Abstract
Background
Pyogenic liver abscesses (PLA) are increasingly managed by percutaneous treatment instead of surgery, but there are limited data about postdischarge outcomes. Postdischarge services and factors predicting poor outcomes have not been described.
Methods
We performed a retrospective, descriptive case series at a single center assessing treatment and outpatient follow-up for patients treated for PLA from 2007 to 2012. We reviewed the electronic medical record for patient characteristics and outcomes. Data for care received at other facilities were not available. In our analysis, we compared patients with malignancy with those without and attempted to determine predictors of emergency department (ED) visits and hospital readmissions.
Results
Of 125 patients identified with PLA, 12 had surgical drainage, 23 had percutaneous aspiration, 78 had percutaneous drainage (PD), 11 had no drainage, and 1 was made comfort measures only. Seventy (60%) were discharged with a drain, and 31 (25%) were discharged on intravenous (IV) antibiotics. After discharge, 46 (38%) had ED visits and 36 (30%) were readmitted within 30 days of discharge. Fourteen (12%) had complications from antibiotics, and 4 (13%) had complications from peripherally inserted center catheter lines. A total of 8 patients, 5 in-hospital and 3 postdischarge, died. In our analysis of risk factors for 90-day postdischarge ED visit/readmission, only malignancy was a predictor.
Conclusions
Pyogenic liver abscess patients have intense postdischarge needs (drain management, IV antibiotics) and a high rate of ED visits and readmissions. Although PD provides source control without surgery, ambulatory needs are now more complex, requiring multidisciplinary collaboration.
Keywords: hepatic abscess, liver abscess, percutaneous drainage, postdischarge outcomes, readmissions
The overall incidence of pyogenic liver abscesses (PLA) is increasing in the United States [1, 2], likely related to aging of the population, an increase in hepatobiliary diseases and malignancies, and increased adoption of biliary procedures [1–4]. Despite this increase in incidence, mortality has decreased dramatically [2], with a current estimated case-fatality rate of 5.6% in the United States [1]. Some of this decline has been attributed to the widespread adoption of percutaneous drainage as first-line therapy in place of open surgical drainage (SD) [1, 2, 5].
Several studies have compared open SD of PLAs with percutaneous catheter drainage (PD) and found that PD of simple, uncomplicated PLAs is superior to SD [6–9]. One other intervention that is gaining popularity is percutaneous needle aspiration (PA). Percutaneous needle aspiration has been found to be equally efficacious with comparable morbidity and mortality to PD [10, 11], and some groups recommend it because it is more cost- effective with superior patient comfort [11]. Still, the optimal management of larger abscesses or more complicated cases is still debated, with some advocating for open SD in abscesses larger than 5 cm [12], multiloculated abscesses [13], or multiple abscesses [14]. Other studies recommended open SD in cases of PLA rupture or with patients that require surgical correction of an underlying pathology (ie, cholecystitis) [4, 15, 16].
Multiple studies have analyzed the factors that lead to treatment failure or other complications to predict which patients require more aggressive, early treatment [8, 9, 16–22]. However, most of these studies have focused on the inpatient setting, looking at treatment failures, morbidity, and mortality before discharge. Because patients with PLAs are generally not disease-free upon discharge from the hospital, they often require prolonged antibiotic courses, follow-up imaging, and drain care. Multiple providers are involved and have to coordinate care in the outpatient setting. We sought to better understand these postdischarge medical needs.
METHODS
We performed a single-center, retrospective, descriptive study assessing treatment and outpatient follow-up for patients treated for PLA. With institutional review board approval, we used International Classification of Diseases, Ninth Revision (ICD-9) code 572.0 (Abscess of liver) to identify patients treated at our regional academic medical care center, for PLA from 2007 to 2012. Both pediatric and adult patients were included in our analysis. Charts were reviewed to determine whether PLA was present. Pyogenic liver abscess was defined by the presence of a consistent infectious syndrome and characteristic findings on imaging (either ultrasound or computed tomography) with pus found on drainage procedure (if performed). Signs and symptoms suggestive of an infectious syndrome included fevers, chills, abdominal pain, and leukocytosis, but not all elements needed to be present for inclusion. If no drainage procedure was performed, then patients needed to clinically and radiographically improve on antibiotic therapy. Cases of cholecystitis or cholangitis without abscess and liver abnormalities that were not infectious were excluded.
