Abstract
Although in Bacillus thuringiensis the cry genes coding for the insecticidal crystal proteins are plasmid-borne and are usually associated with mobile genetic elements, several aspects related to their genomic organization, diversification, and transmission remain to be elucidated. Plasmids of B. thuringiensis and other members of the Bacillus cereus group (n = 364) deposited in GenBank were screened for the presence of cry1 genes, and their genetic environment was analyzed using a comparative bioinformatic approach. The cry1 genes were identified in 27 B. thuringiensis plasmids ranging from 64 to 761 kb, and were predominantly associated with the ori44, ori60, or double orf156/orf157 and pXO1-16/pXO1-14 replication systems. In general, the cry1 genes occur individually or as a part of an insecticidal pathogenicity island (PAI), and are preceded by genes coding for an N-acetylmuramoyl-l-alanine amidase and a putative K+(Na+)/H+ antiporter. However, except in the case of the PAI, the latter gene is disrupted by the insertion of IS231B. Similarly, numerous mobile elements were recognized in the region downstream of cry1, except for cry1I that follows cry1A in the PAI. Therefore, the cassette involving cry1 and these two genes, flanked by transposable elements, named as the cry1 cassette, was the smallest cry1-carrying genetic unit recognized in the plasmids. Conservation of the genomic environment of the cry1 genes carried by various plasmids strongly suggests a common origin, possibly from an insecticidal PAI carried by B. thuringiensis megaplasmids.
Keywords: Bacillus thuringiensis, cry1 genes, IS231, IS232, pathogenicity island, insertion sequences
Introduction
Bacillus thuringiensis is a Gram-positive, spore-forming bacterium, known for the production of parasporal crystal inclusions composed of insecticidal Cry proteins. These toxins create a heterogeneous family of 74 different types of proteins (Cry1–Cry74) (Crickmore etal. 2016) harmful for various insect genera, including Lepidoptera, Coleoptera, Diptera, Hemiptera, and even some nematodes and snails (Palma etal. 2014). Within these toxins, Cry1 represent the most abundant group, accounting for ∼17% of all Cry toxins (Crickmore etal. 2016), and exhibit activity against pests causing the highest damages in crops and forests. For example, Cry1A are toxic to Lepidoptera, whereas Cry1B and Cry1I have dual activity against Lepidoptera and Coleoptera. The Cry–host specificity is partly a consequence of prerequisite conditions for the Cry activation. For instance, the Cry1A crystals must be first solubilized in the alkaline midgut of Lepidoptera and subsequently processed into active forms by the proteolytic digestion of specific serine proteases (Palma etal. 2014). However, other factors associated with toxins processing or stability in the insects midgut, aside from the receptor binding, can influence Cry specificity and activity (reviewed by Jurat-Fuentes and Crickmore 2017).
In general, most cry genes are plasmid-borne and are usually flanked by various mobile elements such as insertion sequences (IS231, IS232, IS240, ISBt1, and ISBt2) and transposons (Tn4430 or Tn5401) (Mahillon etal. 1994; Léonard etal. 1997; Mahillon and Chandler 1998; Schnepf etal. 1998). For example, cry1A genes are frequently found within a composite transposon structure flanked by IS232 (Menou etal. 1990; Murawska etal. 2014). In addition, the cry genes can be organized in operons and/or co-localized with other toxin genes, for example those of the vegetative insecticidal proteins (vip), forming insecticidal pathogenicity islands (PAI) (Aronson 1993; Zhu etal. 2015). This particular genetic environment of the cry genes is believed to be responsible for their amplification in bacterial cells, and to facilitate their recombination and exchange among plasmids, providing a source of novel specificities in crystal-producing strains (Aronson 1993; Léonard etal. 1997; Schnepf etal. 1998; Palma etal. 2014). However, the actual transfer mechanisms and the role of the transposable elements in the evolution of cry-carrying plasmids remain to be elucidated. Similarly, little attention has been paid to non-toxin genes located in the immediate cry genetic environment, while, as a part of insecticidal PAIs, they may contribute to the virulence or be implicated with certain B. thuringiensis specific traits, for example activation of spore germination in an alkaline pH (Abdoarrahem etal. 2009).
The aim of the study was to investigate the genetic and genomic environments of the cry1 genes. To this end, a comparative analysis of the cry1 loci was performed using the various B. thuringiensis plasmids deposited in GenBank.
Materials and Methods
In total, a set of 406 complete plasmids from Bacillus thuringiensis and other Bacillus cereus group members deposited in GenBank were screened for the presence of cry1 genes using the B. thuringiensis Toxin_scanner (Ye etal. 2012) (supplementary table S1, Supplementary Material online). A genetic environment of cry1 was visualized with Easyfig tool (Sullivan etal. 2011). In addition, ISfinder (Siguier etal. 2006) and PHASTER (Arndt etal. 2016) website tools were used in order to identify mobile elements and prophage regions, respectively. DNA sequence alignment and analysis were performed with CLC Genomic Workbench software (CLC Bio), and Blast2GO software was used for functional annotation of proteins (Conesa etal. 2005).
