Abstract
Immune reconstitution inflammatory syndrome can present as a paradoxical reaction after initiation of antiretroviral treatment in patients with severe immunosuppression and underlying infections. Immune reconstitution inflammatory syndrome has often been associated with mycobacteria, and the clinical response to traditional treatment with corticosteroids is not always satisfactory. Consequently, administration of an infliximab biosimilar could lead to an improvement in the clinical status of these patients.
Keywords: HIV, infliximab biosimilar, IRIS, mycobacteria
Immune reconstitution inflammatory syndrome (IRIS) is one of the most stressful situations for both clinicians and patients with human immunodeficiency virus (HIV) infection and pre-existing underlying infections who have initiated antiretroviral therapy (ART) [1].
Immune reconstitution inflammatory syndrome has been associated with antigenic burden, host genetic susceptibility, and the degree of immune restoration during the first months of therapy (especially in severely immunosuppressed patients) [2]. In HIV-infected patients, this paradoxical disorder takes the form of an exaggerated immune response to pathogen-specific antigens of pre-existing treated or occult infections that overlaps with an inflammatory response. Although not yet fully understood, the inflammatory response is mediated by high levels of inflammatory cytokines, including interferon gamma and tumor necrosis factor alpha (TNF-α) [3].
Mycobacteria are the
pathogens most frequently associated with IRIS, especially in low-income countries [4] and countries where HIV infection is often diagnosed late. Clinical management of IRIS depends on presentation and disease severity. Life-threatening presentations require treatment with systemic corticosteroids, although clinical response is not always as satisfactory as expected [5].
Infliximab, a monoclonal antibody that binds to TNF-α and inhibits its functional activity, is commonly used for the treatment of rheumatic diseases such as rheumatoid/psoriatic arthritis [6], ankylosing spondylitis, and inflammatory bowel disease. It recently proved successful in 3 HIV-infected patients with mycobacterial IRIS [7].
In recent years, the death of patents has led to research into and development of biosimilar agents [8]. Infliximab biosimilars, such as CT-P13, have been used for the treatment of rheumatic disease and inflammatory bowel disease, with clinical response rates, safety profiles, and pharmacokinetic outcomes that are similar to those of conventional infliximab [9, 10]. Little information is available on the use of infliximab biosimilars to treat mycobacterial IRIS in HIV-infected persons. We report on 2 HIV-infected patients with severe IRIS and an unsatisfactory response to corticosteroids who were treated with an infliximab biosimilar.
CASE REPORTS
Patient 1 was a 33-year-old African American male who was diagnosed with HIV-1 infection 3 months before admission to hospital because of high fever, weight loss, asthenia, and large and painful cervical and axillary lymphadenopathies. His initial CD4+ T-cell count was 101/mm3, and his HIV-1 viral load was 244 800 copies/mL. Computed tomography (CT) showed a miliary pattern throughout the lungs, with enlarged cervical and axillary lymph nodes and possible splenic involvement, suggesting a diagnosis of disseminated tuberculosis (TB). Fine-needle aspiration of a cervical lymph node revealed acid-fast bacilli, and culture confirmed the presence of drug-sensitive Mycobacterium tuberculosis. Therapy was started with 300 mg of isoniazid, 600 mg of rifampicin, 1500 mg of pyrazinamide, and 1200 mg of ethambutol. The fever resolved several days later, and the patient’s clinical condition improved.
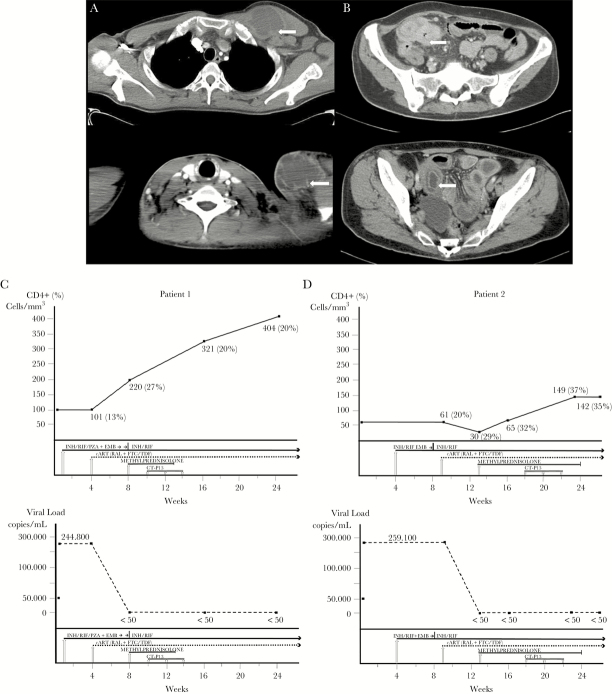
After 3 weeks of anti-TB therapy, the patient initiated 400 mg of raltegravir twice daily and coformulated emtricitabine 200 mg and tenofovir 300 mg daily. Four weeks later, the patient came to our unit with fever and enlarged, rubbery, and painful cervical and axillary lymph nodes (Figure 1A). The CD4+ T-cell count at the time was 220/mm3 and the HIV-1 viral load was less than 50 copies/mL. Therapy with naproxen and methylprednisolone 60 mg daily was initiated, although no clinical improvement was observed in the following 2 weeks. Cervical and axillary lymph nodes were drained 1–2 times weekly. Microbiological studies were performed to rule out other possible pathogens. The results were negative except for M tuberculosis. In the absence of a clinical response, the patient was given an infliximab biosimilar (CT-P13) at a dose of 5 mg/kg every 2 weeks (total of 3 infusions). During the third infusion, he experienced an anaphylactic reaction with angioedema and hypotension despite premedication (hydrocortisone 200 mg and paracetamol 1 g), although this resolved with 0.5 mg of adrenaline, 200 mg of hydrocortisone, and 1000 mL infused saline solution. In the weeks that followed, the fever resolved and the lymph nodes decreased considerably in size until they were no longer palpable. The CD4+ T-cell count and viral load over time are shown in Figure 1C.
