Abstract
Background
Large cell neuroendocrine tumor (LCNEC) of the lung is a rare and aggressive tumor similar to small cell lung cancer (SCLC). Thus, it is often treated similarly to SCLC in the front-line setting with a platinum doublet. However, treatment for patients beyond the first line remains undefined.
Case presentation
We report the case of a patient with stage IB LCNEC (PD-L1 negative but positive for PD-L1 amplification and tumor mutation burden high) who progressed after adjuvant chemotherapy after surgery and subsequent therapy with an antibody drug conjugate targeting a neuroendocrine-specific cell surface marker but achieved a significant and durable response with pembrolizumab, a humanized IgG4 monoclonal anti-PD-1 antibody.
Conclusions
Immunotherapy with checkpoint inhibitors is an effective treatment option for patients with metastatic LCNEC, even if PD-L1 expression is negative.
Keywords: Large cell neuroendocrine tumor, Pembrolizumab, PD-L1, Tumor mutation burden, Immunotherapy
Background
Pulmonary large cell neuroendocrine carcinoma (LCNEC) is a rare and aggressive tumor, diagnosed based on high-grade features of greater than 10 mitotic figures in 2 mm2 and the presence of neuroendocrine markers [1]. Its prognosis and treatment mirror that of small cell lung cancer (SCLC), with a 5-year survival rate for stage IV disease of less than 5% [1]. Compared with SCLC, however, LCNECs tend to present peripherally rather than centrally and are more likely to be early stage at diagnosis. Although there is no prospective studies guiding treatment, extrapolation based on studies performed in SCLC recommend surgical resection for early, localized disease [2]. The GFPC 0302, a phase II study, showed that the cisplatin and etoposide combination yielded similar outcome in LCNEC as in SCLC, with a median progression-free survival (PFS) of 5.2 months and overall survival (OS) of 7.7 months [3]. Recent genomic profiling efforts also revealed similarities between SCLC and LCNEC, with frequent concurrent loss of function mutations in TP53 and RB1 (~40%) [4–6]. In addition, this group harbors higher rates of MYC amplification (~15% in contrast to 6% in SCLC) [6]. The median tumor mutation burden (TMB) of LCNEC and SCLC are similar at 9.9 mutations/megabases, reflecting that both disease entities are commonly associated with the higher mutational load seen in smoking induced malignancies [6]. A second group, however, does not have TP53 and RB1 but instead possesses frequent mutations in KRAS, STK11, NOTCH1–4, and KEAP1, which are more frequently seen in non-small cell lung cancer (NSCLC) [4]. The implication for treatment and responses between these two subsets are not yet clear. Given the dismal poor overall survival rates in LCNECs, new treatment approaches for the disease are needed. Although there have been a paucity of data on PD-L1 expression in LCNEC, the recent successes of checkpoint inhibitors in relapsed SCLC after platinum chemotherapy in the phase I/II CheckMate 032 trial suggest that this might be a promising class of agents in LCNEC as well [7].
Case Presentation
We report a case of a 64 year-old Asian man with a 40 pack-year history of smoking who presented with hemoptysis. Chest X-ray revealed a 3 cm right upper lobe nodule. CT of the chest showed a lobulated 4.2 × 4.2 × 4.8 cm lesion located in the posterior segment of the right upper lobe abutting the T3 vertebra and a 3.5 mm nodule in the right lower lobe which was deemed non-specific. CT guided biopsy initially suggested lung adenocarcinoma that was CK7 positive, TTF-1, CK5/6, 20 and p63 negative. In addition to moderate amount of centrilobar emphysema, PET-CT confirmed a FDG avid lesion spanning 4.7 × 4.9 cm with SUV of 15.8 in the right upper lobe abutting the major fissure, a 5 mm nodule in the right lower lobe too small to characterize, and greater than 1 cm level 10–14 lymph nodes with SUV of 3.3. Subsequent brain MRI was negative. A right upper lobectomy and lymph node dissection were performed. A total of 15 lymph nodes spanning levels 2, 3, 4, 7, 10, and 11 were examined and all were negative for cancer. Pathology showed a 4.7 cm poorly differentiated carcinoma with possible squamous differentiation, 1.5 cm and 1 cm from the parenchymal and bronchial margin respectively, pT2aN0M0 stage IB. Focal large blood vessel invasion was identified but without lymphatic, perineural or pleural invasion. Immunohistochemical staining was negative for TTF-1, Napsin, p40, CK5/6, and the androgen receptor but positive for CK7, synaptophysin and CD56, consistent with a large cell neuroendocrine carcinoma of pulmonary origin (Fig. 1). Greater than 20 mitosis were seen in 10 high power field. Because the tumor was high risk based on the Encore Clinical Multi-Gene Assay for Early Stage Lung Cancer, which predicts an 5-year overall survival of 44.6% [8, 9], the patient received 4 cycles of adjuvant cisplatin and docetaxel chemotherapy based on the TAX 326 study [10].