Results
Strains and Plasmids Characteristics
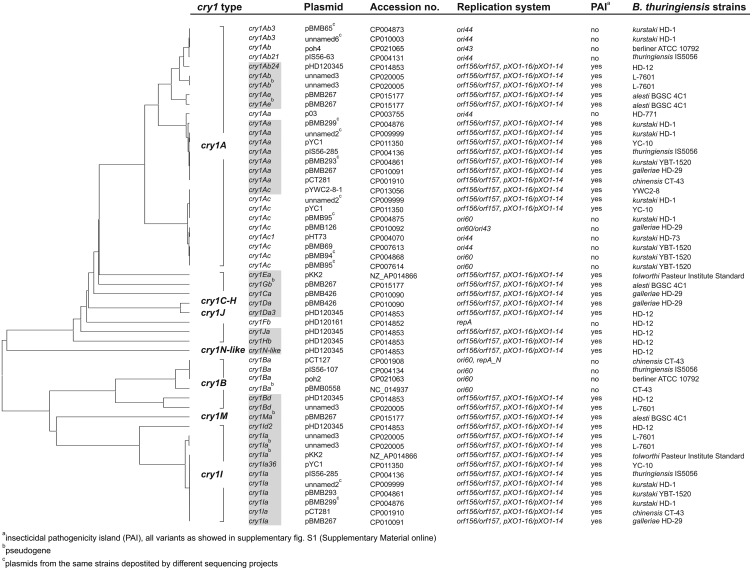
The cry1-carrying plasmids were detected in 15 B. thuringiensis strains (table 1). However, it should be noted that in the case of B. thuringiensis serovar (sv.) kurstaki strains HD-1 and YBT-1520 the same plasmids from different sequencing projects were included into analyses, since notable discrepancies were observed in their sequences (see below).
Table 1.
Characteristics of B. thuringiensis Strains and Their cry1-Carrying Plasmids
| B. thuringiensis serovar and strain |
Isolation |
STa | Plasmid |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Year | Source | Name | Accession Number | Size (bp) | Replication type | Insecticidal PAIb | cry1 genec,d | Other insecticidal toxin genes | ||
| kurstaki YBT-1520 | China | 1990 | Soil | 8 | pBMB69 | CP007613 | 69,416 | ori44 | No | cry1Ac | No |
| pBMB293e | CP004861 | 293,574 | orf156/orf157 | Yes | (cry1Aa, cry1Ia)c | cry2Ab, cry2Aa, vip3Aa | |||||
| pXO1-16/pXO1-14 | |||||||||||
| pBMB95 | CP007614 | 94,637 | ori60 | No | cry1Ac | No | |||||
| pBMB94 | CP004868 | 94,568 | ori60 | No | cry1Ac | No | |||||
| kurstaki HD-1 | United States | 1967 | Pectinophora gossypiella | 8 | pBMB95 | CP004875 | 95,983 | ori60 | No | cry1Ac | No |
| pBMB65 | CP004873 | 65,873 | ori44 | No | cry1Ab3 | No | |||||
| unnamed6 | CP010003 | 69,317 | ori44 | No | cry1Ab3 | No | |||||
| pBMB299 | CP004876 | 299,843 | orf156/orf157 | Yes | (cry1Aa, cry1Ia)c | cry2Ab, cry2Aa, vip3Aa | |||||
| pXO1-16/pXO1-14 | |||||||||||
| unnamed2 | CP009999 | 317,336 | orf156/orf157 | Yes | cry1Ac, (cry1Aa, cry1Ia)c | cry2Ab, cry2Aa, vip3Aa | |||||
| pXO1-16/pXO1-14 | |||||||||||
| thuringiensis IS5056 | Poland | 2005 | Soil | 10 | pIS56-63 | CP004131 | 63,864 | ori44 | No | cry1Ab21 | No |
| pIS56-107 | CP004134 | 107,431 | ori60 | No | cry1Ba | No | |||||
| pIS56-285 | CP004136 | 285,459 | orf156/orf157 | Yes | (cry1Aa, cry1Ia)c | cry2Ab, cry2Aa, vip3Aa | |||||
| pXO1-16/pXO1-14 | |||||||||||
| chinensis CT-43 | China | NDf | NDf | 10 | pCT127 | CP001908 | 127,885 | ori60, repA_N | No | cry1Ba | No |
| pCT281 | CP001910 | 281,231 | orf156/orf157 | Yes | (cry1Aa, cry1Ia)c | cry2Ab, cry2Aa, vip3Aa | |||||
| pXO1-16/pXO1-14 | |||||||||||
| galleriae HD-29 | Czechoslovakia | 1970 | Dendrolimus sibericus | 15 | pBMB267 | CP010091 | 267,359 | orf156/orf157 | Yes | (cry1Aa, cry1Ia)c | cry2Ab, vip3Aa |
| pXO1-16/pXO1-14 | |||||||||||
| pBMB426 | CP010090 | 426,282 | orf156/orf157-like | Yes | (cry1Ca, cryDa)c | No | |||||
| pXO1-16/pXO1-14 | |||||||||||
| pBMB126 | CP010092 | 126,898 | ori60, ori43 | No | cry1Ac | No | |||||
| HD-12 | United States | 2012 | Soil | 23 | pHD120345 | CP014853 | 345,196 | orf156/orf157 | Yes | (cry1Hb, cry1Bb, cry1Ab24, cry1I-like, cry1Ja, cry1Id2, cry1Da3)c | cry2Ad, vip2Af, vip1Ca, vip2Ac, vip1Ba-like |
| pXO1-16/pXO1-14 | |||||||||||
| pHD120161 | CP014852 | 161,353 | repA | No | cry1Fb | No | |||||
| YC-10 | China | 2010 | Nicotiana tabacum roots | 8 | pYC1 | CP011350 | 761,374 | orf156/orf157 | Yes | cry1Ac, (cry1Aa, cry1Ia36)c | cry2Aa, cry2Ab35, vip3Aa |
| pXO1-16/pXO1-14 | |||||||||||
| YWC2-8 | China | 2007 | Soil | 8 | pYWC2-8-1 | CP013056 | 250,706 | orf156/orf157 | Yes | (cry1Ac)c | cry2Aa |
| pXO1-16/pXO1-14 | |||||||||||
| HD-771 | NDf | NDf | NDf | 12 | p03 | CP003755 | 69,876 | ori44 | No | cry1Aa | No |
| kurstaki HD-73 | NDf | NDf | NDf | 8 | pHT73 | CP004070 | 77,351 | ori44 | No | cry1Ac1 | No |
| alesti BGSC 4C1 | Czechoslovakia | 1987 | Bombyx mori | 12 | pBMB267 | CP015177 | 267,609 | pXO1-16/pXO1-14 | Yes | (cry1Ae, cry1Aed, cry1Gbd, cry1Mad)c | cry2Ab, vip3Aa |
| tolworthi Pasteur Institute Standard | NDf | NDf | NDf | pKK2 | NZ_AP014866 | orf156/orf157 | (cry1Ea, cry1Iad)c | cry2Aa | |||
| pXO1-16/pXO1-14 | |||||||||||
| CT-43 | NDf | NDf | NDf | NDf | pBMB0558 | NC_014937 | 109,464 | ori60 | No | cry1Bad | No |
| L-7601 | China | 2015 | NDf | 197 | unnamed3 | CP020005 | 408,071 | orf156/orf157 | Yes | (cry1Ab, cry1Ia, cry1Abd, cry1Iad, cry1Bd)c | vip3Ah |
| pXO1-16/pXO1-14 | |||||||||||
| berliner ATCC 10792 | Israel | NDf | Ephestia kuhniella | 10 | poh4 | CP021065 | 86,488 | ori43 | No | cry1Ab | No |
| poh2 | CP021063 | 113,294 | ori60 | No | cry1Ba | No | |||||
ST, sequence type determined by MLST (http://mlstoslo.uio.no/, last accessed April 3, 2017).
