Abstract
Background & Aims
Bcl-xL, an anti-apoptotic Bcl-2 family protein, is overexpressed in 90% of pancreatic ductal adenocarcinoma (PDAC) cases. However, Bcl-xL expression in pancreatic intraepithelial neoplasias (PanINs) and its significance in PDAC carcinogenesis remain unclear. The aim of this study was to elucidate the significance of Bcl-xL expression in PanINs.
Methods
We investigated the expression levels of Bcl-xL in pancreas-specific KrasG12D (P-KrasG12D) mice and human PanINs and PDAC. We examined the impact of Bcl-xL expression on Kras-mutated pancreatic neoplasia using Bcl-xL–overexpressing P-KrasG12D mice and Bcl-xL–knockout P-KrasG12D mice.
Results
In P-KrasG12D mice, the number of PanINs increased and their grades progressed with age. In total, 55.6% of these mice developed PDAC at 12–14 months. According to the immunohistochemistry of mouse pancreas and human resected specimens, Bcl-xL expression was increased significantly in PanIN-1 compared with that in normal pancreatic ducts, and augmented further with the progression of pancreatic neoplasia in PanIN-2/3 and PDAC. Oncogene-induced senescence was observed frequently in PanIN-1, but rarely was detected in PanIN-2/3 and PDAC. Bcl-xL overexpression significantly accelerated the progression to high-grade PanINs and PDAC and reduced the survival of P-KrasG12D mice. Bcl-xL overexpression in P-KrasG12D mice suppressed oncogene-induced senescence in PanIN-1 and inhibited apoptosis in PanIN-3. Bcl-xL deficiency in P-KrasG12D mice induced cellular senescence in PanIN-2/3.
Conclusions
Bcl-xL expression increases with the progression from PanIN-1 to PDAC, whereas oncogene-induced senescence decreases. Bcl-xL overexpression increases PDAC incidence rates by inhibiting oncogene-induced senescence and apoptosis in PanINs. Conversely, Bcl-xL deficiency induced senescence in PanINs. Anti–Bcl-xL treatments may have the potency to suppress the progression from PanINs to PDAC.
Keywords: Kras, PanINs, Bcl-2 Family Protein
Abbreviations used in this paper: IHC, immunohistochemistry; KO, knockout; P-KrasG12D, Pdx1-Cre LSL-KrasG12D; PanIN, pancreatic intraepithelial neoplasia; PDAC, pancreatic ductal adenocarcinoma; SA–β-gal, senescence-associated β-galactosidase; siRNA, small interfering RNA; Tg, transgenic; TUNEL, terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling
Summary.
Bcl-xL expression increased with the progression of pancreatic neoplasia from pancreatic intraepithelial neoplasia (PanIN)-1 to pancreatic ductal adenocarcinoma. Bcl-xL overexpression in low-grade PanINs inhibits oncogene-induced senescence, whereas it inhibits apoptosis in high-grade PanINs, accelerating carcinogenesis. Bcl-xL deficiency increased senescence in PanINs.
Pancreatic ductal adenocarcinoma (PDAC) is one of the most highly aggressive forms of human cancer, with <5% of patients surviving after 5 years, and the early detection of PDAC remains difficult.1, 2 The number of patients with PDAC has increased steadily, and PDAC has increased to the fourth leading cause of cancer-related death in the United States.3 Pancreatic intraepithelial neoplasias (PanINs) are the most frequent and well-characterized precursor lesions of PDAC.4, 5 PanINs are classified into PanIN-1, 2, and 3, depending on the degree of cytologic and architectural atypia.6 Kras mutations, the most frequent genetic alteration of PDAC, are identified in more than 90% of low-grade PanIN-1/2, as well as high-grade PanIN-3 and PDAC in human beings.7, 8 A pancreas-specific Kras mutant mouse model, which develops PanINs and PDAC over time,9 is suitable for studying both PanINs and PDAC.
Bcl-xL is one of the anti-apoptotic Bcl-2 family proteins.10 Bcl-xL inhibits Bak or Bax activation, which is an essential event for apoptosis execution.11, 12 Previous reports have clarified that increased Bcl-xL expression is observed in 90% of PDAC cases based on immunohistochemical analysis using tumor tissues of PDAC patients.13, 14 However, the expression levels in PanINs remain unclear. The impact of Bcl-xL overexpression on PDAC carcinogenesis also remains uncharacterized.
In the present study, Bcl-xL expression showed a graded increase with the progression of pancreatic neoplasia from PanIN-1 to PDAC. Bcl-xL overexpression accelerated PDAC carcinogenesis with the decrease of senescence in PanIN-1 and apoptosis in PanIN-3, reducing survival in Kras mutant mice. Conversely, Bcl-xL deficiency induced senescence in PanINs. This study showed the function of Bcl-xL and its effect on the process of pancreatic carcinogenesis.
Materials and Methods
Genetically Engineered Mouse Models and Mouse Strains
The Pdx1-Cre transgenic (Tg) strain on a C57BL/6/FVB background and the LSL-KrasG12D knock-in strain on a C57BL/6/129 background were obtained from the Mouse Models of Human Cancer Consortium (National Cancer Institute–Frederick, Bethesda, Maryland). The LSL-KrasG12D allele is expressed at endogenous levels after Cre-mediated excision of a transcriptional stopper element.9, 15 A heterozygous hemagglutinin-hBcl-xL transgenic strain expressing the human Bcl-xL gene under the regulation of the CAG promoter on a C57BL/6/129 background (Bcl-xL Tg mice) using a hemagglutinin-tagged human Bcl-xL expression plasmid (pcDNA3HAbcl-xL) was described previously.16 Mice carrying a bcl-x gene with 2 loxP sequences in the promotor region and a second intron (bcl-xflox/flox mice) were described previously.17 These strains were intercrossed to produce the experimental cohorts. Mice were genotyped by polymerase chain reaction analysis (primer sequences are provided in the Supplementary Materials and Methods section). All mice were maintained with free access to food and water in a specific pathogen-free facility. They were treated with humane care under approval from the Animal Care and Use Committee of Osaka University Medical School.
Histologic Evaluation
Formalin-fixed, paraffin-embedded mouse pancreas tissues were sectioned (4 μm) and stained with H&E. PanIN lesions and carcinoma were classified according to histopathologic criteria.18 To quantify the progression of PanIN lesions, the number and their grades from each sample were obtained from 10 high-power (×200) microscopic fields. The area of normal-appearing pancreatic tissue was measured using the Hybrid Cell Count analysis of a digital microscope image (BZ-X700; Keyence, Osaka, Japan). Terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining, which is used to detect apoptotic cells,19, 20 was performed as previously described.21
Immunohistochemistry
The sections of mouse pancreas were processed further for immunohistochemistry (IHC). The sections were immersed in 1× Target Retrieval Solution (pH 6.0) (Dako, Glostrup, Denmark) for Bcl-xL and p21 staining. The samples were heated to 120°C in a decloaking chamber for antigen retrieval. The endogenous peroxidase activity was quenched by incubating with 3% hydrogen peroxide in methanol for 30 minutes. After sections were blocked in 5% goat serum for 1 hour, they were incubated with antibodies against Bcl-xL (1:200, #2764; Cell Signaling Technology, Danvers, MA) and p21 (1:400, sc-471; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. After rinsing with phosphate-buffered saline, the slides were incubated with secondary antibody (SignalStain Boost IHC detection reagent; Cell Signaling Technology) for 30 minutes. After washes with phosphate-buffered saline, the sections were incubated with 3,3’-diaminobenzidine for 30 seconds and counterstained with hematoxylin. In every staining set, negative controls were applied by omission of the primary antibody.
Evaluation of the Immunohistochemistry Results
Immunohistochemical staining for Bcl-xL was defined as a detectable immunoreaction in the cytoplasm. The number of positive cells was estimated semiquantitatively. The quantity of the immunostaining was evaluated as follows: 0 corresponded to no positive immunostaining, 1 corresponded to less than 20%, 2 corresponded to 20%–75%, and 3 corresponded to more than 75% positive cells.22 The intensity of the immunostaining was evaluated as follows: 0 corresponded to no staining, 1 corresponded to weak staining, 2 corresponded to moderate staining, and 3 corresponded to intense staining.23 A combined total score for the immunostaining was calculated by adding both the quantitative score and the intensity score. The total score for each sample was the average score obtained from 10 high-power (magnification, ×200) microscopic fields.
Senescence-Associated β-Galactosidase Assay
To assess senescence in vivo, mouse pancreatic cryosections were stained for senescence-associated β-galactosidase (SA–β-gal) activity according to a previously described procedure.24 Briefly, the frozen pancreas sections were fixed in 0.2% glutaraldehyde with 2% formaldehyde for 1 minute and incubated overnight in SA–β-gal solution from the Senescence β-Galactosidase Staining Kit (Cell Signaling Technology) at 37°C. This staining kit also was used for the evaluation of senescence in vitro according to the manufacturer's protocol. A total of approximately 500 cells was counted in 4 randomly selected fields to determine the percentage of senescent cells.