Fig. 1.
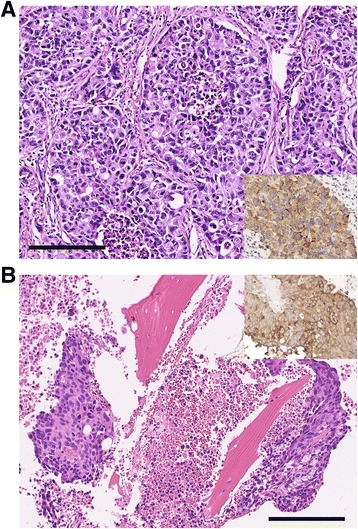
Pathologic findings. a Section from the initial surgical resection specimen shows a malignant neoplasm composed of solid nests of epithelioid cells with large, irregular, hyperchromatic nuclei, occasional prominent nucleoli, and abundant eosinophilic cytoplasm. There are numerous mitotic figures, and necrosis is seen in the centers of some of the tumor nests. b Biopsy of the iliac bone lesion shows metastatic tumor with similar histologic features. Note abundant tumor cell necrosis and fragments of bone within the biopsy. Insets in both panels demonstrate positive immunohistochemical staining for synapthophysin. [200× magnification; bar – 200 μm]
Within 4 months of completing adjuvant chemotherapy, the patient developed excruciating right hip pain. PET-CT revealed hypermetabolism at the right acetabulum and the inferolateral iliac wing (SUV 10) with cortical irregularity and “moth-eaten” erosion, concerning for metastatic infiltration. Biopsy of the right ilium showed a high-grade tumor identical to patient’s resected LCNEC. CK7, synaptophysin and CD56 were positive. At this point, the original surgical specimen was sent for molecular profiling (FoundationOne, Foundation Medicine, Cambridge). Genomic alterations identified included CD274 (PD-L1) amplification, MYC amplification, STK11 (LKB), APC, RB1, and TP53 mutations (Table 1). Tumor mutation burden was high at 24.76 mutations/megabases. Initial PD-L1 staining using the SP142 antibody was negative (Foundation Medicine, Cambridge) and independent confirmatory testing with a second 22C3 antibody (Cancer Genetics, Los Angeles) also showed <1% PD-L1 expression on the tumor cells. However, the tumor was positive for a neuroendocrine specific cell surface marker so patient received palliative radiation to the right hip and was enrolled in a phase I/II study of an antibody drug conjugate targeting this specific cell surface protein.
Table 1.
Genomic Alterations Identified by Hybrid Capture
| Gene Alteration | Loss or Gain of Function | Predictor of Response to Pembrolizumab |
|---|---|---|
| CD274 (PD-L1) amplification | Gain | Yes |
| STK11 S216F and LOH | Loss | Negative predictor |
| MYC amplification | Gain | No |
| APC E2516 | Loss | No |
| RB1 | Loss | No |
| TP 53 R158 L | Loss | No |
Unfortunately, after one dose of the experimental drug, patient’s disease progressed with increase in both the size and number of pulmonary nodules. Two new pancreatic lesions were also identified (Fig. 2). Patient stopped the trial and started on pembrolizumab. After 1 cycle of pembrolizumab, all visible lesions shrunk and no new lesions were seen (Fig. 2). Patient remains on pembrolizumab with continued improvement of the disease 6 months after.
Fig. 2.
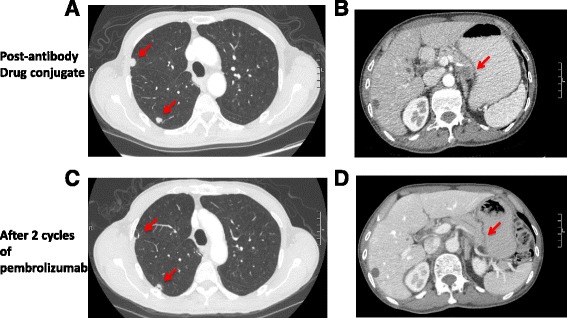
CT findings. a CT chest after 1 cycle of the antibody drug conjugate with new pulmonary nodules. b CT abdomen after 1 cycle of an experimental antibody drug conjugate against a neuroendocrine cell surface marker with a new pancreatic lesion. c Improvement in lung nodules after 2 cycles of pembrolizumab. d Resolution of the pancreatic lesion after 2 cycles of pembrolizumab
Conclusion and Discussion
LCNECs have been found in many organs, including the lung, stomach, uterus, ovary, and bladder [11–14]. Genomic profiling using a hybrid capture based assay revealed multiple alterations including CD274 (PD-L1) amplification, MYC amplification, TP53 mutation, RB1 loss, and mutations in the APC and STK11 (LKB) genes. On retrospective research review, there was loss of heterozygosity (LOH) at the STK11 locus in addition to the mutation identified, thus conferring likely a complete loss of function of STK11. TMB was high averaging 24.76 mutations per megabases. Surprisingly, PD-L1 protein expression was negative by immunohistochemistry. Genomic profiling suggested that the patient’s tumor more closely resembles the small cell type with high mutation burden, TP53 and RB1 loss, and MYC amplification. However, the presence of STK11 mutation in this patient’s tumor is more commonly associated in LCNECs that resemble NSCLC and differs from a recent genomic profiling report of 300 LCNEC where RB1 alterations was mutually exclusive to STK11 mutations (p < 0.001), reflecting the underlying heterogeneity and possible polyclonality of this patient’s disease [6].