PAI, Pathogenicity Island.
cry1 genes in brackets are located in the insecticidal PAI as showed in supplementary fig. S1, Supplementary Material online.
Pseudogene.
Identical plasmid sequence is deposited in GenBank under Acc. number CP007615.
ND, not determined.
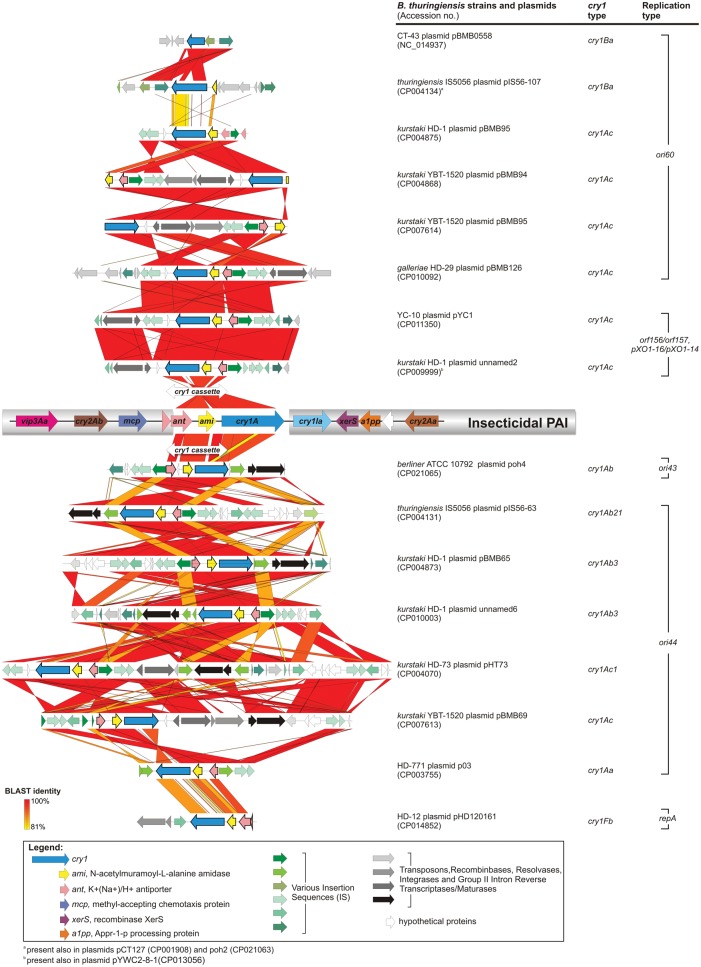
Eight B. thuringiensis strains have only one cry1 bearing plasmid, whereas two or even three plasmids with cry1 were noted in three (CT-43, HD-12, ATCC 10792) and four (HD-1, HD-29, YBT-1520, and IS5056) strains, respectively (table 1 and fig. 1). In total, we identified 14 plasmids carrying only a single cry1 gene, cry1A (n = 9), cry1B (n = 4), or cry1F (n = 1), and 12 plasmids with more than one cry1 or other cry genes (table 1). The latter group involved six megaplasmids carrying the PAI, as described by Zhu etal. (2015), which contains the cry1Aa, cry1Ia, cry2Aa, cry2Ab, and vip3Aa genes (hereinafter referred to as the “insecticidal PAI”), and additional seven megaplasmids containing its variants or derivatives (supplementary fig. S1, Supplementary Material online).
Fig. 1.
—Comparison of the cry1 genes from B. thuringiensis plasmids using UPGMA clustering. The cry1 genes located in the insecticidal PAI, as showed in supplementary fig. S1, Supplementary Material online, are highlighted as grey boxes.