Human Samples
Formalin-fixed, paraffin-embedded samples were obtained from 9 patients with PDAC accompanied by PanIN lesions and 7 patients with other types of pancreatic tumors (neuroendocrine tumor, 2; serous cystic neoplasm, 2; solid pseudopapillary neoplasm, 1; epithelial cyst, 1; and hamartoma, 1) who underwent pancreas resection without preoperative therapy between March 2009 and March 2012. The use of resected samples was approved by the Institutional Review Board for Clinical Research at Osaka University Hospital (14435). Written informed consent was obtained from each patient. These samples were sectioned (4 μm) and stained with H&E. The sections also were stained for Bcl-xL immunohistochemistry. The slides were treated by immersion in 1× Target Retrieval Solution (pH 6.0) (Dako) and heated to 120°C in a decloaking chamber. They were incubated with 3% hydrogen peroxide in methanol for 15 minutes to quench the endogenous peroxidase activity. After blocking with 5% goat serum for 1 hour, they were incubated with anti–Bcl-xL antibody (1:200, 2764; Cell Signaling Technology) overnight at 4°C. After the incubation with secondary antibody (SignalStain Boost IHC detection reagent; Cell Signaling Technology) for 30 minutes, the sections were developed with 3,3’-diaminobenzidine for 2 minutes and counterstained with hematoxylin. Negative controls were performed by omitting the primary antibody. The total score for each sample was the average score obtained from 6 high-power (magnification, ×200) microscopic fields.
Cell Lines and Culture Conditions
The human pancreatic cancer cell lines PANC-1 and MIA PaCa-2 were purchased from the American Type Culture Collection (Manassas, VA). These cells were cultured in Dulbecco’s modified Eagle medium containing 10% heat-inactivated fetal bovine serum and antibiotic–antimycotic (Thermo Fisher Scientific, Rockford, IL) in a 5% CO2 atmosphere at 37°C. PANC-1 and MIA PaCa-2 cells were transfected with Bcl-xL small interfering RNAs (siRNAs) (s1920-22; Thermo Fisher Scientific) using Lipofectamine RNAiMAX Transfection Reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Apoptosis Assay and Cell Viability
Cells were assayed for apoptosis by monitoring caspase-3/7 activity using the Caspase-Glo assay (Promega, Madison, WI) according to a previously described procedure.25 Cell viability was evaluated by the water-soluble tetrazolium salt (WST) assay with cell count reagent SF (Nacalai Tesque, Kyoto, Japan).
Western Blot Analysis
The Western blot analysis was performed using a previously described method.26 The following antibodies were used: anti–Bcl-xL antibody, anti–Bcl-2 antibody, anti–Mcl-1 antibody (Cell Signaling Technology), anti-p21 antibody (Santa Cruz Biotechnology), and anti–β-actin antibody (Sigma-Aldrich, St. Louis, MO).
Statistical Analysis
Survival curves were plotted using the Kaplan–Meier method and compared by the log-rank test using Statistics Package for Social Science software version 21.0 (SPSS, Inc, Chicago, IL). The data were presented as the means ± SD or medians and interquartile range, with comparisons using the Student t test or the Mann–Whitney U test, respectively. The incidences of PDAC were compared using the Fisher exact test. P values less than .05 were considered statistically significant.
All authors had access to the study data and reviewed and approved the final manuscript.
Results
Bcl-xL Expression Increases in Both PanINs and PDAC
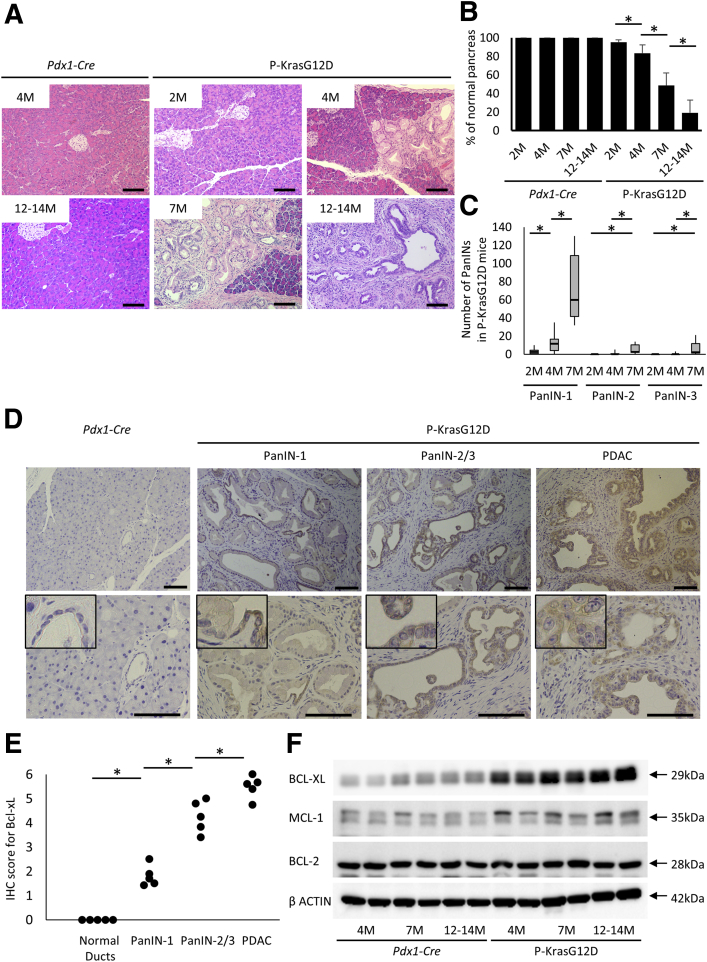
To analyze Bcl-xL expression levels in PanINs and PDAC, pancreas sections were examined in pancreas-specific Kras-mutated mice (Pdx1-Cre LSL-KrasG12D mice [P-KrasG12D mice]). Control littermates of Pdx1-Cre mice did not show any histologic changes in the pancreas from 2 to 14 months (Figure 1A and B). In contrast, P-KrasG12D mice showed sporadic PanIN-1 lesions at 2 months. The number of PanINs and their grade gradually progressed at 4 months and increased further at 7 months (Figure 1A and C). At 12–14 months, widespread low- to high-grade PanINs were detected in all mice, and invasive ductal adenocarcinomas developed in 5 of 9 mice (55.6%) (Figure 1A). The percentage of normal-appearing areas in pancreas sections significantly decreased with age (Figure 1B). Immunohistochemistry showed Bcl-xL overexpression in the cytoplasm of PDACs from P-KrasG12D mice, consistent with previous reports of resected specimens from human beings13, 14 (Figure 1D and E). Of importance, Bcl-xL was overexpressed significantly, even in PanIN-1. Bcl-xL overexpression was augmented in PanIN-2/3 and enhanced further in PDAC (Figure 1D and E). As shown in Western blot analysis, Bcl-xL expression levels were increased in the pancreas of P-KrasG12D mice compared with those in control littermates at 4 months, and gradually increased with age (Figure 1F). We also examined other anti-apoptotic Bcl-2 family proteins, including Mcl-1 and Bcl-2.27 Mcl-1 expression levels were increased slightly in P-KrasG12D mice, whereas Bcl-2 expression levels did not increase (Figure 1F).
Figure 1.
Bcl-xL expression levels are enhanced gradually from PanIN-1 to PDAC in P-KrasG12D mice. (A–C) P-KrasG12D mice (Pdx1-Cre; LSL-KrasG12D) (n = 12 at 2 mo, 10 at 4 mo, 6 at 7 mo, and 9 at 12–14 mo) or wild-type littermates (Pdx1-Cre) (n = 10 at 2 mo, 6 at 4 mo, 4 at 7 mo, and 10 at 12–14 mo) were killed at the indicated months. (A) Representative pictures of H&E staining. Scale bar: 100 μm (original magnification, ×200). (B) The percentage of normal pancreas in defined area. *P < .05. (C) The number of PanINs per defined area in the pancreas sections of P-KrasG12D mice. (D) Representative images of Bcl-xL immunohistochemical staining of pancreas sections in wild-type mice and P-KrasG12D mice at 12–14 months. Scale bar: 100 μm (original magnification, ×200 in the upper row and ×400 in the lower row; width of insets: 50 μm). (E) Total score for the immunostaining of Bcl-xL in normal pancreatic ducts from wild-type mice (n = 5) and PanINs/PDAC from P-KrasG12D mice at 12–14 months (n = 5). (F) Western blot of anti-apoptotic Bcl-2 family proteins in the pancreas of P-KrasG12D mice.
