Abstract
Alteration and activation of recepteur d'origine nantais (RON) expression is known to be associated with cancer progression and decreased survival in various types of human cancer, including pancreatic cancer. Therefore, in the present study, RON expression levels were determined in resected left-sided pancreatic cancer to evaluate the potential oncological role of RON in the clinical setting of distal pancreatic cancer. From January 2005 to December 2011, a total of 57 patients underwent radical distal pancreatectomy for left-sided pancreatic cancer. Ductal adenocarcinoma was confirmed in all patients. Among these patients, 17 patients who received preoperative neoadjuvant treatment and 7 patients without available paraffin-embedded tissue blocks were excluded from the present study. RON expression in a the pancreatic cancer cell lines ASPC-1, BxPC-3, MiaPaCa-3 and Panc-1, as well as in resected left-sided pancreatic cancer specimens was determined by Western blot analysis. RON and vascular endothelial growth factor (VEGF) overexpression in resected left-sided pancreatic cancer was also evaluated by immunohistochemistry using pre-diluted anti-RON and anti-VEGF antibodies. An association was identified between the oncological outcome and RON overexpression. Increased levels of RON expression were observed in two pancreatic cancer cell lines, AsPC-1 and BxPC-3. RON overexpression was detected in specimens from 15/33 patients (45.5%) using immunohistochemistry. No significant association was identified between RON overexpression and VEGF overexpression (25.5 vs. 87.9%; P=0.667). No significant differences in disease-free survival or disease-specific survival associated with RON overexpression were identified. Although the results of previous studies have suggested that RON is a potential target for the treatment of pancreatic cancer, in the present study no association between RON overexpression and any adverse oncological effect was identified.
Keywords: recepteur d'origine nantais, pancreatic cancer, prognosis
Introduction
Recepteur d'origine nantais (RON), a receptor tyrosine kinase belonging to the MET proto-oncogene family (1) shares ~60% structural homology with the c-MET receptor (2). RON is synthesized as a single-chain precursor, pro-RON, which is then cleaved into a 40-kDa α-chain and a 150-kDa β-chain (3). A single disulfide bond links these two chains to form a 180-kDa heterodimer. The α-chain is completely extracellular, and the β-chain has extracellular, transmembrane and intracellular regions containing a functional tyrosine kinase segment as well as multiple regulatory elements. The ligand for RON is macrophage-stimulating factor, also known as hepatocyte growth factor-like protein or scatter factor-2 (4).
Altered RON expression and activation are known to be associated with cancer progression and decreased survival in a number of types of human cancer, including breast (5), colon (6), gastric (7), non-small cell lung (8), bladder (9) and ovary (10) cancer. Research into RON in pancreatic cancer is a relatively recent development. The currently available evidence to support the potential role of RON in carcinogenesis of pancreatic cancer and implications for future targeted therapy in treating pancreatic cancer was previously reviewed (11). RON has been demonstrated to serve important roles in pancreatic cancer carcinogenesis (12–14), epithelial-mesenchymal transition (15,16), tumor migration (17–19), angiogenesis (20,21), cancer stem cells (22) and apoptotic resistance (14,23,24) as a part of the progression of pancreatic cancer, suggesting that RON may be a potential therapeutic target in the treatment of pancreatic cancer. In particular, RON signaling was previously identified to increase VEGF level and promote microtubule formation in BxPC-3 and FG cells, suggesting an specific mechanism for the association of RON with pancreatic cancer progression (21).
Chakedis et al (25,26) identified a novel RON isoform in human pancreatic cancer. Partial splicing of exons 5 and 6 (P5P6) produces a RON isoform that lacks the first extracellular immunoglobulin-plexin-transcription domain (25); RNA sequencing studies revealed that the P5P6 isoform has ligand-independent activity and induces markedly different patterns of gene expression when compared with wild type RON, providing further understanding of RON biology in pancreatic cancer carcinogenesis and exhibiting potential implications for therapeutic strategy (26). RON-specific therapeutic approaches, including tyrosine kinase inhibitors and monoclonal antibodies, have been tested in preclinical and clinical trials to determine their anti-cancer efficacy (27–29). However, their therapeutic efficacy was relatively low. Great effort has since been made to increase the efficacy of monoclonal antibodies against RON for the treatment of pancreatic cancer (30).