Replication System and Size of B. thuringiensis Plasmids Harbouring cry1
As shown in table 1, the majority of plasmids with a single cry1A gene possess the replication systems ori44 (n = 5), ori60 (n = 5), ori43 (n = 1), or both ori43/ori60 (n = 1) (Baum and Gilbert 1991) and are of a size below 100 kb, except for plasmids pBMB126 (126 kb and two ori43/ori60 replication systems) and pHD120161 (161 kb and repA replication type). Similarly, plasmids (n = 4) with a single cry1B have the ori60 replication system and size of 107 kb (pIS56–107), 109 kb (pBMB0558), 113 kb (poh2), and 127 kb (pCT127 and another replication system, repA_N). In contrast, plasmids with several cry genes are larger (from 250 to 761 kb) and are characterized by double orf156/orf157 and pXO1-16/pXO1-14 replication systems (Tang etal. 2007; Pomerantsev etal. 2009; Zheng etal. 2013).
Distribution and Variation of the cry1 Genes
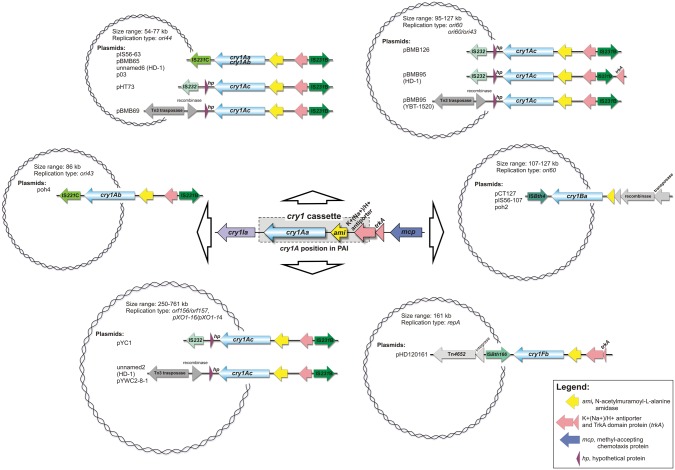
The cry1 genes were represented by 12 types (A–J, M, and N-like) (fig. 1). The cry1A (Aa, Ab, Ac, and Ae) genes constitute the largest group among cry1 (49%) and are present either in the insecticidal PAIs or separately. In addition, all cry1A located in the insecticidal PAI (fig. 2 and supplementary fig. S1, Supplementary Material online) of plasmids with the double orf156/orf157 and pXO1-16/pXO1-14 replication systems, belong to cry1Aa subtype, whereas cry1Ac and cry1Aa-c subtypes are associated with plasmids of the ori60 and ori44 replication systems, respectively (figs. 1 and 2). In contrast, cry1Ia or cry1Id, which represent 21% of the cry1 genes, were found only within the insecticidal PAI, where they are located downstream of cry1A and cry1E (cry1Ia) or cry1D (cry1Id). The cry1N-like, cry1C, cry1E, cry1F, cry1G, cry1H, cry1J, and cry1M genes occur only in individual plasmids, mostly as a part of the insecticidal PAI variants (supplementary fig. S1, Supplementary Material online).
Fig. 2.
—Distribution and variability of the cry1 cassette in B. thuringiensis plasmids.
Genetic Environment of the cry1 Genes
In general, in the insecticidal PAI cry1A is followed by cry1I (fig. 3 and supplementary fig. S1, Supplementary Material online). In contrast, various mobile elements were found downstream of the cry1 genes located outside the PAI or in the remaining plasmids, namely (i) IS231C followed by Tn4430 (pIS56–63, pBMB65, and p03), (ii) IS232 (pHT73, pBMB95 from strain HD1, pBMB126, and pYC1), (iii) a putative transposon (Tn3 family) followed by Tn4652 (pBMB95 from strain YBT-1520) and Tn4430 (pBMB69), (iv) an IS110 family member (pHD120161) (fig. 3 and supplementary fig. S2, Supplementary Material online). Overall, the mobile elements in the direct or more distal cry1 environment include insertion sequences, transposons, and elements associated with DNA integration/recombination (integrases, resolvases) as well as group II intron reverse transcriptase/maturase genes (supplementary fig. S2, Supplementary Material online). More specifically, ISs are represented by four families: (i) IS4 (IS231B, IS231C, IS232S, and ISBth4), (ii) IS21 (IS232), (iii) IS110 (ISBth166), and (iv) IS200/IS605 (ISBth16), while all the Tn transposase genes belong to the Tn3 family. An accumulation of various transposable elements in cry1 proximity is particularly apparent in the ori44-replication plasmids, where they represent up to 46% of the plasmid size (supplementary fig. S2, Supplementary Material online).
Fig. 3.
—The cry1 gene cassette and its genetic environment in B. thuringiensis plasmids. The insecticidal PAI was used as reference. The entirely annotated version is detailed in supplementary fig. S2, Supplementary Material online.
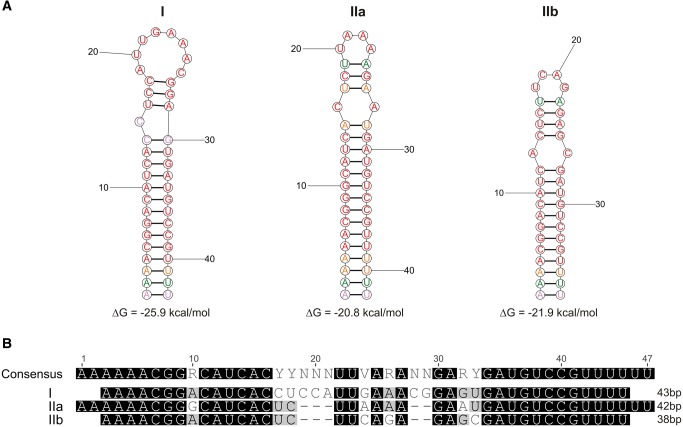
Within the downstream cry1 sequence (except for cry1Ia) we noted the inverted repeat motif described by Wong and Chang (1986) as a positive retroregulator that stabilizes cry1 mRNA. Interestingly, beside the original motif 5′-AAAACGGACATCACCTCC(N8)GGAGTGATGTCCGTTTT-3′, variants characterized by different secondary mRNA structures were also observed (fig. 4 and supplementary fig. S3, Supplementary Material online). In addition, 45 bp downstream of the retroregulator sequence and 127 bp upstream of the cry1Ia promoter sequence, a second inverted repeat structure, 5′-AAGCAGAGATATTTTCA(N8)TGAAAATATCTCTGCTT-3′ was also noted (supplementary fig. S4, Supplementary Material online).