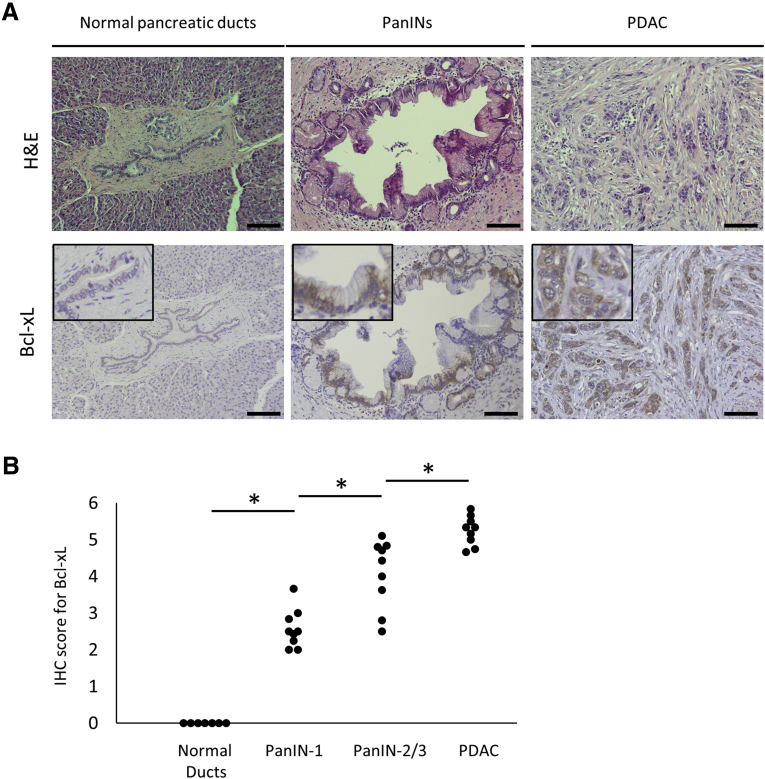
Next, we evaluated Bcl-xL expression levels in PanINs using clinical resected samples. Consistent with the murine results, Bcl-xL also was overexpressed in the cytoplasm of human PanINs and PDAC samples (Figure 2). Total immunostaining scores were increased significantly in human PanIN-1 compared with those in normal human pancreatic ducts, and were increased further with the progression to human PanIN-2/3 and human PDAC (Figure 2). Collectively, Bcl-xL was overexpressed in early stage PanINs and increased with the progression of pancreatic neoplasia.
Figure 2.
Bcl-xL expression levels are increased in human PanINs and PDAC. Human resected pancreatic tissues of 9 patients with PDAC accompanied by PanIN lesions and 7 patients with other types of pancreatic tumors were stained with H&E and anti–Bcl-xL antibody. Given that the pancreatic tissues of 9 patients with PDAC contained many PanINs in the nontumor area, normal pancreatic ducts were evaluated using nontumor areas of pancreatic tissues from 7 patients with other types of pancreatic tumors. (A) Representative images of H&E and Bcl-xL immunohistochemistry staining of pancreas sections. Scale bar: 100 μm (original magnification, ×200); width of insets: 100 μm. (B) Total score for the immunostaining of Bcl-xL. *P < .05.
Bcl-xL Overexpression in Kras-Mutated Cells Promotes PDAC Carcinogenesis
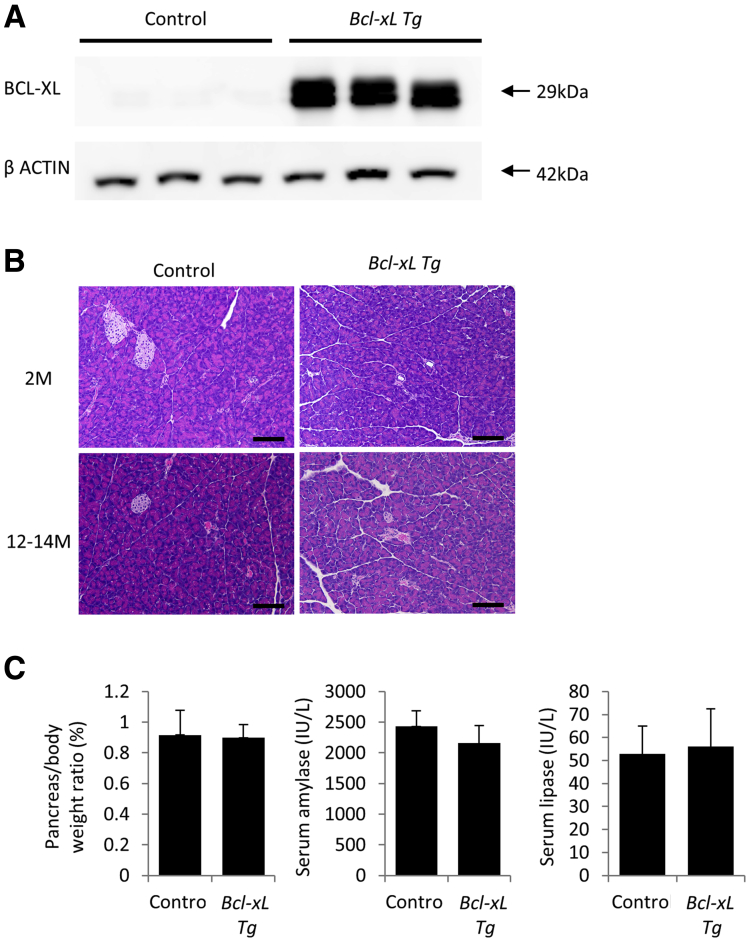
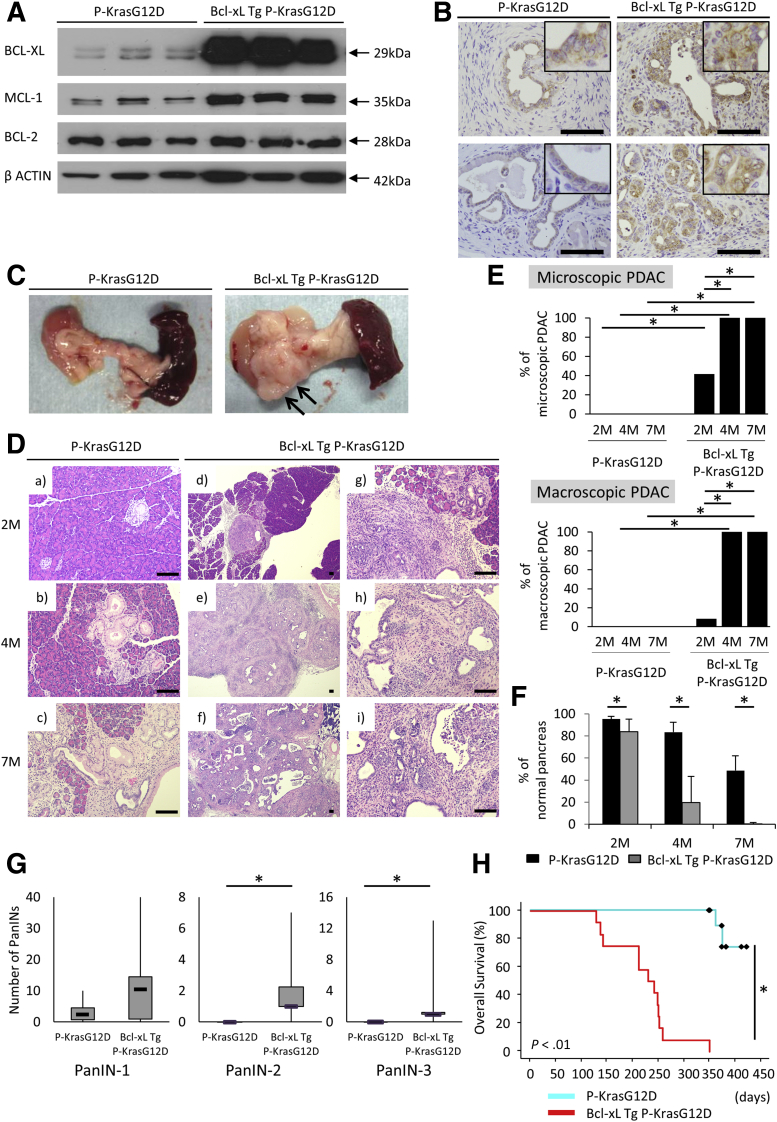
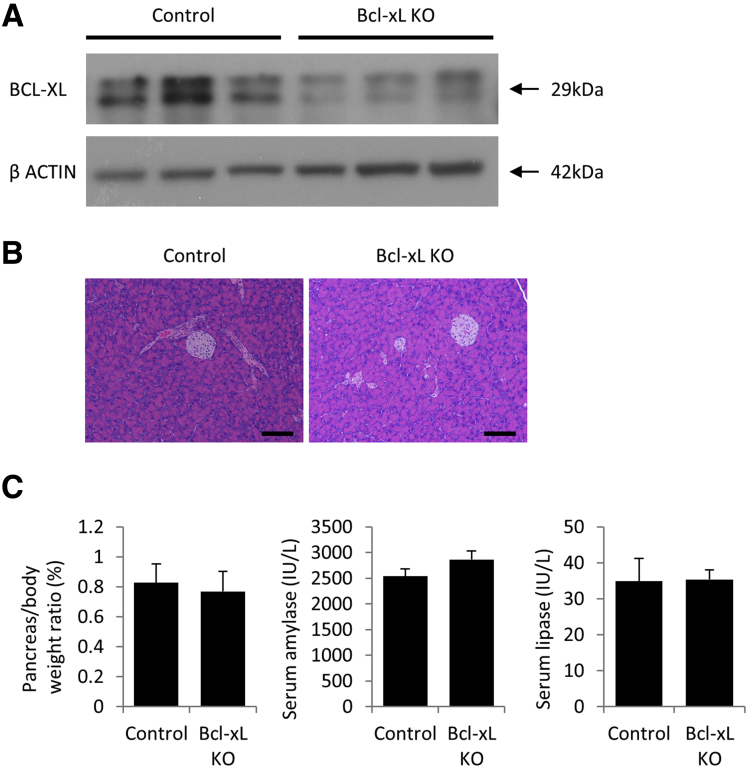
Next, we examined the impact of Bcl-xL overexpression on the development of PanINs and PDAC. For this purpose, we generated Bcl-xL Tg P-KrasG12D mice by mating P-KrasG12D mice and Bcl-xL Tg mice. We confirmed that Bcl-xL expression levels in the pancreas were increased remarkably in Bcl-xL Tg mice compared with those in control wild-type littermates (Figure 3A). In physiological settings, Bcl-xL Tg mice did not show histologic abnormalities of the pancreas (Figure 3B) or any significant differences in pancreas/body weight ratios or serum levels of amylase and lipase compared with littermate wild-type mice (Figure 3C). To examine the impact of Bcl-xL overexpression on carcinogenesis, we compared Bcl-xL Tg P-KrasG12D mice with their P-KrasG12D littermates. Up-regulation of Bcl-xL in the pancreas of Bcl-xL Tg P-KrasG12D mice compared with P-KrasG12D mice was confirmed by Western blot analysis (Figure 4A). Stronger Bcl-xL expression was observed in the cytoplasm of PanINs and PDAC from Bcl-xL Tg P-KrasG12D mice than in that from P-KrasG12D mice, as assessed by immunohistochemistry (Figure 4B). P-KrasG12D mice had no macroscopic or microscopic PDAC at 2, 4, or 7 months (Figure 4C–E). In contrast, 41.7% (5 of 12) of Bcl-xL Tg P-KrasG12D mice developed microscopic PDAC at 2 months, and 8.3% (1 of 12) developed macroscopically visible PDAC (Figure 4D and E). At 4 and 7 months, all had developed macroscopically evident PDAC (Figure 4C–E). H&E staining showed that the area of the normal-appearing pancreas of Bcl-xL Tg P-KrasG12D mice was significantly smaller than that of P-KrasG12D mice (Figure 4F), and the