Abstract
Background
Alkaline thermostable lipase and biosurfactant producing bacteria are very interested at detergent applications, not only because of their eco-friendly characterize, but alsoproduction lipase and biosurfactant by using cheap materials. Ochrobactrum intermedium strain MZV101 was isolated as washing powder resistant, alkaline thermostable lipase and biosurfactant producing bacterium in order to use at detergent applications.
Methods
O. intermedium strain MZV101 produces was lipase and biosurfactant in the same media with pH 10 and temperature of 60 °C. Washing test and some detergent compatibility character of lipase enzyme and biosurfactant were assayed. The antimicrobial activity evaluated against various bacteria and fungi.
Results
Lipase and biosurfactant produced by O. intermedium strain MZV101 exhibited high stability at pH 10–13 and temperature of 70–90 °C, biosurfactant exhibits good stability at pH 9–13 and thermostability in all range. Both lipase and biosurfactant were found to be stable in the presence of different metal ions, detergents and organic solvents. The lipase enzyme extracted using isopropanol with yield of 69.2% and biosurfactant with ethanol emulsification index value of 70.99% and yield of 9.32 (g/l). The single band protein after through from G-50 Sephadex column on SDS-PAGE was calculated to be 99.42 kDa. Biosurfactant O. intermedium strain MZV101 exhibited good antimicrobial activity against Gram-negative bacteria and against various bacterial pathogens. Based upon washing test biosurfactant and lipase O. intermedium strain MZV101considered being strong oil removal.
Conclusion
The results of this study indicate that isolated lipase and biosurfactant with strong oil removal, antimicrobial activity and good stability could be useful for detergent applications.
Graphical abstract
Keywords: Lipase, Biosurfactant, Ochrobactrum intermedium, Washing powder
Background
Many reports have demonstrated that scientists are interested in microorganisms which live and survive from harsh environments such as hot springs, because of their outstanding component, which is more adapted to industrial applications [1–3]. The most important metabolite produced by microorganisms is enzymes like lipase, which can serve as biocatalysts in biotransformation and other industrial processes. Additionally, surface-active agents including, biosurfactants which are produced by various types of microorganisms are widely used in detergent industry due to their eco-friendly characteristics and cost-effective production [4, 5]. Generally, the intensive applications of lipase and biosurfactant include cosmetics, pharmaceutical, agriculture and food industries [5–7]. Considering these metabolites are responsible for cleaning the oily contaminants, they have critical roles in detergent formulations. Besides, the industrial value of biological compounds strongly depends on their stability and adaptability under the production conditions [8, 9]. Therefore, lipases and biosurfactants which are able to function in the temperature range of 30–60 and pH range of 9–12 are used as the major components of enzyme-containing detergent [10, 11]. Other advantages of lipase and biosurfactant for industrial applications include biodegradability, cost-effective production and low toxicity. Since a majority of industrial production is the cost of raw materials applying bacteria which utilize substrates like agro waste and industrial byproducts counts as an economic strategy for industries [12]. For reducing the production cost of microbial metabolites, if the production condition is design based on both compounds, it would be more advantageous for manufacturers. However, since there is a constant striving for energy efficiency, especially with household appliances, the use of lower washing temperatures must be traded off against the hygiene efficacy of laundering which is considerably influenced by the temperature profile [13]. Although washing in low temperature as explained can solve energy striving problem, but laundering at low temperature can influence hygiene. Antimicrobial biosurfactant in laundry detergent can improve hygiene efficiency [14, 15].
We attempted to isolate and identify alkaline thermostable lipase and biosurfactant producing bacterium with antimicrobial activity in the same medium from Gheynarje Nir hot springs in order to use at detergent applications. In the present study, we reported Ochrobactrum intermedium strain MZV101 as lipase and biosurfactant producing bacteriumwhich was isolated in the presence of washing powder and considered its detergent compatibility by examining different environmental factors, metal ions, organic solvents and detergents. Finally, we were studied oil removal ability by washing test for detergent formulation.
Methods
Materials
Chemical compounds with their catalog number which used in this studied are DEAE- Cellulose (30477), Olive oil (75343), Sephadex G-50 column (S5897), Tween 20 (P9416), Tween-80 (P8074) and p-NPP (N2752), Sigma-Aldrich, Germany; Dialysis tubing cellulose membrane flat with 10 mm (D9277) and Rhodamine B (83689), Sigma, Germany; Millipore membrane filter 0.22 μm (GSWP02500) and 0.45 μm (HAWP04700), Merck Millipore, Germany; Acetone (100014), Ammonium sulphate (101217), Barium chloride (101716), Benzene (101783), Calcium chloride (102083), Chloroform (107024), Cobalt (II) chloride anhydrous (802540), Cyclohexane (102817), Ethanol (818760), Ethyl Acetate (100789), Hexane (104373), Isopropanol (113350), Magnesium Chloride (814733), Mercury(II) chloride (104417), Methanol (822283), Sodium deoxycholate (106504), Sodium dodecyl sulfate (817034), Toluene (108327), Triton X-100 (112298), Urea (108486) and Zinc chloride (108816), Merck, Germany. No human or animal was used in this research.
Sampling and media preparation
Sampling was conducted from Gheynarje Nir hot spring Ardebil, Iran (latitude 38° 2′8.98″N; longitude 47°59′0.39″E). Environmental conditions like temperature and pH was recorded as 60 °C and 8.6, respectively.
The medium used for lipase and biosurfactant production was a basal salt medium as described by Schlegel 1961 with some modification [13]. This medium comprised 5 g/l yeast extract, 10 ml of olive oil, 0.2 g/l of MgSO4.7H2O, 0.01 g/l of FeSO4.7H2O, 0.01 g/l of CaCl2.7H2O, 1 g/l of NH4Cl, 0.5 g/l of K2HPO4 and 0.1 ml of trace element solution (70 mg/l of ZnCl2, 100 mg/l of MnCl2.4H2O, 200 mg/l of CoCl2.6H2O, 100 mg/l of NiCl2.6H2O, 20 mg/l of NaMoO8.2H2O, 26 mg/l of Na2SeO3.5H2O and 1 ml of 25% HCl) [13]. We used 1% olive oil as source of carbon, energy and lipase and biosurfactant stimulator. First, 10 ml samples were grown in 250 ml sterile flasks containing 80 ml of basal medium supplemented with 1% of enzyme free detergent powder (Paksan Company, Iran), each culture was adjusted to pH 8, 9 and 10 and incubated in a rotary incubator at a temperature of 60 °C at 120 rpm for 72 h. In order to make solid plate, 20 g/l agar was added to basal medium. Isolated strain was cultured on agar plate incubated at 60 °C for 48 h. Finally, a loop full of isolated strain was added to 100 ml basal medium broth and incubated at 60 °C, pH 10 and agitation speed 180 rpm for 72 h.
Screening and isolation of lipolytic microorganism
Screening of lipase bacteria was obtained on solid plate assay for bacterial lipases according to Kouker and Jaeger’s method [16]. Based on the highest lipase specific activity, one colony was chosen for further investigations.
Lipase activity assay
The lipase activity was determined with p-NPP as substrate according to Winkler and Stuckmann’s [17]. Protein concentration was determined according to Lowry’s method [18].
Screening for biosurfactant production microorganism
Hemolytic activity
Hemolytic assay was used as qualitative method for biosurfactant production on 5% sheep blood agar plate. The clear zone around bacteria colony was considered as biosurfactant activity [19].
Oil spreading assay
Oil spreading assay was measured in 25 cm plastic Petri dish as describe by Willumsen et al. Triton X-100 and water was used as the positive and the negative controls [20].
Drop collapsing test
Drop collapsing test was performed in to the well of a 96-well micro-plate lid according to Bodour and Miller-Maier [21]. A well without surfactant was used as negative control and Triton X-100 with 1 mg/ml concentration was used as standard surfactant for positive control [21].
Emulsification index (%)
Emulification index performed in a test tube as described by Bodour and Maier [21]. Triton X-100 was used as a surfactant for the positive control, and negative control was maintained without surfactant with buffer and heavy petroleum [21].
Antimicrobial activity
Several standard microorganisms strains like gram positive, gram negative bacteria and fungi were cultivated on nutrient agar plate at 37 °C, 24 h for bacterial and 48 h for fungi pathogens. The antimicrobial activity was investigated by disc diffusion method on MHA plate by using Bauer et al. [22] method. The diameter of inhibition zone was measured [22].