However, the data indicating potential associations between RON expression and the clinical outcome of pancreatic cancer are presently limited. Unless this association is confirmed, the recent drive into RON research may be attenuated. Therefore, in the present study, the association between VEGF expression and clinical outcomes with RON expression was evaluated in resected left-sided pancreatic cancer, in order to assess the potential role of RON in the clinical setting of left-sided pancreatic cancer.
Materials and methods
Patient enrollment and review of medical records
From January 2005 to December 2011, a total of 57 patients underwent radical distal pancreatosplenectomy for left-sided pancreatic cancer at Severance hospital, Yonsei University College of Medicine (Seoul, Korea). Ductal adenocarcinoma was confirmed in all patients. A total of 17 patients who received preoperative neoadjuvant treatment and 7 patients for whom paraffin-embedded tissue blocks were unavailable were excluded (Fig. 1). The patients' clinicopathological characteristics, including age, sex, clinical presentation, tumor size, histopathological features and follow-up data were reviewed and recorded. The present study was approved by the Institutional Review Board of Yonsei University College of Medicine.
Figure 1.
Patient eligibility. From January 2005 to December 2011, a total of 57 patients underwent radical distal pancreatosplenectomy for left-sided pancreatic cancer with histological confirmation of ductal adenocarcinoma. Among these patients, 17 patients who received preoperative neoadjuvant treatment and 7 patients for whom paraffin-embedded tissue blocks were unavailable were excluded from the present study.
Cell lines and cell maintenance
The human pancreatic cancer cell lines ASPC-1, BxPC-3, MiaPaCa-2 and Panc-1 were obtained from the Bioevaluation Center (Korea Research Institute of Bioscience and Biotechnology, Daejeon, Korea). ASPC-1 and BxPC-3 cells were maintained in RPMI medium, Panc-1 cells were maintained in Dulbecco's modified Eagle's medium and MiaPaCa-2 cells were maintained in minimal essential medium. All tissue culture media were from Gibco (Thermo Fisher Scientific, Inc.), and were supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin, unless otherwise noted. All cells were grown at 37°C in a humidified incubator containing CO2.
Western blot analysis
Harvested cells were lysed in cell extraction buffer (10 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS and 0.5% deoxycholate). A 40 µg amount of total protein, as quantified with a Bradford assay, was treated with Laemmli sample buffer (Bio-Rad Laboratories, Inc., Hercules, CA, USA), heated at 100°C for 5 min and then resolved by 8% SDS-PAGE. Gels were electroblotted onto nitrocellulose membranes (GE Healthcare Life Sciences, Chalfont, UK). Membranes were blocked with 5% non-fat dry milk in Tris-buffered saline with Tween-20, incubated with antibodies against total RON (cat. no., ab52927; Abcam, Cambridge, UK) and β-actin (cat. no., ab8227; Sigma-Alrich; Merck KGaA, Darmstadt, Germany) overnight at 4°C, and then probed with secondary antibodies (horseradish peroxidase conjugated mouse anti-rabbit IgG; cat. no., sc2357; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature. All antibodies were treated with Dako Antibody Diluent (Agilent Technologies, Santa Clara, CA, USA). The washes were repeated and the membrane was developed using a chemiluminescent agent (GE Healthcare Life Sciences). The whole process was performed in triplicate.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue sections (4 µm) thick were deparaffinized and rehydrated prior to antigen retrieval. Deparaffinization was performed on a rack with the following washes: Xylene for 3 min, xylene 1:1 with 100% ethanol for 3 min, 100% ethanol for 3 min, 95% ethanol for 3 min, 70% ethanol for 3 min, 50% ethanol with 3 min, then a final rinse with tapwater. Immunohistochemical analysis was performed using pre-diluted anti-RON (cat. no., ab52927; Abcam) and anti-vascular endothelial growth factor (VEGF; cat. no., sc-152; Santa Cruz Biotechnology, Inc.) antibodies, according to the manufacturer's protocol. All slides were reviewed by two pathologists blinded to the oncological outcomes and clinicopathological variables. The intensities of RON and VEGF were scored as 0, null; 1+, positive; and 2+, strong positive.