We then reviewed charts to record details of the admission and postdischarge care. We recorded medical comorbidities, abscess size and number, treatment type (SD versus PD versus PA versus antibiotics alone), length of stay, discharge with or without drains, and type of antibiotics at discharge (parenteral versus oral). We also looked at the number of ambulatory visits, emergency department (ED) visits, readmissions, and mortality (as documented in our electronic health record). Readmissions were characterized as related to PLA or unrelated to PLA. Readmissions related to PLA were defined as being secondary to persistent or unresolved infection or secondary to complications from drains, antibiotics, or peripherally inserted center catheter (PICC) lines. Data for care received at other facilities were not available.
In our primary analysis, we compared readmission rates by treatment type. A subanalysis was done for patients with malignancy. The malignancies included in the subanalysis were primarily hepatobiliary and pancreatic malignancies, followed by hepatic metastases. However, there were other primary gastrointestinal (GI), non-GI solid organ, and hematologic malignancies that were also seen.
Statistical analysis was done using a Student t test for quantitative data and χ2 or Fisher exact tests for qualitative data, using QI Macro 2017 for Excel. We used a P value of .05 to denote significance.
RESULTS
One hundred eighty-eight patient admissions were identified using ICD-9 codes over a 6-year period. Twenty-four were excluded after chart review indicated there was not a liver abscess. In total, there were 164 patient admissions with 125 unique patients. Our results were reported in reference to 125 index patient admissions. Of these 125 admissions, 60 of them had a malignancy.
The baseline characteristics of these 125 patients are shown in Table 1, with a comparison of characteristics between the malignancy and nonmalignancy subgroups. Patients with malignancy were significantly older compared with those without malignancy. The percentage of patients with biliary disease was significantly higher in those without malignancy.
Table 1.
Baseline Characteristics of Patients With PLA
| Characteristics | All Patients (n = 125) | Nonmalignancy Subgroup (n = 65) | Malignancy Subgroup (n = 60) | P Values |
|---|---|---|---|---|
| Male, no. (%) | 85 (68) | 48 (74) | 37 (62) | .1447 |
| Age, mean (range) | 61 (13–92) | 58 (13–89) | 65 (40–92) | .021 |
| Race, no. (%) | .9545 | |||
| White | 123 (98) | 64 (98) | 59 (98) | |
| Nonwhite | 2 (2) | 1 (2) | 1 (2) | |
| Comorbidities, no. (%) | ||||
| Diabetes | 30 (24) | 18 (28) | 12 (20) | .3144 |
| Immunosuppression | 8 (6) | 5 (8) | 3 (5) | .5389 |
| IBD | 11 (9) | 8 (12) | 3 (5) | .1496 |
| Cirrhosis | 4 (3) | 4 (6) | 0 (0) | .1202 |
| Liver transplant | 1 (1) | 1 (2) | 0 (0) | 1 |
| Kidney disease | 6 (5) | 1 (2) | 5 (8) | .2096 |
| Biliary disease | 54 (43) | 30 (46) | 14 (23) | .0076 |
Abbreviations: IBD, inflammatory bowel disease; PLA, pyogenic liver abscesses.