DNA extractions, PCR amplification, sequencing and analysis
Isolated bacterial were inoculated in a medium without olive oil containing 1 g/l sodium acetate, 1 g/l sucrose and incubated in a rotary shaker at 60 °C for 48 h. Furthermore, bacteria were cultivated on a solid plate containing 1 g/l sodium acetate, 1 g/l sucrose and 15 g/l agar in order to obtain single pure colonies, and incubated at 60 °C for 24 h. DNA extraction was carried out according to the Bust n’ Grab protocol [23]. The quality of extracted DNA was evaluated using nano drop spectrophotometer readings (Thermo Scientific, Wilmington, DE). The 16S rDNA gene sequencing amplification were conduct using universal primer forward 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and primer reverse 1492R (5′-GGCTACCTTGTTACGACTT-3′). Thereafter, PCR product was purified using high pure PCR purification kit according to the manufacturer’s instructions. Nucleotide sequencing of the amplified fragments was performed with dideoxy chain termination method (SEQLAB, Germany). DNA sequences were analyzed using BLAST program (https://blast.ncbi.nlm.nih.gov/Blast.cgi) [24]. The phylogenetic tree was drawn using molecular evolutionary genetic analysis version 5.0 software (MEGA, Germany). A phylogenetic tree was constructed by using the neighbor-joining method and it was analyzed based on 16S rDNA gene sequences compared to available sequences in the GenBank [25].
Effect of different factors on lipase and biosurfactant stability
The effect of different parameters on lipase activity was measured by incubating the extracellular enzyme with p-Nitrophenyl Palmitate as substrate for 4 h. In order to study biosurfactant emulsification index values were incubated free cellular biosurfactant in mixture of 2 ml of heavy petroleum for 24 h.
Effect of various pH
Lipase and biosurfactant stability was calculated in different pH range of 5–13 at 37 °C according to the same temperature of Winkler and Stuckmann’s protocol assay [17]. The buffers used in this study are (0.05 M), citrate–phosphate buffer pH 5–6, Tris-HCl buffer pH 7–9, NaHCO3-NaOH buffer pH 10–11 and KCl-NaOH buffer pH 12–13.
Effect of temperature
The effect of temperature was studied at a wide range from 4 to 90 °C in optimal pH 9 on Lipase and biosurfactant stability O. intermedium strain MZV101.
Effect of different metal ions
The effect of various reagents was investigated by adding reagents at a concentration of 1 mM of metal ions (BaCl2, CaCl2, CoCl2, HgCl2, MgCl2 and ZnCl2) to the enzyme and biosurfactant reaction mixture was incubated in optimal pH 10, and temperature of 60 °Ϲ [26]. We incubated the lipase enzyme for 4 h and biosurfactant for 24 h.
Effect of different organic solvents and detergents
The lipase enzyme and biosurfactant were incubated in the presence of organic solvent at a final concentration of 0.1% acetone, benzene, chloroform, cyclohexane, ethanol, ethyl Acetate, hexane, methanol, toluene and detergents 1% SDS, 25% Tween-20, 25% Tween- 80 and 25% Triton X-100 at pH 10 and at a temperature of 60 °C [27].
Solvent purification lipase and biosurfactant
Lipase and biosurfactant production was carried out in same basal medium and after 72 h incubation at 60 °C. The culture broth was centrifuged at 13300×g for 10 min at room temperature. In order to achieve the best result, various precipitation methods such as ammonium sulphate with acetone [27] chilled ethanol [28] and isopropanol were studied. At the final step of each method, pellets were filtered through a sterile 0.45 μm millipore membrane. The mentioned precipitation methods for both lipase and biosurfactant were the same.
The precipitate was resuspended in 2 ml of 0.1 M Tris-HCl buffer at pH 10 and dialyzed against 0.1 M Tris buffer at pH 10 for 2 h at room temperature. The concentrated enzyme was loaded on a sephadex G-50 column (2 cm × 150 cm) according to manufacture instructions. The fraction containing protein was determined by specific activity. Purity of protein was evaluated by using SDS-PAGE gel as describe by Laemmli [29].
Washing test
For the investigation of washing performance, white fabric cotton (5 cm × 5 cm) was degreased in boiling chloroform for 4 h and stained with 0.5 ml mixture of olive oil and benzene (100 mg/ml concentration) [11]. The white fabric was stained twice and air dried at room temperature. The stained pieces of cottons were incubated in washing solutions as mentioned in Table 1. They were incubated on 120 rpm shaker for 1 h at 37 °C. The cotton clothes were rinsed with 100 ml distilled water for 3 min at 37 °C. Olive oil was extracted from cotton fabric with petroleum ether (bp 40–60 °C) for 4 h in Soxhlet extractor [26]. The weights of olive oil were measured before and after washing for each treatment. The efficiency of oil removal was calculated by following equation:
Table 1.
Composition of washing solutions
| Volume (ml) | ||||||
|---|---|---|---|---|---|---|
| Solutions | B | B + L | B + S | B + D + L | B + D + S | B + D + L + S |
| 0.1 M Tris buffer pH = 8.5 | 40 | 40 | 40 | 40 | 40 | 40 |
| Lipase | – | 10 | – | 10 | – | 10 |
| Biosurfactant | – | – | 10 | – | 10 | 10 |
| 1% Detergent solution | – | – | – | 40 | 40 | 40 |
| Distilled water | 60 | 50 | 50 | 10 | 10 | – |
B = 0.1 M Tris buffer pH = 8.5
L = lipase (1000 U)
S = biosurfactant (1000 U)
D = 1% Detergent solution
The stained fabrics were incubated in 250 ml flasks containing 100 ml washing solutions as mentioned below. All tests were repeated at least in triplicate independent experiments
Wb = Weight of oil before washing.
Wa = Weight of oil after washing.
Statistical analysis
Experimental data were repeated at least three times. Results are reported as the mean standard deviation. The obtained data were used analyzed using repeated measure ANOVA and significant differences were determined using mauchly’s test of sphericity. The results with a p-value less than <0.05 were considered as significant differences. Statistically analysis was performed using IBM SPSS statistics version 22 software (IBM, USA).
Results and discussion
Screening and isolation of lipolytic and biosurfactant microorganism
Detergent formulations processes are performed under extremely high pH and temperature along with other addictive. The structure of thermophlic enzymes from living microorganism in hot springs are naturally stable and active at high temperatures [30]. Thermophilic enzymes from this microorganism are function at highly alkaline conditions, salinity and other harsh conditions [31]. Although all isolated bacteria are not capable to become commercially available [32]. So, screening of new lipase and biosurfactant producing bacteria are still required [33].
Our sampling site was a hot spring with 60 °C and pH 8.6. Such extreme ecosystem is one of the places in where resistant bacterial groups to harsh conditions could be found. These bacteria might produce metabolites that are suitable for industrial applications. As the favorable cleaning condition for washing powders is at high temperature and alkaline pH, therefore, having the components with high stability to temperature and pH could enhance the cleaning efficiency of detergents when applied as additive in the formulations.
Eighteen strains were isolated from culture with pH 10.In this study, we observed significant clear zone around bacteria colony. The hemolytic assay is not the specific method for screening biosurfactant producing microorganism and has limitation. Therefore, the hemolytic assay should be used as a primary method and drop collapse test, oil spreading assay and emulsification assay methods were studied to determine biosurfactant production [34].Only six strains were positive in lipase and biosurfactant. One strain was chosen for further investigations base on the fact that it had the highest emulsification index (E24 = 62.2%), and lipase specific activity of 1.8 U/mg Protein and antimicrobial activity. The isolated strains showed the positive result for drop collapse assay, oil spreading assay. Same results were reported by Nalini et al. (2013) from Serratia rubidaea SNAU02 with positive blood hemolysis, drop collapse assay and emulsification index of 52.2% [35].
DNA extractions, PCR amplification, sequencing and analysis
According to 16S rDNA sequencing, biochemical and morphological tests (Table 2), the isolated strain MZV101 was identified to be closely related to Ochrobactrum intermedium with 99% homology via DNA BLAST in NCBI GenBank and it was recorded with the number KX619441access in NCBI. A phylogenetic tree of strain MZV101 based on 16S rDNA using neighbor-joining method is shown in Fig. 1 [25]. Ochrobactrum is a gram-negative, strictly aerobic, catalase and oxidase positive and usually single-cell [36]. O. intermedium 2745–2 was the first environmental strain isolated from formation water in China [33]. The optimum growth temperature for O. intermedium strains are 20 to 37 °C and optimum are pH 7–7.3 [37]. In this study, O. intermedium strain MZV101 was isolated from a hot spring at a temperature of 60 °C and pH of 8.6 for detergent application. Lipase from Pseudomonas sp. and Bacillus sp. are commonly used in detergents, also Candida and Chromobacterium [38]. They are reports regarding the production of biosurfactant from Ochrobactrum anthropi strain, Ochrobactrum sp. 1C, Ochrobactrum anthropi strain 2/3, Ochrobactrum sp. strain BS-206 (MTCC 5720) [2, 39–41]. However, Mishra et al. reported biosurfactant and lipase production from O. intermedium strain P2 [42].
Table 2.
