Abstract
Modern pharmacological research has revealed that andrographolide has various functions, including anti-bacterial, anti-inflammatory and anti-viral effects, immunoregulation, treating cardiovascular and cerebrovascular diseases, and prevention and treatment of alcoholic liver injury. The present study investigated whether andrographolide suppresses the proliferation of human colon cancer cell through the Toll-like receptor 4 (TLR4)/nuclear factor (NF)-κB/matrix metalloproteinase-9 (MMP-9) signaling pathway. The MTT assay and lactate dehydrogenase assay were used to evaluate the anticancer effects of andrographolide on cell proliferation and cytotoxicity in human colon cancer SW620 cells. Flow cytometry was used to analyze the anticancer effects of andrographolide on apoptosis by Annexin V-fluorescein isothiocyanate/propidium iodide kit. The effects of andrographolide on the activity of caspase-3/9 were measured using ELISA. Western blot analysis was also used to analyze the protein expression of TLR4, myeloid differentiation primary response gene 88 (MyD88), NF-κB-p65 and MMP-9. In the present study, it was found that andrographolide suppressed the cell proliferation, augmented cytotoxicity, evoked cell apoptosis and activated caspase-3/9 activities in human colon cancer SW620 cells. The results revealed that the anti-proliferation effects of andrographolide on the SW620 cells was associated with the inhibition of TLR4, MyD88, NF-κB-p65 and MMP-9 signaling activation. The results suggest that andrographolide is a promising drug for treatment of human colon cancer via suppression of the TLR4/NF-κB/MMP-9 signaling pathway.
Keywords: andrographolide, human colon cancer, TLR4, NF-κB, MMP-9
Introduction
Colon cancer poses a significant risk to the health of individuals. In recent years, with changes in dietary patterns, the morbidity and mortality rates of colon cancer have significantly increased (1). In cancer progression, the invasion and metastasis of colon cancer markedly affects the prognosis of colon cancer patients and is a common leading cause of cancer-associated mortality (2). The metastasis and infiltration of cancer are associated with various factors. Proteases secreted by cancer, including matrix metalloproteinases, (MMPs) have important roles (3).
As a nuclear transcription factor with numerous regulatory functions, nuclear factor (NF)-κB exists in various cells in a dipolymer or heterodimer form (4). The p50/p65 form is the most common combination form (5). Various studies have shown that NF-κB participates in the transcription of genes regulating apoptosis and hyperplasia (6). It is well-known that apoptosis and hyperplasia are closely associated with the occurrence of cancer.
Toll-like receptor (TLR) is a type I transmembrane protein with >10 family members (7). As an important bridge between innate immune defense and acquired immunity, it can trigger signal transduction, which further leads to the release of inflammatory mediators by identifying pathogen-associated molecular patterns and certain endogenous ligands (8). A previous study found that TLR may play certain roles in pathogenesis and bio-immunotherapy (9).
Andrographolide is a diterpene lactone extracted from Andrographis paniculata and is also present in Jacobinia suberecta. The molecular formula of andrographolide is C20H30O5 and it is one of the active components of A. paniculata extract, comprising up to 1.84% of the extract (10,11). Andrographolide has been produced as a raw material with antipyretic and analgesic effects (12). Previous studies have suggested that andrographolide has various functions, including anti-bacterial, anti-inflammatory and anti-viral effects, immunoregulation, treating cardiovascular and cerebrovascular diseases, and prevention and treatment of alcoholic liver injury (13,14). The present study investigated the effects of andrographolide on the proliferation of human colon cancer cell through the TLR4/NF-κB/MMP-9 signaling pathway.
Materials and methods
Cell line and culture conditions
The human colon cancer SW620 cell line was procured from Shanghai Institute of Cell Resource Center of Life Science (Shanghai, China) and cultured with Dulbecco's modified Eagle's medium (DMEM; Mediatech, Inc., Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS; Mediatech, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a 5% CO2 atmosphere.