number of PanIN-2/3 lesions in the pancreas of Bcl-xL Tg P-KrasG12D mice at 2 months was significantly larger than that of P-KrasG12D mice at 2 months (Figure 4G). All Bcl-xL Tg P-KrasG12D mice died within 1 year; their median survival was 7.8 months (Figure 4H), which was significantly shorter than their P-KrasG12D littermates. These data suggest that Bcl-xL overexpression accelerates carcinogenesis of Kras-mutated pancreatic neoplasia, reducing survival in P-KrasG12D mice.
Figure 3.
Bcl-xL–overexpressing mice do not show any phenotype under physiological conditions. Bcl-xL Tg mice and their control littermates were killed at 2 and 12 months. (A) Western blot analysis of Bcl-xL in the pancreas at 2 months. (B) Representative images of H&E staining of pancreas sections at 2 and 12–14 months. Scale bar: 100 μm (original magnification, ×200). (C) Pancreas/body weight ratio and serum levels of amylase and lipase at 2 months (n = 5 in Bcl-xL Tg mice, n = 4 in control mice).
Figure 4.
Bcl-xL overexpression accelerates the incidence rate of PDAC and reduces survival time in P-KrasG12D mice. P-KrasG12D mice and Bcl-xL Tg P-KrasG12D mice were killed at the indicated months. (A) Western blot of anti-apoptotic Bcl-2 family proteins in the pancreas at 4 months. (B) Representative images of pancreas sections at 4 months stained for Bcl-xL. Scale bar: 100 μm (original magnification, ×400); width of insets: 50 μm. (C) Macroscopic pictures of the pancreas at 4 months. Arrows, macroscopically evident tumors. (D) Representative images of H&E staining of pancreas sections. Scale bar: 100 μm (original magnification for A–F, ×40; G–I, ×200). (E) The ratio of microscopic and macroscopic PDAC ∗P < .05. (F) The percentage of normal pancreas at 2 months (n = 12 per each), 4 months (n = 10 or 11), and 7 months (n = 6 per each). *P < .05. (G) The number of PanINs per defined area in the pancreas sections of P-KrasG12D mice and Bcl-xL Tg P-KrasG12D mice (n = 12 per group) at 2 months ∗P < .05. (H) Kaplan–Meier survival analysis of P-KrasG12D (n = 11) and Bcl-xL Tg P-KrasG12D mice (n = 12). *P < .05.
Bcl-xL Overexpression Suppresses Oncogene-Induced Senescence in Low-Grade PanINs and Apoptosis in High-Grade PanINs
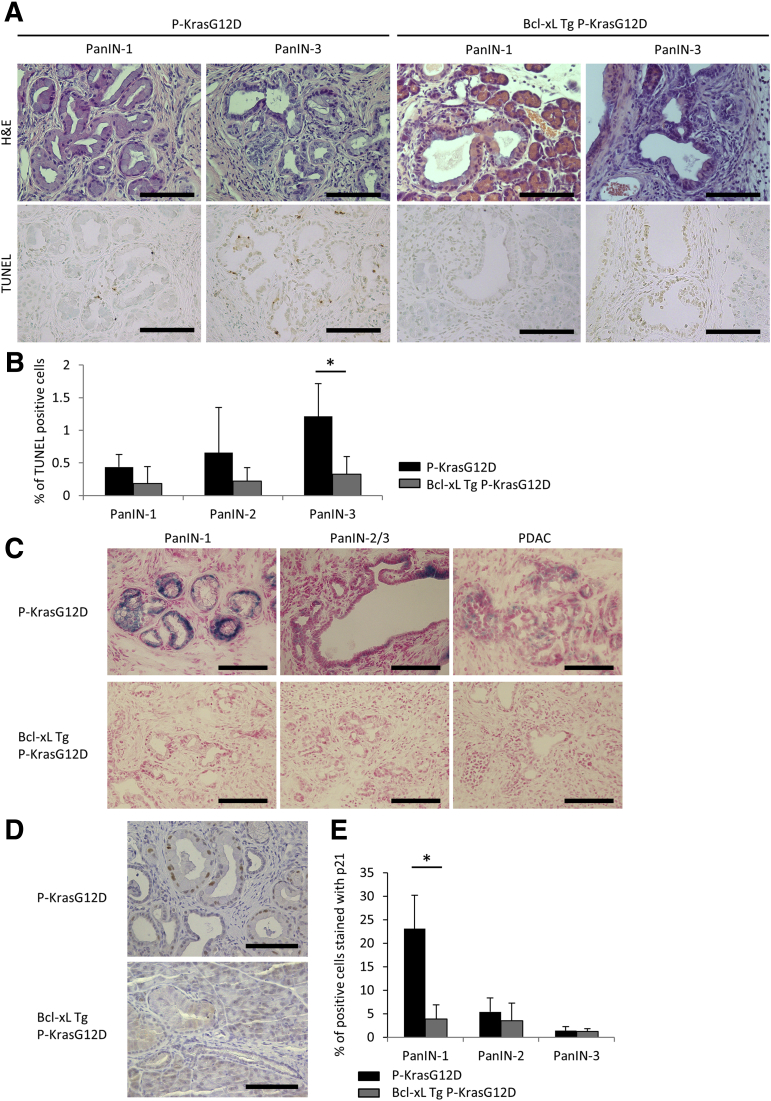
To address the mechanisms by which Bcl-xL overexpression promotes PDAC carcinogenesis, we examined the effect of apoptosis in PanIN lesions and PDAC. We compared P-KrasG12D mice at 12–14 months with Bcl-xL Tg P-KrasG12D mice at 2 months because both had low-grade PanINs as well as high-grade PanINs and PDAC. The percentage of TUNEL-positive cells was 0.44% ± 0.19% in PanIN-1, 0.66% ± 0.69% in PanIN-2, and 1.21% ± 0.50% in PanIN-3 of P-KrasG12D mice (Figure 5A and B). The frequency of TUNEL-positive cells in PanIN-1 and PanIN-2 did not decrease significantly with Bcl-xL overexpression, whereas the number of TUNEL-positive cells decreased significantly in PanIN-3 (Figure 5A and B). These data suggest that Bcl-xL overexpression might not have an effect on apoptosis in low-grade PanIN lesions. In contrast, Bcl-xL overexpression suppressed apoptosis in high-grade PanIN-3.
Figure 5.