Statistical analysis
Statistical analysis was performed using SPSS 23 software (IBM SPSS, Armonk, NY, USA). Continuous variables are presented as the mean ± standard deviation and categorical variables are expressed as the frequency (%). Univariate analysis was performed using a χ2 test, and Student's t-test was used for statistical assessment of the association between clinicopathological characteristics and RON overexpression. Survival curves were obtained by the Kaplan-Meier method, and differences in survival between groups were compared with the log rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
RON expression in pancreatic cancer cell lines and resected left-sided pancreatic cancer
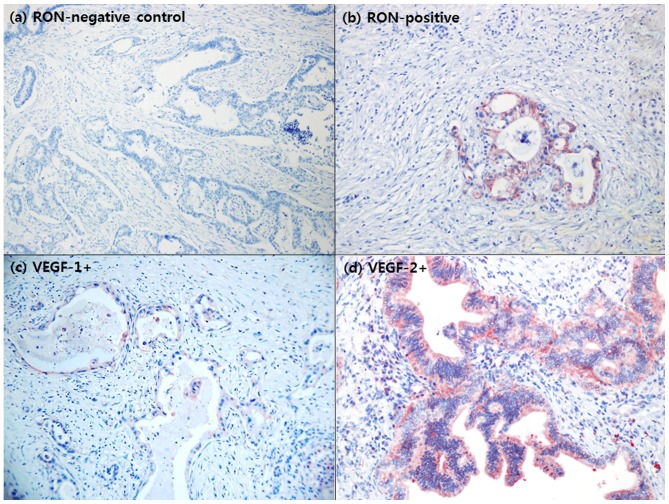
RON protein expression was determined by Western blot analysis in pancreatic cancer cell lines (Fig. 2). All cell lines evaluated expressed RON at various levels. In particular, AsPC-1 and BxPC-3 were identified to exhibit increased expression of both the RON α- and β-chains. By contrast, MiaPaCa-2 and Panc-1 cells were identified to exhibited relatively decreased levels of RON expression. In immunohistochemistry studies, 15/33 patients (45.5%) with resected left-sided pancreatic cancer were identified to overexpress RON (Fig. 3A and b).
Figure 2.

Western blot analysis of RON α- and β-chain expression in pancreatic cancer cell lines. The levels of RON protein were determined by Western blot analysis in pancreatic cancer cell lines. AsPC-1 and BxPC-3 exhibited increased levels of expression of the RON α- and β-chains. However, MiaPaCa-2 and Panc-1 exhibited relatively decreased levels of RON expression. RON, recepteur d'origine nantais.
Figure 3.
Immunohistochemical staining for RON and VEGF expression in resected pancreatic cancer. (A) RON-negative control. (B) RON-positive. Samples from 15/33 patients (45.5%) exhibited RON overexpression by immunohistochemical staining. (C) VEGF 1+. (D) VEGF 2+. A total of 29 patients (87.9%) were determined to exhibit VEGF overexpression. No association between RON overexpression and VEGF overexpression was identified. RON, recepteur d'origine nantais; VEGF, vascular endothelial growth factor.
