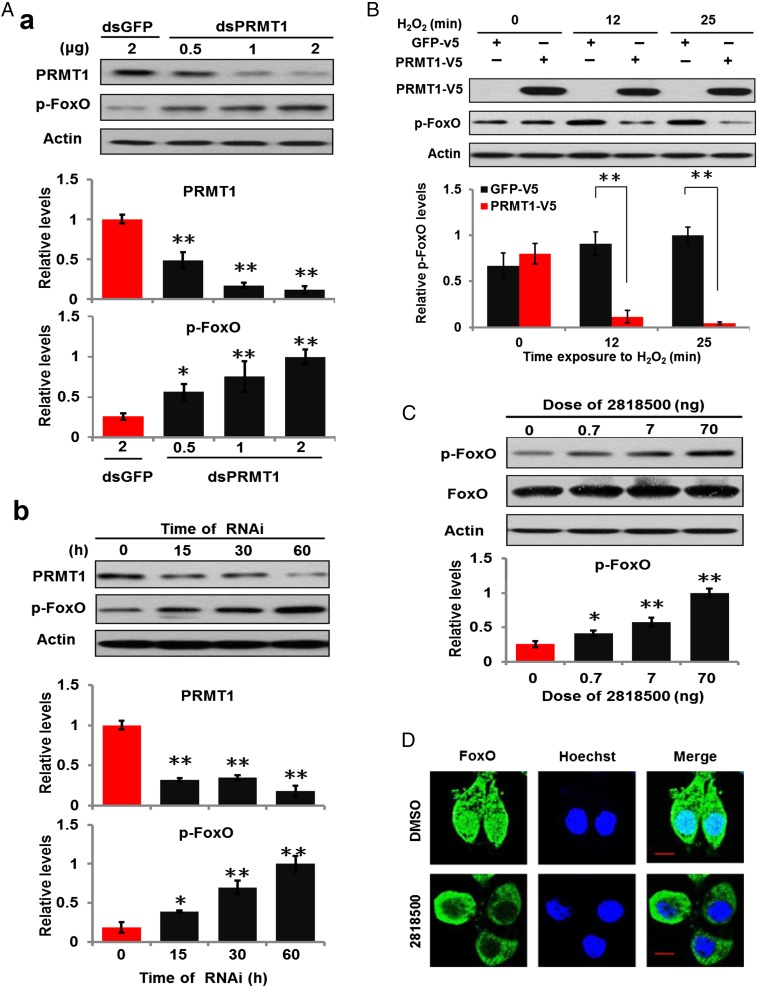
We further investigated changes in PRMT1 expression in response to ROS signaling. Cell experiments indicated that PRMT1 responds to oxidative stress (SI Appendix, Fig. S5). We then overexpressed Akt or PRMT1 in HzAm1 cells and treated the cells with a generator of oxidative stress, H2O2. Oxidative stress increased binding of both Akt and PRMT1 to FoxO, but not to GFP (SI Appendix, Fig. S6). However, when Akt and PRMT1 were cotransfected into HzAm1 cells and subsequently treated with H2O2 we observed increased PRMT1 and decreased Akt binding to FoxO (Fig. 3D). We thus conducted sequential methylation and phosphorylation assays using GST-FoxO (147–334 amino acids) as substrate and observed that Akt-mediated phosphorylation of FoxO was blocked by prior PRMT1 methylation (Fig. 3E), as previously reported (26). Furthermore, we conducted phosphorylation assays in HzAm1 cells using dsRNA against PRMT1; silencing PRMT1 expression by 50–88% resulted in increased levels of p-FoxO (Fig. 4A). When we transfected PRMT1 or GFP into HzAm1 cells and treated with H2O2, PRMT1 overexpression resulted in down-regulation of p-FoxO (Fig. 4B). Injection of a selective PRMT1 inhibitor 2818500 (27) into day-1 diapause-destined pupae increased p-FoxO levels in the brains (Fig. 4C), and treatment of HzAm1 cells with the PRMT1 inhibitor resulted in an increase in nuclear export of FoxO (Fig. 4D), as reported (17). Taken together, this evidence suggests that PRMT1 actively responds to ROS signaling and that FoxO is methylated by PRMT1 to prevent phosphorylation.
