Abstract
Squamous cell carcinoma of the head and neck (HNSCC) and of the lung (LSCC) share some important risk factors, but differ substantially in terms of prognosis and treatment. A pulmonary nodule developing in patients with surgically cured HNSCC may pose a diagnostic dilemma. Markers able to distinguish these two common malignancies would be of major clinical importance. In this work we compared the spectrum of antinuclear antibodies (ANA) from 22 patients with SCCL to that of 40 patients with HNSCC. Patient sera were used to probe immunoblots of nuclear extracts from all four major lung cancer cell types, normal lung fibroblasts, cells cultured from a HNSCC, and keratinocytes cultured from the field cancerization. The ability to classify retrospectively LSCC from HNSCC based on serum ANA reactivities was determined by recursive partitioning analyses. We found that while both malignancies share reactivities to a small group of nuclear antigens, other reactivities are directed against proteins uniquely or preferentially expressed in either SCCL or in SCCHN cells. Our work shows that autoimmunity is a prominent feature of squamous cell carcinoma and suggests that molecular characterization of nuclear antigens recognized by ANAs may lead to the discovery of markers valuable to distinguish LSCC from HNSCC.
Keywords: Serologic markers, Autoimmunity, Antinuclear antibodies, Lung cancer, Head and neck cancer, Keratinocytesx
1. Introduction
Cancers of the lung and of the head and neck are important worldwide problems [1–5]. Although the treatment of stage I and II of head and neck cancer is often successful, the high rate of recurrences and the fact that a substantial number of cured patients develop a second primary, often lung cancer or other upper aerodigestive (UADT) cancer are major concerns [6–10]. Lung cancer is the leading cause of cancer death worldwide and despite the advances in biomarker development and cancer treatment; its prognosis has improved minimally. The dismal prognosis of lung cancer has been attributed in the past to the inadequacy of traditional screening procedures, which detect predominantly well-established tumors and usually fails to identify early lesions, which could be successfully treated [11]. The development of new biomarkers for early diagnosis of lung and of head and neck cancers and the novel techniques currently used for early diagnosis and screening of pre-malignant lung lesions promise to change this situation in the future [12–22].
Our laboratory has reported that the sera of lung cancer patients frequently contain high titer antinuclear antibodies, which are not present in age- and sex-matched sera from normal subjects [23–25]. We found associations between some of these ANAs and cancer cell type and patient outcome, suggesting the potential value of autoantibodies as specific diagnostic and prognostic markers for lung cancer. Here we focus our investigation on squamous cell carcinoma, one of the major lung cancer types and the most prominent malignancy of the head and neck. A pulmonary nodule developing in a patient with a ‘cured’ HNSCC may be metastatic from the original HNSCC or a second primary lung cancer. In our search for markers useful to differentiate these two conditions we compared the repertoire of serum ANAs from patients with LSCC to that from patients with HNSCC. We report that these two groups of cancer patients share reactivities to a small number of nuclear antigens, while other reactivities are directed to nuclear proteins uniquely or preferentially expressed by LSCC or by HNSCC.
2. Materials and methods
2.1. Study groups
In this work, we compared the serum reactivity with nuclear antigens on immunoblots from 22 patients with LSCC to the reactivity observed in the sera of 40 patients with HNSCC. All lung cancer patients were biopsy-proven with locally advanced or metastatic cancer (American Joint Committee on cancer stages IIIA, IIIB and IV). All sera from cancer patients were obtained before the initiation of the therapy. Non-cancer control sera were obtained from 104 subjects recruited from the Rheumatology Clinic at Wayne State University, who had neither a history of cancer nor diagnoses known to have an immune pathogenesis. Demographics as well as clinical characteristics of the LSCC and control group have been reported [24]. Sera from 40 patients with biopsy-proven HNSCC were obtained before initiation of treatment. The average age of the patients with HNSCC was 58.7 years (35–71), 10 were females and 30 were males. The stage was variable: I [1], II [2], IV [33] and in four the stage was unknown. All subjects in both cancer patient groups agreed to participate in the study and were recruited at the Detroit Medical Center Hematology/Oncology clinic by MK (LSCC) and by JE (HNSCC). Samples from both cancer groups were stored at −70°C until use.
2.2. Cell lines
Human lung cell lines and HeLa cells obtained from the American Type Culture Collection (ATCC) (Rockville, MD) were cultured according to specifications described for each cell line. Lung cell lines, HTB-119 (small cell carcinoma), HTB-182 (LSCC), HTB-177 (large cell carcinoma), CRL-5800 (adenocarcinoma), and HTB-157 (normal lung fibroblast) were used. The characteristics of these cell lines have been described [24]. The ‘x’ cell line was derived from a HNSCC and the ‘m’ cell line was cultured from keratinocytes from pathologically normal tissue surrounding the HNSCC tumor, both lines kindly provided by JE. All cell lines were sub-cultured to obtain sufficient material to produce nuclear extracts.
