Abstract
Golgi glycoprotein 73 (GP73) is a type II Golgi transmembrane protein and a potential novel marker for the diagnosis of primary hepatocellular carcinoma (PHC). However, its reliability as a serum marker for the diagnosis of PHC following transcatheter arterial chemoembolization (TACE) remains unknown. The aim of the present study was to evaluate the diagnostic value of serum GP73 levels in patients with PHC, to determine its diagnostic accuracy in patients with PHC following TACE. Reverse transcription-quantitative polymerase chain reaction analysis was used to measure GP73 expression in PHC and adjacent para-carcinomatous liver tissue in 40 patients with PHC, and 15 normal liver samples from benign hepatic tumors. The associations between GP73 expression levels with clinicopathological characteristics of the patients were also analyzed. Serum GP73 levels were detected by ELISA in 68 patients with PHC following TACE and 29 healthy individuals. The levels of serum GP73 were tested 2 days prior to intervention, and 7 and 30 days following TACE. GP73 mRNA expression levels in PHC were significantly higher than in the corresponding para-carcinomatous liver and normal liver samples. High expression levels of GP73 mRNA were associated with tumor size, vascular invasion and tumor differentiation, suggesting augmented tumor invasion and metastasis. The expression levels of serum GP73 were markedly higher in the patients with PHC compared with healthy individuals. Serum GP73 levels in the 68 patients with PHC were higher compared with the 29 normal controls [152.5 (76.4–284.5) compared with 49.3 (12.6–26.7) µg/l], and the difference was statistically significant (P<0.01). High levels of serum GP73 were associated with tumor differentiation. The levels of Barcelona clinic liver cancer stage A, B and C were 92.12 (38.9–135.2), 122.9 (55.2–178.5), and 162.55 (110.8–232.9) µg/l, respectively (P<0.05). The serum GP73 levels 7 days following TACE [99.2 (66.7–150.8)] were significantly lower than prior to TACE, and the difference was statistically significant (P<0.05). In the group of 49 patients with serum α-fetoprotein (AFP) levels <400 µg/l, the serum GP73 levels were >132 µg/l. Serum GP73 levels may serve as a potential independent diagnostic marker for PHC, and the combined evaluation of serum GP73 and AFP may increase the diagnostic efficiency of PHC. Significant overexpression of GP73 mRNA was associated with aggressive PHC. However, further research is required to confirm the potential of GP73 as a diagnostic marker.
Keywords: Golgi membrane protein 73, hepatocellular carcinoma, reverse transcription polymerase chain reaction, clinical pathological features, transcatheter arterial chemoembolization
Introduction
Hepatocellular carcinoma (PHC) is the fifth most common cancer and the third leading cause of cancer-associated mortality globally (1). In China, PHC is a leading cause of cancer-associated mortality (2). PHC is usually diagnosed at an advanced stage, and early diagnosis is of particular importance. Golgi glycoprotein 73 (GP73) is a type II Golgi transmembrane protein (3) and a potential novel marker for the diagnosis of PHC. A previous study has confirmed GP73 as a serum marker of liver cancer, and it is possible to use its gene expression to monitoring early recurrence (2). It is possible to monitor its protein levels by immunohistochemistry (4). The expression of GP73 mRNA levels and clinical characteristics are less well reported. Transcatheter arterial chemoembolization (TACE) is the preferred treatment for unresectable PHC. A previous study suggested that tumor necrosis following intervention is more likely to cause relapse and metastasis, due to the numerous viable tumor cells remaining following TACE therapy (5). Nevertheless, this evaluation of the efficacy of TACE was focused on radiological assessment with no uniform standard determination of residual tumor cells and a lack of objective laboratory parameters, thereby hindering optimal opportunity of one more TACE treatment following the first TACE. This will often induce early and easy recurrence. Serum α-fetoprotein (AFP) is widely used to detect primary liver tumors, but this technique demonstrates poor sensitivity and specificity (6). The present study used reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis to examine the levels of GP73 mRNA expression in PHC and its association with clinicopathological characteristics. In addition, ELISA was used to investigate the importance of GP73 and the involvement of AFP in assessing the efficacy of TACE in primary liver cancer.
Materials and methods
Ethical statement
The present study was approved by the Ethics Committee of the Fourth Hospital of Hebei University (Shijiazhuang, China). All patients provided written, informed consent.
