Abstract
We describe a case of fatal acute liver failure due to echovirus 9 in the setting of persistent B-cell depletion and hypogammaglobulinemia 3 years after rituximab therapy. Metagenomic next-generation sequencing further specified the etiologic agent. Early recognition may provide an opportunity for interventions including intravenous immunoglobulin and liver transplantation.
Keywords: acute liver failure, echovirus, metagenomic next-generation sequencing, rituximab
Enteroviruses are a common cause of infection, accounting for more than 10–15 million symptomatic cases per year in the United States alone. Clinical presentation ranges from a mild febrile illness to severe disease, particularly in neonates or immunocompromised patients [1].
Rituximab is a monoclonal antibody directed against CD20 that is commonly used to treat a variety of B-cell malignancies and autoimmune diseases. It causes peripheral B-cell depletion and hypogammaglobulinemia that can persist for months, thus leading to an increased risk of numerous infections [2]. We describe an unusual case of fatal acute liver failure due to echovirus 9 infection in a patient with persistent B-cell depletion 3 years after rituximab therapy.
CASE REPORT
A 19-year-old man with a history of minimal change disease developed acute liver failure after a prolonged period of illness (Supplemental Figure). At age 15, he was diagnosed with nephrotic syndrome and found to have biopsy-proven minimal change disease. He experienced relapses after corticosteroid therapy and was treated with 2 infusions of rituximab at age 16. Complete remission was achieved, although he had persistent absence of CD19 B cells in his peripheral blood 11 months after the last rituximab treatment.
Eight months before admission, he presented with productive cough progressing to fevers, retro-orbital headache, and photophobia. A lumbar puncture revealed a cerebrospinal fluid (CSF) white blood cell count of 26 cells/µL (62% polymorphonuclear cells, 27% lymphocytes, 11% monocytes), 90 mg/dL protein (normal 15–45 mg/dL), and normal glucose. Cerebrospinal fluid culture was negative for bacterial growth. No viral studies were performed on the CSF. Human immunodeficiency virus (HIV) 1 and 2 antibody screen was negative. The patient was treated with empiric acyclovir and improved.
Four months before admission, he presented with intermittent fevers and myalgias. Diagnostic evaluation was notable for new liver function test (LFT) abnormalities including aspartate aminotransferase (AST) 575 U/L (normal 10–45 U/L), alanine aminotransferase (ALT) 775 U/L (normal 10–65 U/L), total bilirubin 3.7 mg/dL (normal 0.1–1.5 mg/dL), and alkaline phosphatase (ALP) 120 IU/L (normal 35–115 IU/L). Negative studies included hepatitis A immunoglobulin (Ig)M, hepatitis B surface antigen and core IgM, hepatitis C antibody, antinuclear antibody (ANA), antismooth muscle antibody (ASMA), rapid influenza A and B antigens, mononucleosis screen, repeat HIV 1 and 2 antibody screen, cytomegalovirus (CMV) IgM and IgG, and Epstein-Barr virus (EBV) IgM. Epstein-Barr virus IgG was positive. Imaging included a normal computed tomography scan of the abdomen and pelvis. His total serum IgG was low at 489 mg/dL (normal 758–1612 mg/dL). He improved symptomatically with supportive care.
One month before admission, he developed bilateral tinnitus, hearing loss, and mild positional vertigo. Evaluation 2 weeks later revealed sensorineural hearing loss, and shortly thereafter he developed fevers, nausea, and vomiting. New liver function tests showed an AST peak of 1216 U/L, ALT 1752 U/L, total bilirubin 3.8 mg/dL, and ALP 149 IU/L. Repeat hepatitis and HIV 1 and 2 antibody screens, ANA, ASMA, and CMV and EBV serologies remained negative. Hepatitis C ribonucleic acid (RNA), herpes simplex virus (HSV) IgM, antimitochondrial antibody, and antidouble-stranded deoxyribonucleic acid antibody were also negative. Drug screens including acetaminophen were negative except for cannabinoids. There was no evidence in blood of copper or iron overload, and alpha-1 antitrypsin was normal. Abdominal ultrasound showed hepatomegaly with patent flow in the portal and hepatic circulation. He was treated with 60 mg of prednisone per day and his LFTs initially improved. However, he experienced acute recurrence of fever with new right upper quadrant pain. By this time, he had developed acute liver failure with mild encephalopathy and AST 7821 U/L, ALT 5936 U/L, total bilirubin 7.3 mg/dL, ALP 213 IU/L, INR 6.4 (normal 0.0–3.5), and platelet count 16 K/µL (normal 150–450 K/µL). He was transferred to our facility for further management.