Cell viability assay and cytotoxicity
SW620 cells were inoculated onto 96-well plates at a density of 1×104 cells per well and treated with dimethyl sulfoxide (DMSO; Merck KGaA, Darmstadt, Germany) or 0, 5, 10, 15, 20 or 25 µM andrographolide (Sigma-Aldrich; Merck KGaA) for 12, 24 and 48 h. Subsequent to processing, the supernatant was assessed using an MTT assay (Beyotime Institute of Biotechnology, Haimen, China) and cultured for 4 h. The supernatant was discarded and DMSO (0.5 mg/ml) was added to each well for 20 min at 37°C. The cytotoxicity of andrographolide in SW620 cells was measured using Triton X-100 (1%) and a detergent, leading to a complete release of the LDH enzyme (Sigma-Aldrich; Merck KGaA). The absorbance values were determined using a DTX 880 Multimode Detector (Beckman Coulter, Inc., Brea, CA, USA) at 550 nm.
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) assay for cell apoptosis examination
SW620 cells were inoculated onto 96-well plates at a density of 2×106 cells per well and treated with DMSO or 20 µM andrographolide for 24 h. The supernatant of the cell culture was collected and incubated with Annexin V-FITC (BD Biosciences, San Jose, CA, USA) for 20 min in the dark. PI (BD Biosciences) was added into every well and the wells were incubated for 20 min in the dark. Apoptosis was detected using flow cytometry using a FACSCalibur flow cytometer (BD Biosciences).
Activity of caspase-3/9
SW620 cells were inoculated onto 96-well plates at a density of 2×106 cells per well, and the cells were treated with DMSO or 20 µM andrographolide for 24 h. The supernatant of the cell culture was collected and incubated with Ac-DEVD-pNA or Ac-LEHD-pNA for 4 h to assess the activity of caspase-3 and caspase-9, respectively. The activity of caspase-3/9 was detected using a DTX 880 Multimode Detector (Beckman Coulter) at 405 nm.
Western blot analysis
SW620 cells were inoculated onto 96-well plates at a density of 1×104 cells per well and treated with DMSO or 20 µM andrographolide for 24 h. The supernatant of the cell culture was collected and harvested with lysis buffer. Bradford assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to determine the protein content. Protein (50 µg) from each sample was separated on a 10–12% SDS-PAGE gel (Beyotime Institute of Biotechnology) and transferred to a polyvinylidene fluoride membrane (Bio-Rad Laboratories, Inc.). Membranes were blocked with Tris-buffered saline with Tween-20 containing 5% non-fat milk and incubated with rabbit monoclonal anti-TLR4 (cat. no. sc-10741; dilution, 1:400; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-myeloid differentiation primary response gene 88 (cat. no. sc-11356, MyD88; dilution, 1:400; Santa Cruz Biotechnology, Inc.), anti-NF-κB-p65 (cat. no. sc-109; dilution, 1:400; Santa Cruz Biotechnology, Inc.), anti-MMP-9 (cat. no. sc-10737; dilution, 1:400; Santa Cruz Biotechnology, Inc.) and β-actin (cat. no. sc-7210; dilution, 1:2,000; Santa Cruz Biotechnology, Inc.) overnight at 4°C. Membranes were incubated with horseradish peroxidase-conjugated secondary antibody (cat. no. sc-2357; dilution, 1:3,000; Bio-Rad Laboratories, Inc.) at 37 °C for 1 h and detected using a BeyoECL Star enhanced chemiluminescence kit (Beyotime Institute of Biotechnology).