PD-L1 amplification, protein levels, TMB, and STK11 mutations have all been implicated as biomarkers predictive of response to checkpoint inhibitors. While high PD-L1 amplification, protein levels, and TMB are generally associated with responses to checkpoint inhibitors, STK11 mutations are associated with decreased efficacy, at least in NSCLC [15–17]. Of these, PD-L1 amplification has been reported in only 2–4% of lung cancer based on The Cancer Genome Atlas (www.cbioportal.org) and is usually concordant with PD-L1 protein expression, although this is not the case for our patient [18, 19]. Within the SCLC literature, the incidence of PD-L1 alteration is highly controversial. Mutation was only identified in 1.6% of SCLC in the COSMIC dataset, 0/11 large cell carcinomas, and 0/54 carcinoid-endocrine tumors of the lung. Others reported PD-L1protein detection in ~70–80% of the cases (cancer.sanger.ac.uk) [20–23]. One reason for this apparent discrepancy may be attributed to the often lack of large and high quality biopsy specimen for this disease.
Here we present an index case where the a patient with LCNEC demonstrated a robust response to checkpoint inhibitors after failing several prior lines of therapy, suggesting that immunotherapy is effective in this class of disease. Interestingly, this patient had a robust response to checkpoint inhibitors despite the tumor harboring homozygous loss of function mutations in STK11 and being PD-L1 protein expression negative by immunohistochemistry. This example underscores the lack of concordance amongst current biomarkers for predicting responses to checkpoint inhibitor therapies and the need to identify more robust biomarkers. It also suggests that LCNEC may have inherently different biology compared to SCLC and adenocarcinomas of the lung. Future studies should incorporate composite biomarkers to increase the predictive power.
Acknowledgements
Not applicable
Funding
VEW is a Damon Runyon fellow.
Availability of data and materials
All of the relevant data is presented within this manuscript. Frequency of PD-L1 mutations and amplifications in lung cancer are available in the TCGA and the COSMIC database (cbioportal.com and cancer.sanger.ac.uk).
Abbreviations
- LCNEC
Large cell neuroendocrine carcinoma
- NSCLC
Non-small cell lung cancer
- OS
overall survival
- PFS
Progression-free survival
- SCLC
Small cell lung cancer
- TMB
Tumor mutation burden
Authors’ contributions
VEW contributed to the conception, design, and writing. AU contributed to histopathological analysis and figure production. SA and VM contributed to the interpretation of data. RA and DJ were involved in the care of the patient. All authors were involved in revising the manuscript and have given final approval of the version to be published.
Ethics approval and consent to participate
This case report does not qualify as human research and did not require IRB approval.
Consent for publication
Written informed consent was obtained from the patient for publication of the details and accompanying images in this manuscript. The consent form is held in the patients’ clinical files and available for review by the Editor of this journal.
Competing interests
Lee Albacker, Siraj Ali, and Vincent Miller are employees of Foundation Medicine.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Victoria E. Wang, Phone: (617)935-1649, Email: victoria.wang@ucsf.edu
Anatoly Urisman, Email: Anatoly.urisman@ucsf.edu.
Lee Albacker, Email: lalbacker@foundationmedicine.com.
Siraj Ali, Email: sali@foundationmedicine.com.
Vincent Miller, Email: vmiller@foundationmedicine.com.
Rahul Aggarwal, Email: Rahul.aggarwal@ucsf.edu.
David Jablons, Email: David.jablons@ucsf.edu.