On admission the patient reported headache and lethargy. Presenting neurologic exam was nonfocal except for bilateral hearing loss. Lumbar puncture was not performed in the setting of marked coagulopathy. High-dose intravenous acyclovir and methylprednisolone were started empirically. Additional diagnostic tests included negative polymerase chain reaction tests (PCR) in plasma for adenovirus, CMV, EBV, hepatitis B and C, human herpesvirus 6, HIV, HSV, parvovirus B19, and varicella-zoster virus (VZV). Repeat portal and hepatic venous imaging again showed patent flow. Total serum IgG remained low at 522 mg/dL (normal 610–1616). His candidacy for liver transplantation was assessed, but he rapidly deteriorated; upper gastrointestinal bleeding in the setting of ongoing coagulopathy and progressive cerebral edema with herniation led to death on hospital day 4. Reverse transcription-PCR studies on plasma samples collected just before death subsequently demonstrated high levels of entero/parechovirus RNA at 270 000 copies/mL.
PATHOLOGY
The brain was diffusely edematous with tonsillar herniation. There was diffuse lymphomononuclear meningoencephalitis, with inflammatory cell infiltrates composed of T lymphocytes and macrophages in the leptomeninges and the parenchyma of cerebrum, brainstem, and cerebellum. Immunohistochemical studies for VZV, HSV I/II, and CMV were negative. Lymph nodes were notable for absence of germinal centers and complete lack of immunostaining for CD19 and CD20.
The liver demonstrated a combination of autolysis and necrosis involving approximately 90% of the lobular parenchyma with relative sparing of periportal areas (Figure 1A). Patchy mixed lobular inflammation was present, predominantly within sinusoidal spaces, with scattered lobular microabscesses, suggestive of acute hepatitis (Figure 1B). The portal tracts also showed mixed inflammation with patchy interface activity (Figure 1C). Overall, the findings were considered to be due to a combination of viral-induced injury and hypoperfusion resulting in shock liver. After report of high levels of entero/parechovirus RNA in antemortem plasma, a sample of fresh liver frozen at autopsy was cultured. This grew enterovirus, which was subsequently confirmed as echovirus 9 by metagenomic whole-genome sequencing.
Figure 1.