Fig. 4.
Effects of PRMT1 on FoxO phosphorylation and localization. (A) PRMT1 knockdown increased endogenous FoxO phosphorylation in HzAm1 cells. (a) Dose-dependent response to PRMT1 RNAi. HzAm1 cells were treated for 60 h with PRMT1 dsRNA. GFP dsRNA treated with 2 μg for 60 h was used as control. (b) Time-dependent response to RNAi. HzAm1 cells were treated with 2 μg PRMT1 dsRNA. Histograms indicate quantification of the protein bands using image software (Gel-Pro Analyzer) and normalized to the levels of actin, using 1 as the highest value. (B) PRMT1 decreased p-FoxO levels under oxidative stress in HzAm1 cells. Recombinant PRMT1 or GFP were transfected into HzAm1 cells, and cells were treated with H2O2 for 0, 12, or 25 min. (C) Effects of PRMT1 inhibitor 2818500 on p-FoxO levels in the brain. Diapause-destined pupae were injected on day 1 with 2818500 and evaluated 48 h later. Each point represents the mean ± SD of three independent replicates. *P < 0.05; **P < 0.01 (determined by an independent t test). (D) Nuclear export of FoxO in response to a PRMT1 inhibitor 2818500. DMSO treatment was the control. (Scale bar, 10 μm.)
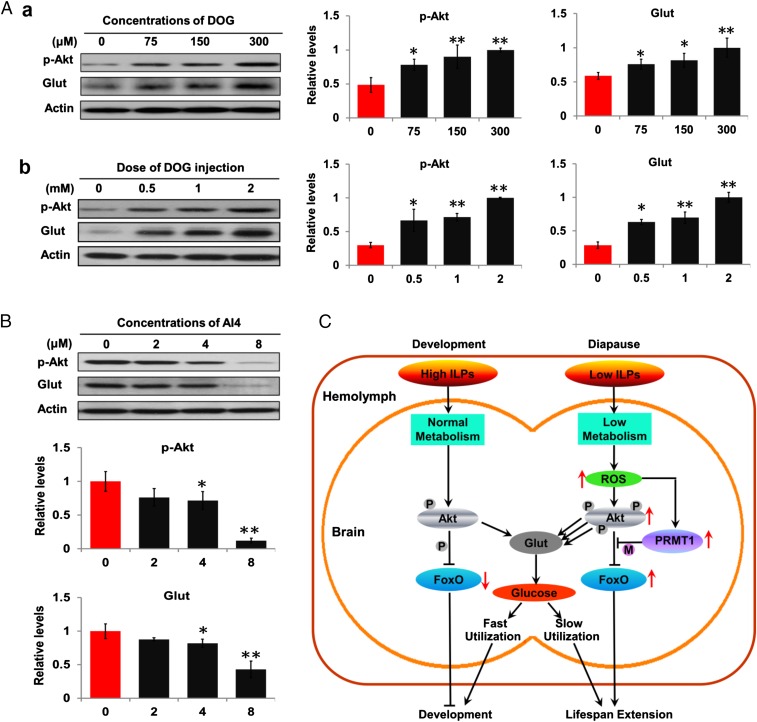
Insulin acts to increase glucose uptake through an Akt-activated glucose transporter (Glut) (28). To probe this portion of the pathway we examined changes in p-Akt and Glut protein levels after treatment of HzAm1 cells with DOG, a glucose derivative known to elevate ROS levels (SI Appendix, Fig. S7), and following injection of DOG into day-6 nondiapause-destined pupae. Both p-Akt and Glut proteins were significantly elevated in both cells and brain in response to this oxidative stress (Fig. 5A). When HzAm1 cells were treated with the Akt inhibitor AI4, both p-Akt and Glut proteins significantly declined (Fig. 5B), indicating that Glut can respond to p-Akt and that Glut is a likely target of Akt in insect diapause.
