Abstract
Current hepatitis C virus (HCV) treatment guidelines recommend treating HCV/human immunodeficiency virus (HIV)-coinfected individuals similar to HCV-monoinfected individuals. Recently inferior response rates to direct acting antiviral (DAA) therapy in HCV/HIV coinfection have been reported. Our German hepatitis C cohort (GECCO) cohort data show that coinfected patients with liver cirrhosis are less likely to achieve viral eradication.
Keywords: chronic hepatitis C, DAA, HCV/HIV coinfection, HCV treatment
Direct-acting antivirals (DAA) against hepatitis C virus (HCV) have impressively improved treatment of chronic hepatitis C infection. Historically, interferon (IFN)-based treatment of HCV in human immunodeficiency virus (HIV)-coinfected individuals led to far lower sustained virologic response (SVR) rates while being associated with more clinical and laboratory toxicities [1]. In contrast, IFN-free DAA combination treatment regimens have led to similar SVR rates in both HCV-monoinfected and HCV/HIV-coinfected individuals in numerous clinical and large phase III HCV licensing trials as well as real-life cohorts [2]. As a consequence, current HCV treatment guidelines recommend treating HCV/HIV-coinfected individuals similar to HCV-monoinfected individuals with regard to indication, selection of DAA, treatment duration, and monitoring [3–5]. The main remaining difference is a higher likelihood for drug-drug interactions between HIV combination antiretroviral therapy (cART) and DAA therapy, which need to be assessed before DAA treatment is initiated. Although coinfected individuals are no longer regarded as a special, difficult-to-treat patient population, HCV relapses after DAA therapy still occur. In recent studies, data from 2 Spanish cohorts have shown slightly lower SVR rates in coinfected patients compared with monoinfected patients (92% of 95% vs 98% of 98%, respectively) [6, 7]. This raises the question of whether there are still potential risk factors in HCV/HIV-coinfected patients that could help to identify those patients at higher risk of DAA treatment failure. Therefore, we assessed the influence of traditional risk factors on treatment outcome of various DAA combination therapies in HCV-monoinfected and coinfected patients of the German hepatitis C cohort (GECCO).
METHODS
The GECCO cohort is a multicenter cohort from 9 sites in Germany. All HCV-monoinfected and coinfected patients with complete follow-up having received 1 of the following DAA regimen were analyzed (n = 1505): pegylated IFN plus ribavirin (RBV) + sofosbuvir (SOF); SOF + RBV; SOF + simeprevir; SOF + daclatasvir ± RBV; SOF + ledipasvir; paritaprevir/ritonavir, ombitasvir ± RBV and ± dasabuvir. Treatment outcome was measured as SVR 12 weeks after end of therapy. Liver cirrhosis was assessed mainly by transient elastography (FibroScan) or aspartate aminotransferase-to-platelet ratio index score. Fisher’s exact, χ2, and Mann-Whitney U tests were used for statistical analysis.
RESULTS
Baseline Characteristics
A total of 952 of 1505 (63%) patients were male, and median age was 52 years (interquartile range [IQR], 45–59). Hepatitis C virus genotype (GT) distribution was as follows: GT1 72%, GT2 4%, GT3 18%, and GT4 6%. A total of 290 of 1505 (20%) patients had high baseline HCV ribonucleic acid ([RNA] >6 million IU/mL). Median baseline alanine aminotransferase (ALT) was 67 U/L (IQR, 43–111). A total of 699 of 1505 (46%) were HCV treatment-experienced (TE). Liver cirrhosis was present in 431 of 1505 (29%). A total of 282 of 1505 (19%) were on opiate substitution therapy (OST). A total of 349 of 1505 (23%) were HIV coinfected. Among these, median CD4 nadir was 206/µL (IQR, 123–360). A total of 69 of 349 (20%) were diagnosed with HIV at Centers for Disease Control and Prevention (CDC) stage C (clinical acquired immune deficiency syndrome), 55 of which were diagnosed at CDC stage C3. A total of 61 of 349 (17%) had baseline CD4 <350/µL, and 53 of 349 (15%) had baseline CD4 <20%. A total of 345 of 349 (99%) were on cART, 318 of 345 (92%) with HIV RNA <40 copies/mL, and median duration of cART before DAA treatment was 6.4 years (2.2–14.7).