Bcl-xL overexpression suppresses oncogene-induced senescence in low-grade PanINs and apoptosis in high-grade PanINs. (A and B) Pancreatic tissues of P-KrasG12D mice at 12–14 months and Bcl-xL Tg P-KrasG12D mice at 2 months were stained with H&E and TUNEL (n = 5 per group). (A) Representative images of H&E and TUNEL staining and (B) the percentage of TUNEL-positive cells. Scale bar: 100 μm (original magnification, ×400). (C–E) Pancreatic tissues of P-KrasG12D mice at 12–14 months and Bcl-xL Tg P-KrasG12D mice at 2 months were stained for SA–β-gal activity and stained with anti-p21 antibody (n = 5 per group). (C) Representative images of staining for SA–β-gal activity. Scale bar: 100 μm (original magnification, ×400). (D) Representative images of p21 staining of pancreas sections and (E) the percentage of p21-positive cells. Scale bar: 100 μm (original magnification, ×400). *P < .05.
Next, we further examined another mechanism of acceleration of carcinogenesis in Bcl-xL Tg P-KrasG12D mice. We examined senescence in P-KrasG12D mice and Bcl-xL Tg P-KrasG12D mice using SA–β-gal activity, which is a specific detector of senescence.28 In 12- to 14-month P-KrasG12D mice, SA–β-gal–positive cells were observed frequently in PanIN-1, but were decreased remarkably in PanIN-2/3 and PDAC (Figure 5C). In contrast, few positive cells were detected in the PanIN-1, PanIN-2/3, and PDAC lesions from Bcl-xL Tg P-KrasG12D mice at 2 months (Figure 5C). Immunostaining for p21, another marker associated with oncogene-induced senescence,29 also showed that the number of p21-positive cells in PanIN-1 lesions was reduced significantly in Bcl-xL Tg P-KrasG12D mice at 2 months compared with that in P-KrasG12D mice at 12–14 months (Figure 5D and 5E). There were few p21-positive PanIN-2/3 and PDAC cells in both mice (Figure 5D and E). These data suggest that oncogene-induced senescence is induced in PanIN-1 of P-KrasG12D mice and that it is inhibited by the increase in Bcl-xL expression levels.
Bcl-xL Deficiency in Kras-Mutated Cells Increases the Number of Senescent Cells in High-Grade PanINs
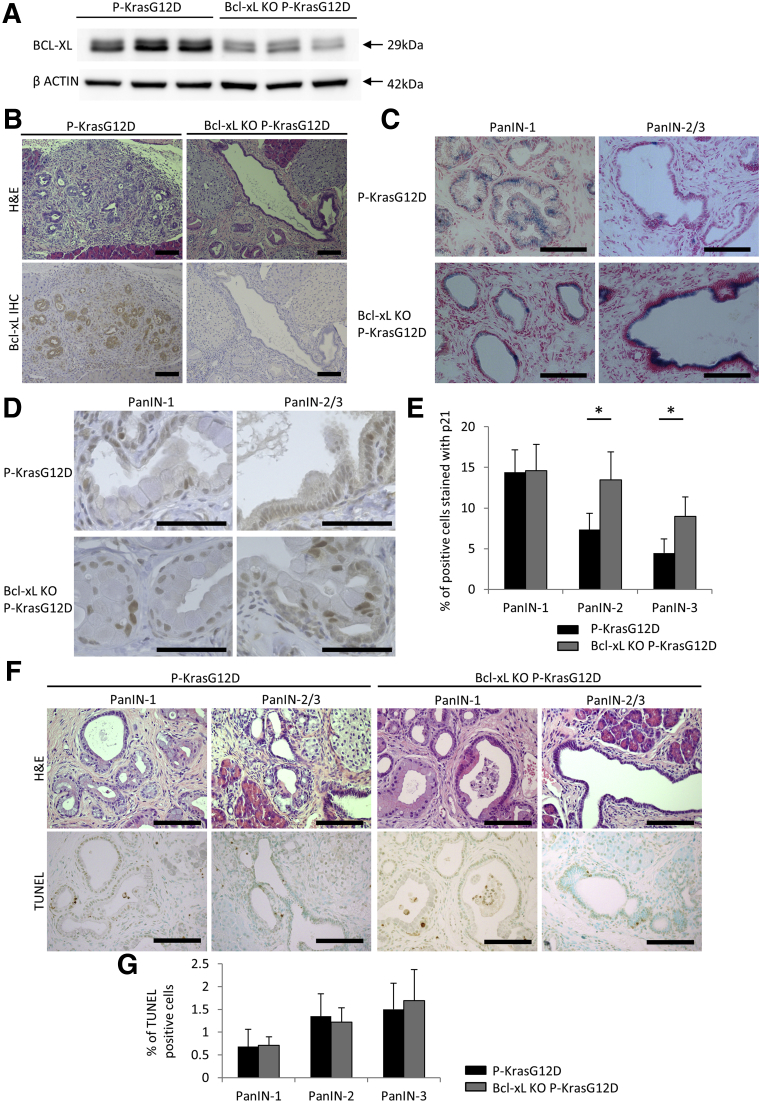
Next, we examined the impact of Bcl-xL deficiency on the induction of senescence in PanINs. Pancreas-specific Bcl-xL knockout mice without the Kras mutation (bcl-xflox/flox Pdx1-Cre mice) did not show any phenotype under physiological conditions (Figure 6). We generated pancreas-specific Kras-mutated and Bcl-xL knockout mice (Bcl-xL knockout [KO] P-KrasG12D mice) by mating P-KrasG12D mice and bcl-xflox/flox mice (Figure 7A and B). SA–β-gal–positive cells were observed frequently in PanIN-1 lesions from both Bcl-xL KO P-KrasG12D mice and P-KrasG12D mice. Positive cells also were detected in PanIN-2/3 lesions from Bcl-xL KO P-KrasG12D mice, but rarely were observed in PanIN-2/3 lesions from P-KrasG12D mice (Figure 7C). According to the immunostaining for p21, the percentage of p21-positive cells in PanIN-2/3 lesions was significantly higher in Bcl-xL KO P-KrasG12D mice than in P-KrasG12D mice, but a significant difference was not detected in the PanIN-1 lesions from these mice (Figure 7D and E). The frequency of TUNEL-positive cells in PanIN lesions of any grade did not increase significantly with Bcl-xL deficiency in P-KrasG12D mice (Figure 7F and G). A lower percentage of Bcl-xL KO P-KrasG12D mice developed PDAC at 12 months than littermate P-KrasG12D mice (35.7% [5 of 14] vs 83.3% [5 of 6]), although the difference did not reach statistical significance (P = .07). These results suggest that Bcl-xL inhibition increases the number of senescent cells in high-grade PanINs, which might suppress the progression from PanINs to PDAC.
Figure 6.
Pancreas-specific Bcl-xL–knockout mice do not show any phenotype under physiological conditions. Bcl-xL KO mice (bcl-xflox/floxPdx1-Cre) and their control littermates (bcl-xflox/flox) were killed at the indicated months. (A) Western blot analysis of Bcl-xL in the pancreas at 2 months. (B) Representative images of H&E staining of pancreas sections at 6 months. Scale bar: 100 μm (original magnification, ×200). (C) Pancreas/body weight ratio and serum amylase and lipase levels at 6 months (n = 11 in Bcl-xL KO mice and n = 7 in control mice).
Figure 7.
Bcl-xL deficiency increases senescence in high-grade PanINs. Bcl-xL KO P-KrasG12D mice (bcl-xflox/floxPdx1-Cre LSL-KrasG12D) (n = 14) and their littermate P-KrasG12D mice (bcl-x+/+Pdx1-Cre LSL-KrasG12D) (n = 6) were killed at 12 months. (A) Western blot of Bcl-xL in the pancreas. (B) Representative images of pancreas sections stained for Bcl-xL. Scale bar: 100 μm (original magnification, ×200). (C) Representative images of staining for SA–β-gal activity. Scale bar: 100 μm (original magnification, ×400). (D) Representative images of p21 staining in pancreas sections and (E) the percentage of p21-positive cells. Scale bar: 50 μm (original magnification, ×1200). *P < .05. (F) Representative images of H&E and TUNEL staining and (G) the percentage of TUNEL-positive cells. Scale bar: 100 μm (original magnification, ×400).