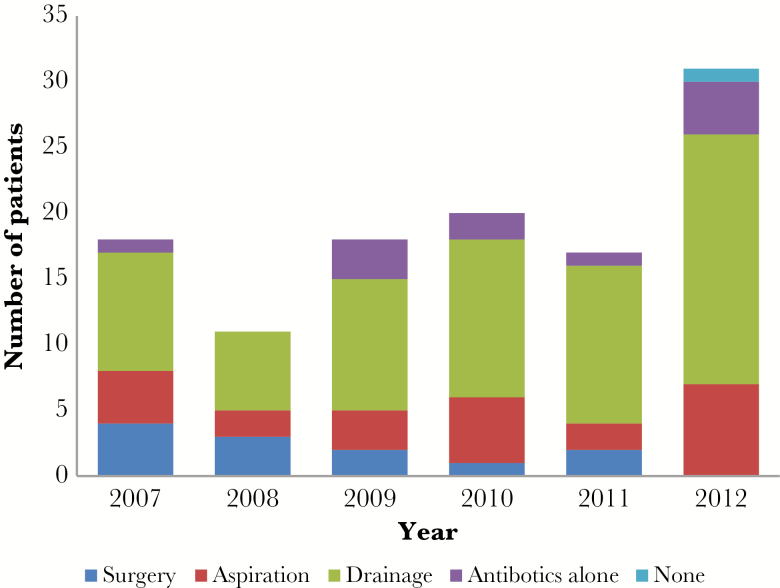
In total, 78 patients (62%) underwent PD, the most common option, followed by 23 patients (18%) that underwent PA, 12 patients (10%) that underwent surgery for abscess drainage, and 11 patients (9%) that were treated with just antibiotics (Table 2). One patient was made comfort measures only per family request shortly after diagnosis of the PLA and died without any treatment. This patient was excluded from additional analysis. Over the 6 years that this study covered, the proportion of SD interventions decreased (Figure 1). There were no cases in which percutaneous methods were used first and then SD was performed afterwards for treatment failure. Five patients died in the hospital as a result of their PLA (Table 2).
Table 2.
Pyogenic Liver Abscess Characteristics and Management
| Characteristics | All Patients (n = 124) | Nonmalignancy Subgroup (n = 64) | Malignancy Subgroup (n = 60) | P Values |
|---|---|---|---|---|
| Abscess size (cm), no. (%) | .1263 | |||
| <5 cm | 35 (28) | 13 (20) | 22 (37) | |
| >5 cm | 54 (44) | 29 (45) | 25 (42) | |
| Unknown | 35 (28) | 22 (34) | 13 (22) | |
| Abscess number, no. (%) | .3778 | |||
| Single | 82 (66) | 40 (63) | 42 (70) | |
| Multiple | 42 (34) | 24 (37) | 18 (30) | |
| Microbiology, no. (%) | ||||
| VRE | 1 (1) | 0 (0) | 1 (2) | .4839 |
| ESBL producers | 2 (2) | 1 (2) | 1 (2) | 1 |
| Escherichia coli | 9 (7) | 5 (8) | 4 (7) | .8246 |
| Klebsiella pneumoniae | 2 (2) | 1 (2) | 1 (2) | 1 |
| Streptococcus anginosus group | 11 (9) | 7 (11) | 4 (7) | .4186 |
| All other Gram positives | 6 (5) | 5 (8) | 1 (2) | .1154 |
| All other Gram negatives | 11 (9) | 5 (8) | 6 (10) | .6491 |
| Anaerobes | 17 (14) | 12 (19) | 5 (8) | .0919 |
| Polymicrobial | 40 (32) | 18 (28) | 22 (37) | .3092 |
| Fungal | 3 (2) | 0 (0) | 3 (5) | .1103 |
| Unknown | 22 (18) | 10 (16) | 12 (20) | .5239 |
| Inpatient intervention, no. (%) | ||||
| Surgery | 12 (10) | 7 (11) | 5 (8) | .6240 |
| Percutaneous aspiration | 23 (19) | 12 (19) | 11 (18) | .9524 |
| Percutaneous drainage | 78 (63) | 39 (61) | 39 (65) | .6398 |
| Antibiotics alone | 11 (9) | 6 (9) | 5 (8) | .8384 |
| Biliary drainage, no. (%) | ||||
| Cholecystectomy | 8 (6) | 7 (11) | 1 (1) | .0357 |
| Percutaneous drainage | 6 (5) | 2 (3) | 4 (7) | .3584 |
| Biliary stenting | 15 (12) | 5 (8) | 10 (17) | .1308 |
| None | 95 (77) | 50 (78) | 45 (75) | .6812 |
| ID consult on initial admission, no. (%) | 78 (63) | 42 (66) | 36 (60) | .5170 |
| Length of stay, mean (range) | 11 (2–108) | 13.5 (2–108) | 9.3 (2–38) | .072 |
| Mortality from PLA in-hospital, no. (%) | 5 (4) | 4 (6) | 1 (2) | .2009 |
Abbreviations: ESBL, extended-spectrum β-lactamase; ID, infectious diseases; PLA, pyogenic liver abscesses; VRE, vancomycin-resistant Enterococcus.