Fig. 4.
—(a) Prediction of the lowest free energy (ΔG in kcal/mol) structure for mRNA of various variants (I, IIa, and IIb) of the cry1 positive retroregulator; the analysis was done using RNAstructure web server (http://rna.urmc.rochester.edu/RNAstructureWeb/Servers/Predict1/Predict1.html, last accessed July 20, 2017). (b) Alignment of the I, IIa, and IIb positive retroregulator mRNA variants.
In contrast, an upstream region of cry1 is occupied by a gene (or its fragment in the case of cry1B) encoding for an N-acetylmuramoyl-l-alanine amidase, that is preceded, except for cry1B, by gene(s) encoding component(s) of a putative K+(Na+)/H+ antiporter (fig. 3). However, in virtually all cases (but not in the PAI), the latter gene is disrupted by the insertion of IS231B and is usually followed by IS232. Therefore, the gene cassette involving cry1 and these two genes or their fragments (hereinafter named as the cry1 cassette) is the smallest cry1-carrying (cry1A, cry1F, or cry1B) genetic unit recognized in different plasmids that possibly originated from the PAI (fig. 2). Interestingly, plasmids pYC1 (strain YC-10) and unnamed 2 (strain kurstaki HD-1) both carry the PAI and the cry1 cassette (fig. 3).
Relatedness of cry1-Carrying Plasmids to Other B. cereus Group Plasmids
For all cry1-carrying plasmids, variants missing the cry1 cassette (supplementary figs. S5–S8, Supplementary Material online) were identified. For instance, plasmids pKK2 (54 kb) and pBMB55 (55 kb) are in fact ∼10 kb smaller cry1-negative variants of the ori44-type cry1-carrying plasmids pIS56-63, pBMB65, pHT73, pBMB69, and p03 (supplementary fig. S5, Supplementary Material online). Similarly, the lack of the cry1 cassette is the only substantial difference between 7 plasmids (72–90 kb) and 12 plasmids (59–89 kb) displaying, respectively, the ori60 or the ori43 replication type, as compared with the cry1A- or cry1B-positive ones (supplementary figs. S6 and S7, Supplementary Material online). Finally, the lack of the large fragment (∼120 kb) containing the insecticidal PAI differentiates pBTBC2 (171 kb) from the PAI-positive megaplasmids pBMB293, pIS56-285, pCT281, or pBMB299 (supplementary fig. S8, Supplementary Material online). Such cry1-negative plasmids were found exclusively in B. thuringiensis strains, except for the plasmid pH308197_73 from the emetic B. cereus strain H3081.97 (supplementary fig. S6, Supplementary Material online). However, it must be stated that certain inconsistencies for the presence of cry1 genes in the same plasmids from different sequencing projects were noted (supplementary fig. S9, Supplementary Material online). For example, the cry1Ac cassette from the 95 kb plasmid pBMB95 (CP004875) of B. thuringiensis sv. kurstaki HD-1 is present in the 317 kb unnamed plasmid (CP009999).
Discussion
The occurrence of various cry1 genes in the proximity of an N-acetylmuramoyl-l-alanine amidase gene (or its fragment), along with K+(Na+)/H+ antiporter pseudogene (i.e., disrupted by IS231B), strongly suggests their common origin. The insecticidal PAI where both genes are complete and where cry1A is adjacent to cry1I instead of a mobile genetic element, seems to be the natural candidate for such ancestor (fig. 2 and supplementary fig. S1, Supplementary Material online). Following this idea, we suggest two independent genetic events that “released” the cry1 cassette from the PAI, (i) a disruption of K+(Na+)/H+ antiporter operon by IS231B, and subsequently (ii) the insertion of an IS or a transposon into the cry1 downstream region. The former event is visible in pKK2, where cry1I is preserved (supplementary fig. S1, Supplementary Material online). In fact, disruption of various genes by IS231 elements is not an unusual phenomenon in B. thuringiensis (Qiu etal. 2010). However, it will be interesting to examine whether the presence of ISs downstream of the cry1 genes is related to the presence of two types of inverted repeat sequences, including one acting as positive retroregulators of the cry1 mRNA (Wong and Chang 1986), since such DNA motifs are common targets for ISs (Tobes and Pareja 2006). Interestingly, the second motif (supplementary fig. S4, Supplementary Material online) is not present in the cry1 cassettes associated with ISs belonging to IS4 family, namely IS231C and ISBth4 (fig. 2), which are flanked by the same direct repeat (5′-TGGCGGTACCC-3′). Further accumulation of numerous transposable elements in this region could be attributed to either homologous recombination or insertion of one transposable element into another. For example, Mahillon etal. (1987) revealed that IS231 transposed into the IR of Tn4430 without affecting the transposon structural integrity. Thus, the formation of such transposon-like structures (Menou etal. 1990; Mahillon etal. 1994) may be an important mechanism for the cry1 cassette transposition among various plasmids. This mobility is supported by the observation of cry1-negative plasmids that are otherwise identical to those containing the cry1 cassette (supplementary figs. S5–S7, Supplementary Material online). Therefore, duplication of the cry1 cassette followed by further sequence divergence, may have led to small-scale diversification of Cry1 toxins active against the same insect targets, and explain the presence of different members of the same Cry toxin family in individual bacterial