Figure 1.
Computed tomography image showing a large supraclavicular lymphadenopathy and axillary lymphadenopathy in Patient 1 (A) and partial intestinal obstruction due to significant bowel edema in Patient 2 (B). CD4+ T-cell count and viral load in Patient 1 (C) and Patient 2 (D). Abbreviations: cART, combination antiretroviral therapy; CT-P13, infliximab biosimilar; EMB, ethambutol; FTC/TDF, emtricitabine/tenofovir; INH, isoniazid; PZA, pyrazinamide; RAL, raltegravir; RIF, rifampicin.
Patient 2 was a 44-year-old white woman who was diagnosed with HIV-1 infection in 2003 but who had not taken ART in the last 2 years (personal decision). In 2015, she was admitted to our hospital with a long history of fever and diarrhea. The initial CD4+ T-cell count was 61/mm3 (20% CD4+), and HIV-1 viral load was 259 100 copies/mL. The abdominal CT scan revealed pancolitis, and analysis of the biopsy specimen taken during colonoscopy revealed granulomas with acid-fast bacilli. Treatment was initiated with 300 mg of isoniazid, 600 mg of rifampicin, 1500 mg of pyrazinamide, and 1200 mg of ethambutol. The fever resolved 1 week later, and the diarrhea improved. Several weeks later, the microbiologist reported that culture of a colon biopsy sample yielded Mycobacterium species. The microorganism was confirmed as Mycobacterium bovis by nucleic acid amplification techniques, and pyrazinamide was withdrawn.
After 5 weeks of antimycobacterial therapy, treatment was started with 400 mg of raltegravir twice daily and coformulated emtricitabine 200 mg and tenofovir 300 mg daily. The patient came to our unit 4 weeks later with abdominal pain and clinical symptoms indicating partial obstruction of the intestine that required hospitalization (Figure 1B). The CD4+ T-cell count at the time was 30 cells/mm3 (30% CD4+), and HIV-1 viral load was <50 copies/mL. The patient was given 40 mg of methylprednisolone, and the episode resolved. While the dose of corticosteroids was being tapered over the following weeks, the patient had to be admitted on several occasions because of partial obstruction of the intestine. Given the impossibility of reducing corticosteroids owing to worsening of the patient’s condition and after ruling out other possible pathogens with additional microbiological studies, treatment with an infliximab biosimilar (CT-P13) was started at 5 mg/kg, every 2 weeks (total of 3 infusions). The patient’s clinical condition gradually improved. Laboratory values returned to normal, with no symptoms suggestive of relapse of mycobacterial disease. Methylprednisolone was tapered without problems until it was withdrawn 6 weeks after the first dose of infliximab biosimilar. No adverse events related to the infliximab biosimilar were recorded. The progress of the CD4+ T-cell count and HIV-1 viral load over time is shown in Figure 1D.
DISCUSSION
Mycobacterial IRIS remains one of the many clinical challenges facing the initiation of ART in patients diagnosed late, especially among those with a CD4+ T-cell count below 200/mm3. The pooled estimated incidence of TB-associated IRIS in HIV-infected patients initiating ART was reported to be 18% in a meta-analysis of 40 studies and 1048 cases, with a mortality rate of 2% attributed directly to TB-IRIS and a morbidity rate that required hospitalization in up to 25% of cases. The preferred treatment was corticosteroids in 38% of cases and nonsteroidal anti-inflammatory drugs in 28% [11]. The morbidity of IRIS has been reduced by early administration of corticosteroids [12], which mainly led to a reduction in levels of various cytokines, including TNF-α.
Treatment for the more severe and life-threatening clinical forms of IRIS has not undergone any innovative changes in recent decades beyond the use of corticosteroids, although the response to conventional high-dose therapy does not always generate the desired results, thus making clinical management challenging [13].