There was no statistically significant difference in baseline parameters between HCV-monoinfected and coinfected individuals except for sex (P ≤ .001), GT (P ≤ .001), baseline HCV RNA (P ≤ .001), and liver cirrhosis (P = .003). Compared with monoinfected patients, coinfected patients were more likely to be male (55% vs 89%), less likely to be infected with GT3 (21% vs 9%), more likely to be infected with GT4 (3% vs 18%), more likely to have high baseline HCV RNA (17% vs 27%), and less likely to suffer from liver cirrhosis (31% vs 22%).
Virologic Response
Overall SVR rate was 95% (1425 of 1505), 95% (1096 of 1156) in HCV-monoinfected patients, and 94% (329 of 349) in HCV/HIV-coinfected patients (P = .684). Ten patients stopped treatment (1 nonresponse, 2 viral breakthroughs, 7 toxicities). Three reinfections and 67 relapsers were noted.
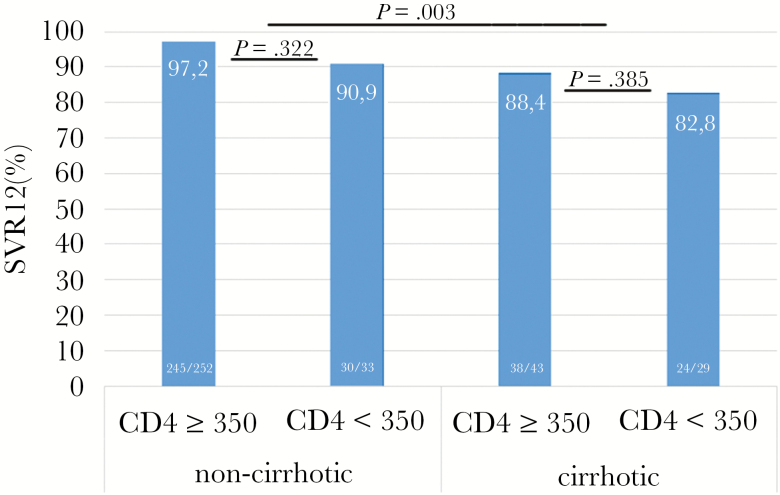
Among the 349 coinfected patients, neither sex (P = .708), age (P = .876), GT (P = .594), high HCV RNA (P = .873), ALT (P = .262), TE (P = .360), CD4 nadir (P = .473), nor OST (P = .391) were statistically significantly associated with SVR in univariate analysis. However, coinfected patients with CD4 <350/µL (P = .012), CD4 <20% (P = .005), and liver cirrhosis (P = .003) were less likely to achieve SVR (see Figure 1). In multivariate analysis, only liver cirrhosis (P = .02; odds ratio = 3.5; 95% confidence interval, 1.2–9.9) remained statistically significantly associated with non-SVR in coinfected patients.
Figure 1.
Sustained virologic response 12 weeks after end of therapy (SVR12) according to cirrhosis status and CD4 T-cell count (/µL).
Safety
Treatment was stopped in 7 patients due to clinical adverse events including dyspnea, rash, panic attacks, insomnia, and nausea. No grade 3 or 4 laboratory adverse event was reported.
DISCUSSION
Our cohort data confirm that HCV/HIV-coinfected patients can be cured from HCV in the overwhelming majority of cases with IFN-free DAA combination therapy. Overall SVR rate in our cohort of 349 HCV/HIV-coinfected patients was 94%. However, HCV cure was not achieved in all patients, particularly not in patients with suboptimal immune function as defined by CD4 T-cell count (the standard clinical surrogate marker in HIV-related immunodeficiency) below 350/µL and/or liver cirrhosis. In patients with both CD4 count <350/µL and liver cirrhosis, SVR rates dropped to 82.8%.