2.3. Nuclear extracts and immunoblotting
Nuclear extracts were prepared as described previously [24], and separated by SDS-PAGE [26]. Gels of different percentages were run to maximize discrimination between antigens of both low and high molecular weights. Proteins were transferred to nitrocellulose as described by Towbin et al. [27]. Patient sera were diluted 1:500 in buffer [10 mM NaPO4 (pH 7.5), 0.2 Triton X-100, 0.15 M NaCl, 1 mM EGTA, and 1 mM NaN3], incubated with membranes at 22 °C for 2 h, and then washed three times for 3–5 min with 1 × GB [50 mM triethanolamine–HCl (pH 7.4), 100 mM NaCl, 2 mM K2-EDTA, 0.5% Triton X-100, and 0.1% SDS]. Sheep antihuman IgG (Amersham, Arlington, IL) and goat antihuman IgM (Sigma, St. Louis, MO) horseradish peroxidase-linked secondary antibodies were used at 1:2500 for 1 h at 22 °C, and washes were repeated as before. Bound antibodies were detected by enhanced chemiluminiscence reagents (Amersham). Exposure time was 60 s. Developed films were scanned for computer analysis. Intensities and band positions were determined using IPLab Spectrum software (Scanalytics, Fairfax, VA). All bands with pixel intensities 20 units more than background were scored as positive. We chose this arbitrary cut-off because under these conditions nuclear reactivities were, on the average, absent in more than 97% of normal sera. Although, 67% of the LSCC and 60% of the HNSCC cancer patient sera showed reactivity at 1:500 dilution or greater, only one normal serum showed reactivity at a dilution greater than 1:500 under the same conditions. Band detection was consistent when assessed by three different operators.
Calibration of immunoblots was performed using molecular standards (Life Technologies, Inc., Gaitersburg, MD). A negative serum from a non-cancer patient was tested in each assay to control for background. A serum from a patient with CREST syndrome was reacted with each substrate as a control to verify protein integrity, transfer, and activity of detecting reagents. Molecular masses of antigens larger than 40 kDa were estimated to the nearest 5 kDa, and molecular masses smaller than 40 kDa were estimated to the nearest 2 kDa. Consistent estimates of molecular masses were obtained by two or more independent measurements. Considering reactivity toward all antigens, more than 97% of nuclear reactivities detected in cancer patient sera were not present in the sera of the non-cancer control group.
2.4. Statistical analyses
For purposes of the analyses that follow, we were interested in merely identifying differences in band sizes on immunoblots. We made no assumption about the identity of each antigen since more than one nuclear antigen can exhibit the same electrophoretic mobility. The ability of ANA reactivities to recognize bands of identical kDa to predict LSCC or HNSCC was determined by CART analyses as described previously [23,24] using CART software (Salford Systems, San Diego, CA). CART is particularly useful when there may exist many variables with a high degree of association among themselves, in this case the ANAs. CART does not assume that a function of a linear combination of covariates affects the outcome, and was developed to allow cross-validation within the same data set [28–30]. Learning and cross-validated trees were grown using 10-fold validation as recommended, which conservatively overestimates the true error rate. The overall correctly predicted percentages of the learning and cross-validated trees were determined and the predictive antigens were selected by CART in their descending order of importance [28–30]. For the trees predicting diagnosis, sensitivity is defined as the percentage of patients with disease who were correctly predicted as having LSCC or HNSCC. Specificity is defined as the percentage of normal subjects who were correctly predicted as not having these malignancies.
We performed CART analysis on ANA reactivities that were unique to each nuclear extract. We defined unique nuclear reactivity as the binding of an autoantibody of the IgG or IgM class to a nuclear antigen band identified in only one of the nuclear extracts [HeLa (h), normal lung fibroblast (n), small cell carcinoma (s), LSCC (q), adenocarcinoma of the lung (a), large cell carcinoma (l), HNSCC (x), or keratinocyte from the field cancerization (m)].