Phenotypic characteristics of isolated bacteria O. intermedium strain MZV101
| Test |
O.intermedium
Strain MZV101 |
O.intermedium
strain LMG3301 |
|---|---|---|
| Growth at 37 °C | + | + |
| H2S production/Utilization of: | ||
| Glycine | + | + |
| D-Alanine | + | + |
| L-Aspartate | + | + |
| D-Arabinose | + | + |
| L- Arabinose | + | + |
| Mannitol | + | + |
| Sorbitol | + | + |
| D-Lyxose | + | + |
| D-Fructose | + | + |
| Mannitol | – | – |
| D- Sorbbitol | + | + |
| Glycerol | – | – |
| Sucrose | + | + |
| Hydrolysis of: | ||
| Gelatin | – | – |
| Urease test after 24 h | – | – |
| Urease test after 48 h | – | – |
| Utilization of: | ||
| Citrate | + | + |
| Sensitivity to antibiotics | ||
| Amoxicillin (25 mg) | R | R |
| Colistin (50 mg) | R | R |
| Chloramphenico (30 mg) | R | R |
| Tetracycline (30 mg) | R | R |
Morphological, physiological and biochemical characteristics of O. intermedium strain MZV101 had comparison with O. intermedium strain LMG3301. Results are scored as (+) positive, (−) negative and (R) resistance. Data were obtained from velasco et al. study [36]
Fig. 1.
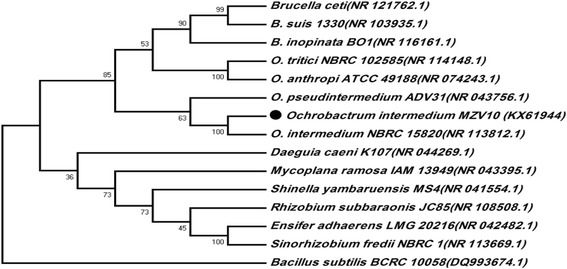
Phylogenetic tree. The Phylogenetic tree is generated on the basis of 16S rDNA gene sequence of isolated O. intermedium strain MZV101 using neighbor-joining method. According to 16S rDNA gene sequencing, BLAST in NCBI GenBank (access number KX619441) and strain was closely related to Ochrobactrum intermedium
Effect of different factors on lipase and biosurfactant stability
Effect of pH
High pH could modify protein structure and consequently changes the enzyme activity. The optimum lipase bacteria activity reported to be over ranges of pH 5–9 [43]. Generally, laundering performed at alkaline conditions, thus alkaline lipase were prefer for laundry detergent [33]. So, enzyme function at high pH is essential in detergent formulation [44]. O. intermedium strain MZV101 lipase maintains its activity and stability at pH 5–13 with maximum activity at pH 9 at 37 °C (Fig. 2). Lipase enzyme O. intermedium strain MZV101 has 80% stability at pH 10–13 and minimum stability between pH 6–8. The enzyme also remains stable after 24 h in all indicated pH range at room temperature. Chen et al. have declare that alkaline lipase from Achetobacter radioresistens was stable in optimum pH 10.0 at 30 °C and as a good potential for detergent application [45]. This report is in agreement with our results. Accordingly, other studies revealed high relative activity at lower pH from our obtained data in this study. For instance, Golaki et al. (2015) have been reported 70% relative activity of lipase 3646 from thermophilic indigenous Cohnella sp. A01 was stable in the pH range of 7 to 9 and maximal activity was obtained at pH 8.5 [46].
Fig. 2.
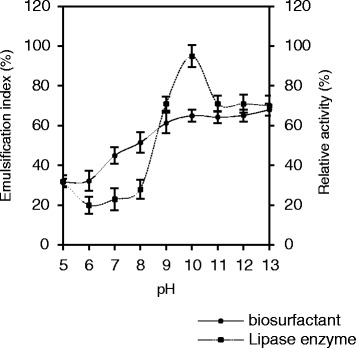
Effect of pH on lipase and biosurfactant stability of O. intermedium strain MZV101.The test were studded in pH 5–13 in temperature 37 °C which incubated 4 h for lipase and 24 h for biosurfactant. Results were represented as mean ± S.D. and all tests are examined at least in triplicate independent experiments. Error bar has indicated standard deviations shown in all figures
Biosurfactant with high stable ability in the presence of alkaline condition is required for the detergent additive because pH of laundry is generally between ranges of 9.0–12.0 [47, 48]. Many reports have been revealed the important role of bacteria biosurfactant as laundry detergents additives [12, 47, 49]. High stability was observed from pH 10–13 (E24 average = 65.58%), on the other hand, emulsification index values decreased from pH 5–6 (E24 average = 31.84%) (Fig. 2).These results showed that emulsification index values are accompanied with pH. These results are in agreement with those by Shavandi et al. who reported that emulsification activity was sensitive to pH variations [50]. From our same species, Ferhat et al. reported that biosurfactant from Ochrobactrum sp. 1C the highest (E24 average = 95%) at pH 7 to 11 was observed, while emulsification values decrease to (E24 = 70.58%) at pH 4 and temperature 37 °C [39]. Bhattacharya et al. have declaim that biosurfactant from Ochrobactrum sp. C1 the highest emulsification index values (E24 = 69.42%) were observed at low pH 7.3 and temperature 36.4 °C [51].
Effect of temperature
Lipase enzyme with activity and stability at the wide range of temperature is favoring characteristic for detergent applications, at high temperatures due to their responses provides fewer microbial contamination threats, high solubility of substrates and low viscosity of the reaction elements [52]. At low temperature can accomplish with synthetic unstable compounds and improves oil elimination from fabric; consequently, reduces energy consumption [52–54]. According to test results, lipase of O. intermedium strain MZV101 had remained active and stable at temperature range of 4–90 °C (Fig. 3). The lipase of O. intermedium strain MZV101 remains 80% active at 70–90 °C and with an optimal activity at 60 °C for 1 h at pH 10. In contrast to other lipases, lipase enzyme of O. intermedium strain MZV101 remained active even after 4 h at 60 °C [5]. Similarly, 60% relative activity for lipase 36,464 from thermophilic indigenous Cohnella sp. A01 at 60 °C and pH 10 for 180 min has been reported [46]. Lipases from Bacillus megaterium AKG-1 and Acinetobacter sp. have reported to be in the optimum temperatures range of 50–60 °C [55, 56]. Recently, García-Silvera et al. have described that lipase stability from Serratia marcescens wild type and three mutant strains in temperature over range of 5 to 55 °C with an optimum in temperature 50 °C and stable at pH 6 to 10 with optimum pH 8 for 1 h were obtained for detergent formulation and biodiesel [52]. Comparably with our results, Bora et al. have mentioned that the lipase from Bacillus sp. DH4 with optimum activity at temperature 60 °C and pH 9 as an additive for detergent formulation [57]. Cherif et al. have explained a high alkaline lipase produced by Staphylococcus sp. strain ESW the optimum activity was at temperature 60 °C and pH 12 and it was an ideal choice in detergent formulations [24].
Fig. 3.
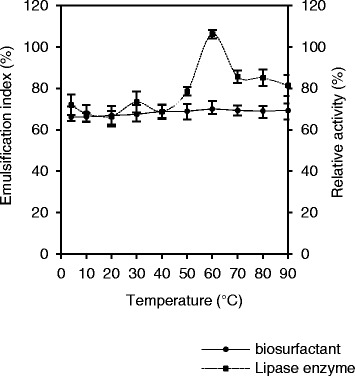
Effect of temperature on lipase and biosurfactant stability of O. intermedium strain MZV101. Experiments were measured in temperature 10–90 °C in optimal pH 10 which incubated 4 h for lipase and 24 h for biosurfactant
Nevertheless, its emulsification index values increased with temperatures between 4 °C (E24 = 66.25%) to 60 °C (E24 = 70.41%), and this value remain stable between temperatures of 70–90 °C (E24 average = 69.34%) (Fig. 3). According to results, biosurfactant of O. intermedium strain MZV101 at wide range of temperature (4–90 °C) had remained stable and had no significant effect on emulsification index. Ferhat et al. reported that biosurfactant produced by Brevibacterium sp. 7G, it emulsification index values remain stable between 20 and 100 °C [39]. Mukherjee reported that biosurfactant produced by Bacillus subtilis DM-03 and DM-04 have thermal stability at pH 7–12 and 80 °C for 60 min, also compatibility and stability with commercial laundry detergents [48]. Comparable with our data, Sajna et al. mentions that biosurfactant from Pseudozyma sp. NII 08165 as an additive for laundry detergent at pH 8–12 and temperature 80 °C was stable for 2 h [47]. It has been described that biosurfactant from Bacillus subtilis PF1with good stability at pH 6–11 and temperature 30–60 °C and emulsification index (E24 = 100%) at pH 10–11 and 30 °C as laundry detergent additive [12]. In contrast to our data, other studies found stable biosurfactant as detergent additive in low pH and temperature. For instance, Hirata et al. [59] have reported that glycolipid biosurfactant from Candida bombicola ATCC22214 was active at temperature 20 °C and pH 8.94 as biodegradable low-foaming surfactants with high detergency ability [58].