RON and VEGF overexpression in resected left-sided pancreatic cancer
Specimens from 29 patients (87.9%) exhibited VEGF overexpression (Fig. 3C and d). No association between RON and VEGF expression was identified in resected left-sided pancreatic cancer (P=0.381; Table I).
Table I.
Association between RON and VEGF overexpression.
| RON overexpression | ||||
|---|---|---|---|---|
| Parameter | 0 | 1+ | P-value | |
| VEGF | 0 | 3 | 1 | 0.381 |
| overexpression | 1+ | 8 | 8 | |
| 2+ | 7 | 6 | ||
RON, recepteur d'origine nantais; VEGF, vascular endothelial growth factor.
Clinical validation of the oncological role of RON expression in resected left-sided pancreatic cancer
No association between clinical oncological parameters and overexpression in resected left-sided pancreatic cancer was identified (P>0.05; Table II). In particular, no significant differences in tumor stage (P=0.981), tumor size (P=0.2000), node stage (P=0.898), perineural invasion (P=1.000) and lymphovascular invasion (P=0.919) were identified.
Table II.
Association between RON overexpression and clinical oncological parameters.
| RON overexpression | |||
|---|---|---|---|
| Oncological parameter | No | Yes | P-value |
| CA19-9, U/ml ± SD | 1304.7±4671.5 | 603.3±1475.6 | 0.581 |
| Tumor size, cm ± SD | 3.1±0.9 | 2.7±0.9 | 0.200 |
| T stage | 0.981 | ||
| T1 | 0 | 0 | |
| T2 | 1 | 1 | |
| T3 | 16 | 13 | |
| T4 | 1 | 1 | |
| N stage | 0.898 | ||
| N0 | 8 | 7 | |
| N1 | 10 | 8 | |
| LNR | 0.4±1.3 | 0.1±0.1 | 0.384 |
| Differentiation | 0.750 | ||
| Well | 4 | 3 | |
| Moderate | 11 | 11 | |
| Poor | 2 | 1 | |
| None | 1 | 0 | |
| LVI | 0.919 | ||
| No | 10 | 9 | |
| Yes | 6 | 5 | |
| PNI | 1.000 | ||
| No | 8 | 7 | |
| Yes | 8 | 7 | |
| R-status | 0.727 | ||
| R0 | 16 | 12 | |
| R1 | 1 | ||
| R2 | 1 | 1 | |
| Postadjuvant chemotherapy | 0.475 | ||
| No | 4 | 5 | |
| Yes | 14 | 10 | |
RON, recepteur d'origine nantais; CA19-9, cancer antigen 19-9; SD, standard deviation; T, tumor; N, node; LNR, lymph node ratio; LVI, lymphovascular invasion; PNI, perineural invasion; R, residual tumor.
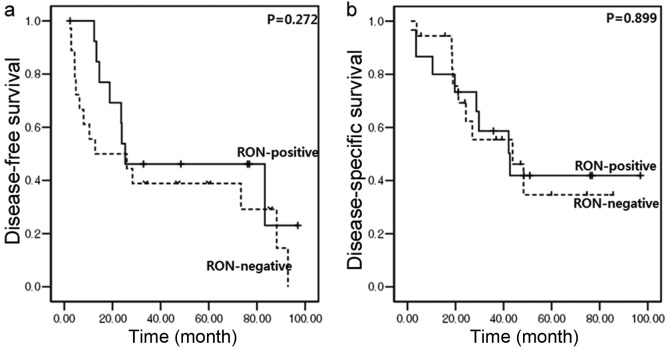
In addition, RON overexpression did not cause any oncological effect on tumor recurrence and overall survival. In resected left-sided pancreatic cancer, no significant differences in disease-free survival (median, 12.8 months [95% CI (confidence interval), 0–45.1] vs. median, 25.3 months (95% CI, 0–67.4); P=0.272; Fig. 4A) or disease-specific survival [median, 43.8 months (95% CI, 16.6–70.9) vs. median, 42.7 months (95% CI, 22–63.3); P=0.899; Fig. 4B] between RON-positive and RON-negative patients were identified.