Our findings are in line with a recent report from a large Spanish HCV treatment cohort [7]. This prospective multicenter study enrolled all patients who consecutively attended 33 Infectious Diseases Units throughout Spain and who initiated DAA-based therapy since October 2011. Overall, 404 HCV-monoinfected and 423 HCV/HIV-coinfected patients receiving IFN-free DAA therapy were enrolled as well as 276 monoinfected and 173 coinfected patients receiving DAA-based, IFN-containing HCV therapy. The overall SVR rate in patients receiving IFN-free DAA therapy was 98% in monoinfected patients versus 95% in coinfected patients. Apart from the fact that the regimens used were different and could well explain a difference of 3% in SVR rates, it is important to note that coinfected patients were far more likely to be cirrhotic (64% vs 52%), which taken together with our findings indicate that underlying liver cirrhosis may indeed impair response to DAA therapy in a subset of coinfected patients. Unfortunately, no data on immune status were reported by the Spanish colleagues, which would have helped to support our finding that liver cirrhosis in combination with low immune function are the 2 driving forces behind lower HCV cure rates in coinfection. Similar data from another Spanish cohort have recently been published and also showed lower SVR rates among coinfected patients with higher liver fibrosis/cirrhosis compared with monoinfected patients (92% vs 98%) [6]. In the Italian Icona cohort, SVR rates were also lower among coinfected patients with (decompensated) cirrhosis (86%) [8]. Additional support comes from the Madrid-CoRe—a prospective registry of all coinfected adults undergoing DAA therapy in hospitals from the Madrid Regional Health Service [9]. Of 2030 enrolled coinfected patients, 37.1% were suffering from compensated cirrhosis and an additional 7.2% were already suffering from decompensated cirrhosis. Although the overall SVR rate was satisfying with 92%, and even patients with compensated cirrhosis experienced HCV cure in 91.5%, only 80.8% of patients with very advanced liver disease—decompensated cirrhotics—reached SVR. Again, no data on immune status were reported unfortunately.
However, a wealth of data exists which demonstrates that treatment responses are in fact not significantly different between HCV-monoinfected and HCV/HIV-coinfected individuals [2], as reflected in current treatment recommendations of various HCV guidelines [3–5]. Support also comes from the German Hepatitis C-Registry (Deutsches Hepatitis C-Register [DHC-R]). A total of 5657 HCV-monoinfected and 488 HCV/HIV-coinfected subjects were included into their analysis. No significant difference in overall SVR rates was observed between the 2 treatment groups across 4 GTs, SVR rates among cirrhotics were 87.8 in monoinfected and 89.3 in coinfected patients [10]. However, in contrast to our and the Spanish cohort, cirrhosis was far less frequent in the HCV/HIV-coinfected patient group compared with monoinfected patients in the DHC-R (17.2% in coinfected vs 29.4 in monoinfected). Another analysis suggesting similar HCV cure rates regardless of underlying HIV infection comes from the United States, which compared SVR rates from clinical coinfection trials to outcome data reported from real-world cohorts and showed comparable SVR rates ranging from 91% to 100% in both clinical trials and cohorts [11].
How to solve the dilemma? We speculate that there is a small proportion of coinfected patients that will respond worse to current DAA therapy because they suffer from both liver cirrhosis and potentially related impaired immunity although receiving fully active cART. The low CD4 T helper cell counts can potentially be explained by lymphopenia due to splenomegaly, which itself results from advanced liver cirrhosis accompanied by portal hypertension. Our findings should by no means lead to DAA therapy being withheld from those at utmost need of HCV cure−HCV/HIV-coinfected patients with advanced liver cirrhosis. On the contrary, our findings should support and highlight the need for access to DAA therapy for all HCV-infected individuals and initiation of HCV treatment before the onset of advanced liver fibrosis let alone cirrhosis as currently recommended by HCV treatment guidelines [3–5].
CONCLUSIONS
In summary, despite considerably improved safety and efficacy of treatment of chronic hepatitis C with DAA in HCV/HIV coinfection, liver cirrhosis remains as a risk factor for DAA treatment failure in patients with CD4 count <350/µL. More importantly, low CD4 cell counts coincided with liver cirrhosis probably due to splenomegaly causing lymphopenia. This highlights the need for early initiation of DAA therapy in HCV/HIV-coinfected patients before the onset of higher liver fibrosis/cirrhosis to allow for optimal rates of viral eradication and to substantially reduce morbidity and mortality in this patient population.