Effect of different metal ions
Lipase enzyme with detergent compatibility acts differently in presence of metal ions [54]. Various studies have demonstrated that the activity of lipase enzyme depends on the presence of Ca2+, our study shows that lipase O. intermedium strain MZV101 can be activated in the absence of Ca2+ [49, 59]. Lipase of O. intermedium strain MZV101 was 55% inhibited by Mg2+ and 33% by Hg2+ and Ba2+, while it was activated toward Co2+ (Table 3). Kanwar et al. (2006) have reported lipase from Bacillus coagulans MTCC-6375 an average relative activity 112.93% on the presence of 1 mM of Mg2+, Ca2+ and Hg2 +, while activity decreases to 39.7% toward 1 mM of Co2+ [60]. Sekhon et al. have explained that an extracellular lipase isolated from Bacillus megaterium AKG-1 in existence of metal ions Co2+, Ca2+ and Mg2+ stimulated the enzyme activity to 276, 325, 250% respectively, whereas Hg2+ and Zn2+ inhibits lipase enzyme activity to 58% [56].
Table 3.
Effect of metal ions on biosurfactant and lipase stability of O. intermedium strain MZV101
| Metal ions | Emulsification index (%) | Relative activity (%) |
|---|---|---|
| Control | 100 | 100 |
| BaCl2 | 95.99 | 78.8 |
| CaCl2 | 96.27 | 97.41 |
| CoCl2 | 95.79 | 114.58 |
| HgCl2 | 95.49 | 81.52 |
| MgCl2 | 94.01 | 48.68 |
| NaCl | 94.38 | 87.70 |
| ZnCl2 | 95.67 | 80.70 |
The experimental were tested with final concentration of 1 mM on lipase and biosurfactant stability of O. intermedium strain MZV101 in optimal pH 10, and temperature of 60 °C incubated respectively for 4 h and 24 h. In this study, results are expressed as mean ± S.D. and all tests were examined at least in triplicate independent experiments
High stability of biosurfactant O. intermedium strain MZV101 was observed in the presence of Ca2+, Mg2+, Zn2+, Hg2+ and Co2+ (E24 average = 95.53%) (Table 3). In this study, results revealed that other metal ions had no significant effect on enzyme and biosurfactant stability (Table 3). It is a very important factor for detergent formulate that enzyme and biosurfactant stay stable at presence of metal ions [61]. As pointed by Kanna et al. that biosurfactant from Pseudomonas putida MTCC 2467 after 48 h incubation in presence of metal ions Ca2+, Mg2+ and Hg2+ with 20 (g/l) concentration, it activity was less than control and no significant difference in effect between metal ions [62]. Maneerat et al. reported that crude biosurfactant from Acinetobacter calcoaceticus subsp. anitratus SM7 also remained stable in the presence of low concentration of Ca2+ and Mg2+ metal ions [61]. The findings of the present study are in line with the results in the preceding paragraph. Ochoa-Loza et al. have observed lower activity by biosurfactant from Pseudomonas aeruginosa ATCC 9027 in the presence of Zn2+, Ca2+, Hg2+ Mg2+, and Co2+ with 0. 5 mM concentration at pH 6.9 and room temperature incubation for 2 h [63]. This is in inconsistent with our obtained data in this research.
Effect of different organic solvents and detergents
Lipase enzyme with removal ability could not be effective as detergent addictive component [11]. Functional alkaline lipase in the presence of other detergent and ingredients are necessary specifications for detergent application [32]. Toida et al. have reported that the activity of lipase dramatically reduced in the presence of 1% SDS [64]. In contrast to other reports, lipase produced by O. intermedium strain MZV101 not only was stable in the presence of 1% SDS, but even showed 30% increase of relative activity. Recently, the reports have shown that addition of Tween-20 increase lipolytic activity of some lipase enzymes. In this study, the lipase enzyme has about 70–80% in the presence of Tween- 80, benzene and Chloroform as seen in Table 1 [64]. Lipase enzyme activity has exhibited an average activity of 117.03% in the presence of ethyl acetate, toluene and cyclohexane (Table 4). Lipase from Pseudomonas fluorescens P21 has been found to be stable toward toluene, benzene, cyclohexane and hexane with residual activity 66.7, 68, 80 and 94.1% respectively after incubation for 2 h [65]. These results are in line with our obtained data in this research. Lipase enzyme strain MZV101 was shown 35.11% relative activity in existence of Triton X-100. Several authors have been reported solvent tolerant lipases produced by Pseudomonas sp. and Burkholderia sp. [37, 65]. In contrast to our results, Rathi et al. have described that alkaline lipase from Burkholderia cepacia RGP-10 for detergent formulation remains 80% active in presence Triton X-100, while 33, 57 and 40% relative activity toward SDS, Tween-20 and Tween-80 respectively at 37 °C and pH 11 for 1 h [37]. 80–75% inhibition was shown toward acetone, ethanol and methanol after incubation for 4 h. Relatively close to our observations, Bose et al. have explained that 25, 50 and 75 (v/v%) methanol caused approximately 90–97% inhibited lipase activity from Pseudomonas aeruginosa AAU2 activity after 24 h and 48 h of incubation [66]. Equally, Lipase from Pseudomonas fluorescens P21 has been reported to be unstable toward acetone and methanol [65].
Table 4.
Effect of 0.1% organic solvents and 25% detergents on biosurfactant and lipase of O. intermedium strain MZV101 stabilities
| Organic solvents/detergents | Emulsification index (%) | Relative activity (%) |
|---|---|---|
| Control | 100 | 100 |
| Acetone | 94.59 | 20.00 |
| Benzene | 88.23 | 77.46 |
| Chloroform | 88.98 | 44.01 |
| Cyclohexane | 87.83 | 112.67 |
| Dimethyl | 82.45 | 88.02 |
| Ethanol | 92.99 | 23.05 |
| Ethyl Acetate | 91.21 | 107.18 |
| Hexane | 86.75 | 101.01 |
| Methanol | 93.23 | 25.07 |
| Toluene | 87.56 | 131.26 |
| 1% SDS | 98.65 | 122.53 |
| Tween-20 | 92.34 | 117.74 |
| Tween- 80 | 91.23 | 80.98 |
| Triton X-100 | 85.30 | 35.11 |
The tested were studied on lipase stability of O. intermedium strain MZV101 in optimal pH 10 and in optimal temperature 60 °C incubated for 4 h and same mentioned condition was used for biosurfactant O. intermedium strain MZV101 incubated for 24 h. Results were represented as mean ± S.D. and all tests are examined at least in triplicate independent experiments
The maximum emulsifying activity values were obtained by Acetone, Methanol and Ethanol (E24 average = 93.60%). A slight decrease in emulsification activity of biosurfactant O. intermedium strain MZV101 was observed at 1% SDS (E24 = 97.89%), 25% Tween-20 (E24 = 94.34%) and 25% Tween-80 (E24 = 91.23%). 25% Triton X-100 shows the most inhibition of biosurfactant activity (E24 = 85.31%) (Table 1). The result is in comparable with that of Ben Ayed et al. (2013) who have declare that biosurfactants from Bacillus mojavensis A21 in presence of SDS and Tween-80 with concentration 5 mg/ml at 20 °C incubated for 24 h was observed to be (E24 average = 71%) [67]. In another study, Kim et al. have declare that biosurfactants from Nocardia sp. L-417 was stable with maximum emulsification activity in presence of Triton X-100 and less activity by Tween-80 and SDS at 30 °C and pH 6 for 50 min [68]. No significant differences were observed between emulsifying activity values (E24 average = 87.43%), inhibition by the addition of Benzene, Chloroform, Cyclohexane and Hexane. Darvishi et al. have explain that a new microbial consortium of Enterobacter cloacae and Pseudomonas sp. ERCPPI-2 with emulsifying activity values at the presence of hexane (E24 = 43.4%) and cyclohexane (E24 = 57.5%) was observed [69]. Our study shows an inferior value with hexane and cyclohexane by O. intermedium strain MZV101.
Solvent purification lipase and biosurfactant
Lipase enzyme in detergent application dose not required high level of purity [70]. Abdel-Mawgoud et al. (2010) reported the purity level of biosurfactant related to final product application [71]. The biosurfactant and lipase of O. intermedium strain MZV101 were precipitated with three methods; 30–80% ammonium sulphate with acetone, ethanol and isopropanol precipitation. In order to precipitate the lipase enzyme, we attempted all mentioned methods. Enzyme precipitation failed except isopropanol which is even less common method for nucleic acid precipitation [72]. Various concentration of isopropanol from 50 to 90% was investigated. Although precipitation by organic solvents required 80–90% alcohol concentration to precipitate lipase enzyme [73], we obtain best result by 60% chilled isopropanol with (56.30 U/mg and yield 51.71%) (Table 5).
Table 5.