Figure 4.
Oncological effect of RON expression in resected left-sided pancreatic cancer. (A) No significant difference in disease-free survival was identified between RON-positive patients and RON-negative patients (median, 12.8 months vs. 25.3 months; P=0.272). (B) No significant difference in disease-specific survival was identified between RON-positive patients and RON-negative patients (median, 43.8 months vs. 42.7 months; P=0.899). RON, recepteur d'origine nantais.
Discussion
A previous study has suggested a potential oncological role for RON expression in pancreatic cancer progression (10); however, there have been limited studies concerning the oncological effect of RON overexpression in resected pancreatic cancer. To the best of our knowledge, only a single study has been published: Tactacan et al (31) assessed RON expression in a total of 492 pancreatic cancer patients and evaluated the association between RON expression and patient outcomes and clinicopathological variables. The study identified that increased RON expression was a biomarker for poor prognosis in a training set. However, the study failed to identify that RON expression was not prognostic in the larger validation set. In addition, no association was identified between RON expression and tumor stage (P=0.123), tumor size (P=0.629) lymph node metastases (P=0.942), grade (P=0.332), perineural invasion (P= 0.335) or vascular invasion (P=0.210), leading to the conclusion that RON is not a prognostic marker for resectable pancreatic cancer. When looking at their patient population, >80% of the patients had pancreatic head cancer, therefore allowing the possibility of unintended contamination by other periampullary cancers from the ampulla of Vater and distal bile ducts. To avoid this potential selection bias in the present study, the study population consisted only of patients with resected left-sided pancreatic cancer.
Thomas et al (21) demonstrated that RON signaling resulted in mitogen-activated protein kinase-mediated VEGF secretion by pancreatic cancer cells and in the promotion of microtubule formation, suggesting that RON signaling may also positively regulate an angiogenic mediator, VEGF, to promote cancer progression in pancreatic cancer. This may explain the results of another study where treatment with gemcitabine plus the anti-VEGF monoclonal antibody bevacizumab failed to provide an oncological benefit over gemcitabine treatment alone (32). However, in the present study, no association between RON and VEGF expression in resected left-sided pancreatic cancer was identified (P=0.381). Our understanding of the regulation of angiogenesis in pancreatic cancer therefore remains limited.
The clinical data of the present study also failed to reveal an oncological role for RON in resected left-sided pancreatic cancer. Following assessment of the potential association of RON expression with clinicopathological characteristics, RON overexpression was not identified to be associated with tumor size, pathological node stage, pathological tumor stage, tumor differentiation, perineural invasion or lymphovascular invasion (P>0.05). In addition, there were no significant oncological differences in terms of disease-free and disease-specific survival.
There are a number of potential reasons for the current missing link between RON expression and oncological outcome in resected left-sided pancreatic cancer, as follows: i) Pancreatic cancer harbors multiple genetic mutations and various dysregulated signaling pathways the contribute to cancer progression. ii) It is known that there are multiple splice variants of the RON receptor (33–35), including RON Δ165, RON Δ160, RON Δ155, RON Δ170, RON Δ110 and RON Δ52. To the best of our knowledge, the presence of these variant types of the RON receptor has not been investigated, and their individual oncogenic capability has not been evaluated in pancreatic cancer. In addition, it is impossible to discriminate between these variant types of RON receptors using current immunohistochemistry techniques. In particular, a truncated form of the RON receptor (short-form RON), lacking a majority of the extracellular domain (36), may not be detectable by conventional routine immunohistochemistry. However, this short-form RON is constitutively active, leading to pathogenesis and cancer progression in pancreatic cancer. iii) The potential role of the microenvironment also requires consideration. It is well-known that severe fibrosis and a desmoplastic reaction including fibroblasts, immune cells, endothelial cells and neural cells are associated with pancreatic cancer. Evidence from a previous study suggests that interactions occur between the microenvironment and pancreatic cancer cells, facilitating pancreatic cancer pathogenesis (37). It was not possible to replicate the potential contribution of this ‘harmony’ in the present study. iv) Finally, the present study was based on a retrospective study design with a small number of patients. Therefore, selection bias was unavoidable.