Bcl-xL Inhibition Induces Oncogene-Induced Senescence and Apoptosis in PDAC
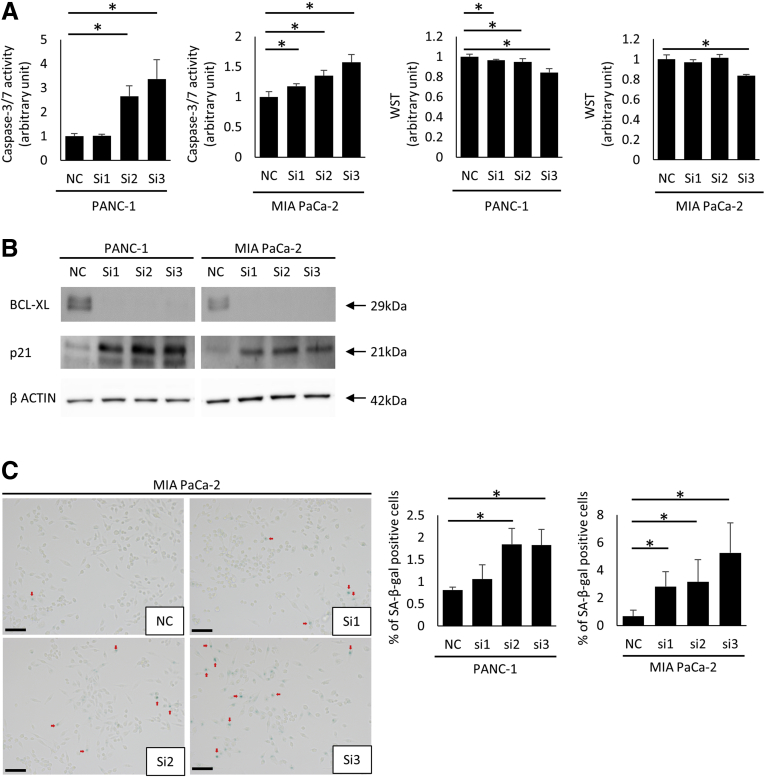
Finally, to examine the possibility of apoptotic induction and oncogene-induced senescence by Bcl-xL inhibition in PDAC, PANC-1 and MIA PaCa-2 cells were treated with siRNAs against Bcl-xL. siRNA-mediated knockdown of Bcl-xL led to a significant increase in caspase-3/7 activity and a significant decrease in cell viability (Figure 8A). Bcl-xL knockdown increased p21 expression levels, as evaluated by Western blot analysis (Figure 8B). Bcl-xL knockdown also significantly increased the number of β-galactosidase–positive cells (Figure 8C). Collectively, these data suggest that Bcl-xL inhibition efficiently induces oncogene-induced senescence and apoptosis in PDAC.
Figure 8.
Oncogene-induced senescence is induced by Bcl-xL inhibition in vitro. PANC-1 and MIA Paca-2 cells were transfected with Bcl-xL siRNAs (si1, si2, si3) or negative control siRNA (NC) for 24 hours. After changing the medium, cells were incubated for an additional 24 hours (caspase-3/7 activity) and for 48 hours (WST assay, Western blot analysis, and SA–β-gal activity). (A) Caspase-3/7 activity in culture supernatants and cell viability was determined by the WST assay (n = 4). (B) Western blot of Bcl-xL and p21. (C) Representative images of PDAC cells stained for SA–β-gal activity and the percentage of SA–β-gal–positive cells. Scale bar: 100 μm. *P < .05.
Discussion
Bcl-xL overexpression has been observed in several types of cancer30, 31 and is associated with tumor growth, malignant biological behaviors, and survival.32, 33, 34 According to previous studies of PDAC, Bcl-xL up-regulation is observed frequently in human samples13, 14, 35 and is associated with a poorer prognosis,23 but the effect of Bcl-xL overexpression during PDAC carcinogenesis and whether Bcl-xL expression is observed in precancerous PanIN lesions has not been investigated completely. As shown in this study, Bcl-xL expression was increased significantly in the cytoplasm of PanIN-1 lesions compared with that in normal pancreatic ducts, and was augmented gradually with the progression of pancreatic neoplasia in Kras mutant mouse models and human samples (Figures 1D–F and 2A and B). We report that Bcl-xL expression increases in PanINs as well as PDAC.
As clearly shown in the present study, Bcl-xL overexpression led to the acceleration of carcinogenesis in Kras-mutated pancreatic neoplasia. All Bcl-xL Tg P-KrasG12D mice developed grossly evident PDAC at as early as 4 months, whereas P-KrasG12D mice showed only microscopic PanIN lesions (Figure 4C–E). Genetically engineered mouse models of PDAC are based commonly on Kras mutations,9 and various mutations or deletions of genes, such as p53,36 Ink4a,15 and transforming growth factor-β,37 result in rapid carcinogenesis of Kras-mutated pancreatic neoplasia. However, no murine PDAC models have been reported to harbor gene mutations or deletions in Bcl-2 family proteins. This study showed accelerated pancreatic carcinogenesis using a mouse model of Bcl-2 family proteins. The results showing that 41.7% (5 of 12) of Bcl-xL Tg P-KrasG12D mice developed microscopic PDAC at as early as 2 months (Figure 4D and E) and the number of microscopic PanIN-2/3 lesions was increased significantly compared with that in P-KrasG12D mice (Figure 4G) suggest that Bcl-xL up-regulation in the presence of Kras mutation causes the rapid progression of low-grade PanINs to high-grade PanINs and PDAC, reducing the survival of Bcl-xL Tg P-KrasG12D mice (Figure 4H). The overexpression of anti-apoptotic proteins, including Bcl-xL, in tumor cells directly accelerates tumor proliferation by inhibiting apoptosis.32, 38 Together with the in vitro findings that siRNA-mediated Bcl-xL knockdown in Kras-mutated PDAC cells decreased cell viability and increased apoptosis (Figure 8A), accelerated tumor cell proliferation caused by Bcl-xL overexpression, also may contribute to the reduced survival. Bcl-xL staining recently was detected in the nucleus and cytoplasm of PDAC.35 As reported by Choi et al,39 nuclear Bcl-xL promotes the epithelial–mesenchymal transition, migration, invasion, and stemness of cell lines derived from pancreatic neuroendocrine tumors and breast cancer. In the present study, we observed Bcl-xL immunostaining in the cytoplasm of PanINs and PDAC, consistent with previous immunohistochemical studies of PDAC,23, 40 but we did not detect immunopositivity in the nucleus (Figures 1D, 2A, and 4B). However, there remains a possibility that nuclear Bcl-xL, which may be expressed in undetectable levels by immunohistochemistry, affects the development of PDAC in Bcl-xL Tg P-KrasG12D mice.
In the present study, we addressed 2 possible mechanisms by which Bcl-xL overexpression accelerated the progression to high-grade PanINs and PDAC in Kras mutant mice: inhibition of apoptosis and inhibition of oncogene-induced senescence. Bcl-xL is an anti-apoptotic protein,27 and its overexpression is expected to inhibit PanIN apoptosis, resulting in the acceleration of carcinogenesis. A small number of apoptotic cells was observed in PanIN-1 lesions from P-KrasG12D mice (Figure 5A and B), consistent with a previous report of resected samples from human beings.41 Bcl-xL overexpression in Kras-mutated neoplasia did not significantly decrease the number of apoptotic cells in PanIN-1 lesions (Figure 5A and B). In contrast, the number of apoptotic cells was suppressed significantly by Bcl-xL overexpression in PanIN-3 (Figure 5A and 5B). Kras mutation induces oncogene-induced senescence,42 which is regarded as a tumor-suppressive effect because the suppression of senescence leads to the rapid progression to high-grade PanINs and PDAC.43, 44 In the present study, oncogene-induced senescent cells were observed frequently in PanIN-1 lesions from P-KrasG12D mice, but not in PanIN-2/3 and PDAC lesions (Figure 5C–E). Importantly, Bcl-xL overexpression strongly suppressed the oncogene-induced senescence observed in the PanIN-1 lesions from P-KrasG12D mice (Figure 5C–E). These data suggest that the suppressive effect of Bcl-xL overexpression on oncogene-induced senescence shows a greater impact on low-grade PanINs than high-grade PanINs. As shown by Yosef et al,45 joint inhibition of Bcl-w and Bcl-xL, but not single inhibition of Bcl-xL, eliminates senescent fibroblasts by inducing apoptosis. In the present study, Bcl-xL overexpression decreased the number of senescent cells in PanIN-1 lesions from P-KrasG12D mice without inducing apoptosis (Figure 5). Although Yosef et al45 described the effect of Bcl-xL on apoptosis in senescent cells, we reported the effect of Bcl-xL on the regulation of senescence in PanINs. Collectively, Bcl-xL overexpression in PanINs promotes progression to PDAC by inhibiting apoptosis, particularly in high-grade PanINs, and oncogene-induced senescence in low-grade PanINs.