Statistical analysis
The data were presented as the mean ± standard deviation and analyzed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance was used for multi-group comparisons with a Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Andrographolide suppresses cell proliferation of SW620 cells
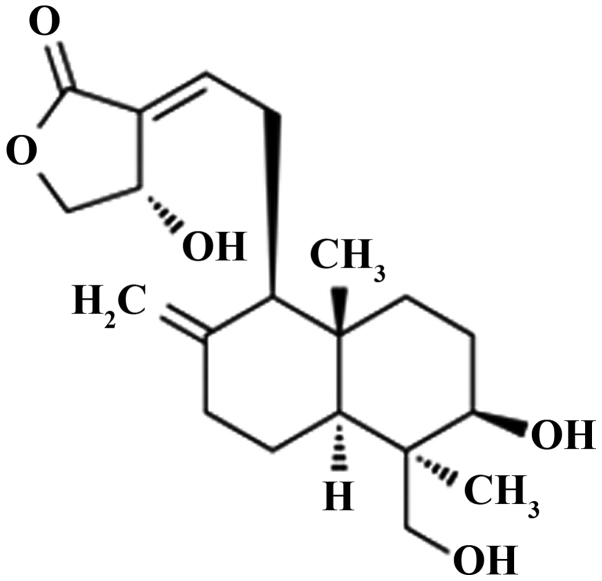
The chemical structure of andrographolide is shown in Fig. 1. To examine the anticancer effect of andrographolide on SW620 cells, the cells were treated revealed with DMSO or 0, 5, 10, 15, 20 and 25 µM andrographolide for 24 h. As shown in Fig. 2, andrographolide could suppress the cell proliferation of SW620 cell in dose-dependent manner compared to the controls or DMSO group. In particular, 15, 20 and 25 µM andrographolide significantly inhibited the proliferation of SW620 cells compared to the control or DMSO groups.
Figure 1.
The chemical structure of andrographolide.
Figure 2.
Andrographolide suppresses cell proliferation of human colon cancer SW620 cells. Andrographolide suppresses cell proliferation at (A) 12, 24 and 48 h time-points at an andrographolide concentration of 20 µM and (B) increasing concentrations of andrographolide at 24 h in SW620 cells. **P<0.001 compared with the control group. And, andrographolide; DMSO, dimethyl sulfoxide.
Andrographolide promotes cytotoxicity of SW620 cells
In the present study, the cytotoxic effect of andrographolide on human colon cancer SW620 cells was analyzed. As shown in Fig. 3, 15, 20 and 25 µM andrographolide significantly enhanced the cytotoxicity of SW620 cells compared with the control or DMSO group.
Figure 3.
Andrographolide is cytotoxic in SW620 cells. **P<0.001 compared with the control group. And, andrographolide; DMSO, dimethyl sulfoxide.
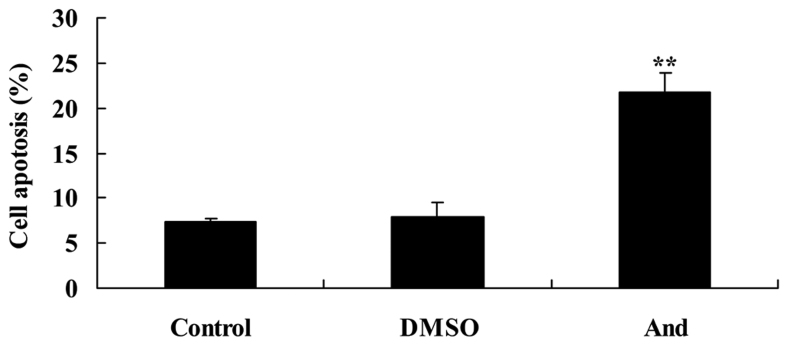
Andrographolide induces apoptosis of SW620 cells
For flow cytometry, SW620 cells treated with 20 µM andrographolide were stained with Annexin V-FITC/PI and evaluated for the apoptosis rate. As shown in Fig. 4, 20 µM andrographolide significantly induces the apoptosis rate of SW620 cells compared with the control or DMSO groups.
Figure 4.
Andrographolide induces apoptosis of SW620 cells. **P<0.001 compared with the control group. And, andrographolide; DMSO, dimethyl sulfoxide.