3. Results
To compare the spectrum of antinuclear antibodies in the sera from patients with LSCC and HNSCC we probed immunoblots of nuclear protein extracts prepared from HeLa, normal lung fibroblasts, small cell carcinoma, LSCC, adenocarcinoma, large cell carcinoma, HNSCC, and keratinocytes, with sera from LSCC or HNSCC patients, or from normal control subjects. The use of these cell types as sources of nuclear antigens allowed us to detect autoantigens that are uniquely or preferentially expressed in each cell type. Representative immunoblots of protein extracts from LSCC and HNSCC cells probed with sera from patients with LSCC and HNSCC and from non-cancer control subjects are shown in Figs. 1 and 2. We found that at a dilution of 1:500, IgG reactivities to a spectrum of antigens of different molecular masses were revealed using sera from LSCC and HNSCC patients but not using non-cancer control sera. Similar results were obtained for IgM antibodies (data not shown). Nuclear reactivities to all lung cancer and HNSCC antigens as well as to normal lung fibroblasts and keratinocytes were considered, and CART analysis software was used to select antigens that had predictive value to classify LSCC cases from normal subjects (Fig. 3), HNSCC from normal subjects (Fig. 4), and LSCC from HNSCC (Fig. 5). In the analysis of data from HNSCC versus non-cancer controls, the overall percentage of correctly predicted subjects was 68% with a specificity of 95% and a sensitivity of 40%, while in the analysis of data from LSCC versus non-cancer controls the percentage of correctly predicted subjects was 77% with a specificity of 95% and a sensitivity of 46%.
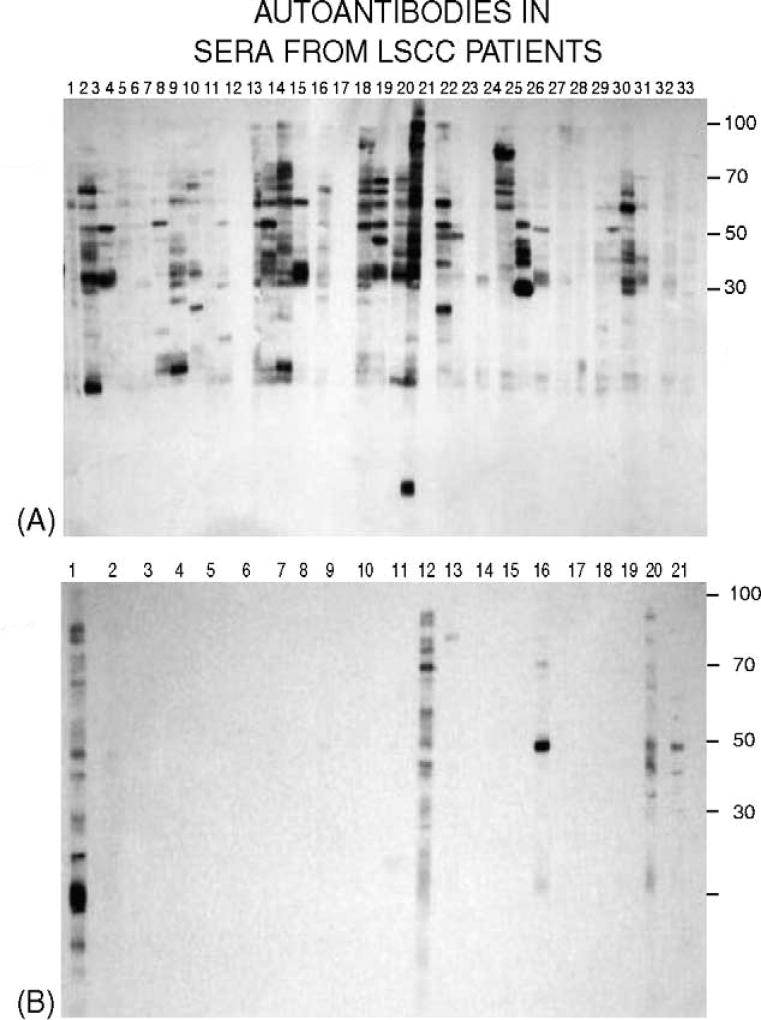
Fig. 1.
(A) Immunoblots of nuclear extracts from LSCC cells (HTB-182 human lung squamous cell carcinoma from the ATCC) probed with sera from LSCC patients and (B) with sera from subjects without cancer. Patient and control sera were diluted 1:500. Secondary antibody, sheep anti-human IgG, diluted 1:2500.