Figure 1.
Management of pyogenic liver abscesses at study institution, 2007–2012.
Most of the abscesses for which sizes were available were greater than 5 cm (43%), and the majority of cases were single abscesses (66%). Microbiology data was defined in all but 23 cases, with most abscesses (32%) being polymicrobial. The most common single organisms seen were Streptococcus anginosus group and Escherichia coli. There was a fairly low incidence of more resistant organisms with only 1 case of vancomycin-resistant Enterococcus (VRE) and only 2 cases of extended-spectrum β-lactamase (ESBL)-producing organisms. There were no significant differences between patients with malignancy and those without malignancy.
The discharge characteristics are shown in Table 3. Of the 120 patients that survived to discharge, 81 patients (65%) were discharged on oral antibiotics, 31 patients (25%) were discharged on parenteral antibiotics, 13 patients (10%) completed their treatment in the hospital, and 70 patients (56%) were discharged with a drain still in place in the liver abscess cavity. The mean length of stay was 11 days, although there was a wide range, from 2 to 108 days (Table 2). The antibiotic route, presence of a drain, and length of stay did not differ between patients with malignancy and those without malignancy.
Table 3.
Discharge Characteristics of Patients with PLA
| Characteristics | All Patients (n = 120) | Nonmalignancy Subgroup (n = 61) | Malignancy Subgroup (n = 59) | P Values |
|---|---|---|---|---|
| Discharge antibiotics, no. (%) | .2079 | |||
| IV | 31 (25) | 20 (31) | 11 (18) | |
| PO | 80 (65) | 37 (58) | 43 (72) | |
| None | 9 (10) | 4 (11) | 5 (10) | |
| Drain at discharge, no. (%) | .3387 | |||
| Drain in | 70 (56) | 33 (51) | 37 (62) | |
| No drain | 50 (44) | 28 (49) | 22 (38) |
Abbreviations: IV, intraveneous; PLA, pyogenic liver abscesses; PO, oral.
Table 4 shows the postdischarge healthcare use for the study patients. Upon discharge, the mean postdischarge antibiotic duration was 49.6 days, although there was a great deal of variability. The decision on duration of antibiotics was made initially by the inpatient treating physician, but decisions on continuation or not of antibiotics were often made in outpatient follow-up, not always by the same physician. Of the 112 patients that were discharged on antibiotics, 14 (12%) had an adverse event related to the antibiotics. Of the 31 patients discharged with a PICC line, 4 (13%) had a complication related to the PICC line. The mean number of follow-up appointments was 4.8, although this only included follow-up appointments within our system. Thirty-eight percent of patients had ED visits, 30% of patients had 30-day all-cause readmissions, and 23% of patients had 31- to 90-day all-cause readmissions (Table 4). Three patients died as a result of PLA after discharge.
Table 4.
Postdischarge Healthcare Utilization and Outcomes
| Variable | All Patients (n = 120) | Nonmalignancy Subgroup (n = 61) | Malignancy Subgroup (n = 59) | P Values |
|---|---|---|---|---|
| Length of antibiotic course, mean (range) | 51.2 | 53.9 | 48.4 | .681 |
| Follow-up appointments, mean (range) | 4.8 (0–33) | 4.2 (0–21) | 5.4 (0–33) | .114 |
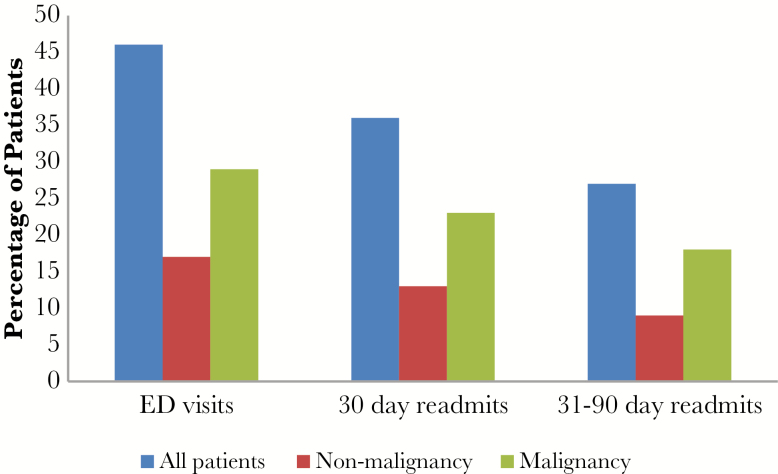
| ED visits, all cause, no. (%) | 46 (38) | 17 (28) | 29 (49) | .017 |
| 30-day readmits, all cause, no. (%) | 36 (30) | 13 (21) | 23 (39) | .035 |
| 31- to 90-day readmits, all cause, no. (%) | 27 (23) | 9 (15) | 18 (31) | .038 |
| Antibiotic complications, no. (%) | 14 (12) | 7 (11) | 7 (12) | .947 |
| PICC complications, no. (%)* | 4 (13) | 2 (10) | 2 (18) | .601 |
| Postdischarge mortality from liver abscess, no. (%) | 3 (3) | 0 (0) | 3 (5) | .0745 |
Abbreviations: ED, emergency department; PICC, peripherally inserted center catheter.