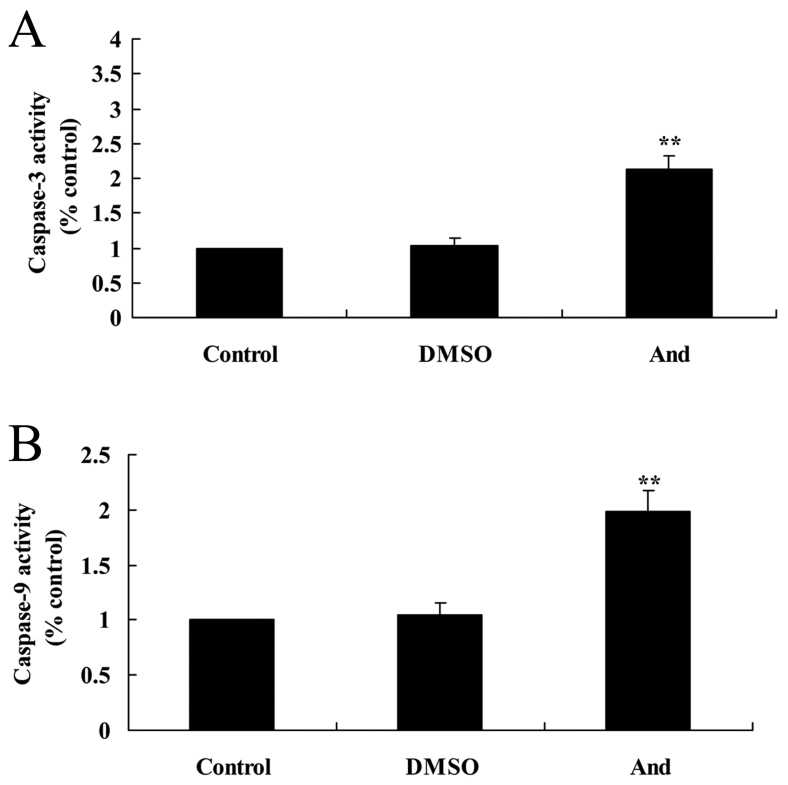
Andrographolide induces caspase-3/9 activities of SW620 cells
The anticancer effect of andrographolide on SW620 cells was demonstrated by caspase-3/9 activity. As shown in Fig. 5, 20 µM andrographolide significantly increased the activity of caspase-3/9 in SW620 cells compared with the control and DMSO groups.
Figure 5.
Andrographolide induces caspase-3/9 activity in SW620 cells. Andrographolide induces (A) caspase-3 and (B) caspase-9 activity in human colon cancer SW620 cells. **P<0.001 compared with control group. And, andrographolide; DMSO, dimethyl sulfoxide.
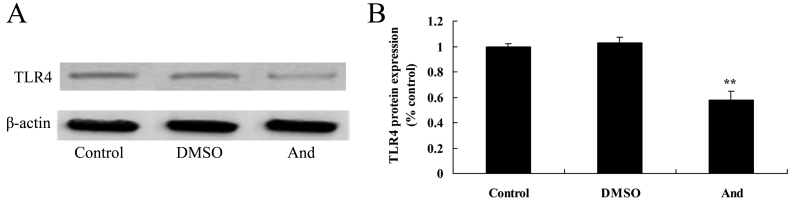
Andrographolide induces the protein expression of TLR4 in SW620 cells
Western blot analysis was used to detect TLR4 protein expression in SW620 cells (Fig. 6). As compared with the control or DMSO groups, the protein expression of TLR4 in SW620 cells was significantly suppressed by treatment with 20 µM andrographolide.
Figure 6.
Andrographolide induces the protein expression of TLR4 in SW620 cells, as shown by (A) western blot analysis and (B) quantification of TLR4 protein expression in SW620 cells. **P<0.001 compared with control group. And, andrographolide; DMSO, dimethyl sulfoxide; TLR4, Toll-like receptor 4.