Clinical data
A total of 40 PHC tissue samples with corresponding para-cancer tissue samples and 15 normal liver tissue samples were obtained from 55 patients admitted to the Department of Hepatobiliary Surgery at the Fourth Hospital of Hebei University from October 2013 to June 2014. None of the patients were diagnosed with metastasis prior to surgery. Tissue samples were obtained from 40 patients with pathologically confirmed PHC, and the para-cancer tissues were obtained within 2.0 cm from the border of the cancerous tissue. The samples were frozen in liquid nitrogen within 30 min of dissection. The patients with PHC included 29 males and 11 females, aged 39–74 (52±11.3) years. According to the Edmondson grading system (7), 28 patients demonstrated low to medium differentiation (level I–III), and the other 12 patients demonstrated high differentiation (level IV). The median diameter of the tumors was 6.33 cm (range, 1.9–11.6 cm), and 11 cases demonstrated vascular invasion. All patients had complete clinicopathological data, and did not receive radiotherapy or chemotherapy prior to surgery. Histopathological diagnosis revealed the presence of 31 cases of PHC and 9 cases of bile duct carcinoma. All patients underwent AFP examination. The 15 normal samples were obtained from normal liver tissue adjacent to benign liver tumors, including 12 males and 3 females with an average age of 45.3 years (range, 32–66 years).
Clinical TACE data comprised of 68 patients with PHC at the Fourth Hospital of Hebei Medical University, all of whom were hospitalized for the first time between May 2014 and January 2015), including 45 males and 23 females. Patients were 39–78 years old, with an average age of 53.2 years. According to the Barcelona clinic liver cancer (BCLC) staging system (8), 29 cases were stage A, 26 stage B, and 13 stage C. The patients were diagnosed with PHC with a history of chronic liver disease, following imaging and examination of the tumor marker AFP. All patients met the criteria of level A or B in the Child-Pugh staging system (9), life quality Kamofsky score (10) >70, and no absolute contraindications to TACE. The patients demonstrated no significant difference in transaminases, alkaline phosphatase, γ-glutamine transpeptidase (γ-GT), albumin (PA), blood routine or kidney function compared with the control group, and had not received any prior treatment (chemotherapy, radiotherapy or immunotherapy). The peripheral blood of patients with liver cancer was isolated 2 days prior to surgery and 7 and 30 days following surgery, and was stored at −80°C. The data of 29 healthy people that came to the Fourth Hospital of Hebei Medical University from September 2014 to January 2015 for health examinations were used as controls, including 17 males and 12 females with an average age of 41.6 years (range, 23–64 years).
RT-qPCR was used to determine mRNA expression
As per the manufacturer's protocol, the tissue samples stored at −80°C were cut into sections (0.3–0.5 cm), placed in 1.5 m centrifuge tubes, and 1 ml RNA iso Plus reagent (Takara Bio, Inc., Otsu, Japan) was added, followed by centrifugation at 4°C at 19,185 × g for 15 min. Absorbance (A) at 260 nm and 280 nm (A260 and A280) was measured to calculate the RNA concentration. First strand cDNA was synthesized using a reverse transcription kit (PrimeScript™ RT reagent kit with gDNA Eraser; Takara Bio, Inc.) in a 36-µl reaction volume, according to the manufacturer's protocol. Primer sequences used were as follows: GP73 forward, 5′-GTGTGAGGAGCGAATAGAAGAGG-3′ and reverse, 5′-GTCTCTGGTCGTTGTTTTCACT-3′; GAPDH forward, 5′-CTGGGCTACACTGAGCACC-3′ and reverse, 5′-AAGTGGTCGTTGAGGGCAATG-3′. All primers were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). PCR amplification was performed in a 50 µl reaction volume, using SYBR® Premix EX Taq™ (Takara Bio, Inc.). Thermocycling conditions were as follows: 95°C for 3 min, and 45 cycles of melting at 95°C for 30 sec, followed by 57°C for 30 sec. Gel electrophoresis images were acquired and stored (UVP, LLC, Upland, CA, USA), and A values of the GP73 and GAPDH bands were calculated using the 2−ΔΔCq method (11). Relative expression of GP73 was represented as the ratio of AGP73 to AGAPDH.