isolates (e.g., Cry1Aa, Cry1Ab, and Cry1Ac in B. thuringiensis sv. kurstaki HD1 or Cry1Aa and Cry1Ab in B. thuringiensis IS5056) (Murawska etal. 2013; Palma etal. 2014). In addition, diversification of the cry1 genes also involves their positive retroregulator sequence that improves the cry1 mRNA stability, which may be associated with insecticidal efficacy of certain B. thuringiensis strains (fig. 4). Furthermore, the presence of cry1 cassettes, predominantly located in a limited number of plasmid types (i.e., ori44 and ori60), lends further support to the mobilization of cry-carrying plasmids between B. thuringiensis strains as the mechanism which explains why identical copies of cry genes are distributed among geographically separated isolates (Palma etal. 2014). This is the case for B. thuringiensis strain Na205-3 isolated in Spain (Palma etal. 2014a) and B. thuringiensis strain CT-43 isolated in China (He etal. 2011) that share some, but not all, cry-carrying plasmids with B. thuringiensis strain IS5056 from Poland (Swiecicka etal. 2008; Murawska etal. 2013) (table 1). In addition, cry1I, cry1M, and cry1N-like encoding smaller toxins, that is, devoid of C-terminal part responsible for crystallization, appeared to be associated with a gene encoding a “XerS” tyrosine recombinase and limited to plasmids with the PAI (supplementary fig. S6, Supplementary Material online). Although, those proteins are technically naturally truncated versions (65–70 kDa) of the 130–140 kDa toxins, we did not observe genetic events that might support their evolution neither by (i) a fragmentation or disruption of the most related cry1B genes nor (ii) in a manner characteristic for cry10A, cry39A, and cry40A, where ORFs encoding N-terminal and C-terminal parts of toxin are separated by short non-coding region (de Maagd etal. 2003). Nevertheless, the presence of xerS also in proximity of genes encoding Cry3A, toxic for Coleoptera, might be connected with dual Lepidoptera/Coleoptera activity of Cry1I, that is, as the result of domain swapping, since domains I and II of those toxins are phylogenetically related (de Maagd etal. 2001).
Considering the above observations, the genetic environment of cry1 should be perceived from the perspective of the insecticidal PAI, where the N-acetylmuramoyl-l-alanine amidase and K+(Na+)/H+ antiporter genes are preceded by an ORF encoding for a putative methyl-accepting chemotaxis protein (MCP). Consequently, this genetic context qualifies those genes as potential virulence factors or at least as part of B. thuringiensis adaptation machinery to its pathogenic lifestyle (Jensen etal. 2003; Raymond etal. 2010). However, an ad hoc explanation of this thesis is challenging, since these companion genes are associated with fundamental physiological processes, such as cell wall maintenance, ion transport and chemotaxis. Their contribution to the development of B. thuringiensis under the alkaline pH of insect midgut, may nevertheless be connected to potassium transport and net accumulation of K2CO3 (Dow 1984). For instance, the involvement of K+/H+ antiporter in alkaline pH homeostasis has been reported in several bacteria, and the presence of the proper antiporter is sufficient to enable a non-alkaliphilic bacterium to grow at extremely high pH (Padan etal. 2005). In addition, certain ion antiporters participate to spore germination in Bacillus spp. This is the case for B. cereus for which GerT significantly contributes to spore outgrowth from the germinated state during alkaline or Na+ stress (Senior and Moir 2008). Similarly, it has been demonstrated that loss of the K+ or NH4+ transporter may affect endospore formation and germination in an alkaliphilic Bacillus pseudofirmus (Wei etal. 2003). A stimulation of spore germination in B. thuringiensis by an alkaline pH has also been reported in several studies (Benoit etal. 1995; Du and Nickerson 1996; Bhattacharya 1999; Abdoarrahem etal. 2009), and could be considered as an adaptation that enable to germinate at the appropriate time in the insect guts (Du and Nickerson 1996; Jensen etal. 2003).
The PAI K+(Na+)/H+ antiporter proteins showed 80 and 62% identity with the YhaU and YhaT from Bacillus subtilis, respectively (Fujisawa etal. 2004). Interestingly, yhaU is strongly induced by alkaline pH and salt-induced stress and this antiporter may extrude K+ and . Similarly, one MCP has been recognized as necessary for optimal pH homeostasis and for normal chemotaxis responses in the alkaliphilic B. pseudofirmus OF4 (Fujinami etal. 2007). However, it should be noted that the K+(Na+)/H+ antiporter and MCP from the PAI are not plasmid-specific proteins, and that homologs are present in the B. thuringiensis chromosome (supplementary figs. S11 and S12, Supplementary Material online).
Certain N-acetylmuramoyl-l-alanine amidases have been shown as important enzymes for germination in Bacillus spp. (Makino and Moriyama 2002; Wu etal. 2015). It has also been revealed that the peptidoglycan hydrolases of B. thuringiensis are more active at high pH (Raddadi etal. 2004). These enzymes may contribute to B. thuringiensis virulence as an “evasin” which would ensure successful colonization of B. thuringiensis in the insect hemocoel before the host develops an immunological response, as it has been proposed for the AmiA amidase of Bacillus anthracis (Mesnage and Fouet 2002).