Extraction of biosurfactant and lipase from O. intermedium strain MZV101
| Precipitation methods | Emulsification index (%) | Biosurfactant yield (%) | Specific activity (U/mg) | Lipase enzyme yield (%) |
|---|---|---|---|---|
| Ammonium sulphate | 60.11 | 48.04 | 0.00 | 0.00 |
| Acetone | 62.87 | 38.21 | 0.00 | 0.00 |
| Ethanol | 70.99 | 50.32 | 0.00 | 0.00 |
| Isopropanol | 0.00 | 0.00 | 56.30 | 51.71 |
| dialysis | 69.45 | 48.63 | 55.00 | 49.62 |
Results of solvent extraction and dialysis biosurfactant and lipase of O. intermedium strain MZV101 were represented as mean ± S.D. All tests were examined at least in triplicate independent experiments
Even though these results differ from some earlier studies, Ameri et al. found that 80% pre-chilled ethanol with 39.8% yield was the best method to precipitate thermoalkalophilic lipase from bacterial strain Bacillus atrophaeus FSHM2 [74], and also Raza et al. (2017) found that 80% ammonium sulfate with 5.3 fold was the most suitable method to precipitate the lipase from Staphylococcus aureus for detergent industry [75], we found that this method can completely inactive the lipase from O. intermedium strain MZV101.
Isopropanol was unable to precipitate biosurfactant O. intermedium strain MZV101.The best result (E24 = 70.99% and yield = 49.32%) was obtained from ethanol and also good results were obtained with ammonium sulphate (E24 = 60.11% and yield = 48.04%) and acetone (E24 = 62.87% and yield = 38.21%) (Table 5). It has been reported that biosurfactant from Nocardia sp. L-417 was stable by extraction of ammonium sulphate fractionation and chilled acetone in two primary purification steps [68]. The best results obtained by cold ethanol precipitation (E24 = 70.99% and yield = 50.32%). Same result was reported by Ramasamy et al. for cold ethanol precipitation of the biosurfactant produced by biosurfactant Ochrobactrum anthropi MP3 [76]. Our experiments corroborate with previous results Salleh et al. who explain that organic solvent extraction was found as the best giving and the highest recovery at 89.70% (w/v) purification method for biosurfactant produced by Pseudomonas aeruginosa USM AR-2. Additionally, they were obtained good results with ammonium sulphate/acetone method precipitation [77].
Both enzyme and biosurfactant purification were continued by short dialysis against 0.1 M Tris buffer pH 9 and insignificant purity of biosurfactant and lipase was observed (Table 5). At the final step of lipase purification, the following results were obtained after the investigation of suitable column: DEAE- Cellulose column with a recovery of 5.241%, 2.649 fold, and G-10 Sephadex column with a recovery of 7.082%, 3.038 fold. Finally, the best result with a recovery of 48.452% and 19.095 fold was obtained from G-50 Sephadex column. The single protein band molecular mass was estimated on SDS-PAGE to be 99.42 kDa (Fig. 4). Comparable with our results, Kanwar et al. (2006) have mentioned high molecular mass of approximately 103 kDa on SDS-PAGE for lipase from Bacillus coagulans MTCC-6375 [60]. Furthermore, Dosanjh et al. have reported higher molecular mass 112 kDa from Bacillus GK 8 [78]. In contrast, Kumar et al. have describe lower molecular mass 31 kDa from B. coagulans BTS-3 [79] and Huang et al. have published protein mass 62-kDa lipase from Geotrichum marinum [80].
Fig. 4.
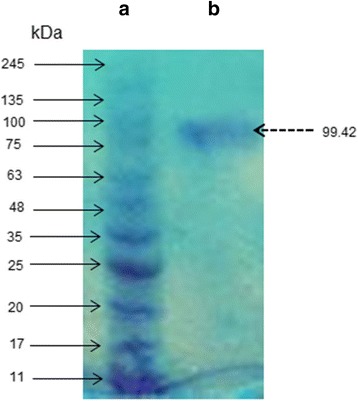
SDS-PAGE gel of Lipase enzyme O. intermedium strain MZV101. (A) standard marker proteins, (B) Single protein band become visible at 99.42 kDa on 12% sodium dodecyl sulfate-polyacrylamide gel after stained with coomassie blue G 250
Antimicrobial activity
Antibacterial detergents experimentally were proved to have bacteristatic activity, inhibit their growth and kill bacteria at a specific concentration [81]. Antimicrobial biosurfactant in detergents are very effective against some Gram-positive and Gram-negative bacteria specially, it could be very helpful against multi-drug-resistant pathogens [37, 82], and on the other hand, it can cause antimicrobial resistances in bacteria. Gharaei-Fathabad et al. reported that biosurfactant from B. circulans has antimicrobial activity against Gram negative and Gram positive pathogens and semi pathogenic microbial strains including MDR strain [83]. In this study, purified biosurfactant of Ochrobactrum intermedium strain MZV101 were used. The result of antimicrobial activity was investigated against Gram-positive and Gram-negative bacteria by measuring the zone of inhibition (Table 6).
Table 6.
Biosurfactant as an antimicrobial agent produced by O. intermedium strain MZV101
| Microorganism | Zone of inhibition diameter (mm) |
|---|---|
| Gram positive | |
| Bacillus subtilis (ATCC 465) | 3 |
| Bacillus cereus (ATCC11778) | 4 |
| Enterococcus faecalis (ATCC11778) | 3 |
| Staphylococcus aureus (ATCC 25923 s) | 2 |
| Streptococcus pyogenes (ATCC 8668) | 4 |
| Gram negative | |
| Escherichia coli (ATCC 25922) | 14 |
| Pseudomonas aeruginosa (ATCC 85327) | 10 |
| Proteus vulgaris (ATCC 13315) | 12 |
| Salmonella typhi (PTCC 1609) | 11 |
| Fungi | |
| Aspergillus niger (N 402) | 1 |
| Candida albicans (ATCC 10231), | 0.5 |
| Klebsiella pneumoniae (ATCC 10031) | 1.5 |
| Saccharomyces cerevisiae (BY 4743) | 1 |
MHA plate was incubated at 37 °C, 24 h for bacterial and 48 h for fungi pathogens and the diameter of inhibition zone was measured [22]
Higher antimicrobial activity with diameter of 10–14 mm clear zone was observed against the Gram-negative bacteria. The highest inhibition of zone were obtained with Escherichia coli (ATCC 25922) (14 mm), Proteus vulgaris (ATCC 13315) (12 mm) and Salmonella typhi (PTCC 1609) (11 mm). Biosurfactant of O. intermedium strain MZV101 demonstrated a weak antimicrobial activity with 2–4 mm diameter of clear zone against Gram-positive bacteria, and also the same result was observed against fungi. Noparat et al. have reported high antimicrobial activity against Gram-positive and lower activity against Gram-negative of biosurfactant produced by Ochrobactrum anthropi strain 2/3 against several pathogens and this is in contrast to the results achieved in our study from biosurfactant of O. intermedium strain MZV101 [41]. The inhibition of zone biosurfactant by isolated bacteria O. intermedium strain MZV101 against S. aureus (ATCC 25923 s) and Pseudomonas aeruginosa (ATCC 85327) were recorded to be in respect 2 mm and 15 mm. Biosurfactant from Ochrobactrum sp. 1C has reported to obtained (11.1937 mm) inhibition zone with S. aureus (ATCC 9144) and (15.9468 mm) with P. aeruginosa (ATCC) [39].
Washing test
The washing test of the lipase and biosurfactant O. intermedium strain MZV101 isolated in the present study was evaluated by their ability to remove olive oil stain from cloth. The solution of 1% detergent and Tris buffer could only remove 16.11% and buffer solution alone had insignificant removal ability. At alkaline condition, fatty acids were more easily eliminate from fabric but they stay on fabric because they are unable to saponify by the alkaline solution. Thus, by addition of lipase enzyme they could remove easily from fabric [15]. With the combination of lipase, detergent and buffer, high oil removal of 76.34% was observed and also the presence of lipase O. intermedium strain MZV101 without detergent shows 67.63% olive oil removal. Thirunavukarasu et al. (2008) had reported that the percentage of olive oil removal lipase produced by Cryptococcus sp. S-2 with other detergents from fabric was higher than 21.1% [65]. Grbavčić et al. reported good oil stain removal with the addition of lipase from P. aeruginosa in washing formulation [11]. It has been demonstrated that combination of extracelluar lipase from Micrococcus sp. ML-1 with different commonly used detergents could enhance the removal of greasy stains from textile [84]. Also, lipase from Ralstonia pickettii has been reported in the presence of detergent improves the removal of oil by 24–27% over treatment with detergent alone [15].
An oil removal about 60.11% was obtained in a solution containing buffer solution and isolated biosurfactant and also less olive oil removal of 23% was observed in solution without biosurfactant O. intermedium strain MZV101. Recently, it has been reported that by additional of CLP biosurfactants from B. subtilis strain DM-04 could enhance 9–12% elimination of oily stain from fabric [48]. Additionally, Jain et al. have mentioned that biosurfactant from an alkaliphilic bacterium Klebsiella sp. strain RJ-03, it alone removed 80% oil and with various laundry detergents have enhanced oil removal to (17–20%) which improved the washing performance [85].