*Of the 31 patients discharged with a PICC.
In comparing patients with malignancy with those without, there was a significantly higher rate of ED visits, 30-day all-cause readmissions, and 31- to 90-day all-cause readmission for patients with malignancies (Figure 2). However, the mortality rate was nonsignificantly higher for patients without malignancies (Table 4).
Figure 2.
Percentage of patients treated for pyogenic liver abscesses who had emergency department (ED) visits and readmissions.
We then performed a univariate analysis comparing the patients with ED visits or readmissions (65 patients) to those who had none (56 patients). This analysis excluded the 4 patients who died during their first admission. We found a significantly higher rate of malignancy among those with ED visits or readmission to those with none (Table 5). There were no other characteristics associated with a higher readmission rate. When we analyzed the readmissions that were classified as related to PLA or management of PLA compared with those related to another issue, and there were no significant differences between the groups (Table 6).
Table 5.
Risk Factors for 90-Day Postdischarge ED Visit or Readmission Among Patients Treated for PLA
| Variable | Patients With ED Visits or Readmissions (n = 65) |
Patients Without ED Visits or Readmissions (n = 55) |
P Values |
|---|---|---|---|
| Age, mean (range) | 61 (13–91) | 62 (23–92) | .995 |
| Comorbidities, no. (%) | |||
| Malignancy | 42 (63) | 17 (31) | .003 |
| Biliary disease | 23 (35) | 12 (22) | .103 |
| Abscess size >5 (cm), mean (%)* | 27 (50) | 26 (47) | .322 |
| Microbiology unknown, no. (%) | 12 (18) | 10 (18) | .969 |
| Inpatient intervention, no. (%) | |||
| Surgery | 8 (12) | 2 (4) | .087 |
| Percutaneous aspiration | 10 (15) | 13 (24) | .253 |
| Percutaneous drainage | 40 (62) | 36 (65) | .657 |
| Antibiotics alone | 7 (11) | 4 (7) | .508 |
| ID consult, no. (%) | 41 (63) | 37 (67) | .631 |
| Discharge with IV antibiotics, no. (%) | 17 (26) | 14 (25) | .931 |
| Discharge with drain, no. (%) | 40 (62) | 30 (55) | .439 |
| Length of stay <5 days | 20 (31) | 21 (38) | .394 |
| Length of antibiotic course <30 days | 25 (38) | 23 (42) | .863 |
| Follow-up appointments <3 within 90 days, no. (%) | 32 (49) | 25 (45) | .680 |
Abbreviations: ED, emergency department; ID, infectious diseases; IV, intraveneous; PLA, pyogenic liver abscesses.
*Of 88 patients in which the abscess size was documented.
Table 6.