TACE
Using a modified Seldinger technique (12), the right femoral artery was punctured and the guide wire was inserted, followed by the 5F sheath, and then a 5F-RH catheter was inserted for celiac trunk angiography and indirect portal venography. Superior mesenteric artery, left gastric artery and right phrenic artery angiography were also performed when necessary. Then, the catheter was inserted into the target vessel (certain patients required the use of Progreat microcatheters, purchased from Terumo Corporation, Tokyo, Japan), and 1,000 mg tegafur (Hao Chuang Co., Ltd., Hainan, China), 150 mg oxaliplatin (Jiangsu Hengrui Co., Ltd., Lianyungang, China) and 200 mg leucovorin (Hainan SIDA Pharmaceutical Co., Ltd., Hainan, China) were slowly perfused, followed by 8–25 ml ultra-fluid lipiodol (Jiangsu Hengrui Co., Ltd.) containing 15 mg hydroxycamptothecin for embolization of the artery supporting the tumor. Choice and volume of the embolization agent were based on the cross-sectional area of the embolization and size of the tumor, as well as preoperative liver function status of the patients.
Assessment of serum GP73 levels in the blood samples by ELISA and AFP levels by chemiluminescence immunoassay
Serum GP73 levels were assessed using the Human Golgi glycoprotein 73 (GP73) ELISA kit (Beijing Hotgen Bioech Co., Ltd., Beijing, China) according to the manufacturer's protocol. For each test, samples (20 µl) were added to a 96-well plate. The experiment was performed in triplicate and independently repeated three times. Optical density values at 450 nm and 630 were read using a multiskan MK3 microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA). AFP levels were determined using the Cobas 601 enhanced chemiluminescence immunoassay analyzer and supporting kit [Human αFP (alpha-fetoprotein) kit; YZB/GEM 1177–2011; Roche Diagnostic GmbH], according to the manufacturer's protocol.
Statistical analysis
The data were analyzed using SPSS 19.0 statistical software (IBM Corp., Armonk, NY, USA). Quantitative data were expressed as the mean ± standard deviation. The Mann-Whitney U-test was used for comparisons between the groups. Spearman correlation coefficients were used to analyze the associations between GP73 expression levels and clinicopathological data. P<0.05 was considered to indicate a statistically significant difference.
Results
GP73 mRNA expression levels are higher in cancer tissues than para-cancer or normal tissues
GP73 mRNA expression was detected in all 40 samples of primary liver cancer and corresponding para-cancer liver tissues, as well as in normal liver tissue, but the levels differed between the PHC tissues, corresponding para-cancer liver tissues and normal liver tissue. The relative levels of GP73 mRNA were 2.35+0.17 in primary liver cancer, which was significantly higher than that in para-cancer liver tissue (0.93+0.05) and normal liver tissue (0.53+0.04; Table I; Figs. 1–4).
Table I.
Relative expression levels of GP73 mRNA.
| Group | Number of patients | GP73 mRNA expression levels |
|---|---|---|
| Liver cancer tissue | 40 | 2.35±0.17 |
| Para-cancer tissue | 40 | 0.93±0.15a |
| Normal liver tissue | 15 | 0.53±0.24a |
P<0.05 vs. liver cancer tissue. GP73, Golgi glycoprotein 73.
Figure 1.
RNA electrophoresis image. Lane 1, 2 and 3 were loaded with test samples, and lane M was loaded with the M-DL2000 marker.
Figure 4.
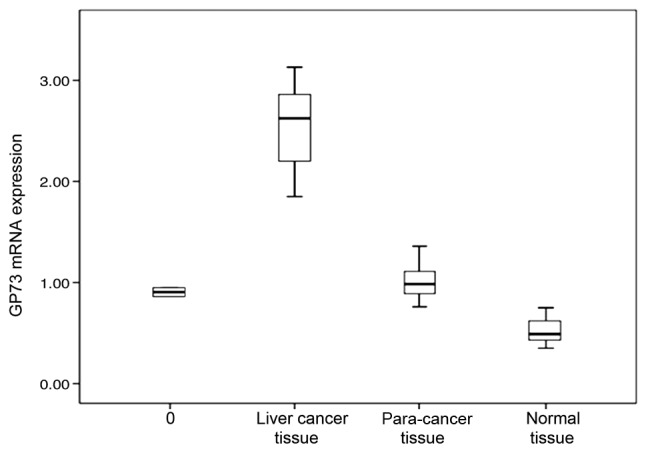
GP73 mRNA expression levels in different tissues. GP73, Golgi glycoprotein 73.