RON overexpression failed to result in an adverse oncological effect in resected left-sided pancreatic cancer, despite previous studies suggesting that RON may be a potential target in the treatment of pancreatic cancer. Further clinical studies validating the potential oncological role of RON are required, and consideration of the multifactorial and heterogeneous nature of pancreatic cancer is also required.
Acknowledgements
The present study was supported by a faculty research grant from Yonsei University College of Medicine (grant no, 6-2013-0041).
References
- 1.Ronsin C, Muscatelli F, Mattei MG, Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene. 1993;8:1195–1202. [PubMed] [Google Scholar]
- 2.Park M, Dean M, Kaul K, Braun MJ, Gonda MA, Vande Woude G. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci USA. 1987;84:6379–6383. doi: 10.1073/pnas.84.18.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang MH, Wang D, Chen YQ. Oncogenic and invasive potentials of human macrophage-stimulating protein receptor, the RON receptor tyrosine kinase. Carcinogenesis. 2003;24:1291–1300. doi: 10.1093/carcin/bgg089. [DOI] [PubMed] [Google Scholar]
- 4.Wang MH, Ronsin C, Gesnel MC, Coupey L, Skeel A, Leonard EJ, Breathnach R. Identification of the ron gene product as the receptor for the human macrophage stimulating protein. Science. 1994;266:117–119. doi: 10.1126/science.7939629. [DOI] [PubMed] [Google Scholar]
- 5.Lee WY, Chen HH, Chow NH, Su WC, Lin PW, Guo HR. Prognostic significance of co-expression of RON and MET receptors in node-negative breast cancer patients. Clin Cancer Res. 2005;11:2222–2228. doi: 10.1158/1078-0432.CCR-04-1761. [DOI] [PubMed] [Google Scholar]
- 6.Lee CT, Chow NH, Su PF, Lin SC, Lin PC, Lee JC. The prognostic significance of RON and MET receptor coexpression in patients with colorectal cancer. Dis Colon Rectum. 2008;51:1268–1274. doi: 10.1007/s10350-008-9297-1. [DOI] [PubMed] [Google Scholar]
- 7.Song YA, Park YL, Kim KY, Myung E, Chung CY, Cho SB, Lee WS, Jung YD, Kweon SS, Joo YE. RON is associated with tumor progression via the inhibition of apoptosis and cell cycle arrest in human gastric cancer. Pathol Int. 2012;62:127–136. doi: 10.1111/j.1440-1827.2011.02765.x. [DOI] [PubMed] [Google Scholar]
- 8.Han WL, Li WD, Hu J, Rusidanmu A, Chen LF, Shen L, Zheng SS. Expression of the recepteur d'originenantais receptor tyrosine kinase in non-small cell lung cancer and its clinical significance. Chin Med J (Engl) 2012;125:1110–1114. [PubMed] [Google Scholar]
- 9.Cheng HL, Liu HS, Lin YJ, Chen HH, Hsu PY, Chang TY, Ho CL, Tzai TS, Chow NH. Co-expression of RON and MET is a prognostic indicator for patients with transitional-cell carcinoma of the bladder. Br J Cancer. 2005;92:1906–1914. doi: 10.1038/sj.bjc.6602593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrandina G, Martinelli E, Petrillo M, Prisco MG, Zucconi A, Santaguida S, Zannoni G, Scambia G, Ferlini C. Prognostic role of the recepteur d'origine nantais (RON) expression in ovarian cancer patients. Gynecol Oncol. 2008;111:237–243. doi: 10.1016/j.ygyno.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Kang CM, Babicky ML, Lowy AM. The RON receptor tyrosine kinase in pancreatic cancer pathogenesis and its potential implications for future targeted therapies. Pancreas. 2014;43:183–189. doi: 10.1097/MPA.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camp ER, Yang A, Gray MJ, Fan F, Hamilton SR, Evans DB, Hooper AT, Pereira DS, Hicklin DJ, Ellis LM. Tyrosine kinase receptor RON in human pancreatic cancer: Expression, function, and validation as a target. Cancer. 2007;109:1030–1039. doi: 10.1002/cncr.22490. [DOI] [PubMed] [Google Scholar]