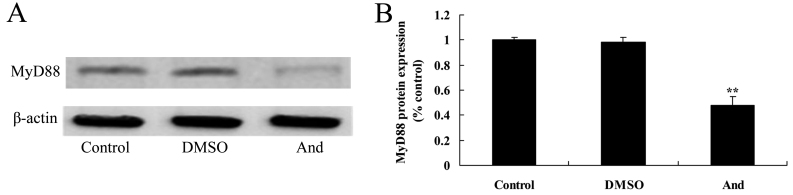
Andrographolide induces the protein expression of MyD88 SW620 cells
In order to explore the effect of andrographolide on MyD88 protein expression in SW620 cells, western blot analysis was performed to detect whether MyD88 participates in the anticancer effect of andrographolide. The results showed that treatment with andrographolide significantly inhibited MyD88 protein expression in SW620 cells compared to the control or DMSO groups (Fig. 7).
Figure 7.
Andrographolide induces the protein expression of MyD88 in SW620 cells. The protein expression of MyD88 was determined using (A) western blot analysis and (B) quantification. **P<0.001 compared with control group. MyD88, myeloid differentiation primary response gene 88; DMSO, dimethyl sulfoxide; And, andrographolide.
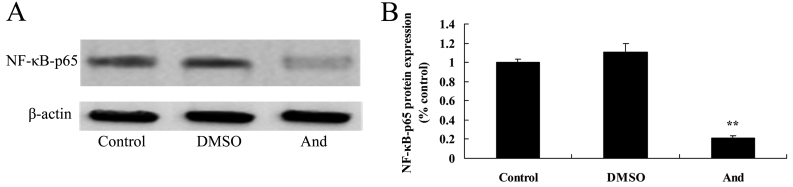
Andrographolide induces the protein expression of NF-κB-p65 in SW620 cells
To research the mechanism of andrographolide on human colon cancer, NF-κB-p65 was examined using western blot analysis, as shown in Fig. 8. Treatment with 20 µM andrographolide significantly attenuated the protein expression of NF-κB-p65 in SW620 cells compared to the control or DMSO groups.
Figure 8.
Andrographolide induces the protein expression of NF-κB-p65 in SW620 cells. The protein expression of NF-κB-p65 was determined using (A) western blot analysis and (B) quantification. **P<0.001 compared with control group. NF-κB, nuclear factor-κB; DMSO, dimethyl sulfoxide; And, andrographolide.
Andrographolide induces MMP-9 protein expression in SW620 cells
To further investigate the mechanism of andrographolide in human colon cancer cells, the protein expression of MMP-9 was investigated using western blot analysis. As shown in Fig. 9, 20 µM andrographolide treatment significantly reduced MMP-9 protein expression in SW620 cells compared to the control and DMSO groups.
Figure 9.
Andrographolide induces the protein expression of MMP-9 in SW620 cells. The protein expression of MMP-9 was determined using (A) western blot analysis and (B) quantification. **P<0.001 compared with control group. MMP-9, matrix metalloproteinase-9; DMSO, dimethyl sulfoxide; And, andrographolide.
Discussion
Colon cancer is a common malignant cancer with increasing morbidity and mortality rates (15). During the occurrence and progression of colon cancer, the cellular mechanisms involved change continuously. Metastasis and infiltration refers to the direct and discontinuous process as well as the growth at other regions, including separation, invasion, recurrence, adhesion and growth (16). The metastasis and invasion of malignant cancers is complicated and polytropic, and is regulated by numerous genes. Different cancers have specific biological features (17). However, the degradation of extra-cellular matrix and basement membrane is prerequisite of infiltration and metastasis of cancers. Therefore, certain studies showed that andrographolide sensitizes the cytotoxicity of human colorectal carcinoma cells (12), prevents human breast cancer (18) and suppressed cell growth of A549 non-small cell lung cancer (19). The present study demonstrates that andrographolide suppresses proliferation, induces apoptosis and increases the caspase-3/9 activity of SW620 cells. Therefore, andrographolide may be a new drug for the treatment of human colon cancer.