Biosurfactant by reducing surface tension along with other enzymes is able to remove better oily stains [7]. The best result of 82.33% oil removal was obtained in combination of Lipase, biosurfactant, detergent and buffer. Hence, from the results of washing test, it can be concluded that lipase and biosurfactant O. intermedium strain MZV101 with good oil removal from white cotton can use as addictive washing component in laundry detergents. Same results have reported by Bhange et al. which biosurfactant and enzyme along with other detergent component could improve the stain removal efficiency [12].
Conclusion
In this study, as main goal we demonstrated that enzyme and biosurfactant produced by isolated bacteria Ochrobactrum intermedium strain MZV101 had high pH and temperature stability and they remain stable at presence of other metal ions and organic solvent.
The enzyme can be activated in the absence of Ca2 + .They show stability in the presence of detergents and lipase enzyme activity had increased towards of 1% SDS.
Hence, Ochrobactrum intermedium strain MZV101 with strong oil removal capability and also antimicrobial characterization is promising candidate for laundry detergent. Lipase and biosurfactant production by bacteria in a single low cost medium, fast production with good yield not only could solve economic problems but also eco-friendly detergent additives can protect our environmental.
During our study, we found isolation of microorganism in washing powder could be an efficient method for isolating compatible strain for detergent application, and also isopropanol as a new alternative organic solvent for lipase enzyme precipitation instead of usual agents used before. Our aim was to precipitate lipase and biosurfactant with same suitable method to save money and time in detergent application; therefore, this step required further investigation.
Acknowledgments
This research was funded by Department of Microbiology and Microbial Biotechnology, Faculty of Biological Sciences and Technology, university of Shahid–Beheshty, Tehran, Iran. Authors are grateful to the Department of Microbiology and Microbial Biotechnology, for their support.
Availability of data and materials
All data generated or analysed during this study are included in this published article and it is no further data in Additional file 1.
Highlights
Sampling from hot spring with high temperature and pH
Bacteria primarily cultivated and isolated in laundry washing powder
Lipase enzyme and biosurfactant were both resistance to detergent and they showed high efficiency in presence of detergent.
High temperature and pH stability lipase enzyme and biosurfactant
Enzyme precipitated with isopropanol, different usual organic solvent
Enzyme and biosurfactant as additives detergent with antimicrobial activity and oil removal are good candidate for detergent application
Abbreviations
- BLAST
Basic Local Alignment Search Tool
- DEAE- Cellulose
Diethyl Aminoethyl –Cellulose
- kDa
Kilodalton
- MHA
Muller-Hinton Agar
- NCBI
- p-NPP
para-Nitrophenyl Palmitate
- SDS-PAGE
Sodium Dodecyl Sulfate – Polyaryl Amidee Gel Electrophoresis
Authors’ contributions
MZ, GE and HS were designed the experiments, analyzed the data and MZ have drafted the manuscript. GE has approved the final version of the manuscript to be published. All authors read and approve the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mina Zarinviarsagh, Email: zarinmina@yahoo.com.
Gholamhossein Ebrahimipour, Email: g-ebrahimi@sbu.ac.ir.
Hossein Sadeghi, Email: sadeghi.hsn88@gmail.com.
References
- 1.Dalmaso GZL, Ferreira D, Vermelho AB. Marine Extremophiles: a source of Hydrolases for biotechnological applications. Mar Drugs. 2015;13:1925–1965. doi: 10.3390/md13041925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiraldi C, De Rosa M. The production of biocatalysts and biomolecules from extremophiles. Trends Biotechnol. 2002;20:515–521. doi: 10.1016/S0167-7799(02)02073-5. [DOI] [PubMed] [Google Scholar]
- 3.Wilson ZE, Brimble MA. Molecules derived from the extremes of life. Nat Prod Rep. 2009;26:44–71. doi: 10.1039/B800164M. [DOI] [PubMed] [Google Scholar]
- 4.Calvo C, Silva-Castro GA, Uad I, García Fandiño C, Laguna J, González-López J. Efficiency of the EPS emulsifier produced by Ochrobactrum anthropi in different hydrocarbon bioremediation assays. J Ind Microbiol Biotechnol. 2008;35:1493–1501. doi: 10.1007/s10295-008-0451-5. [DOI] [PubMed] [Google Scholar]
- 5.Charpentier S, Amiche M, Mester J, Vouille V, Le Caer J-P, Nicolas P, Delfour A. Structure, synthesis, and molecular cloning of Dermaseptins B, a family of skin peptide antibiotics. J Biol Chem. 1998;273:14690–14697. doi: 10.1074/jbc.273.24.14690. [DOI] [PubMed] [Google Scholar]
- 6.Hassanshahian M. Isolation and characterization of biosurfactant producing bacteria from Persian gulf (Bushehr provenance) Mar Pollut Bull. 2014;86:361–366. doi: 10.1016/j.marpolbul.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Ramnani P, Kumar SS, Gupta R. Concomitant production and downstream processing of alkaline protease and biosurfactant from bacillus licheniformis RG1: bioformulation as detergent additive. Process Biochem. 2005;40:3352–3359. doi: 10.1016/j.procbio.2005.03.056. [DOI] [Google Scholar]
- 8.Donio MBS, Ronica SFA, Viji VT, Velmurugan S, Jenifer JA, Michaelbabu M, Citarasu T. Isolation and characterization of halophilic bacillus sp. BS3 able to produce pharmacologically important biosurfactants. Asian Pac J Trop Med. 2013;6:876–883. doi: 10.1016/S1995-7645(13)60156-X. [DOI] [PubMed] [Google Scholar]
- 9.Gudiña EJ, Rangarajan V, Sen R, Rodrigues LR. Potential therapeutic applications of biosurfactants. Trends Pharmacol Sci. 2013;34:667–675. doi: 10.1016/j.tips.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 10.de Carvalho CCCR. Enzymatic and whole cell catalysis: finding new strategies for old processes. Biotechnol Adv. 2011;29:75–83. doi: 10.1016/j.biotechadv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Grbavčić S, Bezbradica D, Izrael-Živković L, Avramović N, Milosavić N, Karadžić I, Knežević-Jugović Z. Production of lipase and protease from an indigenous Pseudomonas Aeruginosa strain and their evaluation as detergent additives: compatibility study with detergent ingredients and washing performance. Bioresour Technol. 2011;102:11226–11233. doi: 10.1016/j.biortech.2011.09.076. [DOI] [PubMed] [Google Scholar]
- 12.Bhange K, Chaturvedi V, Bhatt R. Simultaneous production of detergent stable keratinolytic protease, amylase and biosurfactant by Bacillus Subtilis PF1 using agro industrial waste. Biotechnol Rep. 2016;10:94–104. doi: 10.1016/j.btre.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlegel HG, Kaltwasser H, Gottschalk G. A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies. Archiv fur Mikrobiologie. 1961;38:209–222. doi: 10.1007/BF00422356. [DOI] [PubMed] [Google Scholar]
- 14.Bockmühl DP. Laundry hygiene-how to get more than clean. J Appl Microbiol. 2017;122:1124–1133. doi: 10.1111/jam.13402. [DOI] [PubMed] [Google Scholar]
- 15.Hemachander C, Puvanakrishnan R. Lipase from Ralstonia pickettii as an additive in laundry detergent formulations. Process Biochem. 2000;35:809–814. doi: 10.1016/S0032-9592(99)00140-5. [DOI] [Google Scholar]
- 16.Kouker G, Jaeger KE. Specific and sensitive plate assay for bacterial lipases. Appl Environ Microbiol. 1987;53:211–213. doi: 10.1128/aem.53.1.211-213.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler UK, Stuckmann M. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia Marcescens. J Bacteriol. 1979;138:663–670. doi: 10.1128/jb.138.3.663-670.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Rodrigues LR, Teixeira JA, van der Mei HC, Oliveira R. Isolation and partial characterization of a biosurfactant produced by Streptococcus Thermophilus a. Colloids Surf B: Biointerfaces. 2006;53:105–112. doi: 10.1016/j.colsurfb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Willumsen PA, Karlson U. Screening of bacteria, isolated from PAH-contaminated soils, for production of biosurfactants and bioemulsifiers. Biodegradation. 1996;7:415–423. doi: 10.1007/BF00056425. [DOI] [Google Scholar]