References
- 1.Naidoo J, et al. Large cell Neuroendocrine carcinoma of the lung: Clinico-pathologic features, treatment, and outcomes. Clinical lung cancer. 2016;17:e121–e129. doi: 10.1016/j.cllc.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veronesi G, et al. Large cell neuroendocrine carcinoma of the lung: a retrospective analysis of 144 surgical cases. Lung Cancer. 2006;53:111–115. doi: 10.1016/j.lungcan.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Le Treut J, et al. Multicentre phase II study of cisplatin-etoposide chemotherapy for advanced large-cell neuroendocrine lung carcinoma: the GFPC 0302 study. Ann Oncol : official J European Soc Med Oncol. 2013;24:1548–1552. doi: 10.1093/annonc/mdt009. [DOI] [PubMed] [Google Scholar]
- 4.Rekhtman N, et al. Next-generation sequencing of pulmonary large cell Neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin Cancer Res : an Official J Am Assoc Cancer Res. 2016;22:3618–3629. doi: 10.1158/1078-0432.CCR-15-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyoshi T, et al. Genomic profiling of large-cell Neuroendocrine carcinoma of the lung. Clin Cancer Res : an Official J Am Assoc Cancer Res. 2017;23:757–765. doi: 10.1158/1078-0432.CCR-16-0355. [DOI] [PubMed] [Google Scholar]
- 6.Chae YK, et al. Genomic alterations (GA) and tumor mutational burden (TMB) in large cell neuroendocrine carcinoma of lung (L-LCNEC) as compared to small cell lung carcinoma (SCLC) as assessed via comprehensive genomic profiling (CGP). J Clin Oncol 35, Abstr 8517; 2017.
- 7.Antonia SJ, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. The Lancet Oncology. 2016;17:883–895. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 8.Kratz JR, et al. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet. 2012;379:823–832. doi: 10.1016/S0140-6736(11)61941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kratz JR, Van den Eeden SK, He J, Jablons DM, Mann MJ. A prognostic assay to identify patients at high risk of mortality despite small, node-negative lung tumors. JAMA. 2012;308:1629–1631. doi: 10.1001/jama.2012.13551. [DOI] [PubMed] [Google Scholar]
- 10.Fossella F, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003;21:3016–3024. doi: 10.1200/JCO.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 11.Kamboj M, et al. Neuroendocrine carcinoma of gall bladder: a series of 19 cases with review of literature. J Gastrointestinal Cancer. 2015;46:356–364. doi: 10.1007/s12029-015-9745-9. [DOI] [PubMed] [Google Scholar]
- 12.Liu DJ, et al. Clinicopathological, treatment, and prognosis study of 43 gastric neuroendocrine carcinomas. World J Gastroenterol. 2017;23:516–524. doi: 10.3748/wjg.v23.i3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ki EY, Park JS, Lee KH, Bae SN, Hur SY. Large cell neuroendocrine carcinoma of the ovary: a case report and a brief review of the literature. World J Surg Oncol. 2014;12:314. doi: 10.1186/1477-7819-12-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vora M, Lacour RA, Black DR, Turbat-Herrera EA, Gu X. Neuroendocrine tumors in the ovary: histogenesis, pathologic differentiation, and clinical presentation. Arch Gynecol Obstet. 2016;293:659–665. doi: 10.1007/s00404-015-3865-0. [DOI] [PubMed] [Google Scholar]
- 15.Inoue Y, et al. Clinical significance of PD-L1 and PD-L2 copy number gains in non-small-cell lung cancer. Oncotarget. 2016;7:32113–32128. doi: 10.18632/oncotarget.8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizvi NA, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyama S, et al. STK11/LKB1 Deficiency promotes Neutrophil recruitment and Proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res. 2016;76:999–1008. doi: 10.1158/0008-5472.CAN-15-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George J, et al. Genomic amplification of CD274 (PD-L1) in small-cell lung cancer. Clin Cancer Res. 2017;23:1220–1226. doi: 10.1158/1078-0432.CCR-16-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda S, et al. PD-L1 is Upregulated by simultaneous amplification of the PD-L1 and JAK2 genes in non-small cell lung cancer. J Thoracic Oncol. 2016;11:62–71. doi: 10.1016/j.jtho.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Chang YL, Yang CY, Huang YL, Wu CT, Yang PC. High PD-L1 expression is associated with stage IV disease and poorer overall survival in 186 cases of small cell lung cancers. Oncotarget. 2017;8:18021–18030. doi: 10.18632/oncotarget.14935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schultheis AM, et al. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer. 2015;51:421–426. doi: 10.1016/j.ejca.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Ishii H, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thoracic Oncol. 2015;10:426–430. doi: 10.1097/JTO.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 23.Komiya T, Madan R. PD-L1 expression in small cell lung cancer. Eur J Cancer. 2015;51:1853–1855. doi: 10.1016/j.ejca.2015.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the relevant data is presented within this manuscript. Frequency of PD-L1 mutations and amplifications in lung cancer are available in the TCGA and the COSMIC database (cbioportal.com and cancer.sanger.ac.uk).