A previous study investigating colon cancer and laryngeal carcinoma suggested that the expression of TLR2 or TLR4 in cancer cells or infiltrating inflammatory cells are likely to be closely associated with immune escape of cancer (20). Continuous activation of TLR2 and TLR4 signal pathways is considered to be an important factor in the progression of malignant cancers from chronic inflammation (21). In the present study, treatment with andrographolide significantly suppressed TLR4 and MyD88 protein expressions in SW620 cells. Zhang et al also showed that andrographolide suppresses the tumor growth of insulinoma through the TLR4/MyD88/NF-κB signaling pathway (22).
NF-κB, which locates to the cytoplasm, is a transcription factor that is most common in its p50/p65 form. Subsequent to combining with inhibitor of κB (IκB-α), NF-κB locates to the cytoplasm. Under the activity of IκB kinase, IκB-α can be phosphorylated and separated from NF-κB (23). NF-κB combines with promoters of target genes and induces the synthesis of mRNA. This participates in the immune response to infection and inflammation, and immunoregulation (24). A recent study suggested that NF-κB has is associated with the occurrence, infiltration and metastasis of cancers, such as apoptosis, cell cycle control and differentiation (25). In the present study, treatment with 20 µM andrographolide significantly attenuated the protein expression of NF-κB-p65 in SW620 cells. Peng et al also reported that andrographolide suppresses NF-κB expression in nasopharyngeal carcinoma cells (26).
The MMP family consists of enzymes that can break down the extra-cellular matrix. MMP-9 is produced by neutrophil granulocytes, macrophages, malignant cells and capillary endothelial cells (27). MMP-9 can promote cancer cells to have infiltration invasion through the degradation and destruction of the basilar membrane or new vessels to enhance cancer growth and dissemination (28). The present study found that treatment with andrographolide significantly reduced MMP-9 protein expression in SW620 cells. Luo et al (29) also suggested that andrographolide inhibits MMP-9 and NF-κB activity in lung cancer H3255 cells.
In conclusion, the present study demonstrated that andrographolide suppresses proliferation and induces apoptosis of SW620 cells through suppression of the TLR4/MyD88/NF-κB/MMP-9 signaling pathway. It is therefore suggested that andrographolide may be a promising drug candidate for treatment of human colon cancer.
Acknowledgements
The present study was supported by Science and Technology Key Project of Liaoning Province, China (grant no. 2012225016).
References
- 1.Mathis KL, Green EM, Sargent DJ, Delaney C, Simmang CL, Nelson H. Surgical quality surrogates do not predict colon cancer survival in the setting of technical credentialing: A report from the prospective COST trial. Ann Surg. 2013;257:102–107. doi: 10.1097/SLA.0b013e318260a8e6. [DOI] [PubMed] [Google Scholar]
- 2.Kim SK, Kim SY, Kim JH, Roh SA, Cho DH, Kim YS, Kim JC. A nineteen gene-based risk score classifier predicts prognosis of colorectal cancer patients. Mol Oncol. 2014;8:1653–1666. doi: 10.1016/j.molonc.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang TH, Chiu YH, Chan YL, Chiu YH, Wang H, Huang KC, Li TL, Hsu KH, Wu CJ. Prophylactic administration of fucoidan represses cancer metastasis by inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in Lewis tumor-bearing mice. Mar Drugs. 2015;13:1882–1900. doi: 10.3390/md13041882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miao Z, Zhao T, Wang Z, Xu Y, Song Y, Wu J, Xu H. CARMA3 is overexpressed in colon cancer and regulates NF-κB activity and cyclin D1 expression. Biochem Biophys Res Commun. 2012;425:781–787. doi: 10.1016/j.bbrc.2012.07.152. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka S, Toki T, Yokoyama M, Shimizu H, Yamasaki T, Yoneda Y, Muro F, Yasukochi T, Iimura S, Morishita K. A novel inhibitor of I-kappaB kinase beta ameliorates experimental arthritis through downregulation of proinflammatory cytokines in arthritic joints. Biol Pharm Bull. 2014;37:87–95. doi: 10.1248/bpb.b13-00628. [DOI] [PubMed] [Google Scholar]