- 21.Bodour AA, Miller-Maier RM. Application of a modified drop-collapse technique for surfactant quantitation and screening of biosurfactant-producing microorganisms. J Microbiol Methods. 1998;32:273–280. doi: 10.1016/S0167-7012(98)00031-1. [DOI] [Google Scholar]
- 22.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 23.Harju S, Fedosyuk H, Peterson KR. Rapid isolation of yeast genomic DNA: bust n’ grab. BMC Biotechnol. 2004;4:8–8. doi: 10.1186/1472-6750-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherif S, Mnif S, Hadrich F, Abdelkafi S, Sayadi S. A newly high alkaline lipase: an ideal choice for application in detergent formulations. Lipids Health Dis. 2011;10:221. doi: 10.1186/1476-511X-10-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 26.Kanjanavas P, Khuchareontaworn S, Khawsak P, Pakpitcharoen A, Pothivejkul K, Santiwatanakul S, Matsui K, Kajiwara T, Chansiri K. Purification and characterization of organic solvent and detergent tolerant lipase from thermotolerant bacillus sp. RN2. Int J Mol Sci. 2010;11:3783–3792. doi: 10.3390/ijms11103783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanwar L, Gogoi BK, Goswami P. Production of a pseudomonas lipase in n-alkane substrate and its isolation using an improved ammonium sulfate precipitation technique. Bioresour Technol. 2002;84:207–211. doi: 10.1016/S0960-8524(02)00061-5. [DOI] [PubMed] [Google Scholar]
- 28.Lin S-C, Jiang H-J. Recovery and purification of the lipopeptide biosurfactant of Bacillus Subtilis by ultrafiltration. Biotechnol Tech. 1997;11:413–416. doi: 10.1023/A:1018468723132. [DOI] [Google Scholar]
- 29.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Vieille C, Burdette DS, Zeikus JG. Thermozymes. Biotechnol Annu Rev. 1996;2:1–83. doi: 10.1016/S1387-2656(08)70006-1. [DOI] [PubMed] [Google Scholar]
- 31.Morita RY. Extremes of biodiversity. Bioscience. 1999;49:245–248. doi: 10.2307/1313521. [DOI] [Google Scholar]
- 32.Jurado E, Bravo V, Luzón G, Fernández-Serrano M, García-Román M, Altmajer-Vaz D, Vicaria JM. Hard-surface cleaning using lipases: enzyme–surfactant interactions and washing tests. J Surfactant Deterg. 2007;10:61–70. doi: 10.1007/s11743-006-1009-z. [DOI] [Google Scholar]
- 33.L-j C, X-w J, Zhang F, Zheng B-W, Shu F-C, Wang Z-L, Cui Q-F, Dong H-P, Zhang Z-Z, Hou D-J, She Y-H. Isolation and characterization of a crude oil degrading bacteria from formation water: comparative genomic analysis of environmental Ochrobactrum intermedium isolate versus clinical strains. J Zhejiang Univ Sci B. 2015;16:865–874. doi: 10.1631/jzus.B1500029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter V, Syldatk C, Hausmann R. Screening concepts for the isolation of biosurfactant producing microorganisms. In: Sen R, editor. Biosurfactants. New York: Springer New York; 2010. pp. 1–13. [DOI] [PubMed] [Google Scholar]
- 35.Nalini S, Parthasarathi R. Biosurfactant production by Serratia rubidaea SNAU02 isolated from hydrocarbon contaminated soil and its physico-chemical characterization. Bioresour Technol. 2013;147:619–622. doi: 10.1016/j.biortech.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 36.Velasco J, Romero C, Lopez-Goni I, Leiva J, Diaz R, Moriyon I. Evaluation of the relatedness of Brucella spp. and Ochrobactrum anthropi and description of Ochrobactrum intermedium sp. nov., a new species with a closer relationship to Brucella spp. Int J Syst Bacteriol. 1998;48:759–768. doi: 10.1099/00207713-48-3-759. [DOI] [PubMed] [Google Scholar]
- 37.Rathi P, Saxena RK, Gupta R. A novel alkaline lipase from Burkholderia cepacia for detergent formulation. Process Biochem. 2001;37:187–192. doi: 10.1016/S0032-9592(01)00200-X. [DOI] [Google Scholar]
- 38.Hasan F, Shah AA, Hameed A. Industrial applications of microbial lipases. Enzym Microb Technol. 2006;39:235–251. doi: 10.1016/j.enzmictec.2005.10.016. [DOI] [Google Scholar]
- 39.Ferhat S, Mnif S, Badis A, Eddouaouda K, Alouaoui R, Boucherit A, Mhiri N, Moulai-Mostefa N, Sayadi S. Screening and preliminary characterization of biosurfactants produced by Ochrobactrum sp. 1C and Brevibacterium sp. 7G isolated from hydrocarbon-contaminated soils. Int Biodeterior Biodegrad. 2011;65:1182–1188. doi: 10.1016/j.ibiod.2011.07.013. [DOI] [Google Scholar]
- 40.Kumar CG, Sujitha P, Mamidyala SK, Usharani P, Das B, Reddy CR. Ochrosin, a new biosurfactant produced by Halophilic ochrobactrum sp. strain BS-206 (MTCC 5720): purification, characterization and its biological evaluation. Process Biochem. 2014;49:1708–1717. doi: 10.1016/j.procbio.2014.07.004. [DOI] [Google Scholar]
- 41.Noparat P, Maneerat S, Saimmai A. Utilization of palm oil decanter cake as a novel substrate for biosurfactant production from a new and promising strain of Ochrobactrum anthropi 2/3. World J Microbiol Biotechnol. 2014;30:865–877. doi: 10.1007/s11274-013-1493-z. [DOI] [PubMed] [Google Scholar]
- 42.Mishra S, Singh SN. Microbial degradation of n-hexadecane in mineral salt medium as mediated by degradative enzymes. Bioresour Technol. 2012;111:148–154. doi: 10.1016/j.biortech.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 43.Silva JA, Macedo GP, Rodrigues DS, Giordano RLC, Gonçalves LRB. Immobilization of Candida Antarctica lipase B by covalent attachment on chitosan-based hydrogels using different support activation strategies. Biochem Eng J. 2012;60:16–24. doi: 10.1016/j.bej.2011.09.011. [DOI] [Google Scholar]
- 44.Banat IM. The isolation of a thermophilic biosurfactant producing bacillus SP. Biotechnol Lett. 1993;15:591–594. doi: 10.1007/BF00138546. [DOI] [Google Scholar]
- 45.Chen S-J, Cheng C-Y, Chen T-L. Production of an alkaline lipase by Acinetobacter radioresistens. J Ferment Bioeng. 1998;86:308–312. doi: 10.1016/S0922-338X(98)80135-9. [DOI] [Google Scholar]
- 46.Golaki BP, Aminzadeh S, Karkhane AA, Yakhchali B, Farrokh P, Khaleghinejad SH, Tehrani AA, Mehrpooyan S. Cloning, expression, purification, and characterization of lipase 3646 from thermophilic indigenous Cohnella sp. A01. Protein Expr Purif. 2015;109:120–126. doi: 10.1016/j.pep.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Sajna KV, Sukumaran RK, Jayamurthy H, Reddy KK, Kanjilal S, Prasad RBN, Pandey A. Studies on biosurfactants from Pseudozyma sp. NII 08165 and their potential application as laundry detergent additives. Biochem Eng J. 2013;78:85–92. doi: 10.1016/j.bej.2012.12.014. [DOI] [Google Scholar]
- 48.Mukherjee AK. Potential application of cyclic lipopeptide biosurfactants produced by Bacillus Subtilis strains in laundry detergent formulations. Lett Appl Microbiol. 2007;45:330–335. doi: 10.1111/j.1472-765X.2007.02197.x. [DOI] [PubMed] [Google Scholar]
- 49.Sayari A, Agrebi N, Jaoua S, Gargouri Y. Biochemical and molecular characterization of Staphylococcus Simulans lipase. Biochimie. 2001;83:863–871. doi: 10.1016/S0300-9084(01)01327-X. [DOI] [PubMed] [Google Scholar]
- 50.Shavandi M, Mohebali G, Haddadi A, Shakarami H, Ashrafossadat N. Emulsification potential of a newly isolated biosurfactant-producing bacterium, Rhodococcus sp. strain TA6. Colloids Surf B. 2011;82:477–482. doi: 10.1016/j.colsurfb.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Bhattacharya M, Biswas D, Sana S, Datta S. Biodegradation of waste lubricants by a newly isolated Ochrobactrum sp. C1. 3 Biotech. 2015;5:807–817. doi: 10.1007/s13205-015-0282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.García-Silvera EE, Martínez-Morales F, Bertrand B, Morales-Guzmán D, Rosas-Galván NS, León-Rodríguez R, Trejo-Hernández MR. Production and application of a thermostable lipase from Serratia Marcescens in detergent formulation and biodiesel production. Biotechnol Appl Biochem. 2017. doi:10.1002/bab.1565. [DOI] [PubMed]