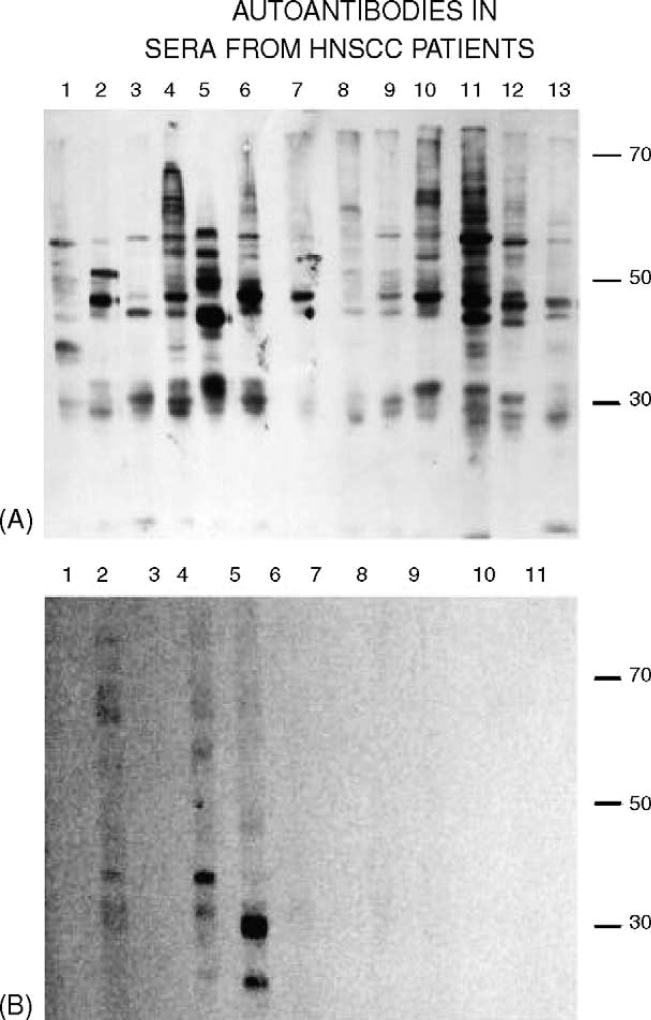
Fig. 2.
(A) Immunoblots of nuclear extracts from HNSCC cells probed with sera from patients with HNSCC and (B) with non-cancer control sera. Conditions related to primary and secondary antibodies were as in Fig. 1.
Fig. 3.
Tree to distinguish sera from LSCC patients (n = 22) from non-cancer control sera (n = 40) using all eight antigen sets (h, s, q, a, l, n, x, m). Antigen variables selected by CART analyses are presented and labeled with the first letter designating the reactive antigen set, the second letter (g or m) designating the reactive isotype IgG or IgM, and the following number designating the antigen molecular mass in kDa. The fraction of cases correctly identified over the total number of cases is included for each terminal node.
Fig. 4.
Tree to distinguish sera from patients with HNSCC (n = 40) from non-cancer control sera (n = 40) using all eight antigen sets (h, s, q, a, l, n, x, m). Antigen variables selected by CART analyses are presented and labeled as in Fig. 3.
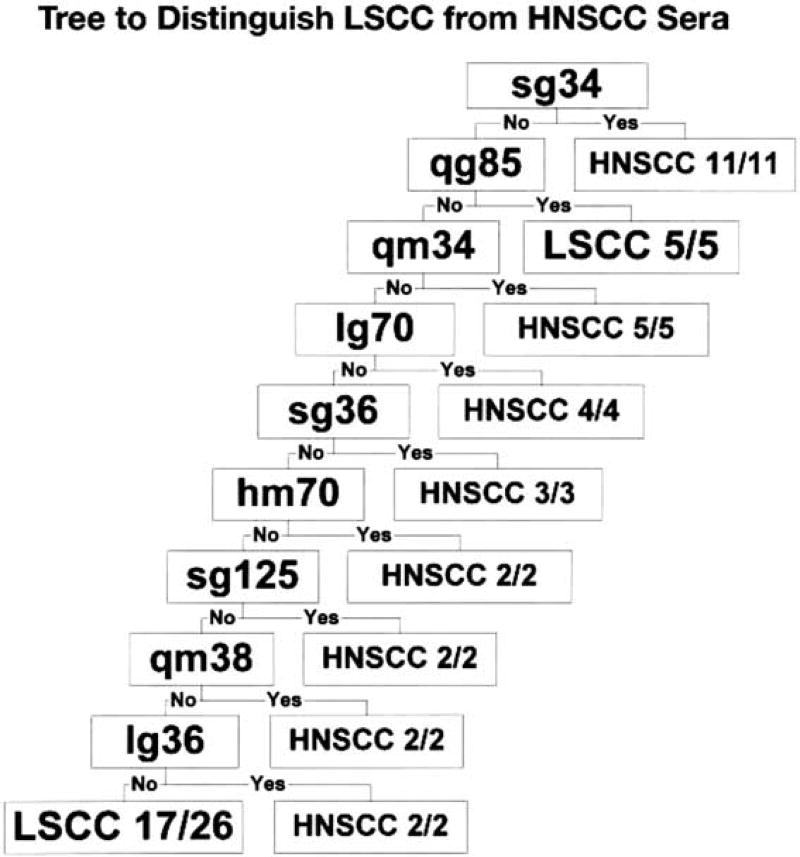
Fig. 5.