- 6.Miller JA, Kirkley KA, Padmanabhan R, Liang LP, Raol YH, Patel M, Bialecki RA, Tjalkens RB. Repeated exposure to low doses of kainic acid activates nuclear factor kappa B (NF-κB) prior to seizure in transgenic NF-κB/EGFP reporter mice. Neurotoxicology. 2014;44:39–47. doi: 10.1016/j.neuro.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin SC, Liao EC, Chiu CL, Chang CY, Tsai JJ. Der p2 internalization by epithelium synergistically augments toll-like receptor-mediated proinflammatory signaling. Allergy Asthma Immunol Res. 2015;7:393–403. doi: 10.4168/aair.2015.7.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ojha D, Mukherjee H, Mondal S, Jena A, Dwivedi VP, Mondal KC, Malhotra B, Samanta A, Chattopadhyay D. Anti-inflammatory activity of Odina wodier Roxb, an Indian folk remedy, through inhibition of toll-like receptor 4 signaling pathway. PLoS One. 2014;9:e104939. doi: 10.1371/journal.pone.0104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charrier E, Cordeiro P, Brito RM, Harnois M, Mezziani S, Herblot S, Le Deist F, Duval M. Impaired interferon-alpha production by plasmacytoid dendritic cells after cord blood transplantation in children: Implication for post-transplantation toll-like receptor ligand-based immunotherapy. Biol Blood Marrow Transplant. 2014;20:1501–1507. doi: 10.1016/j.bbmt.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Aromdee C. Andrographolide: Progression in its modifications and applications-a patent review (2012–2014) Expert Opin Ther Pat. 2014;24:1129–1138. doi: 10.1517/13543776.2014.956084. [DOI] [PubMed] [Google Scholar]
- 11.Bera R, Ahmed SK, Sarkar L, Sen T, Karmakar S. Pharmacokinetic analysis and tissue distribution of andrographolide in rat by a validated LC-MS/MS method. Pharm Biol. 2014;52:321–329. doi: 10.3109/13880209.2013.836544. [DOI] [PubMed] [Google Scholar]
- 12.Lin HH, Shi MD, Tseng HC, Chen JH. Andrographolide sensitizes the cytotoxicity of human colorectal carcinoma cells toward cisplatin via enhancing apoptosis pathways in vitro and in vivo. Toxicol Sci. 2014;139:108–120. doi: 10.1093/toxsci/kfu032. [DOI] [PubMed] [Google Scholar]
- 13.Yang T, Sheng HH, Feng NP, Wei H, Wang ZT, Wang CH. Preparation of andrographolide-loaded solid lipid nanoparticles and their in vitro and in vivo evaluations: Characteristics, release, absorption, transports, pharmacokinetics and antihyperlipidemic activity. J Pharm Sci. 2013;102:4414–4425. doi: 10.1002/jps.23758. [DOI] [PubMed] [Google Scholar]
- 14.Roy DN, Sen G, Chowdhury KD, Biswas T. Combination therapy with andrographolide and d-penicillamine enhanced therapeutic advantage over monotherapy with d-penicillamine in attenuating fibrogenic response and cell death in the periportal zone of liver in rats during copper toxicosis. Toxicol Appl Pharmacol. 2011;250:54–68. doi: 10.1016/j.taap.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Dong M, Yang G, Liu H, Liu X, Lin S, Sun D, Wang Y. Aged black garlic extract inhibits HT29 colon cancer cell growth via the PI3K/Akt signaling pathway. Biomed Rep. 2014;2:250–254. doi: 10.3892/br.2014.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y, Ge H, Li S. Haematoporphyrin based photodynamic therapy combined with hyperthermia provided effective therapeutic vaccine effect against colon cancer growth in mice. Int J Med Sci. 2012;9:627–633. doi: 10.7150/ijms.