Univariate Analysis of Risk Factors for 90-Day Readmissions Secondary to PLA Among Patients Treated for PLA
| Variable | Readmissions Secondary to PLA or Treatment (n = 33) | Unrelated or No Readmissions (n = 87) |
P Values |
|---|---|---|---|
| Age, mean (range) | 62 (15–89) | 62 (13–92) | |
| Comorbidities, no. (%) | |||
| Malignancy | 13 (39) | 46 (53) | .187 |
| Biliary disease | 10 (30) | 25 (29) | .866 |
| Abscess size >5 (cm), mean (%)* | 15 (45) | 38 (44) | .911 |
| Microbiology unknown, no. (%) | 6 (18) | 17 (20) | .979 |
| Inpatient intervention, no. (%) | |||
| Surgery | 4 (12) | 6 (7) | .355 |
| Percutaneous aspiration | 6 (18) | 17 (20) | .866 |
| Percutaneous drainage | 21 (64) | 55 (63) | .966 |
| Antibiotics alone | 2 (6) | 9 (10) | .468 |
| ID consult, no. (%) | 21 (64) | 57 (66) | .847 |
| Discharge with IV antibiotics, no. (%) | 10 (30) | 21 (24) | .491 |
| Discharge with drain, no. (%) | 23 (70) | 47 (54) | .120 |
| Length of stay <5 days | 12 (36) | 29 (33) | .755 |
| Length of antibiotic course <30 days | 13 (39) | 34 (39) | .975 |
| Follow-up appointments <3 within 90 days, no. (%) | 16 (48) | 45 (52) | .894 |
Abbreviations: ID, infectious diseases; IV, intraveneous; PLA, pyogenic liver abscesses.
*Of 88 patients in which the abscess size was documented.
DISCUSSION
Our data confirm the shift towards percutaneous treatment for management of PLA. Over the 6 years described in this study, there was a decline in SD procedures with none performed in 2012. The decision about whether to perform surgery was made at the discretion of the attending surgeon, so the decrease in SD procedures likely represents an overall change in practice patterns. However, despite this shift, the patients continued to have intense outpatient needs as well as an overall high rate of ED visits and readmissions, with more than 50% of patients in our study having an ED visit or readmission within 90 days.
An analysis of all readmissions showed that malignancy was the only factor identified that significantly predicted an increased risk of ED visit or readmission. However, when the admissions were broken down as to whether they were related to PLA or PLA treatment versus unrelated, there was no significant association. This suggests that patients with malignancies have an overall high rate of readmission, likely secondary to their underlying disease. As a result, they warrant close attention and careful discharge planning. This correlates with other studies that have found increased in-hospital morbidity among patients with malignancies [6, 15, 20, 22]. However, we were unable to find any factors that would predict readmission specifically related to PLA or PLA treatment, making it difficult to predict which patients are at the highest risk.
There has been a debate about the optimal treatment of PLA, particularly in cases of large, multiple, or complicated abscesses [6, 7, 10–12]. However, we did not find that the intervention used impacted readmissions or ED visits. Although SD can more rapidly remove infected hepatic debris and necrotic tissue, there may still be postsurgical complications resulting in readmission, including complications related to drains, antibiotics, or the surgery itself. In contrast to other studies, there were no cases of SD being used as salvage therapy in the setting of failed PD or PA in our sample. In addition, the majority of patients completed prolonged antibiotic courses, with the mean duration of 49.6 days. This may minimize some of the differences between the treatment interventions. Therefore, the optimal treatment modality should be made on a case-by-case basis based on patient characteristics.
In terms of microbiology, most of the cases in our study were polymicrobial, with a low proportion with unknown microbiology. Given the high likelihood of polymicrobial abscesses, patients should be empirically started on broad-spectrum coverage that includes Gram-positive, Gram-negative, and anaerobic coverage. There was no association between having undefined microbiology and readmissions, suggesting that empiric regimens are generally acceptable for treatment if an organism cannot be identified. There was a low number of highly resistant organisms, such as VRE and ESBL-producing Gram negatives, seen in our study, which probably contributes to the success of empiric regimens.
Overall, 8 patients died as a result of PLA, 5 in the hospital and 3 after discharge. We opted to follow patients out for 120 days after discharge with the assumption that by that point, all patients should have completed treatment for PLA. This gives an overall mortality rate of 7% from PLA, comparable with rates reported in some other studies [1, 12, 23]. However, it is possible that this mortality rate is an underestimate because patients who died at home or at other institutions may have not been captured in our medical record. It is interesting that the patient population without malignancy had a higher mortality rate than those patients with malignancy. This is in contrast to other studies that have shown a higher mortality rate in patients with malignancy [20–22]. Perhaps patients with malignancy in our study have a higher level of outpatient visits and monitoring, allowing diagnosis to occur earlier, before development of septic shock.