Tree to distinguish sera from patients with HNSCC (n = 40) from sera from LSCC patients (n = 22). Antigen variables selected by CART are presented and labeled as in Fig. 3.
The data indicated that autoantibodies directed to nuclear antigens have the potential to distinguish LSCC as well as HNSCC from normal subjects without cancer, respectively (Figs. 3 and 4). Moreover, comparing the reactivities of the two cancer groups, CART analysis of immunoblots using all antigens and probed with sera from 22 patients with LSCC and 40 patients with HNSCC indicated that the antigens selected could differentiate LSCC from HNSCC (Fig. 5). This differentiation had an overall percentage of correctly predicted subjects with these malignancies of 85% using the identified antigens. As presented in Fig. 6, when considering all antigens, CART analyses picked a set of unique antigens with predicting ability for LSCC and a set of different antigens with predicting ability for HNSCC, while a small group of antigens selected by CART had predicting ability for both malignancies. To be noted, three of these autoantigens were derived from HeLa cells and one from LSCC, while none were derived from the other three lung cell types or from non-cancer cell lines.
Fig. 6.
Diagrammatic summary of the results of two independent CART analyses identifying variables (nuclear antigens) listed in order of predicting ability for the diagnoses of HNSCC and LSCC from subjects without cancer, respectively. Antigen designation follows the nomenclature described in Fig. 3.
4. Discussion
Four tumor types account for 95% of all lung malignancies, small cell carcinoma, large cell carcinoma, adenocarcinoma, and LSCC [31]. While LSCC accounts for about one-third of all lung cancer cases, the majority of head and neck cancers are HNSCC [6–10]. LSCC and HNSCC share similar risk factors [1–7]. While in lung cancer cigarette smoking has been identified as the prime etiologic factor [2–5,31], most cases of HNSCC also occur in patients with an extensive history of tobacco exposure [6,7]. N-nitrosamines found in tobacco are known to produce methyl-DNA adducts. Methylnitrosamine-1-(3-pyridyl)-1-butanone (NKK) is believed to be involved in the induction of lung cancer in smokers [32] However, the risk for HNSCC increases 10-fold in those subjects that smoke and drink alcohol heavily [33]. Our approach demonstrates that many of the nuclear antigens recognized by LSCC and by HNSCC patient sera are unique, i.e., they exhibit tumor specificity and are potential diagnostic markers to distinguish LSCC from HNSCC. There are several reports in support of the hypothesis that tumor-associated antigens can be valuable as diagnostic markers in lung cancer [34–42]. The anti-Hu system has long been recognized as an example of autoantibodies used as markers of small cell lung carcinoma [38,41]. More recently the report of Hanash et al., on the common occurrence of annexin I and II autoantibodies in lung cancer sera using proteomics technology offered considerable promise of producing valuable diagnostic markers for lung cancer [12].
Analyses of the pattern of autoantigens recognized by LSCC and HNSCC patient sera show that a small group of nuclear antigens are recognized by ANAs found in the sera of both malignancies (Fig. 6). It is intriguing that three of the four shared antigens selected by CART with predicting ability were expressed in HeLa cells, which are also derived from a squamous cell carcinoma [43]. This finding suggests that part of the autoimmune response may reflect some basic antigenic commonalities in squamous cell cancers.
The majority of the autoantigens selected by CART; however, were exclusively recognized by antibodies from either LSCC or HNSCC patient sera. These results suggest that ANAs may be useful to differentiate LSCCL from HNSCC, and may reflect both differences and similarities in the underlying molecular changes that occur in these two tumor types. The heterogeneity in the antigenic profile found in these malignancies is in agreement with reported molecular and clinical differences between LSCC and HNSCC.
At the molecular level, Olshan et al. reported that the mutational pattern observed in HNSCC cancer appears to be different from that found in lung cancer [44]. For example, the G–T transversion, a mutation type considered to be characteristic of exogenos DNA-damaging agents including tobacco smoke carcinogens, varied among tobacco-related cancer sites. Boyle et al., however, reported that the spectrum of codon hotspots in HNSCC is similar to that in LSCC and 64% of mutations in p53 occur at G nucleotides, suggesting that carcinogens from tobacco smoke are involved in the etiology of HNSCC [45]. Genetically determined susceptibility factors seem also to play an important role in both SCCL and SCCHN cancer [46,47].