- 13.Babicky ML, Maruyama K, Jaquish D, French R. RON overexpression accelerates tumorigenesis and induces metastasis in a KRAS mutant mouse model of pancreatic cancer. J Am Coll Surg. 2011;213:S131. doi: 10.1016/j.jamcollsurg.2011.06.314. (Suppl) [DOI] [Google Scholar]
- 14.Thomas RM, Toney K, Fenoglio-Preiser C, Revelo-Penafiel MP, Hingorani SR, Tuveson DA, Waltz SE, Lowy AM. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res. 2007;67:6075–6082. doi: 10.1158/0008-5472.CAN-06-4128. [DOI] [PubMed] [Google Scholar]
- 15.Kalluri R. EMT: When epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Jaquish DV, Yu PT, Shields DJ, French RP, Maruyama KP, Niessen S, Hoover HA, Cheresh D, Cravatt B, Lowy AM. IGF1-R signals through the RON receptor to mediate pancreatic cancer cell migration. Carcinogenesis. 2011;32:1151–1156. doi: 10.1093/carcin/bgr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajeshkumar NV, Rasheed ZA, Garcia-Garcia E, López-Rios F, Fujiwara K, Matsui WH, Hidalgo M. A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model. Mol Cancer Ther. 2010;9:2582–2592. doi: 10.1158/1535-7163.MCT-10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peace BE, Toney-Earley K, Collins MH, Waltz SE. Ron receptor signaling augments mammary tumor formation and metastasis in a murine model of breast cancer. Cancer Res. 2005;65:1285–1293. doi: 10.1158/0008-5472.CAN-03-3580. [DOI] [PubMed] [Google Scholar]
- 21.Thomas RM, Jaquish DV, French RP, Lowy AM. The RON tyrosine kinase receptor regulates vascular endothelial growth factor production in pancreatic cancer cells. Pancreas. 2010;39:301–307. doi: 10.1097/MPA.0b013e3181bb9f73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padhye SS, Guin S, Yao HP, Zhou YQ, Zhang R, Wang MH. Sustained expression of the RON receptor tyrosine kinase by pancreatic cancer stem cells as a potential targeting moiety for antibody-directed chemotherapeutics. Mol Pharm. 2011;8:2310–2319. doi: 10.1021/mp200193u. [DOI] [PubMed] [Google Scholar]
- 23.Camp ER, Liu W, Fan F, Yang A, Somcio R, Ellis LM. RON, a tyrosine kinase receptor involved in tumor progression and metastasis. Ann Surg Oncol. 2005;12:273–281. doi: 10.1245/ASO.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Logan-Collins J, Thomas RM, Yu P, Jaquish D, Mose E, French R, Stuart W, McClaine R, Aronow B, Hoffman RM, et al. Silencing of RON receptor signaling promotes apoptosis and gemcitabine sensitivity in pancreatic cancers. Cancer Res. 2010;70:1130–1140. doi: 10.1158/0008-5472.CAN-09-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakedis J, French R, Babicky M, Jaquish D, Howard H, Mose E, Lam R, Holman P, Miyamoto J, Walterscheid Z, Lowy AM. A novel protein isoform of the RON tyrosine kinase receptor transforms human pancreatic duct epithelial cells. Oncogene. 2016;35:3249–3259. doi: 10.1038/onc.2015.