Our data demonstrate that PLA patients have intense ambulatory care needs, so as survival improves, areas for improvement in the management of PLA will move to the outpatient setting. The increasing number of percutaneous procedures performed as inpatients means that patients will continue to need interventional radiology follow up upon discharge. More than 50% of patients in our study were discharged with drains in place, and at our institution, percutaneously placed drains continue to be managed by interventional radiology after discharge. A potential area for improvement is to form ambulatory care teams, working in collaboration with interventional radiology. More data are needed to determine the optimal duration of drain placement and the interval at which the drain is checked. Another potential area of study is to assess the antibiotic course to determine optimal duration. For patients followed by infectious disease, our standard practice is a minimum of 4 weeks of antibiotics with discontinuation based on radiologic resolution or stability of the abscess cavity. However, patients followed by other providers, such as primary care, surgery, and oncology, may have much more variable courses of antibiotics. Although prolonged courses of antibiotics may decrease the risk of relapses and treatment failure, it does pose an increased risk of adverse effects related to antibiotics. We did not specifically look at the development of resistance on therapy, but this could be another complication of prolonged courses of antibiotics. In addition, we had a high number of patients discharged on oral antibiotics (65%), most on fluoroquinolones either with or without metronidazole. Although this takes away the risk of PICC lines in the outpatient setting, they will continue to need close monitoring, particularly for QT prolongation and because quinolone use is limited by the rising prevalence of multidrug resistance Gram-negative rods.
The advantage of our study is that it focused specifically on outpatient follow up as well as readmissions, areas not analyzed in other studies. In addition, although we were unable to capture all the outpatient follow up, our institution is the only academic medical care center in a large geographic area. Eighty-five percent of the patients that survived to discharge received at least part of their follow-up care at our institution. Patients that require specialty care, including interventional radiology or infectious disease, generally continue to follow up within our institution.
There are some limitations to our study. It was a retrospective chart review. Given that, the rate of adverse effects related to antibiotics may be an underestimate. Because this was a single-center study, we were not able to capture outpatient follow-up as well as ED visits that occurred outside our institution. The record of ED visits, readmissions, and mortality may be an underestimate. If that is the case, there may be factors that actually significantly increase the risk of readmission that we were unable to identify. In addition, our patient population was very homogenous, being almost entirely white. Given that there are microbiologic and epidemiologic differences in PLAs worldwide [3], it is possible that some of the characteristics of our study would not generalize as well.
CONCLUSIONS
In conclusion, patients with PLA have intense ambulatory care needs and a high rate of ED visits and readmissions. Patients with malignancy have an even higher rate of ED visits and readmissions, whereas the type of intervention used to treat PLA did not influence the rate of ED visits and readmission. It is important to recognize the continued needs of patients with PLA, even after discharge. Further research is needed to elucidate how outcomes can be improved for these patients. In the interim, clinicians should be cognizant of the complexities of treating PLAs and consider moving towards a more multidisciplinary approach.