On clinical grounds there are also differences between LSCC and HNSCC in terms of prognosis and response to treatment. The discovery of a pulmonary nodule in a patient with HNSCC presents a diagnostic dilemma [7–10]. One of the major unresolved problems in this field relates to the nature of this new lung lesion, which can be a new primary tumor, a hematogenous spread or metastases from the original HNSCC. This is not a trivial question since the overall prognosis of lung cancer remains extremely poor. While the majority of patients with LSCC have advanced disease at the time of diagnosis and die from the consequences of the disorder, the prognosis of HNSCC has improved through the development of new treatment protocols. Thus, a serologic method allowing to make this distinction can potentially influence patient prognosis and could guide treatment strategies. We propose that serum recognition of autoantigens uniquely expressed in these tumors is an avenue that could contribute to solving this problem. Even successfully treated patients with early stage HNSCC face a constant annual 4–7% risk of developing potentially fatal second primary tumors, mostly in foci of smoking related carcinogenesis, including aerodigestive tract (UADT) and bladder cancers [8–10]. It is generally accepted that the primary therapy and related rate of survival for patients with HNSCC will very likely improve as a consequence of continuing advances in diagnostic methods [6,7]. Our data support the view that genetic and molecular diversity may be responsible for the clinical and prognostic differences between LSCC and HNSCC. It is possible that serologic markers, such as autoantibodies, may allow the early diagnosis of a second primary or a recurrence of the original cancer, thus improving the outcome of these patients. In support of this conclusion, it has become apparent that some autoantibodies are tumor restricted and may have potential as biomarkers for the diagnosis of cancer [12,23–25,36,38,39]. The accumulation of many genetic and epigenetic abnormalities found in clinically evident cancers [48,49] suggests the possibility of a multiplicity of abnormal gene products that could elicit an autoantibody response. The literature indicates that autoantibodies are prevalent in lung and other cancers and that they may occur prior to the clinical appearance of the malignancy [39,50] thus being potential biomarkers of the disease.
More recently, using a T7 bacteriophage system and a high throughput method our laboratory has reported that high titer antibodies present in breast cancer patient sera led to the cloning of a panel of autoantigens commonly recognized by sera from patients with breast cancer which appear to be potential biomarkers for the early diagnosis of breast cancer [51]. Similarly, this work shows that autoimmunity is a prominent feature of HNSCC and LSCC and suggests that molecular characterization of nuclear antigens recognized by high titer autoantibodies may lead to the discovery of proteins with diagnostic value to distinguish LSCC from HNSCC.
Acknowledgments
This work was supported by grants from the Karmanos Cancer Institute, the Lupus Foundation, the Flora Temple Fund and Mrs. Mary Webber Parker.
References
- 1.Scoggin CH. Pulmonary neoplasms. In: Wyngaarten JB, Smith LH, Bennett JC, editors. Cecil, Textbook of medicine. 19. WB Saunders; 1992. pp. 435–43. [Google Scholar]
- 2.Fry WA, Menck HR, Winchester DP. The national cancer data base report on lung cancer. Cancer. 1999;77:1947–55. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1947::AID-CNCR27>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 3.Reis LAG, Kosary CL, Hankey BF, et al. SEER cancer statistics review. Bethesda, MD: National Cancer Institute; 1999. [Google Scholar]
- 4.Coleman WB, Tsongalis GJ. Cancer epidemiology. In: Coleman WB, Tsongalis GJ, editors. The molecular basis of human cancer. Totowa, N J: Humana Press; 2002. pp. 3–22. [Google Scholar]
- 5.Sankaranarayanan R, Masuyer E, Swaminathan R, et al. Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Res. 1998;18:4779–86. [PubMed] [Google Scholar]