GP73 mRNA expression levels are associated with tumor size, vascular invasion and tumor differentiation
GP73 expression in liver cancer tissue demonstrated no significant associations with age, sex, number of tumors, serum AFP or history of hepatitis (P>0.05; Table II), but demonstrated significant associations with tumor size, vascular invasion and tumor differentiation (P<0.05; Table II).
Table II.
GP73 mRNA expression levels in liver cancer, and its associations with clinicopathological characteristics.
| Clinicopathological characteristic | n | GP73 mRNA expression levels (RU) | P-value |
|---|---|---|---|
| Age | 0.31 | ||
| ≤65 | 34 | 2.15±0.13 | |
| >65 | 6 | 2.21±0.18 | |
| Sex | 0.42 | ||
| Male | 29 | 2.32±0.08 | |
| Female | 11 | 2.33±0.16 | |
| Tumor size, cm | 0.01 | ||
| ≤3 | 21 | 2.17±0.13 | |
| >3 | 19 | 2.42±0.09 | |
| Tumor number | 0.59 | ||
| 1 | 31 | 2.36±0.11 | |
| ≥2 | 9 | 2.39±0.09 | |
| Differentiation | 0.02 | ||
| Well differentiated | 12 | 2.15±0.13 | |
| Moderately and poorly differentiated | 28 | 2.37±0.08 | |
| Angioinvasion | 0.01 | ||
| Yes | 11 | 2.41±0.15 | |
| No | 29 | 2.17±0.11 | |
| AFP, ng/ml | 0.85 | ||
| ≤400 | 10 | 2.34±0.11 | |
| >400 | 30 | 2.31±0.12 | |
| HBV | 0.56 | ||
| + | 33 | 2.35±0.13 | |
| − | 7 | 2.33±0.08 |
GP73, Golgi glycoprotein 73; AFP, α-fetoprotein; HBV, hepatitis B virus.
Serum GP73 levels differ between patients with cancer and controls, differ between BCLC stage, and are associated with clinical stage
Prior to TACE, serum GP73 levels of the 68 patients with primary liver cancer [152.5 µg/l (76.4–284.5 µg/l)] were significantly higher compared with the normal control [49.3 µg/l (12.6–26.7 µg/l); P<0.01]. Significant differences in GP73 levels were also detected between BCLC stage A, B and C liver cancers [92.12 µg/l (38.9–135.2 µg/l), 122.9 µg/l (55.2–178.5 µg/l), 162.55 µg/l (110.8–232.9 µg/l), respectively; P<0.05]. These results indicated that serum GP73 levels were correlated with clinical stage (P<0.05; r=0.27), but were not significantly affected by the sex or age of the patient.
Serum GP73 levels decreased following TACE
Serum GP73 levels of the 68 patients with primary liver cancer significantly decreased to 99.2 µg/l (66.7–150.8 µg/l; P<0.05) 7 days following TACE; and serum GP73 levels of the 28 patients with BCLC Stage A liver cancer decreased to 76.5 µg/l (59.2–107.2 µg/l; P<0.01) 7 days following TACE.
AFP-negative patients have a GP73 high detection rate
Among the 65 patients with primary liver cancer, 43 had AFP levels >400 µg/ml, and the other 22 had AFP levels <400 µg/ml. Among those with AFP levels <400 µg/l, 49 (75.3%) patients had a GP73 level ≥132 µg/l, suggesting a high detection rate of GP73 for AFP-negative patients, which may help to improve the accuracy of liver cancer diagnosis for patients with negative AFP.
Patients with a good response to TACE had lower serum GP73 levels than those with a poor response
Serum GP73 levels were rechecked 30 days following TACE, and upper abdominal computerized tomography or digital subtraction hepatic arteriography was performed. The results indicated that 31 patients had poor lipiodol retention, the lesion was active with intrahepatic metastasis, and serum GP73 levels were an average of 183.2 µg/l (79.5–235.2 µg/l). The other 34 cases achieved a good response to TACE (good lipodol retention and no indication of intrahepatic metastasis), and their GP73 levels were 115.2 µg/l (63.4–148.2 µg/l), which was significantly lower than those with a poor response (P<0.05).