Acknowledgments
We acknowledge Drs. Michael Calderwood and Alicia Zbehlik for assistance in editing this manuscript.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Meddings L, Myers RP, Hubbard J et al. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol 2010; 105:117–24. [DOI] [PubMed] [Google Scholar]
- 2. Huang CJ, Pitt HA, Lipsett PA et al. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg 1996; 223:600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cerwenka H. Pyogenic liver abscess: differences in etiology and treatment in Southeast Asia and Central Europe. World J Gastroenterol 2010; 16:2458–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mischinger HJ, Hauser H, Rabl H et al. Pyogenic liver abscess: studies of therapy and analysis of risk factors. World J Surg 1994; 18:852–7; discussion 858. [DOI] [PubMed] [Google Scholar]
- 5. Christein JD, Kendrick ML, Que FG. What affects mortality after the operative management of hepatic abscess? HPB 2006; 8:175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mezhir JJ, Fong Y, Jacks LM et al. Current management of pyogenic liver abscess: surgery is now second-line treatment. J Am Coll Surg 2010; 210:975–83. [DOI] [PubMed] [Google Scholar]
- 7. Ferraioli G, Garlaschelli A, Zanaboni D et al. Percutaneous and surgical treatment of pyogenic liver abscesses: observation over a 21-year period in 148 patients. Dig Liver Dis 2008; 40:690–6. [DOI] [PubMed] [Google Scholar]
- 8. Alvarez Pérez JA, González JJ, Baldonedo RF et al. Clinical course, treatment, and multivariate analysis of risk factors for pyogenic liver abscess. Am J Surg 2001; 181:177–86. [DOI] [PubMed] [Google Scholar]
- 9. Pang TC, Fung T, Samra J et al. Pyogenic liver abscess: an audit of 10 years’ experience. World J Gastroenterol 2011; 17:1622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giorgio A, de Stefano G, Di Sarno A et al. Percutaneous needle aspiration of multiple pyogenic abscesses of the liver: 13-year single-center experience. AJR Am J Roentgenol 2006; 187:1585–90. [DOI] [PubMed] [Google Scholar]
- 11. Yu SC, Ho SS, Lau WY et al. Treatment of pyogenic liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Hepatology 2004; 39:932–8. [DOI] [PubMed] [Google Scholar]
- 12. Tan YM, Chung AY, Chow PK et al. An appraisal of surgical and percutaneous drainage for pyogenic liver abscesses larger than 5 cm. Ann Surg 2005; 241:485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hope WW, Vrochides DV, Newcomb WL et al. Optimal treatment of hepatic abscess. Am Surg 2008; 74:178–82. [PubMed] [Google Scholar]
- 14. Chou FF, Sheen-Chen SM, Chen YS, Chen MC. Single and multiple pyogenic liver abscesses: clinical course, etiology, and results of treatment. World J Surg 1997; 21:384–8. [DOI] [PubMed] [Google Scholar]
- 15. Barakate MS, Stephen MS, Waugh RC et al. Pyogenic liver abscess: a review of 10 years’ experience in management. Aust N Z J Surg 1999; 69:205–9. [DOI] [PubMed] [Google Scholar]
- 16. Cerwenka H, Bacher H, Werkgartner G et al. Treatment of patients with pyogenic liver abscess. Chemotherapy 2005; 51:366–9. [DOI] [PubMed] [Google Scholar]
- 17. Chen W, Chen CH, Chiu KL et al. Clinical outcome and prognostic factors of patients with pyogenic liver abscess requiring intensive care. Crit Care Med 2008; 36:1184–8. [DOI] [PubMed] [Google Scholar]
- 18. Ruiz-Hernández JJ, León-Mazorra M, Conde-Martel A et al. Pyogenic liver abscesses: mortality-related factors. Eur J Gastroenterol Hepatol 2007; 19:853–8. [DOI] [PubMed] [Google Scholar]
- 19. Chen SC, Huang CC, Tsai SJ et al. Severity of disease as main predictor for mortality in patients with pyogenic liver abscess. Am J Surg 2009; 198:164–72. [DOI] [PubMed] [Google Scholar]
- 20. Yeh TS, Jan YY, Jeng LB et al. Pyogenic liver abscesses in patients with malignant disease: a report of 52 cases treated at a single institution. Arch Surg 1998; 133:242–5. [DOI] [PubMed] [Google Scholar]
- 21. Chen SC, Lee YT, Tsai SJ et al. Clinical outcomes and prognostic factors of cancer patients with pyogenic liver abscess. J Gastrointest Surg 2011; 15:2036–43. [DOI] [PubMed] [Google Scholar]
- 22. Wong WM, Wong BC, Hui CK et al. Pyogenic liver abscess: retrospective analysis of 80 cases over a 10-year period. J Gastroenterol Hepatol 2002; 17:1001–7. [DOI] [PubMed] [Google Scholar]
- 23. Foo NP, Chen KT, Lin HJ, Guo HR. Characteristics of pyogenic liver abscess patients with and without diabetes mellitus. Am J Gastroenterol 2010; 105:328–35; doi:10.1038/ajg.2009.586. [DOI] [PubMed] [Google Scholar]
- 24. Tan L, Zhou HJ, Hartman M et al. Laparoscopic drainage of cryptogenic liver abscess. Surg Endosc 2013; 27:3308–14. [DOI] [PubMed] [Google Scholar]