- 6.Forastiere A, Koch W, Trotti A, Sidransky D. Medical progress: head and neck cancer. N Engl J Med. 2001;345:1890–900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 7.Leong PP, Rezai B, Koch WM, et al. Distiguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma. J Natl Cancer Inst. 1998;90:972–7. doi: 10.1093/jnci/90.13.972. [DOI] [PubMed] [Google Scholar]
- 8.Dhooge IJ, DeVos M, Van Cauwenberge PB. Multiple primary malignant tumors in patients with head and neck cancer: results of a prospective study and future perpectives. Laryngoscope. 1998;108:250–6. doi: 10.1097/00005537-199802000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Narayana A, Vaughan AT, Fisher SG, et al. Second primary tumors in laryngeal cancer: results of long-term follow up. Int J Radiat Oncol Biol Phys. 1998;42:557–62. doi: 10.1016/s0360-3016(98)00250-8. [DOI] [PubMed] [Google Scholar]
- 10.Fontana RS, Sanderson DR, Woolner LB, et al. Screening for lung cancer: a critique of the Mayo lung project. Cancer. 1991;67:1155–64. doi: 10.1002/1097-0142(19910215)67:4+<1155::aid-cncr2820671509>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Manser RL, Irving LB, Byrnes G, et al. Screening for lung cancer: a systematic review and meta-analysis of controlled trials. Thorax. 2003;58:784–9. doi: 10.1136/thorax.58.9.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brichori FM, Misek DE, Yim AM, et al. An immune response manifested by the common occurrence of annexins I and II autoantibodies and high circulating levels of IL-6 in lung cancer. Proc Natl Acad Sci U S A. 2001;98:9824–9. doi: 10.1073/pnas.171320598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niklinski J, Chyczewski L, Becker HD, et al. Molecular genetic abnormalities in premalignant lung lesions: biological and clinical implications. Eur J Cancer Prev. 2001;10:213–26. doi: 10.1097/00008469-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Mulshine JL. Reducing lung cancer risk. Early detection. Chest. 1999;116:493S–6S. doi: 10.1378/chest.116.suppl_3.493s. [DOI] [PubMed] [Google Scholar]
- 15.Wadsworth JT, Sommers K, Stack B, et al. Identification of patients with head and neck cancer using serum protein profiles. Arch Otolaryngol-Head Neck Surg. 2004;130:98–104. doi: 10.1001/archotol.130.1.98. [DOI] [PubMed] [Google Scholar]
- 16.Selvaggi GI, Novello S, Torri V, et al. Epidermal growth factor receptor overexpression correlates with poor prognosis in completely resected stage I–IIIA non-small lung cancer. Ann Oncol. 2004;15:28–32. doi: 10.1093/annonc/mdh011. [DOI] [PubMed] [Google Scholar]
- 17.Brown J, Xu H, Nishitani J, et al. Potential biomarkers for head and neck squamous cell carcinoma. Laryngoscope. 2003;113:393–400. doi: 10.1097/00005537-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Wong I, Lo Y, Johnson P. Epigenetic tumor markers in plasma and serum: biology and applications to molecular diagnosis and disease monitoring. Ann N Y Acad Sci. 2001;945:36–50. [PubMed] [Google Scholar]
- 19.Gonzalez HE, Gujratio M, Frederick M, et al. Identification of 9 genes differentially expressed in head and neck squamous cell carcinoma. Arch Otolaryngol. 2003;129:754–9. doi: 10.1001/archotol.129.7.754. [DOI] [PubMed] [Google Scholar]
- 20.Verma M, Dunn BK, Ross S, et al. Early detection and risk assessment proceedings and recommendations from the workshop on epigenetics in cancer prevention. Ann N Y Acad Sci. 2003;983:298–319. doi: 10.1111/j.1749-6632.2003.tb05984.x. [DOI] [PubMed] [Google Scholar]
- 21.Koch W. Genetic markers in the clinical care of head and neck cancer: slow in coming. Arch Otolaryngol-Head Neck Cancer. 2001;129:367–8. doi: 10.1001/archotol.129.3.367. [DOI] [PubMed] [Google Scholar]
- 22.Brown JJ, Xu H, Nishitani J, et al. Potential biomarkers for head and neck squamous cell carcinoma. Laryngoscope. 2003;113:393–400. doi: 10.1097/00005537-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Fernández Madrid F, Granda JL, VandeVord P, et al. Antinuclear antibodies in lung cancer. Cancer Detect Prev. 1998;22:S185. doi: 10.1016/j.cdp.2004.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernández Madrid F, VandeVord PJ, Yang X, et al. Antinuclear antibodies as potential markers of lung cancer. Clin Cancer Res. 1999;5:1393–400. [PMC free article] [PubMed] [Google Scholar]
- 25.Fernández Madrid F, Tomkiel JE. Antinuclear antibodies as potential markers in the diagnosis and prognosis of lung cancer. In: Shoenfeld Y, Gershwin ME, editors. Cancer and autoimmunity. Elsevier Science; 2000. pp. 151–8. [Google Scholar]