Tumor necrosis factor-α plays an important role in mycobacterial infection; its deficiency is associated with a higher incidence of TB infection, although excessive production of TNF by CD4+ T cells after mycobacterial antigen stimulation produces a more exaggerated inflammatory response that may result in mycobacterial IRIS [14]. In this context, infliximab has been used in non-HIV patients with TB and severe paradoxical reaction of the central nervous system and lymph nodes [15]. Favorable results were recently reported in 3 cases of mycobacterial IRIS in HIV-infected patients [7].
We decided to start early treatment with an infliximab biosimilar in 2 patients because the response to corticosteroids was not what we expected, ie, no clinical improvement despite aggressive drainage of lymph nodes in one case and worsening of clinical status after tapering corticosteroids in the other case. We also based our decision on available data on use of infliximab in HIV-infected patients with TB-IRIS, which showed promising results for the clinical response, even with no reduction in inflammatory marker values.
Although our experience is limited (2 patients), it is consistent with that reported by other authors, namely, progressive clinical improvement of mycobacterial IRIS that enabled us to reduce the dose of corticosteroids or withdraw the drugs altogether. Furthermore, during treatment with the infliximab biosimilar, HIV-1 viral load remained undetectable (<50 copies/mL), and CD4+ T-cell counts increased over time.
It is noteworthy that one of the patients in the present report experienced an anaphylactic reaction, with potentially relevant clinical implications. This obliges us to weigh up the risks and benefits of infliximab biosimilars based on the specific clinical situation of each patient, because short-term use of these drugs may be associated with severe adverse effects that are not present with corticosteroids or nonsteroidal anti-inflammatory drugs. They may even carry a greater risk of predisposition to certain tumors [9]. The use of inexpensive anti-TNF biosimilars could promote the development of clinical trials that objectively verify the validity and safety of such treatments while simultaneously enabling greater accessibility to treatment of corticosteroid-refractory mycobacterial IRIS in less-developed regions.
CONCLUSIONS
Despite the paucity of evidence from case reports, both infliximab and biosimilars could be a promising alternative for patients with severe corticosteroid-refractory mycobacterial IRIS. Further research with clinical trials and observational cohort studies should be carried out to confirm these promising results.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis 2009; 48:101–7. [DOI] [PubMed] [Google Scholar]
- 2. Breton G, Duval X, Estellat C et al. Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin Infect Dis 2004; 39:1709–12. [DOI] [PubMed] [Google Scholar]
- 3. Sereti I, Rodger AJ, French MA. Biomarkers in immune reconstitution inflammatory syndrome: signals from pathogenesis. Curr Opin HIV AIDS 2010; 5:504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bell LC, Breen R, Miller RF et al. Paradoxical reactions and immune reconstitution inflammatory syndrome in tuberculosis. Int J Infect Dis 2015; 32:39–45. [DOI] [PubMed] [Google Scholar]
- 5. Meintjes G, Scriven J, Marais S. Management of the immune reconstitution inflammatory syndrome. Curr HIV/AIDS Rep 2012; 9:238–50. [DOI] [PubMed] [Google Scholar]
- 6. Markham A, Lamb HM. Infliximab: a review of its use in the management of rheumatoid arthritis. Drugs 2000; 59:1341–59. [DOI] [PubMed] [Google Scholar]
- 7. Hsu DC, Faldetta KF, Pei L et al. A paradoxical treatment for a paradoxical condition: infliximab use in three cases of mycobacterial IRIS. Clin Infect Dis 2016; 62:258–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mellstedt H. Clinical considerations for biosimilar antibodies. EJC 2013; 11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Komaki Y, Yamada A, Komaki F et al. Efficacy, safety and pharmacokinetics of biosimilars of anti-tumor necrosis factor-α agents in rheumatic diseases: a systematic review and meta-analysis. J Autoimmun 2017; 79:4–16. [DOI] [PubMed] [Google Scholar]
- 10. Komaki Y, Yamada A, Komaki F et al. Systematic review with meta-analysis: the efficacy and safety of CT-P13, a biosimilar of anti-tumour necrosis factor-α agent (infliximab), in inflammatory bowel diseases. Aliment Pharmacol Ther 2017; 45:1043–57. [DOI] [PubMed] [Google Scholar]
- 11. Namale PE, Abdullahi LH, Fine S et al. Paradoxical TB-IRIS in HIV-infected adults: a systematic review and meta-analysis. Future Microbiol 2015; 10:1077–99. [DOI] [PubMed] [Google Scholar]
- 12. Meintjes G, Wilkinson RJ, Morroni C et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2010; 24:2381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brunel AS, Reynes J, Tuaillon E et al. Thalidomide for steroid-dependent immune reconstitution inflammatory syndromes during AIDS. AIDS 2012; 26:2110–2. [DOI] [PubMed] [Google Scholar]
- 14. Dorhoi A, Kaufmann SH. Tumor necrosis factor alpha in mycobacterial infection. Semin Immunol 2014; 26:203–9. [DOI] [PubMed] [Google Scholar]
- 15. Blackmore TK, Manning L, Taylor WJ, Wallis RS. Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis 2008; 47:e83–5. [DOI] [PubMed] [Google Scholar]