- 53.Kilcawley KN, Wilkinson MG, Fox PF. A novel two-stage process for the production of enzyme-modified cheese. Food Res Int. 2006;39:619–627. doi: 10.1016/j.foodres.2005.12.006. [DOI] [Google Scholar]
- 54.Holmes B, Popoff M, Kiredjian M, Kersters K. Ochrobactrum anthropi gen. Nov., sp. nov. from human clinical specimens and previously known as group Vd. Int J Syst Bacteriol. 1988;38:406–416. doi: 10.1099/00207713-38-4-406. [DOI] [Google Scholar]
- 55.Ahmed EH, Raghavendra T, Madamwar D. An alkaline lipase from organic solvent tolerant Acinetobacter sp. EH28: application for ethyl caprylate synthesis. Bioresour Technol. 2010;101:3628–3634. doi: 10.1016/j.biortech.2009.12.107. [DOI] [PubMed] [Google Scholar]
- 56.Sekhon A, Dahiya N, Tiwari RP, Hoondal GS. Properties of a thermostable extracellular lipase fromBacillus megaterium AKG-1. J Basic Microbiol. 2005;45:147–154. doi: 10.1002/jobm.200410498. [DOI] [PubMed] [Google Scholar]
- 57.Bora L, Kalita MC. Production of thermostable alkaline lipase on vegetable oils from a thermophilicBacillus sp. DH4, characterization and its potential applications as detergent additive. J Chem Technol Biotechnol. 2008;83:688–693. doi: 10.1002/jctb.1853. [DOI] [Google Scholar]
- 58.Hirata Y, Ryu M, Oda Y, Igarashi K, Nagatsuka A, Furuta T, Sugiura M. Novel characteristics of sophorolipids, yeast glycolipid biosurfactants, as biodegradable low-foaming surfactants. J Biosci Bioeng. 2009;108:142–146. doi: 10.1016/j.jbiosc.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 59.Rosenstein R. Staphylococcal lipases: biochemical and molecular characterization. Biochimie. 2000;82:1005–1014. doi: 10.1016/S0300-9084(00)01180-9. [DOI] [PubMed] [Google Scholar]
- 60.Kanwar SS, Ghazi IA, Chimni SS, Joshi GK, Rao GV, Kaushal RK, Gupta R, Punj V. Purification and properties of a novel extra-cellular thermotolerant metallolipase of Bacillus Coagulans MTCC-6375 isolate. Protein Expr Purif. 2006;46:421–428. doi: 10.1016/j.pep.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Maneerat S, Kulnaree P. Isolation of biosurfactant-producing marine bacteria and characteristics of selected biosurfactant. 2007. [Google Scholar]
- 62.Kanna R, Gummadi SN, Kumar GS. Production and characterization of biosurfactant by pseudomonas putida MTCC 2467. J Biol Sci. 2014;14:436–445. doi: 10.3923/jbs.2014.436.445. [DOI] [Google Scholar]
- 63.Ochoa-Loza FJ, Artiola JF, Maier RM. Stability constants for the Complexation of various metals with a Rhamnolipid biosurfactant. J Environ Qual. 2001;30:479. doi: 10.2134/jeq2001.302479x. [DOI] [PubMed] [Google Scholar]
- 64.Toida J, Arikawa Y, Kondou K, Fukuzawa M, Sekiguchi J. Purification and Characterization of Triacylglycerol Lipase fromAspergillus oryzae. Bioscience, Biotechnology, and Biochemistry. 1998;62:759–63. [DOI] [PubMed]
- 65.Thirunavukarasu K, Edwin Oliver NG, G. Edwinoliver N, Durai Anbarasan S, Mk G, Iefuji H, R. Kamini N. Removal of triglyceride soil from fabrics by a novel lipase from Cryptococcus sp. S-2. 2008.
- 66.Bose A, Keharia H. Production, characterization and applications of organic solvent tolerant lipase by Pseudomonas Aeruginosa AAU2. Biocatal Agric Biotechnol. 2013;2:255–266. [Google Scholar]
- 67.Ben Ayed H, Jridi M, Maalej H, Nasri M, Hmidet N. Characterization and stability of biosurfactant produced by Bacillus mojavensisA21 and its application in enhancing solubility of hydrocarbon. J Chem Technol Biotechnol. 2013;89:1007–1014. doi: 10.1002/jctb.4192. [DOI] [Google Scholar]
- 68.Kim SH, Lim EJ, Lee SO, Lee JD, Lee TH. Purification and characterization of biosurfactants from Nocardia sp. L-417. Biotechnol Appl Biochem. 2000;31:249. doi: 10.1042/BA19990111. [DOI] [PubMed] [Google Scholar]
- 69.Darvishi P, Ayatollahi S, Mowla D, Niazi A. Biosurfactant production under extreme environmental conditions by an efficient microbial consortium, ERCPPI-2. Colloids Surf B: Biointerfaces. 2011;84:292–300. doi: 10.1016/j.colsurfb.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 70.Nerurkar M, Joshi M, Pariti S, Adivarekar R. Application of lipase from marine bacteria Bacillus Sonorensis as an additive in detergent formulation. J Surfactant Deterg. 2013;16:435–443. doi: 10.1007/s11743-012-1434-0. [DOI] [Google Scholar]
- 71.Abdel-Mawgoud AM, Hausmann R, Lépine F, Müller MM, Déziel E. Microbiology monographs. Heidelberg: Springer Berlin; 2010. Rhamnolipids: detection, analysis, biosynthesis, genetic regulation, and bioengineering of production; pp. 13–55. [Google Scholar]
- 72.Jiang L, He L, Fountoulakis M. Comparison of protein precipitation methods for sample preparation prior to proteomic analysis. J Chromatogr A. 2004;1023:317–320. doi: 10.1016/j.chroma.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 73.Karlheinz D, Harald G, Oliver M. Enzyme catalysis in organic synthesis, vol. 2. Third ed: Wiley-VCH Verlag GmbH & Co. KGaA; 2012. p. 212.
- 74.Ameri A, Shakibaie M, Faramarzi MA, Ameri A, Amirpour-Rostami S, Rahimi HR, Forootanfar H. Thermoalkalophilic lipase from an extremely halophilic bacterial strain bacillus atrophaeus FSHM2: purification, biochemical characterization and application. Weinheim. Biocatal Biotransform. 2017;35:151–60.
- 75.Raza FA, Sabri AN, Rehman A, Hasnain S. Characterization of Thermophilic alkaline lipase produced by Staphylococcus Aureus suitable for leather and detergent industries. Iran J Sci Technol Trans A Sci. 2017;41:287–294. doi: 10.1007/s40995-017-0265-2. [DOI] [Google Scholar]
- 76.Ramasamy S, Mathiyalagan P, Chandran P. Characterization and optimization of EPS-producing and diesel oil-degrading Ochrobactrum anthropi MP3 isolated from refinery wastewater. Pet Sci. 2014;11:439–445. doi: 10.1007/s12182-014-0359-9. [DOI] [Google Scholar]
- 77.Salleh SM, Noh NAM, Yahya ARM. Comparitive study: different recovery techniques of rhamnolipid produced by Pseudomonas Aeroginosa USMAR-2. Int Conf Biotechnol Environ Manag IPCBEE. 2011;18:132–135. [Google Scholar]
- 78.Dosanjh NS, Kaur J. Biochemical analysis of a native and Proteolytic fragment of a high-molecular-weight Thermostable lipase from a Mesophilic bacillus sp. Protein Expr Purif. 2002;24:71–75. doi: 10.1006/prep.2001.1528. [DOI] [PubMed] [Google Scholar]
- 79.Kumar S, Kikon K, Upadhyay A, Kanwar SS, Gupta R. Production, purification, and characterization of lipase from thermophilic and alkaliphilic Bacillus Coagulans BTS-3. Protein Expr Purif. 2005;41:38–44. doi: 10.1016/j.pep.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 80.Huang Y, Locy R, Weete JD. Purification and characterization of an extracellular lipase from Geotrichum marinum. Lipids. 2004;39:251–258. doi: 10.1007/s11745-004-1227-1. [DOI] [PubMed] [Google Scholar]
- 81.Rama B, PS P, Vinita Preethi M, Pavithra S. Antimicrobial activities of soap and detergents. Adv Biores. 2011;2:10. [Google Scholar]
- 82.Tauxe RV, Hughes JM. International investigation of outbreaks of foodborne disease. BMJ. 1996;313:1093–1094. doi: 10.1136/bmj.313.7065.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gharaei-Fa E. Biosurfactants in pharmaceutical industry (a mini-review) Am J Drug Discov Devel. 2011;1:58–69. doi: 10.3923/ajdd.2011.58.69. [DOI] [Google Scholar]
- 84.Naz N, Gupta JK, Gupta LK. Application of micrococcal alkaline lipase in commonly used detergents. Indian J Microbiol. 2001;41:177–179. [Google Scholar]
- 85.Jain RM, Mody K, Mishra A, Jha B. Physicochemical characterization of biosurfactant and its potential to remove oil from soil and cotton cloth. Carbohydr Polym. 2012;89:1110–1116. doi: 10.1016/j.carbpol.2012.03.077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and it is no further data in Additional file 1.