Fig. 5.
P-Akt activation of the glucose transporter (Glut). (A) ROS increased Glut expression by elevating p-Akt levels. ROS increased Glut expression by p-Akt in (a) HzAm cells and (b) the brain. HzAm1 cells were cultured with DOG (2-deoxy-glucose) for 18 h. Nondiapause-destined pupae were injected on day 6 with DOG for 48 h. Proteins were extracted from cells or brains for Western blots with p-Akt, Glut, and actin antibodies. (B) P-Akt and Glut levels in response to the p-Akt inhibitor AI4. Histograms indicate quantification of protein bands using image software (Gel-Pro Analyzer) and normalized to levels of actin, using 1 as the highest value. Each point represents mean ± SD of three independent replicates. *P < 0.05; **P < 0.01 (determined by an independent t test). (C) A schematic representation depicting a central role for ROS in regulation of pupal diapause in Helicoverpa armigera. High insulin signaling promotes continuous development through Akt-mediated phosphorylation of FoxO and activation of other downstream genes, including Glut. Low insulin signaling results in low metabolic levels, which induce high ROS levels in diapause individuals. ROS then lead to high levels of Akt, p-Akt, and PRMT1; PRMT1 methylates FoxO to inhibit Akt-mediated FoxO-phosphorylation. High p-Akt levels activate Glut to sense and absorb low glucose levels from the hemolymph into the brain as an energy resource for slow utilization during the long diapause phase. Abundant FoxO leads to life span extension (diapause).
Discussion
Diapause is a complex physiological response, with many signaling pathways participating in the process. Although we have known about roles for prothoracicotropic hormone, ecdysone, the juvenile hormones, and diapause hormone for quite some time (3), many additional signaling pathways are also likely involved. One such prominent pathway is the insulin-signaling pathway (29). In the insulin-signaling pathway, insulin activates Akt, and high p-Akt levels repress FoxO activity and activate other genes that promote development. In contrast, low insulin signaling decreases p-Akt levels, resulting in active FoxO, which regulates life span extension through activation of a variety of downstream genes that generate the diapause phenotype (30). FoxO is thus a well-known regulator of life span extension (31). In the present study, we found that the blood of H. armigera contains low ILP levels, and the brain expresses high FoxO levels in diapause-destined pupae, suggesting that ILPs regulate the developmental timing of insects through genes downstream of FoxO. Interestingly, the upstream signals Akt and p-Akt, which are negative FoxO regulators, are also highly expressed in diapause individuals, indicating that high Akt and p-Akt levels are regulated by factors other than ILPs. High p-Akt levels in diapause individuals are inconsistent with low p-FoxO levels, suggesting that p-Akt does not regulate FoxO phosphorylation in this case. These conflicting data indicate that the life span extension phenotype, diapause, has a distinct regulatory mechanism in the brain, defined by the following observations.
ROS Increase Akt Expression and p-Akt Levels.
ROS generation by exogenous sources such as H2O2 triggers a number of pathways including the ROS-activated PI3K/Akt-signaling pathway (32). The association of aging with ROS has been observed throughout the animal kingdom (33). However, glucose restriction activates ROS (8), and impaired respiration elevates ROS (34) to extend life span in C. elegans. We now suggest a role for ROS in insect diapause. Metabolic depression and glucose restriction are common in diapausing individuals (3, 22), suggesting that ROS are possible regulators of insect diapause. In this study, we showed that ROS levels are higher in brains of diapause-destined pupae than in their nondiapausing counterparts. Glucose restriction can increase ROS activity, and increased ROS activity in nondiapausing pupae delays development. These results suggest the low metabolic rates characteristic of diapause result in increased ROS activity and consequently imply a regulatory role for ROS in insect diapause.