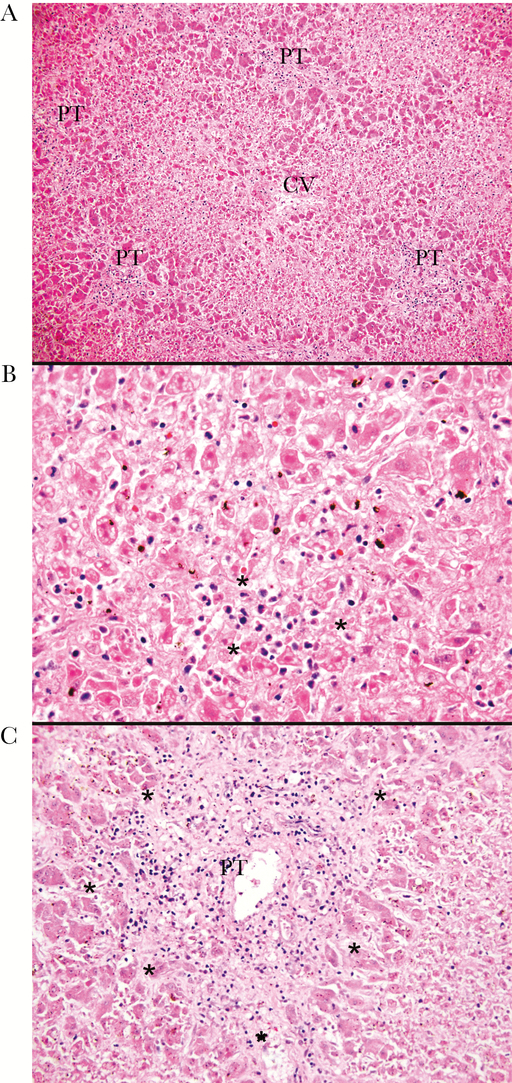
Liver pathology. (A) The liver parenchyma exhibits a combination of autolysis and necrosis involving approximately 90% of the liver with relative sparing of periportal areas; hematoxylin and eosin (H&E) stain, ×100. (B) Patchy mixed lobular inflammation, predominantly involving sinusoidal spaces, with scattered lobular microabscesses (*); H&E stain, ×200. (C) Mixed portal inflammation spilling into the adjacent liver parenchyma (*); H&E stain, ×200. CV, central veins; PT, portal tracts.
METAGENOMIC SEQUENCING
Both the enterovirus-positive plasma sample and culture-positive frozen liver specimen were subjected to metagenomic next-generation sequencing, as described previously [3, 4]. The whole genome from the culture was recovered using iterative de novo assembly from consensus sequences in the 5’UTR, 2C, and 3D derived from mapping to enterovirus B reference sequence (NC_001472). The near complete enterovirus genome from the plasma sample was derived from mapping to the full genome from the culture supernatant. Both genomes are deposited in National Center for Biotechnology Information (NCBI) Nucleotide (KX610685 [tissue culture], KX681481 [plasma]). The 1.36 million metagenomic reads derived from the plasma specimens were aligned using DIAMOND against the NCBI nonredundant database yielding nonphage viral alignments to Enterovirus B species (11 142 reads) [5]. No hepatitis A–C virus reads were detected from the plasma specimen.
A total of 635 267 reads (53% of the metagenomic sequencing library) mapped to the finished viral genome contig recovered from the culture supernatant, yielding 10 920X sequencing coverage of the viral genome. A total of 16 495 reads from the plasma RNA-sequencing sample (1.2% of the metagenomic sequencing library) mapped to the culture supernatant sequencing, offering 271X coverage of the genome. BLASTN analysis of the 7454 nucleotide viral genome revealed it to be 84% identical by nucleotide to the echovirus 9 DM33 strain (Finland, 2012), whereas BLASTP analysis showed 97% amino acid identity to the echovirus 9 MSH/KM812 strain (China, 2010). The sequences obtained directly from plasma and the culture specimen differed by only 2 nonsynonymous changes in the VP1 capsid protein.
DISCUSSION
Acute liver failure secondary to echovirus 9 infection is rarely reported. When recognized, enterovirus-associated hepatitis in healthy adults generally manifests as mild to moderate disease [6]. Enteroviral sepsis and severe hepatitis is more frequently seen in the neonatal population [7], although there have been rare instances of severe hepatitis in immunocompromised adults [8, 9].
Although not definitively proven, it is highly likely that echovirus 9 infection caused the patient’s relapsing meningoencephalitis. There have been increasing reports of severe enteroviral infections, particularly chronic meningoencephalitis, in adults treated with rituximab [2, 10]. Presentation occurred months to years after rituximab administration and was associated with prolonged persistence of hypogammaglobulinemia. Our case is the third reported occurrence of enterovirus-related acute liver failure in a patient treated with rituximab. The absence of CD19 and CD20 cells in this patient’s lymph nodes confirmed that B-cell depletion persisted for 3 years after his rituximab therapy.