In the experiments using Bcl-xL KO P-KrasG12D mice, Bcl-xL deficiency significantly increased the number of senescent cells in PanIN-2/3 lesions from the P-KrasG12D mice (Figure 7C–E). In P-KrasG12D mice, Bcl-xL expression showed a graded increase with the progression from PanIN-1 to PDAC, whereas oncogene-induced senescence was detected frequently in PanIN-1, but rarely found in PanIN-2/3 and PDAC (Figures 1D and E, and 5C–E). Bcl-xL overexpression in PanIN-1 lesions significantly decreased the number of senescent cells (Figure 5C–E). These results suggest that the gradual increase of Bcl-xL expression suppresses senescence in PanINs. Regarding cancer cell senescence, previous studies have shown that Bcl-xL overexpression inhibits senescence by preventing reactive oxygen species generation.46 ABT-737, a small molecule that inhibits Bcl-xL, increases senescence and reactive oxygen species levels.47 In the present study, Bcl-xL knockdown in PDAC cell lines increased the number of senescent cells (Figure 8C). Collectively, Bcl-xL inhibition could induce senescence in PDAC as well as PanINs. Advanced PDAC remains difficult to treat,48 and therapies preventing the progression of PanINs to PDAC could be promising treatments to reduce the incidence of PDAC. Treatments for Bcl-xL may be an efficient preventive therapy for patients at high risk of developing PanINs, such as patients with chronic pancreatitis.49
In conclusion, we clarified that Bcl-xL expression levels increase with the progression of pancreatic neoplasia from PanIN-1 to PDAC, whereas oncogene-induced senescence decreases. Bcl-xL overexpression suppresses oncogene-induced senescence in low-grade PanINs and inhibits apoptosis in high-grade PanINs, promoting the progression to high-grade PanINs and PDAC. Together with the finding that Bcl-xL deficiency in Kras mutant mice increases senescence, Bcl-xL is suggested to be an effective target for the prevention of PDAC by inducing senescence in PanINs.
Acknowledgments
The authors thank Dr Lothar Hennighausen (Laboratory of Genetics and Physiology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Health, Bethesda, MD) for providing the bcl-xflox/flox mice.
Footnotes
Author contributions Study concept and design: K. Ikezawa, H. Hikita, M. Shigekawa, and T. Takehara. Acquisition of data: K. Ikezawa, M. Shigekawa, K. Iwahashi, H. Eguchi, and R. Sakamori. Analysis and interpretation of data: K. Ikezawa, H. Hikita, M. Shigekawa, T. Tatsumi, and T. Takehara. Writing, review, or revision of the manuscript: K. Ikezawa, H. Hikita, and T. Takehara.
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported in part by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (JP16K15429 to T.Tak. and JP25860534 and JP15k19325 to M.S.) and research grant from Bristol-Myers Squibb (to H.H.).
Supplementary Materials and Methods
Genetically Engineered Mouse Models and Mouse Strains
Mice were genotyped by polymerase chain reaction analysis. The oligonucleotide primer sequences were as follows: Kras,
5’-CCTTTACAAGCGCACGCAGACTGTAGA-3’ (sense) and
5’-AGCTAGCCACCATGGCTTGAGTAAGTCTGCA-3’ (antisense); Pdx1-Cre,
5’-CTGGACTACATCTTGAGTTGC-3’ (sense) and 5’-
GGTGTACGGTCAGTAAATTTG-3’ (antisense); Bcl-xL Tg,
5’-GCTGGTTATTGTGCTGTCTC-3’ (sense) and 5’-
GGTAAGTGGCCATCCAAGCTGC-3’ (antisense); and bcl-xflox/flox,
5’-GCCACCTCATCAGTCGGG-3’ (sense) and 5’-TCAGAAGCCGCAATATCCCC-3’ (antisense).
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Tang H., Partyka K., Hsueh P., Sinha J.Y., Kletter D., Zeh H., Huang Y., Brand R.E., Haab B.B. Glycans related to the CA19-9 antigen are increased in distinct subsets of pancreatic cancers and improve diagnostic accuracy over CA19-9. Cell Mol Gastroenterol Hepatol. 2016;2:210–221. doi: 10.1016/j.jcmgh.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Scarlett C.J., Salisbury E.L., Biankin A.V., Kench J. Precursor lesions in pancreatic cancer: morphological and molecular pathology. Pathology. 2011;43:183–200. doi: 10.1097/PAT.0b013e3283445e3a. [DOI] [PubMed] [Google Scholar]
- 5.Basturk O., Hong S.M., Wood L.D., Adsay N.V., Albores-Saavedra J., Biankin A.V., Brosens L.A., Fukushima N., Goggins M., Hruban R.H., Kato Y., Klimstra D.S., Klöppel G., Krasinskas A., Longnecker D.S., Matthaei H., Offerhaus G.J., Shimizu M., Takaori K., Terris B., Yachida S., Esposito I., Furukawa T., Baltimore Consensus Meeting A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39:1730–1741. doi: 10.1097/PAS.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hruban R.H., Takaori K., Klimstra D.S., Adsay N.V., Albores-Saavedra J., Biankin A.V., Biankin S.A., Compton C., Fukushima N., Furukawa T., Goggins M., Kato Y., Klöppel G., Longnecker D.S., Lüttges J., Maitra A., Offerhaus G.J., Shimizu M., Yonezawa S. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 7.Morris J.P., Wang S.C., Hebrok M.K.R.A.S. Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanda M., Matthaei H., Wu J., Hong S.M., Yu J., Borges M., Hruban R.H., Maitra A., Kinzler K., Vogelstein B., Goggins M. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730–733.e9. doi: 10.1053/j.gastro.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hingorani S.R.S., Petricoin E.F., Maitra A., Rajapakse V., King C., Jacobetz M.A., Ross S., Conrads T.P., Veenstra T.D., Hitt B.A., Kawaguchi Y., Johann D., Liotta L.A., Crawford H.C., Putt M.E., Jacks T., Wright C.V., Hruban R.H., Lowy A.M., Tuveson D.A. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 10.Takehara T., Tatsumi T., Suzuki T., Rucker E.B., 3rd, Hennighausen L., Jinushi M., Miyagi T., Kanazawa Y., Hayashi N. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–1197. doi: 10.1053/j.gastro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Hikita H., Takehara T., Kodama T., Shimizu S., Hosui A., Miyagi T., Tatsumi T., Ishida H., Ohkawa K., Li W., Kanto T., Hiramatsu N., Hennighausen L., Yin X.M., Hayashi N. BH3-only protein bid participates in the Bcl-2 network in healthy liver cells. Hepatology. 2009;50:1972–1980. doi: 10.1002/hep.23207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsova P., Gores G.J. Death receptor-mediated cell death and proinflammatory signaling in nonalcoholic steatohepatitis. Gastroenterol Hepatol. 2015;1:17–27. doi: 10.1016/j.jcmgh.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma J., Srinivasan R., Majumdar S., Mir S., Radotra B.D., Wig J.D. Bcl-XL protein levels determine apoptotic index in pancreatic carcinoma. Pancreas. 2005;30:337–342. doi: 10.1097/01.mpa.0000160282.64451.f1. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto Y., Hosotani R., Wada M., Lee J.U., Koshiba T., Fujimoto K., Tsuji S., Nakajima S., Doi R., Kato M., Shimada Y., Imamura M. Immunohistochemical analysis of Bcl-2, Bax, Bcl-X, and Mcl-1 expression in pancreatic cancers. Oncology. 1999;56:73–82. doi: 10.1159/000011933. [DOI] [PubMed] [Google Scholar]
- 15.Aguirre A.J.A., Bardeesy N., Sinha M., Lopez L., Tuveson D.A., Horner J., Redston M.S., DePinho R.A. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kodama T., Takehara T., Hikita H., Shimizu S., Shigekawa M., Li W., Miyagi T., Hosui A., Tatsumi T., Ishida H., Kanto T., Hiramatsu N., Yin X.M., Hayashi N. BH3-only activator proteins Bid and Bim are dispensable for Bak/Bax-dependent thrombocyte apoptosis induced by Bcl-xL deficiency: molecular requisites for the mitochondrial pathway to apoptosis in platelets. J Biol Chem. 2011;286:13905–13913. doi: 10.1074/jbc.M110.195370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hikita H., Kodama T., Shimizu S., Li W., Shigekawa M., Tanaka S., Hosui A., Miyagi T., Tatsumi T., Kanto T., Hiramatsu N., Morii E., Hayashi N., Takehara T. Bak deficiency inhibits liver carcinogenesis: a causal link between apoptosis and carcinogenesis. J Hepatol. 2012;57:92–100. doi: 10.1016/j.jhep.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 18.Hruban R.H., Adsay N.V., Albores-Saavedra J., Anver M.R., Biankin A.V., Boivin G.P., Furth E.E., Furukawa T., Klein A., Klimstra D.S., Kloppel G., Lauwers G.Y., Longnecker D.S., Luttges J., Maitra A., Offerhaus G.J., Pérez-Gallego L., Redston M., Tuveson D.A. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 19.Louhimo J.M., Steer M.L., Perides G. Necroptosis is an important severity determinant and potential therapeutic target in experimental severe pancreatitis. Gastroenterol Hepatol. 2016;2:519–535. doi: 10.1016/j.jcmgh.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mareninova O.A., Sendler M., Malla S.R., Yakubov I., French S.W., Tokhtaeva E., Vagin O., Oorschot V., Lüllmann-Rauch R., Blanz J., Dawson D., Klumperman J., Lerch M.M., Mayerle J., Gukovsky I., Gukovskaya A.S. Lysosome associated membrane proteins maintain pancreatic acinar cell homeostasis: LAMP-2 deficient mice develop pancreatitis. Gastroenterol Hepatol. 2015;1:678–694. doi: 10.1016/j.jcmgh.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hikita H., Takehara T., Shimizu S., Kodama T., Li W., Miyagi T., Hosui A., Ishida H., Ohkawa K., Kanto T., Hiramatsu N., Yin X.M., Hennighausen L., Tatsumi T., Hayashi N. Mcl-1 and Bcl-xL cooperatively maintain integrity of hepatocytes in developing and adult murine liver. Hepatology. 2009;50:1217–1226. doi: 10.1002/hep.23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohuchida K., Mizumoto K., Yamada D., Fujii K., Ishikawa N., Konomi H., Nagai E., Yamaguchi K., Tsuneyoshi M., Tanaka M. Quantitative analysis of MUC1 and MUC5AC mRNA in pancreatic juice for preoperative diagnosis of pancreatic cancer. Int J Cancer. 2006;118:405–411. doi: 10.1002/ijc.21317. [DOI] [PubMed] [Google Scholar]