The N-acetylmuramoyl-l-alanine amidase from the insecticidal PAI has putatively bacteriophage origin, since it shows similarity with the amidase XlyA encoded by the defective prophage PBSX from B. subtilis (Krogh etal. 1998), and because its closest homolog on B. thuringiensis chromosome was identified in a prophage region (supplementary fig. S13, Supplementary Material online). A relationship of the PAI with prophages is also visible in the non-cry genes located between cry1I and cry2Aa. They code for an ADP-ribose 1-phosphate phosphatase and a potential ADP-ribosylase (protein family: pfam14487) (supplementary fig. S1, Supplementary Material online), that share homology with putative gene products of a Geobacillus subterraneus prophage (supplementary fig. S14, Supplementary Material online). Since ADP-ribosylation is used by various bacterial toxins, including the B. thuringiensis Vip1/Vip2 (Palma etal. 2014), they might also represent virulence attributes in this bacterium. As a whole, the insecticidal PAI appears to be a conglomerate of various genetic determinants of chromosomal and prophage origin, intermingled with insecticidal genes. In addition, the PAI might also be a part of a larger (∼120 kb) genomic unit (supplementary fig. S8, Supplementary Material online), containing among others a novel haemolysin operon (Zhu etal. 2015) and an operon encoding spore delaying proteins whose homologs where shown to contribute to cannibalism behaviour in B. subtilis (González-Pastor 2011) (supplementary table S2, Supplementary Material online).
In conclusion, the similarity of genetic environment of various cry1 genes implies their common origin, likely the insecticidal PAI located in ∼300 kb B. thuringiensis megaplasmids. We suggest that two independent insertion events “released” cry1 from the PAI in the form of a cry1 cassette, involving N-acetylmuramoyl-l-alanine amidase and fragment of K+(Na+)/H+ antiporter genes. Hence, cry1 sequences divergence and/or homologous recombination between cry1 genes, including their positive retroregulator, occurring in this shared genetic environment appear to play a central role in the evolution and spread of the cry1 genes.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online.
Funding
This study was supported by grant No. N N302 656640 of Ministry of Science and Higher Education of Poland (I. Swiecicka), and by project numbers N/ST/ZB/16/001/1122 (K. Fiedoruk) and N/ST/ZB/16/004/1122 (T. Daniluk) from the Medical University of Bialystok.
Supplementary Material
Literature Cited
- Abdoarrahem MM, Gammon K, Dancer BN, Berry C.. 2009. Genetic basis for alkaline activation of germination in Bacillus thuringiensis subsp. israelensis. Appl Environ Microbiol. 75(19):6410–6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt D, et al. 2016. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 44(W1):W16–W21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson AI. 1993. The two faces of Bacillus thuringiensis: insecticidal proteins and post-exponential survival. Mol Microbiol. 7(4):489–496. [DOI] [PubMed] [Google Scholar]
- Baum JA, Gilbert MP.. 1991. Characterization and comparative sequence analysis of replication origins from three large Bacillus thuringiensis plasmids. J Bacteriol. 173:5280–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit TG, Newnam KA, Wilson GR.. 1995. Correlation between alkaline activation of Bacillus thuringiensis var. kurstaki spores and crystal production. Curr Microbiol. 31(5):301–303. [Google Scholar]
- Bhattacharya PR. 1999. Activation and germination of spores of Bacillus thuringiensis var. israelensis by alkaline pH and larval (Aedes aegypti) gut fluid. Southeast Asian J Trop Med Public Health 30(2):338–342. [PubMed] [Google Scholar]
- Conesa A, et al. 2005. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21(18):3674–3676. [DOI] [PubMed] [Google Scholar]
- Crickmore N, et al. 2016. Bacillus thuringiensis toxin nomenclature. Availabe from http://www.btnomenclature.info/. Cited 2017 Apr 3.
- de Maagd RA, Bravo A, Crickmore N.. 2001. How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet. 17:193–199. [DOI] [PubMed] [Google Scholar]
- de Maagd RA, Bravo A, Berry C, Crickmore N, Schnepf HE.. 2003. Structure, diversity, and evolution of protein toxins from spore-forming entomopathogenic bacteria. Annu Rev Genet. 37:409–433. [DOI] [PubMed] [Google Scholar]
- Dow JA. 1984. Extremely high pH in biological systems: a model for carbonate transport. Am J Physiol. 246:R633–R636. [DOI] [PubMed] [Google Scholar]
- Du C, Nickerson KW.. 1996. Bacillus thuringiensis HD-73 spores have surface-localized Cry1Ac toxin: physiological and pathogenic consequences. Appl Environ Microbiol. 62(10):3722–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa M, Wada Y, Ito M.. 2004. Modulation of the K+ efflux activity of Bacillus subtilis YhaU by YhaT and the C-terminal region of YhaS. FEMS Microbiol Lett. 231(2):211–217. [DOI] [PubMed] [Google Scholar]
- Fujinami S, et al. 2007. The voltage-gated Na+ channel NaVBP co-localizes with methyl-accepting chemotaxis protein at cell poles of alkaliphilic Bacillus pseudofirmus OF4. Microbiology 153(Pt 12):4027–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Pastor JE. 2011. Cannibalism: a social behavior in sporulating Bacillus subtilis. FEMS Microbiol Rev. 35(3):415–424. [DOI] [PubMed] [Google Scholar]
- He J, et al. 2011. Complete genome sequence of Bacillus thuringiensis subsp. chinensis strain CT-43. J Bacteriol. 193(13):3407–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen GB, Hansen BM, Eilenberg J, Mahillon J.. 2003. The hidden lifestyles of Bacillus cereus and relatives. Environ Microbiol. 5(8):631–640. [DOI] [PubMed] [Google Scholar]
- Jurat-Fuentes JL, Crickmore N.. 2017. Specificity determinants for Cry insecticidal proteins: insights from their mode of action. J Invertebr Pathol. 142:5–10. [DOI] [PubMed] [Google Scholar]