Discussion
Studies on cancer recurrence and metastasis-associated factors are of significance for the prediction of PHC prognosis and anti-metastatic therapy (13). Early diagnosis and treatment of PHC are vital for improving the overall survival rate of patients (14). AFP is an important indicator for early diagnosis of liver cancer and is used to monitor its progression, and thus is widely used in clinical practice. However, 30–40% of liver cancers, in particular those originating from the bile duct, are negative for AFP expression. Furthermore, elevated AFP levels may be detected in patients with cirrhosis or exacerbations of chronic hepatitis (15). Prospective studies analyzing the performance characteristics of AFP for PHC surveillance reported sensitivities of 39–64%, specificities of 76–91% and positive predictive values of 9–32% (16,17). Thus, the clinical value of AFP has been questioned due to its low sensitivity and specificity (16–18), and novel serum markers for liver cancer with higher sensitivity and specificity are being actively sought in current research. GP73 is a novel tumor marker and was originally described as a resident Golgi type II transmembrane protein, with a single, N-terminal transmembrane domain and an extensive, C-terminal coiled-coil domain located on the luminal surface of the Golgi apparatus (19). Its mRNA was first identified in a patient with syncytial giant cell hepatitis (20). Serum GP73 levels gradually increase with the progression of hepatitis, cirrhosis, and liver cancer (2), suggesting that it is an effective indicator for monitoring liver cancer progression.
Using immunohistochemistry, GP73 was previously demonstrated to be highly expressed in primary liver cancer tissue and correlated with tumor differentiation (21). However, the semi-quantitative nature of immunohistochemistry makes it relatively difficult to provide an accurate diagnosis, and it demonstrates considerable false positive and false negative results. In the present study, RT-qPCR was used to determine GP73 mRNA levels in liver cancer tissues, para-cancer tissues and normal liver tissues, as this method is intuitive and reliable. Statistical analysis indicated that GP73 expression was the highest in primary liver cancer, moderate in para-cancer tissue, and the lowest in normal liver tissue (P<0.05), suggesting that high GP73 levels may be involved in the progression of primary liver cancer, and may be used as a marker for the diagnosis of liver cancer and for monitoring its recurrence. The associations between GP73 mRNA expression levels and clinicopathological features of patients with primary liver cancer were investigated, and GP73 expression was revealed to not be affected by age, sex, number of tumors, serum AFP levels or history of hepatitis, but it was associated with tumor size, vascular invasion and tumor differentiation, inferred as increased primary liver cancer burden and risk of metastasis and recurrence (22,23). The association of GP73 expression with these indicators suggested that GP73 may be involved in the progression and metastasis of primary liver cancer, and may be used as a novel serum marker for monitoring primary PHC recurrence following surgery, which may help clinicians to determine the timing of preventive treatment and to improve prognosis and patient survival.
There was a high detection rate in patients with liver cancer who were negative for AFP expression. The combined use of GP73 and AFP may significantly improve the accuracy of early diagnosis of liver cancer. In addition, patients with BCLC stage C had higher serum GP73 levels than patients with BCLC stage B and A, suggesting the involvement of GP73 in progression and metastasis of liver cancer. In addition, dynamic changes of serum GP73 levels also reflected the efficacy of TACE treatment. Serum GP73 levels significantly decreased 7 days following TACE treatment, suggesting that TACE significantly reduced liver tumor burden and delayed tumor progression. Serum GP73 levels in patients with tumor progression was significantly higher than those in remission 30 days following TACE treatment. Acute liver damage and repeated liver tissue regeneration may lead to increased GP73 expression (22), which is then released into the blood and reflects tumor progression. The combined use of the two markers may become an important means for the diagnosis of primary liver cancer. Hu et al (24) studied receiver operating characteristic (ROC) curves comparing Chinese patients with PHC admitted to Xiangya Second Hospital from June 2008 to December 2008, and demonstrated that the Hepatitis B virus-associated PHC area under the (AU) ROC curve for GP73 was 0.89 [95% confidence interval (CI), 0.82–0.97], with a sensitivity of 77.4% and specificity of 83.9%, whereas the AUROC for AFP was 0.77 (95% CI, 0.65–0.89), with a sensitivity of 48.4% and specificity of 96.8%. Zhou et al (25) performed a meta-analysis, which revealed a sensitivity of 76% (GP73, 95% CI, 51–91%) vs. 70% (AFP, 95% CI, 47–86%) and specificity of 86% (GP73, 95%CI, 65–95%) compared with 89% (AFP, 95% CI, 69–96%), respectively, which indicated that serum GP73 has a comparable accuracy to AFP for the diagnosis of PHC. The present study indicated that serum GP73 levels were significantly increased in patients with poor prognosis 30 days following surgery, which may be useful for the assessment of prognosis and disease progression. It is generally recognized that for patients with low AFP expression, AFP levels rarely reflect the efficacy of TACE, while the dynamic changes of GP73 levels accurately reflect the condition of the patient. These results suggested that for patients with AFP levels ≤400 µg/l, the dynamic changes in serum GP73 are valuable in the assessment of TACE efficacy, and should be promoted in clinical examination.