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: Anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110:469–478. doi: 10.1038/bjc.2013.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhai Z, Qu X, Yan W, Li H, Liu G, Liu X, Tang T, Qin A, Dai K. Andrographolide prevents human breast cancer-induced osteoclastic bone loss via attenuated RANKL signaling. Breast Cancer Res Treat. 2014;144:33–45. doi: 10.1007/s10549-014-2844-7. [DOI] [PubMed] [Google Scholar]
- 19.Lim JC, Jeyaraj EJ, Sagineedu SR, Wong WS, Stanslas J. SRS06, a new semisynthetic andrographolide derivative with improved anticancer potency and selectivity, inhibits nuclear factor-kappaB nuclear binding in the A549 non-small cell lung cancer cell line. Pharmacology. 2015;95:70–77. doi: 10.1159/000370313. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Qian D, Ju F, Wang B. Upregulation of Toll-like receptor 2 expression in colorectal cancer infected by human cytomegalovirus. Oncol Lett. 2015;9:365–370. doi: 10.3892/ol.2014.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng J, Guo C, Zhu Y, Pang L, Yang Z, Zou Y, Zheng X. Baicalin down regulates the expression of TLR4 and NFkB-p65 in colon tissue in mice with colitis induced by dextran sulfate sodium. Int J Clin Exp Med. 2014;7:4063–4072. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang QQ, Zhou DL, Ding Y, Liu HY, Lei Y, Fang HY, Gu QL, He XD, Qi CL, Yang Y, et al. Andrographolide inhibits melanoma tumor growth by inactivating the TLR4/NF-κB signaling pathway. Melanoma Res. 2014;24:545–555. doi: 10.1097/CMR.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 23.Lee DH, Nam YJ, Lee CS. Quercetin-3-O-(2′'-galloyl)-α-L-rhamnopyranoside attenuates cholesterol oxidation product-induced apoptosis by suppressing NF-κB-mediated cell death process in differentiated PC12 cells. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:869–881. doi: 10.1007/s00210-015-1120-7. [DOI] [PubMed] [Google Scholar]
- 24.Chen D, Su A, Fu Y, Wang X, Lv X, Xu W, Xu S, Wang H, Wu Z. Harmine blocks herpes simplex virus infection through downregulating cellular NF-κB and MAPK pathways induced by oxidative stress. Antiviral Res. 2015;123:27–38. doi: 10.1016/j.antiviral.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Lv Z, Li C, Zhang W, Jin C, Shao Y, Xuemei D, Qingxi H. Nemo like kinase negatively regulates NF-κB activation and coelomocytes apoptosis in Apostichopus japonicus. Dev Comp Immunol. 2016;54:109–115. doi: 10.1016/j.dci.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Peng T, Hu M, Wu TT, Zhang C, Chen Z, Huang S, Zhou XH. Andrographolide suppresses proliferation of nasopharyngeal carcinoma cells via attenuating NF-κB pathway. Biomed Res Int. 2015;2015:735056. doi: 10.1155/2015/735056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura R, Kuwabara H, Yoneda M, Yoshihara S, Ishikawa T, Miura T, Nozaka H, Nanashima N, Sato T, Nakamura T. Suppression of matrix metalloproteinase-9 by 4-methylumbelliferone. Cell Biol Int. 2007;31:1022–1026. doi: 10.1016/j.cellbi.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Sun R, Tao K, Wang G. The CCL21/CCR7 pathway plays a key role in human colon cancer metastasis through regulation of matrix metalloproteinase-9. Dig Liver Dis. 2011;43:40–47. doi: 10.1016/j.dld.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Luo W, Liu Y, Zhang J, Luo X, Lin C, Guo J. Andrographolide inhibits the activation of NF-κB and MMP-9 activity in H3255 lung cancer cells. Exp Ther Med. 2013;6:743–746. doi: 10.3892/etm.2013.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]