Akt is widely recognized as a key component of the insulin-signaling pathway and is highly responsive to insulin signaling (35). ILP levels are lower in diapause-destined individuals compared with their nondiapausing counterparts. This result is consistent with the finding that a low insulin signal results in diapause through FoxO activation. However, diapause-destined pupal brains express high Akt and p-Akt levels, suggesting that factors other than ILPs regulate Akt expression and phosphorylation in diapause individuals. ROS have been reported to induce increased Akt activity and p-Akt levels (36–38). In the present paper, Akt expression and p-Akt levels in diapause individuals responded to oxidative stress, but not to ILPs, suggesting that ROS function as important regulators of insect diapause by elevating Akt and p-Akt levels.
Inhibition of FoxO Phosphorylation by PRMT1.
Akt-dependent phosphorylation is crucial for the regulation of FoxO function. Akt phosphorylates FoxO to exclude it from the nucleus and promote its degradation (39). However, the observation that high p-Akt and low p-FoxO levels are synchronously present in diapause-destined pupal brains indicates that other mechanisms regulate FoxO activity during diapause in H. armigera. Two recent reports demonstrated that FoxO methylation by PRMT1 can directly block Akt-mediated phosphorylation (26, 40). Thus, we focused on FoxO methylation in insect diapause. Our main findings are as follows: (i) Brains from diapause-destined pupae expressed high levels of ROS-induced PRMT1, and the PRMT1 expression pattern was similar to that of FoxO; (ii) PRMT1 could bind to FoxO and methylate FoxO in vitro; (iii) PRMT1, but not Akt, can effectively bind to FoxO under high oxidative stress; (iv) the down-regulation of PRMT1 levels by RNAi resulted in increased p-FoxO levels, and a methylation inhibitor increased p-FoxO levels in vivo and the nuclear export of FoxO in vitro; and (v) FoxO methylation can counteract Akt-mediated phosphorylation. These results show that FoxO methylation decreases Akt-mediated phosphorylation and that FoxO accumulates, leading to the induction of diapause.
Physiological Significance of High Akt and p-Akt Levels in the Regulation of Diapause.
As described above, strong insulin signals usually promote development, not diapause (13). Our data showed that both Akt and p-Akt levels were abundant in brains of diapause-destined pupae, but FoxO cannot be phosphorylated by p-Akt. This suggests that Akt and p-Akt may have some other biological significance in diapause-destined individuals. Based on the facts that glucose is expressed at low levels in blood of diapausing individuals, while high levels of glucose accumulate in the brain (22), and p-Akt can activate Glut to increase glucose uptake (28), we speculate that high p-Akt levels may be correlated with glucose uptake. Our results showed that Glut is one of the p-Akt target proteins, suggesting that high p-Akt levels are responsible for sensing and absorbing low glucose levels in the blood of diapause individuals by activating Glut, as previously reported (41, 42). In addition, it may be necessary to maintain abundant Akt until postdiapause development is initiated. Specifically, key developmental regulators such as Akt may accumulate before diapause entry so that the animal is capable of rapidly restarting pupal–adult development when diapause is completed (3). The exact function of Akt in diapausing individuals, however, requires further investigation.
High ROS Levels in Pupae Can Lengthen Life Span.
Metabolism-induced ROS production and oxidative damage are considered a primary cause of aging and aging-related diseases (43, 44). However, increasing evidence suggests that the opposite may also be true: chemical inhibition of metabolism (2-deoxy-d-glucose, paraquat) during young adulthood or mutations that affect mitochondrial function can extend life span in an ROS-dependent manner in C. elegans (8, 9). ROS can also increase FoxO levels and act through transportin-1 to extend life span in C. elegans (45). However, many details of the molecular mechanism that extends life span by ROS remain unclear.