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakedis J, French R, Babicky M, Jaquish D, Mose E, Cheng P, Holman P, Howard H, Miyamoto J, Porras P, et al. Characterization of RON protein isoforms in pancreatic cancer: Implications for biology and therapeutics. Oncotarget. 2016;7:45959–45975. doi: 10.18632/oncotarget.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Toole JM, Rabenau KE, Burns K, Lu D, Mangalampalli V, Balderes P, Covino N, Bassi R, Prewett M, Gottfredsen KJ, et al. Therapeutic implications of a human neutralizing antibody to the macrophage-stimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res. 2006;66:9162–9170. doi: 10.1158/0008-5472.CAN-06-0283. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Kaplan-Lefko PJ, Rex K, Yang Y, Moriguchi J, Osgood T, Mattson B, Coxon A, Reese M, Kim TS, et al. Identification of a novel recepteur d'origine nantais/c-met small-molecule kinase inhibitor with antitumor activity in vivo. Cancer Res. 2008;68:6680–6687. doi: 10.1158/0008-5472.CAN-07-6782. [DOI] [PubMed] [Google Scholar]
- 29.Guin S, Yao HP, Wang MH. RON receptor tyrosine kinase as a target for delivery of chemodrugs by antibody directed pathway for cancer cell cytotoxicity. Mol Pharm. 2010;7:386–397. doi: 10.1021/mp900168v. [DOI] [PubMed] [Google Scholar]
- 30.Yao HP, Feng L, Zhou JW, Zhang RW, Wang MH. Therapeutic evaluation of monoclonal antibody-maytansinoid conjugate as a model of RON-targeted drug delivery for pancreatic cancer treatment. Am J Cancer Res. 2016;6:937–956. [PMC free article] [PubMed] [Google Scholar]
- 31.Tactacan CM, Chang DK, Cowley MJ, Humphrey ES, Wu J, Gill AJ, Chou A, Nones K, Grimmond SM, Sutherland RL, et al. RON is not a prognostic marker for resectable pancreatic cancer. BMC Cancer. 2012;12:395. doi: 10.1186/1471-2407-12-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O'Reilly E, Wozniak TF, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: Phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collesi C, Santoro MM, Gaudino G, Comoglio PM. A splicing variant of the RON transcript induces constitutive tyrosine kinase activity and an invasive phenotype. Mol Cell Biol. 1996;16:5518–5526. doi: 10.1128/MCB.16.10.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang MH, Kurtz AL, Chen Y. Identification of a novel splicing product of the RON receptor tyrosine kinase in human colorectal carcinoma cells. Carcinogenesis. 2000;21:1507–1512. doi: 10.1093/carcin/21.5.507. [DOI] [PubMed] [Google Scholar]
- 35.Zhou YQ, He C, Chen YQ, Wang D, Wang MH. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: Generation of different splicing RON variants and their oncogenic potential. Oncogene. 2003;22:186–197. doi: 10.1038/sj.onc.1206075. [DOI] [PubMed] [Google Scholar]
- 36.Bardella C, Costa B, Maggiora P, Patane' S, Olivero M, Ranzani GN, De Bortoli M, Comoglio PM, Di Renzo MF. Truncated RON tyrosine kinase drives tumor cell progression and abrogates cell-cell adhesion through E-cadherin transcriptional repression. Cancer Res. 2004;64:5154–5161. doi: 10.1158/0008-5472.CAN-04-0600. [DOI] [PubMed] [Google Scholar]
- 37.Xu Z, Pothula SP, Wilson JS, Apte MV. Pancreatic cancer and its stroma: A conspiracy theory. World J Gastroenterol. 2014;20:11216–11229. doi: 10.3748/wjg.v20.i32.11216. [DOI] [PMC free article] [PubMed] [Google Scholar]