- Krogh S, Jørgensen ST, Devine KM.. 1998. Lysis genes of the Bacillus subtilis defective prophage PBSX. J Bacteriol. 180(8):2110–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léonard C, Chen Y, Mahillon J.. 1997. Diversity and differential distribution of IS231, IS232 and IS240 among Bacillus cereus, Bacillus thuringiensis and Bacillus mycoides. Microbiology 143(8):2537–2547. [DOI] [PubMed] [Google Scholar]
- Mahillon J, Seurinck J, Delcour J, Zabeau M.. 1987. Cloning and nucleotide sequence of different iso-IS231 elements and their structural association with the Tn4430 transposon in Bacillus thuringiensis. Gene 51(2–3):187–196. [DOI] [PubMed] [Google Scholar]
- Mahillon J, Rezsöhazy R, Hallet B, Delcour J.. 1994. IS231 and other Bacillus thuringiensis transposable elements: a review. Genetica 93(1–3):13–26. [DOI] [PubMed] [Google Scholar]
- Mahillon J, Chandler M.. 1998. Insertion sequences. Microbiol Mol Biol Rev. 62(3):725–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Moriyama R.. 2002. Hydrolysis of cortex peptidoglycan during bacterial spore germination. Med Sci Monit. 8(6):RA119–RA127. [PubMed] [Google Scholar]
- Menou G, Mahillon J, Lecadet MM, Lereclus D.. 1990. Structural and genetic organization of IS232, a new insertion sequence of Bacillus thuringiensis. J Bacteriol. 172(12):6689–6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnage S, Fouet A.. 2002. Plasmid-encoded autolysin in Bacillus anthracis: modular structure and catalytic properties. J Bacteriol. 184(1):331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawska E, Fiedoruk K, Bideshi DK, Swiecicka I.. 2013. Complete genome sequence of Bacillus thuringiensis subsp. thuringiensis strain IS5056, an isolate highly toxic to Trichoplusia ni. Genome Announc. 1(2):e00108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawska E, Fiedoruk K, Swiecicka I.. 2014. Modular genetic architecture of the toxigenic plasmid pIS56-63 harboring cry1Ab21 in Bacillus thuringiensis subsp. thuringiensis strain IS5056. Pol J Microbiol. 63:147–156. [PubMed] [Google Scholar]
- Padan E, Bibi E, Ito M, Krulwich TA.. 2005. Alkaline pH homeostasis in bacteria: new insights. Biochim Biophys Acta 1717(2):67–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma L, Muñoz D, Berry C, Murillo J, Caballero P.. 2014. Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins (Basel) 6(12):3296–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma L, Muñoz D, Murillo J, Caballero P.. 2014a. Draft Genome Sequence of Bacillus thuringiensis serovar tolworthi strain Na205-3, an isolate toxic for Helicoverpa armigera. Genome Announc. 2:e00187–e00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantsev AP, Camp A, Leppla SH.. 2009. A new minimal replicon of Bacillus anthracis plasmid pXO1. J Bacteriol. 191(16):5134–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu N, et al. 2010. Prevalence and diversity of insertion sequences in the genome of Bacillus thuringiensis YBT-1520 and comparison with other Bacillus cereus group members. FEMS Microbiol Lett. 310(1):9–16. [DOI] [PubMed] [Google Scholar]
- Raymond B, Johnston PR, Nielsen-LeRoux C, Lereclus D, Crickmore N.. 2010. Bacillus thuringiensis: an impotent pathogen? Trends Microbiol. 18(5):189–194. [DOI] [PubMed] [Google Scholar]
- Raddadi N, et al. 2004. The autolytic phenotype of Bacillus thuringiensis. J Appl Microbiol. 97(1):158–168. [DOI] [PubMed] [Google Scholar]
- Schnepf E, et al. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev. 62:775–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A, Moir A.. 2008. The Bacillus cereus GerN and GerT protein homologs have distinct roles in spore germination and outgrowth, respectively. J Bacteriol. 190(18):6148–6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M.. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 34(90001):D32–D36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MJ, Petty NK, Beatson SA.. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecicka I, Bideshi DK, Federici BA.. 2008. Novel isolate of Bacillus thuringiensis subsp. thuringiensis that produces a quasicuboidal crystal of Cry1Ab21 toxic to larvae of Trichoplusia ni. Appl Environ Microbiol. 74(4):923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Bideshi DK, Park H-W, Federici BA.. 2007. Iteron-binding ORF157 and FtsZ-like ORF156 proteins encoded by pBtoxis play a role in its replication in Bacillus thuringiensis subsp. israelensis. J Bacteriol. 189(22):8053–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobes R, Pareja E.. 2006. Bacterial repetitive extragenic palindromic sequences are DNA targets for Insertion Sequence elements. BMC Genomics 7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, et al. 2003. Mutational loss of a K+ and NH4+ transporter affects the growth and endospore formation of alkaliphilic Bacillus pseudofirmus OF4. J Bacteriol. 185(17):5133–5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HC, Chang S.. 1986. Identification of a positive retroregulator that stabilizes mRNAs in bacteria. Proc Natl Acad Sci U S A. 83(10):3233–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, et al. 2015. Characterization of the activity of the spore cortex lytic enzyme CwlJ1. Biotechnol Bioeng. 112(7):1365–1375. [DOI] [PubMed] [Google Scholar]
- Ye W, et al. 2012. Mining new crystal protein genes from Bacillus thuringiensis on the basis of mixed plasmid-enriched genome sequencing and a computational pipeline. Appl Environ Microbiol. 78(14):4795–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Peng D, Ruan L, Sun M.. 2013. Evolution and dynamics of megaplasmids with genome sizes larger than 100 kb in the Bacillus cereus group. BMC Evol Biol. 13:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, et al. 2015. Genomic and transcriptomic insights into the efficient entomopathogenicity of Bacillus thuringiensis. Sci Rep. 5:14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.