We speculate that this paradox, an apparent dual role for ROS, may reflect distinct actions of ROS at different life stages: ROS induce the aging process in old individuals, but extend life span in young individuals. For example, in D. melanogaster, consistent increased ROS levels were observed in old individuals and are presumed to contribute to the aging process, but transgenic overexpression of NDI1 (a rotenone-insensitive alternative NADH dehydrogenase from fungi) increased ROS levels in the brain of young adults and extended life span (46). This result indicates that increased ROS levels in the early adult stage can extend life span, although the mechanism is unknown. In addition, epidermal mitochondrial oxidative damage delays features of aging in young mice; however, in older mice, this damage accelerates features of aging (47).
In the present study, we showed that diapause pupae, which are a “nonaging” natural physiological state, produce high levels of ROS in the brain and that ROS increase life span through a distinct signaling pathway using components of the insulin-signaling pathway, as described above. A model for the regulation of developmental timing is proposed in Fig. 5C. In developing individuals, strong insulin signals lead to the progression of development through Akt-mediated phosphorylation of FoxO and activation of downstream genes, including Glut. By contrast, low insulin signals found in diapause-destined pupae promote low metabolic activity, resulting in increased ROS activity. High ROS activity then leads to high levels of Akt, p-Akt, and PRMT1, but PRMT1 inhibits Akt-mediated phosphorylation through FoxO methylation, and abundant FoxO induces pupal life span extension by regulating expression of select downstream genes. High p-Akt levels activate Glut expression, as previously reported (41, 42), to sense and absorb low blood glucose (22) into the brain as an energy resource for slow utilization throughout the long diapause phase, as suggested by the fact that expression and activity of hexokinase, which converts glucose to glucose-6-phosphate (the first rate-limiting enzyme in glycolysis), are low in brains of diapausing pupae and result in low metabolic activity (48).
In summary, ROS have long been considered a primary cause of aging and aging-related diseases (5–7), but only recently have studies suggested that the opposite is also true: that elevated ROS at nonphysiological levels can extend life span at certain phases of the lifecycle (8–11). Our results support the conclusion that physiological levels of ROS are beneficial for extending life span in young individuals, a viewpoint consistent with recent findings in D. melanogaster (46), mice (47), and humans (49).
Materials and Methods
Larvae of Helicoverpa armigera were reared on an artificial diet at 20 ± 1 °C under a light–dark cycle of 14 h light/10 h dark (nondiapause) or under a cycle of 10 h light/14 h dark (diapause). All nondiapausing pupae developed without entering diapause, whereas over 95% of the diapause-programmed pupae entered diapause. Developmental stages were synchronized by collecting pupae on the day of pupation. Pupal brains were dissected in ice-cold 0.75% NaCl and stored at −80 °C until used.
For insulin injection experiments shown in Fig.1C, diapause-programmed pupae were injected on day 1 with differing concentrations of bovine insulin (Sigma) in a volume of 5 μL (0 mM insulin, n = 98; 0.5 mM insulin, n = 94; 1 mM insulin, n = 94; 2 mM insulin, n = 92) and held at 22.5 °C. Pupal development was determined by observing disappearance of the pupal stemmata, a marker for development. For PQ injections shown in Fig. 1F, nondiapause-destined pupae were injected with PQ on day 1 and incubated at 22.5 °C. Developmental delay was determined by examining the location of the pupal stemmata on different days after injection (0 μg PQ, n = 73; 2 μg PQ, n = 71; 4 μg PQ, n = 70; 6 μg PQ, n = 56). For NAC injections shown in Fig. 1G, diapause-programmed pupae were injected on day 1 with a 3-μL NAC solution or H2O as a control (H2O, n = 77; 150 μg NAC, n = 70; 300 μg NAC, n = 53; 450 μg NAC, n = 53) and then kept at 22.5 °C. Pupal developmental status was determined by observing disappearance of the pupal stemmata. For DOG injections shown in SI Appendix, Fig. S7B, nondiapause-programmed pupae were injected on day 6 with 3 μL DOG solution (2 mM) for 48 h or H2O as a control (H2O, n = 45; DOG, n = 45).