Combination with AFP may significantly improve the rate of early diagnosis of PHC, and GP73 levels inpatients with BCLC stage C PHC were significantly higher compared with patients with stage B PHC, which indicated that serum GP73 levels are associated with the progression and metastasis of PHC. Meanwhile, serum GP73 changes are relevant to the effect of TACE. Serum GP73 levels decreased 7 days post-intervention as compared with those recorded prior to treatment, given that TACE significantly reduces the liver tumor load and delays the progression of tumors. GP73 serum levels in patients with disease progression, recorded 30 days post-operation, were significantly higher than those of patients in remission. With disease progression and interventional therapy, normal liver cells are damaged, and acute hepatic injury and repeated liver tissue remodeling may lead to GP73 being released into the blood, subsequently leading to disease progression (26). GP73 sensitivity and specificity were higher compared with that of detection of AFP, and the two may be used in combination as an improved method of diagnosis for primary liver cancer.
The present study indicated that serum GP73 levels significantly increased in patients with a rapid progression of the disease 30 days following TACE, and may be useful in evaluating prognosis and disease progression. For AFP negative patients or those with low concentrations by AFP, the effect of TACE is difficult to predict. The dynamic changes of serum GP73 level may accurately reflect the patient's condition, suggesting its clinical application value in monitoring the effect of TACE treatment in AFP-negative patients with PHC or with AFP levels <400 g/l, and this may be widely applied for clinical detection.
In summary, to the best of our knowledge, this is the first demonstration of GP73 expression at them RNA and serum levels in terms of treatment with TACE, and its association with clinicopathological features of PHC was demonstrated. Manipulation of GP73 expression in patients with PHC patients may lead to the development of novel therapies. Further studies of GP73 functions and mechanisms of its regulation in normal and PHC tissues are warranted.
Figure 2.
Polymerase chain reaction amplification curve for GP73. GP73, Golgi glycoprotein 73; ΔRn, fluorescence signal with baseline subtracted.
Figure 3.
Polymerase chain reaction amplification curve for the internal reference gene, GAPDH. ΔRn, fluorescence signal with baseline subtracted.
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, Zhao H, Chen W, Xu Y, Chi T, et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut. 2010;59:1687–1693. doi: 10.1136/gut.2010.214916. [DOI] [PubMed] [Google Scholar]
- 3.Kladney RD, Bulla GA, Guo L, Mason AL, Tollefson AE, Simon DJ, Koutoubi Z, Fimmel CJ. GP73, a novel Golgi-localized protein uprcgulated by viral infection. Gene. 2000;249:53–65. doi: 10.1016/S0378-1119(00)00136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang F, Gu Y, Li X, Wang W, He J, Peng T. Upregulated Golgi phosphoprotein 2 (GOLPH2) expression in lung adenocarcinoma tissue. Clin Biochem. 2010;43:983–991. doi: 10.1016/j.clinbiochem.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Nagasue N, Galizia G, Kohno H, Chang YC, Hayashi T, Yamanoi A, Nakamura T, Yukaya H. Adverse effects of preoperative hepatic artery chemoembolization for resectable hepatocellular carcinoma: A retrospective comparision of 138 liver resection. Surgery. 1989;106:81–86. [PubMed] [Google Scholar]
- 6.Benowitz S. Liver cancer biomarkers struggling to succeed. J Natl Cancer Inst. 2007;99:590–591. doi: 10.1093/jnci/djk174. [DOI] [PubMed] [Google Scholar]