Benefit of treatment with intravenous Ig (IVIG) has been demonstrated for patients with agammaglobulinemia and chronic enterovirus infections, but there has been no proven efficacy in neonates [11]. Successful treatment of rituximab-associated enteroviral meningoencephalitis in adults has been reported in a few cases. There have been rare instances of successful liver transplantation in neonates as treatment for acute liver failure due to disseminated enterovirus infection [12, 13].
CONCLUSIONS
In conclusion, enterovirus infections should be considered in the differential diagnosis of acute liver failure or meningoencephalitis, particularly in patients with underlying humoral immunodeficiency. Rituximab-induced B-cell depletion can persist for years and is a critical risk factor for these infections. The case further highlights the diagnostic utility of preserving frozen tissue for viral culture and molecular studies as well as the power of metagenomic sequencing to rapidly recover whole genomes direct from patient specimens. Although available therapy is currently limited to IVIG, there is a potential role for liver transplantation if definitive diagnosis can be made sufficiently early.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We thank Dennis Reichenbach, Matthew Yeh, Kelly Smith, and Gordana Juric-Sekhar for expert pathology interpretation. We thank the patient’s mother for permission to publish this case.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. Enterovirus surveillance--United States, 1970–2005. MMWR Surveill Summ 2006; 55:1–20. [PubMed] [Google Scholar]
- 2. Makatsori M, Kiani-Alikhan S, Manson AL et al. . Hypogammaglobulinaemia after rituximab treatment-incidence and outcomes. QJM 2014; 107:821–8. [DOI] [PubMed] [Google Scholar]
- 3. Greninger AL, Naccache SN, Messacar K et al. . A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012-14): a retrospective cohort study. Lancet Infect Dis 2015; 15:671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greninger AL, Zerr DM, Qin X et al. . Rapid metagenomic next-generation sequencing during an investigation of hospital-acquired human parainfluenza virus 3 infections. J Clin Microbiol 2017; 55:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods 2015; 12:59–60. [DOI] [PubMed] [Google Scholar]
- 6. Schleissner LA, Portnoy B. Hepatitis and pneumonia associated with ECHO virus, type 9, infection in two adult siblings. Ann Intern Med 1968; 68:1315–9. [DOI] [PubMed] [Google Scholar]
- 7. Modlin JF. Perinatal echovirus infection: insights from a literature review of 61 cases of serious infection and 16 outbreaks in nurseries. Rev Infect Dis 1986; 8:918–26. [DOI] [PubMed] [Google Scholar]
- 8. Morgan C, Thomson SJ, Legg J, Narat S. A case of fulminant hepatitis due to echovirus 9 in a patient on maintenance rituximab therapy for follicular lymphoma. Case Rep Hematol 2015; 2015:454890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lefterova MI, Rivetta C, George TI, Pinsky BA. Severe hepatitis associated with an echovirus 18 infection in an immune-compromised adult. J Clin Microbiol 2013; 51:684–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grisariu S, Vaxman I, Gatt M et al. . Enteroviral infection in patients treated with rituximab for non-Hodgkin lymphoma: a case series and review of the literature. Hematol Oncol 2016; doi: 10.1002/hon.2365. [DOI] [PubMed] [Google Scholar]
- 11. Abzug MJ, Keyserling HL, Lee ML et al. . Neonatal enterovirus infection: virology, serology, and effects of intravenous immune globulin. Clin Infect Dis 1995; 20:1201–6. [DOI] [PubMed] [Google Scholar]
- 12. Chuang E, Maller ES, Hoffman MA et al. . Successful treatment of fulminant echovirus 11 infection in a neonate by orthotopic liver transplantation. J Pediatr Gastroenterol Nutr 1993; 17:211–4. [DOI] [PubMed] [Google Scholar]
- 13. Miyata I, Hanaoka N, Okabe N et al. . Echovirus 3 as another enterovirus causing life-threatening neonatal fulminant hepatitis. J Clin Virol 2014; 59:132–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