Additional details on polyclonal antibody generation, competitive ELISA, protein extraction and Western blot, construction of overexpression plasmids, vector transfection and cell treatments, coimmunoprecipitation and immunoblot analysis, measurement of ROS generation, RNA interference, immunofluorescence assay, GST pull-down assay, in vitro phosphorylation assay, in vitro methylation assay, in vitro sequential methylation and phosphorylation assay, and gene-specific primers are included in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
This study was supported by National Natural Scientific Foundation of China Grant-in-Aid 31230066 (to W.-H.X.) and US Department of Agriculture (USDA)-National Institute of Food and Agriculture (NIFA) Grant 2015-67013-23416 (to D.L.D.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711042114/-/DCSupplemental.
References
- 1.Hu PJ. Dauer. WormBook: The Online Review of C. elegans Biology. 2007 doi: 10.1895/wormbook.1.144.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aloia RC, Raison JK. Membrane function in mammalian hibernation. Biochim Biophys Acta. 1989;988:123–146. doi: 10.1016/0304-4157(89)90007-5. [DOI] [PubMed] [Google Scholar]
- 3.Denlinger DL, Yocum GD, Rinehart JP. Hormonal control of diapause. In: Gilbert LI, editor. Comprehensive Molecular Insect Science. Elsevier; Amsterdam: 2005. pp. 615–650. [Google Scholar]
- 4.Lu YX, Denlinger DL, Xu WH. Polycomb repressive complex 2 (PRC2) protein ESC regulates insect developmental timing by mediating H3K27me3 and activating prothoracicotropic hormone gene expression. J Biol Chem. 2013;288:23554–23564. doi: 10.1074/jbc.M113.482497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Levine RL. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med. 2002;32:790–796. doi: 10.1016/s0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- 7.Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A. Protein carbonylation in human diseases. Trends Mol Med. 2003;9:169–176. doi: 10.1016/s1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 8.Schulz TJ, et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang W, Hekimi S. Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans. Aging Cell. 2010;9:433–447. doi: 10.1111/j.1474-9726.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- 11.Pan Y, Schroeder EA, Ocampo A, Barrientos A, Shadel GS. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab. 2011;13:668–678. doi: 10.1016/j.cmet.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatar M, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 13.Sim C, Denlinger DL. Insulin signaling and the regulation of insect diapause. Front Physiol. 2013;4:189. doi: 10.3389/fphys.2013.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedrick SM, Hess Michelini R, Doedens AL, Goldrath AW, Stone EL. FOXO transcription factors throughout T cell biology. Nat Rev Immunol. 2012;12:649–661. doi: 10.1038/nri3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broughton SJ, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Keizer PLJ, Burgering BMT, Dansen TB. Forkhead box o as a sensor, mediator, and regulator of redox signaling. Antioxid Redox Signal. 2011;14:1093–1106. doi: 10.1089/ars.2010.3403. [DOI] [PubMed] [Google Scholar]
- 18.Bao B, Xu WH. Identification of gene expression changes associated with the initiation of diapause in the brain of the cotton bollworm, Helicoverpa armigera. BMC Genomics. 2011;12:224. doi: 10.1186/1471-2164-12-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu YX, Xu WH. Proteomic and phosphoproteomic analysis at diapause initiation in the cotton bollworm, Helicoverpa armigera. J Proteome Res. 2010;9:5053–5064. doi: 10.1021/pr100356t. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Lu YX, Xu WH. Integrated proteomic and metabolomic analysis of larval brain associated with diapause induction and preparation in the cotton bollworm, Helicoverpa armigera. J Proteome Res. 2012;11:1042–1053. doi: 10.1021/pr200796a. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Lu YX, Xu WH. Proteomic and metabolomic profiles of larval hemolymph associated with diapause in the cotton bollworm, Helicoverpa armigera. BMC Genomics. 2013;14:751. doi: 10.1186/1471-2164-14-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu WH, Lu YX, Denlinger DL. Cross-talk between the fat body and brain regulates insect developmental arrest. Proc Natl Acad Sci USA. 2012;109:14687–14692. doi: 10.1073/pnas.1212879109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarse K, et al. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 2012;15:451–465. doi: 10.1016/j.cmet.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedford MT, Richard S. Arginine methylation an emerging regulator of protein function. Mol Cell. 2005;18:263–272. doi: 10.1016/j.molcel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Kops GJ, et al. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 26.Yamagata K, et al. Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Mol Cell. 2008;32:221–231. doi: 10.1016/j.molcel.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Dillon MBC, et al. Novel inhibitors for PRMT1 discovered by high-throughput screening using activity-based fluorescence polarization. ACS Chem Biol. 2012;7:1198–1204. doi: 10.1021/cb300024c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leto D, Saltiel AR. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- 29.Sim C, Denlinger DL. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc Natl Acad Sci USA. 2008;105:6777–6781. doi: 10.1073/pnas.0802067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sim C, Kang DS, Kim S, Bai X, Denlinger DL. Identification of FOXO targets that generate diverse features of the diapause phenotype in the mosquito Culex pipiens. Proc Natl Acad Sci USA. 2015;112:3811–3816. doi: 10.1073/pnas.1502751112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martins R, Lithgow GJ, Link W. Long live FOXO: Unraveling the role of FOXO proteins in aging and longevity. Aging Cell. 2016;15:196–207. doi: 10.1111/acel.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finkel T. Redox-dependent signal transduction. FEBS Lett. 2000;476:52–54. doi: 10.1016/s0014-5793(00)01669-0. [DOI] [PubMed] [Google Scholar]
- 33.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010;20:2131–2136. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng WH, Kar S, Quirion R. Insulin-like growth factor-1-induced phosphorylation of the forkhead family transcription factor FKHRL1 is mediated by Akt kinase in PC12 cells. J Biol Chem. 2000;275:39152–39158. doi: 10.1074/jbc.M002417200. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Zhang LL, Shen L, Xu XM, Yu HG. Regulation of AKT gene expression by cisplatin. Oncol Lett. 2013;5:756–760. doi: 10.3892/ol.2013.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Belle JE, et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chetram MA, et al. ROS-mediated activation of AKT induces apoptosis via pVHL in prostate cancer cells. Mol Cell Biochem. 2013;376:63–71. doi: 10.1007/s11010-012-1549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi Y, et al. Asymmetric arginine dimethylation determines life span in C. elegans by regulating forkhead transcription factor DAF-16. Cell Metab. 2011;13:505–516. doi: 10.1016/j.cmet.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Cheng CM, et al. Insulin-like growth factor 1 regulates developing brain glucose metabolism. Proc Natl Acad Sci USA. 2000;97:10236–10241. doi: 10.1073/pnas.170008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kupriyanova TA, Kandror KV. Akt-2 binds to Glut4-containing vesicles and phosphorylates their component proteins in response to insulin. J Biol Chem. 1999;274:1458–1464. doi: 10.1074/jbc.274.3.1458. [DOI] [PubMed] [Google Scholar]
- 43.Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 44.Morais VA, et al. PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science. 2014;344:203–207. doi: 10.1126/science.1249161. [DOI] [PubMed] [Google Scholar]
- 45.Putker M, et al. Redox-dependent control of FOXO/DAF-16 by transportin-1. Mol Cell. 2013;49:730–742. doi: 10.1016/j.molcel.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Scialò F, et al. Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell Metab. 2016;23:725–734. doi: 10.1016/j.cmet.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velarde MC, Demaria M, Melov S, Campisi J. Pleiotropic age-dependent effects of mitochondrial dysfunction on epidermal stem cells. Proc Natl Acad Sci USA. 2015;112:10407–10412. doi: 10.1073/pnas.1505675112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin XW, Xu WH. Hexokinase is a key regulator of energy metabolism and ROS activity in insect lifespan extension. Aging (Albany NY) 2016;8:245–259. doi: 10.18632/aging.100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ristow M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.