Acknowledgments
Potential conflicts of interest. C. B. has received honoraria for consulting or educational lectures from AbbVie, BMS, Gilead, MSD, and ViiV. P. I. has received honoraria for consulting or educational lectures from AbbVie, BMS, Gilead, MSD, Janssen-Cilag, and ViiV. T. L. has received honoraria for consulting or educational lectures from AbbVie, BMS, Gilead, MSD, Janssen-Cilag, and ViiV. K. S. has received honoraria for consulting or educational lectures from AbbVie, BMS, Gilead, Janssen-Cilag, Hexal, MSD, and ViiV. J. S. z. W. has received honoraria for consulting or speaking at educational events from AbbVie, BMS, Gilead, Janssen-Cilag, and MSD. A. B. has received honoraria for consulting or speaking at educational events from AbbVie, BMS, Gilead, Janssen-Cilag, MSD, and ViiV. S. C. has received honoraria for consulting or speaking at educational events from AbbVie, BMS, Gilead, Janssen-Cilag, MSD, and ViiV. J. K. R. has received honoraria for consulting or educational lectures from AbbVie, Bionor, BMS, Cipla, Gilead, Hexal, Janssen-Cilag, MSD, Roche, and ViiV. S. M. has received honoraria for consulting or educational lectures from AbbVie, BMS, Gilead, Janssen-Cilag, MSD, and ViiV. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sulkowski MS. HCV-HIV co-infected patients: no longer a ‘special’ population? Liver Int 2016; 36(Suppl 1):43–6. [DOI] [PubMed] [Google Scholar]
- 2. Naggie S, Muir AJ. Oral combination therapies for hepatitis C virus infection: successes, challenges, and unmet needs. Annu Rev Med 2017; 68:345–58. [DOI] [PubMed] [Google Scholar]
- 3. European AIDS Clinical Society. EACS Guidelines 8.1 Available at: http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html. Accessed 18 December 2016.
- 4. European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2016 Available at: http://www.easl.eu/research/our-contributions/clinical-practice-guidelines/detail/easl-recommendations-on-treatment-of-hepatitis-c-2016. Accessed 18 December 2016. [DOI] [PubMed]
- 5. American Association for the Study of Liver Diseases (AASLD). HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C Available at: http://www.hcvguidelines.org. Accessed 18 December 2016.
- 6. Arias A, Aguilera A, Soriano V et al. Rate and predictors of treatment failure to all-oral HCV regimens outside clinical trials. Antivir Ther 2016. doi: 10.3851/IMP3061. [DOI] [PubMed] [Google Scholar]
- 7. Neukam K, Suárez-Santamaría M, Rivero-Juárez A et al. HIV coinfection impairs the response to DAA-based HCV therapy. In: 51st EASL; April 13–17, 2016 (Abstract LBP513); Barcelona, Spain. [Google Scholar]
- 8. d’Arminio Monforte A, Cozzi-Lepri A, Ceccherini-Silberstein F et al. Access and response to direct antiviral agents (DAA) in HIV-HCV co-infected patients in Italy: data from the Icona cohort. PLoS One 2017; 12:e0177402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gil-Martin A, Gonzalez-García JJ, Cruz-Martos E et al. Real-world outcomes with new HCV antivirals in HIV/HCV-coinfected subjects: Madrid Coinfection Registry(Madrid-CoRE) findings. In: 67th Annual Meeting of the American Association for the Study of Liver Diseases; November 11–15, 2016 (Abstract 78); Boston, Massachusetts. [Google Scholar]
- 10. Rockstroh J, Lutz T, Mauss S et al. SVR12 rates under DAA-based HCV therapy from the National German Cohort Study: does HIV co-infection impair the response to DAA combination therapy? In: 67th Annual Meeting of the American Association for the Study of Liver Diseases; November 11–15, 2016 (Abstract 907); Boston, Massachusetts. [Google Scholar]
- 11. Naggie S, Rosenthal E, Kattakuzhy S et al. Real world effectiveness of ledipasvir/sofosbuvir (LDV/SOF) in patients coinfected with HCV and HIV-1: a comparative analysis of clinical trials with four real world cohorts. In: 67th Annual Meeting of the American Association for the Study of Liver Diseases; November 11–15, 2016 (Abstract 898); Boston, Massachusetts. [Google Scholar]