- 23.Friess H., Lu Z., Andrén-Sandberg A., Berberat P., Zimmermann A., Adler G., Schmid R., Büchler M.W. Moderate activation of the apoptosis inhibitor Bcl-xL worsens the prognosis in pancreatic cancer. Ann Surg. 1998;228:780–787. doi: 10.1097/00000658-199812000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debacq-Chainiaux F., Erusalimsky J.D., Campisi J., Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc. 2009;4:1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu S., Takehara T., Hikita H., Kodama T., Miyagi T., Hosui A., Tatsumi T., Ishida H., Noda T., Nagano H., Doki Y., Mori M., Hayashi N. The let-7 family of microRNAs inhibits Bcl-xL expression and potentiates sorafenib-induced apoptosis in human hepatocellular carcinoma. J Hepatol. 2010;52:698–704. doi: 10.1016/j.jhep.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Shigekawa M., Hikita H., Kodama T., Shimizu S., Li W., Uemura A., Miyagi T., Hosui A., Kanto T., Hiramatsu N., Tatsumi T., Takeda K., Akira S., Takehara T. Pancreatic STAT3 protects mice against caerulein-induced pancreatitis via PAP1 induction. Am J Pathol. 2012;181:2105–2113. doi: 10.1016/j.ajpath.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 27.Adams J.M., Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moir J.A.G., White S.A., Mann J. Arrested development and the great escape–the role of cellular senescence in pancreatic cancer. Int J Biochem Cell Biol. 2014;57:142–148. doi: 10.1016/j.biocel.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Morton J.P., Jamieson N.B., Karim S.A., Athineos D., Ridgway R.A., Nixon C., McKay C.J., Carter R., Brunton V.G., Frame M.C., Ashworth A., Oien K.A., Evans T.R., Sansom O.J. LKB1 haploinsufficiency cooperates with Kras to promote pancreatic cancer through suppression of p21-dependent growth arrest. Gastroenterology. 2010;139:586–597. doi: 10.1053/j.gastro.2010.04.055. 597.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korkolopoulou P., Lazaris ACh, Konstantinidou A.E., Kavantzas N., Patsouris E., Christodoulou P., Thomas-Tsagli E., Davaris P. Differential expression of bcl-2 family proteins in bladder carcinomas. Relationship with apoptotic rate and survival. Eur Urol. 2002;41:274–283. doi: 10.1016/s0302-2838(02)00003-9. [DOI] [PubMed] [Google Scholar]
- 31.Takehara T., Liu X., Fujimoto J., Friedman S.L., Takahashi H. Expression and role of Bcl-xL in human hepatocellular carcinomas. Hepatology. 2001;34:55–61. doi: 10.1053/jhep.2001.25387. [DOI] [PubMed] [Google Scholar]
- 32.Hikita H., Takehara T., Shimizu S., Kodama T., Shigekawa M., Iwase K., Hosui A., Miyagi T., Tatsumi T., Ishida H., Li W., Kanto T., Hiramatsu N., Hayashi N. The Bcl-xL inhibitor, ABT-737, efficiently induces apoptosis and suppresses growth of hepatoma cells in combination with sorafenib. Hepatology. 2010;52:1310–1321. doi: 10.1002/hep.23836. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe J., Kushihata F., Honda K., Sugita A., Tateishi N., Mominoki K., Matsuda S., Kobayashi N. Prognostic significance of Bcl-xL in human hepatocellular carcinoma. Surgery. 2004;135:604–612. doi: 10.1016/j.surg.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Bauer C., Hees C., Sterzik A., Bauernfeind F., Mak'Anyengo R., Duewell P., Lehr H.A., Noessner E., Wank R., Trauzold A., Endres S., Dauer M., Schnurr M. Proapoptotic and antiapoptotic proteins of the Bcl-2 family regulate sensitivity of pancreatic cancer cells toward gemcitabine and T-cell-mediated cytotoxicity. J Immunother. 2015;38:116–126. doi: 10.1097/CJI.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 35.Shi M., He X., Wei W., Wang J., Zhang T., Shen X. Tenascin-C induces resistance to apoptosis in pancreatic cancer cell through activation of ERK/NF-κB pathway. Apoptosis. 2015;20:843–857. doi: 10.1007/s10495-015-1106-4. [DOI] [PubMed] [Google Scholar]
- 36.Hingorani S.R., Wang L., Multani A.S., Combs C., Deramaudt T.B., Hruban R.H., Rustgi A.K., Chang S., Tuveson D.A. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Ijichi H., Chytil A., Gorska A.E., Aakre M.E., Fujitani Y., Fujitani S., Wright C.V., Moses H.L. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-β signaling in cooperation with active Kras expression. Genes Dev. 2006;20:3147–3160. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delbridge A.R.D., Strasser A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015;22:1071–1080. doi: 10.1038/cdd.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi S., Chen Z., Tang L.H., Fang Y., Shin S.J., Panarelli N.C., Chen Y.T., Li Y., Jiang X., Du Y.C. Bcl-xL promotes metastasis independent of its anti-apoptotic activity. Nat Commun. 2016;7:10384. doi: 10.1038/ncomms10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen D., Zheng X., Kang D., Yan B., Liu X., Gao Y., Zhang K. Apoptosis and expression of the Bcl-2 family of proteins and P53 in human pancreatic ductal adenocarcinoma. Med Princ Pract. 2012;21:68–73. doi: 10.1159/000332423. [DOI] [PubMed] [Google Scholar]
- 41.Lüttges J., Neumann S., Jesnowski R., Borries V., Löhr M., Klöppel G. Lack of apoptosis in PanIN-1 and PanIN-2 lesions associated with pancreatic ductal adenocarcinoma is not dependent on K-ras status. Pancreas. 2003;27:e57–e62. doi: 10.1097/00006676-200310000-00021. [DOI] [PubMed] [Google Scholar]
- 42.Grasso D., Garcia M.N., Hamidi T., Cano C., Calvo E., Lomberk G., Urrutia R., Iovanna J.L. Genetic inactivation of the pancreatitis-inducible gene Nupr1 impairs PanIN formation by modulating Kras(G12D)-induced senescence. Cell Death Differ. 2014;21:1633–1641. doi: 10.1038/cdd.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kennedy A.L., Morton J.P., Manoharan I., Nelson D.M., Jamieson N.B., Pawlikowski J.S., McBryan T., Doyle B., McKay C., Oien K.A., Enders G.H., Zhang R., Sansom O.J., Adams P.D. Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Mol Cell. 2011;42:36–49. doi: 10.1016/j.molcel.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guerra C., Collado M., Navas C., Schuhmacher A.J., Hernández-Porras I., Cañamero M., Rodriguez-Justo M., Serrano M., Barbacid M. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–739. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yosef R., Pilpel N., Tokarsky-Amiel R., Biran A., Ovadya Y., Cohen S., Vadai E., Dassa L., Shahar E., Condiotti R., Ben-Porath I., Krizhanovsky V. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 2016;7:11190. doi: 10.1038/ncomms11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung M.S., Jin D.H., Chae H.D., Kang S., Kim S.C., Bang Y.J., Choi T.S., Choi K.S., Shin D.Y. Bcl-xL and E1B-19K proteins inhibit p53-induced irreversible growth arrest and senescence by preventing reactive oxygen species-dependent p38 activation. J Biol Chem. 2004;279:17765–17771. doi: 10.1074/jbc.M305015200. [DOI] [PubMed] [Google Scholar]
- 47.Song J.H., Kandasamy K., Zemskova M., Lin Y.W., Kraft A.S. The BH3 mimetic ABT-737 induces cancer cell senescence. Cancer Res. 2011;71:506–515. doi: 10.1158/0008-5472.CAN-10-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garrido-Laguna I., Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319–334. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]
- 49.LeBlanc J.K., Chen J.H., Al-Haddad M., Luz L., McHenry L., Sherman S., Juan M., Dewitt J. Can endoscopic ultrasound predict pancreatic intraepithelial neoplasia lesions in chronic pancreatitis?: a retrospective study of pathologic correlation. Pancreas. 2014;43:849–854. doi: 10.1097/MPA.0000000000000142. [DOI] [PubMed] [Google Scholar]