- 7.Edmondson HA, Steiner PE. Primary carcinoma of the liver: A study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::AID-CNCR2820070308>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. EASL Panel of Experts on HCC: Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European association for the study of the liver. J Hepatol. 2001;35:421–430. doi: 10.1016/S0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 9.Triantos C, Zisimopoulos K, Tsochatzis E, Vlachogiannakos J, Manolakopoulos S, Rigamonti C, Goulis J, Manesis E, Anastasiou J, Papalexi F, et al. 207 Meld vs cps for prognosis in cirrhosis. Results from a multicentre study. J Hepatol. 2010;52:S89. doi: 10.1016/S0168-8278(10)60209-7. (Suppl 1) [DOI] [Google Scholar]
- 10.Bai H. Manual of tumor diagnosis and treatment for primary physicians. Peking University Medical Press; Beijing: 2008. p. 356. [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta Radiol. 1953;39:368–376. doi: 10.1177/028418515303900502. [DOI] [PubMed] [Google Scholar]
- 13.Saffroy R, Pham P, Reffas M, Takka M, Lemoine A, Debuire B. New perspectives and strategy research biomarkers for hepatocellular carcinoma. Clin Chem Lab Med. 2007;45:1169–1179. doi: 10.1515/CCLM.2007.262. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Wan X, Li Z, Lin C, Zhan Y, Lu X. Golgi protein 73 (GP73), a useful serum marker in liver diseases. Clin Chem Lab Med. 2011;49:1311–1316. doi: 10.1515/CCLM.2011.640. [DOI] [PubMed] [Google Scholar]
- 15.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases: Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 16.Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatol. 1994;19:61–66. doi: 10.1002/hep.1840190111. [DOI] [PubMed] [Google Scholar]
- 17.Pateron D, Ganne N, Trinchet JC, Aurousseau MH, Mal F, Meicler C, Coderc E, Reboullet P, Beaugrand M. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol. 1994;20:65–71. doi: 10.1016/S0168-8278(05)80468-4. [DOI] [PubMed] [Google Scholar]
- 18.Zoli M, Magalotti D, Bianchi G, Gueli C, Marchesini G, Pisi E. Efficacy of a surveillance program for early detection of hepatocellular carcinoma. Cancer. 1996;78:977–985. doi: 10.1002/(SICI)1097-0142(19960901)78:5<977::AID-CNCR6>3.3.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 19.Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, Comunale MA, D'Amelio A, Lok AS, Block TM. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol. 2005;43:1007–1012. doi: 10.1016/j.jhep.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Norton PA, Comunale MA, Krakover J, Rodemich L, Pirog N, D'Amelio A, Philip R, Mehta AS, Block TM. N-linked glycosylation of the liver cancer biomarker GP73. J Cell Biochem. 2008;104:136–149. doi: 10.1002/jcb.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riener MO, Stenner F, Liewen H, Soll C, Breitenstein S, Pestalozzi BC, Samaras P, Probst-Hensch N, Hellerbrand C, Müllhaupt B, et al. Golgi phosphoprotein 2 (GOLPH2) expression in liver tumors and its value as a serummarker in hepatocellular carcinomas. Hepatology. 2009;49:1602–1609. doi: 10.1002/hep.22843. [DOI] [PubMed] [Google Scholar]
- 22.Poon Tung-Ping R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimada K, Sano T, Sakamoto Y, Kosuge T. A long-term follow-up and management study of hepatocellular carcinoma patients surviving for 10 years or longer after curative hepatectomy. Cancer. 2005;104:1939–1947. doi: 10.1002/cncr.21461. [DOI] [PubMed] [Google Scholar]
- 24.Hu JS, Wu DW, Liang S, Miao XY. GP73, a resident Golgi glycoprotein, is sensibility and specificity for hepatocellular carcinoma of diagnosis in a hepatitis B-endemic Asian population. Med Oncol. 2010;27:339–345. doi: 10.1007/s12032-009-9215-y. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Yin X, Ying J, Zhang B. Golgi protein 73 versus alpha-fetoprotein as a biomarker for hepatocellular carcinoma: A diagnostic meta-analysis. BMC Cancer. 2012;12:17. doi: 10.1186/1471-2407-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iftikhar R, Kladney RD, Havlioglu N, Schmitt-Gräff A, Gusmirovic I, Solomon H, Luxon BA, Bacon BR, Fimmel CJ. Disease- and cell-specific expression of GP73 in human liver disease. Am J Gastroenterol. 2004;99:1087–1095. doi: 10.1111/j.1572-0241.2004.30572.x. [DOI] [PubMed] [Google Scholar]