- 26.Laemli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Towbin H, Stahelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breiman L, Friedman JH, Olshen RA, et al. Classification and regression trees. Belmont, CA: Wadsworth; 1984. [Google Scholar]
- 29.Clack LA, Pregibon D. Tree-based models. In: Chambers JM, Haste TJ, editors. Statistical models. S. Pacific grove, CA: Wadsworth; 1992. [Google Scholar]
- 30.Steinberg D, Phillip C. CART: tree structured nonparametric data analysis. San Diego, CA: Salford Systems; 1995. [Google Scholar]
- 31.Rubin E, Farber JL. The respiratory system. In: Rubin E, Farber JL, editors. Pathology. Philadelphia: JB Lippincott Co.; 1994. pp. 557–617. [Google Scholar]
- 32.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 33.Zamboni P, Talamini R, La Vecchia C, et al. Smoking, type of alcoholic beverage and squamous-cell oesophageal cancer in northen Italy. Int J Cancer. 2000;86:144–9. doi: 10.1002/(sici)1097-0215(20000401)86:1<144::aid-ijc23>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 34.Hodson ME, Turner-Warwick M. Autoantibodies in patients with bronchial carcinoma. Thorax. 1975;30:367–70. doi: 10.1136/thx.30.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kornguth SE, Klein R, Appen R, Choate J. Occurrence of anti-retinal ganglion cell antibodies in patients with small cell carcinoma of the lung. Cancer. 1982;50:1289–93. doi: 10.1002/1097-0142(19821001)50:7<1289::aid-cncr2820500711>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Buddle-Steffen C, Anderson NE, Rosenblum MK, et al. Expression of an antigen in small cell lung carcinoma lines detected by antibodies from patients with paraneoplastic dorsal root ganglionopathy. Cancer Res. 1988;48:430–4. [PubMed] [Google Scholar]
- 37.Winter SF, Minna JD, Johnson BE, Takahashi T, Gazdar AF, Carbone DP. Development of antibodies against p53 in lung cancer patients appears to be dependent on the type of p53 mutation. Cancer Res. 1992;52:4168–74. [PubMed] [Google Scholar]
- 38.Dalmau J, Graus F, Rosenblum MK, et al. Anti-Hu associated paraneoplastic encephalomyelitis/sensory neuropathy. A clinical study of 71 patients. Medicine. 1992;71:59–72. doi: 10.1097/00005792-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Frenkel K, Karkoszka J, Kato J, et al. Systemic biomarkers of cancer risk. Cancer Detect Prev. 1996;20:234. [Google Scholar]
- 40.Mooney LA, Perera FP, Van Bennekum AM, et al. Gender differences in autoantibodies to oxidative DNA base damage in cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2001;10:641–8. [PubMed] [Google Scholar]
- 41.Anhalt GJ, Nousari HC. Paraneoplastic autoimmune syndromes. In: Rose NR, Mackay IR, editors. The autoimmune diseases. 3. Academic Press; 1998. pp. 775–8. [Google Scholar]
- 42.Sangrajrang S, Sornprom A, Chernrungroj G, et al. Serum p53 antibodies in patients with lung cancer: correlation with clinicopathologic features and smoking. Lung Cancer. 2003;39:297–301. doi: 10.1016/s0169-5002(02)00509-3. [DOI] [PubMed] [Google Scholar]
- 43.Jones HW, Jr, McKusick VA, et al. George Otto Gey (1899–1970). The He La cell and a reappraisal of its origin. Obstet Gynecol. 1971;38:945–9. [PubMed] [Google Scholar]
- 44.Olshan AF, Weissler MC, Pei H, et al. P53 mutational spectra in head and neck cancer: new data and evaluation of mutational spectra. Cancer Epidemiol Biomarkers Prev. 1997;6:499–504. [PubMed] [Google Scholar]
- 45.Boyle Jo, Hakim J, Koch W, et al. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res. 1993;53:4477–88. [PubMed] [Google Scholar]
- 46.Hu N, Roth MJ, Emmert-Buck MR, et al. Allelic loss in esophageal squamous cell carcinoma patients with and without family history of upper gastrointestinal tract cancer. Clin Cancer Res. 1999;5:3476–82. [PubMed] [Google Scholar]
- 47.Shields PG. Molecular epidemiology of lung cancer. Ann Oncol. 1999;5:S7–11. doi: 10.1093/annonc/10.suppl_5.s7. [DOI] [PubMed] [Google Scholar]
- 48.Wistuba I, Beherens C, Milchgrub S, et al. Sequential abnormalities are involved in the multistage development of squamous cell lung carcinoma. Oncogene. 1999;18:643–50. doi: 10.1038/sj.onc.1202349. [DOI] [PubMed] [Google Scholar]
- 49.Franklin WA, Gazdar AF, Haney J, et al. Widely dispersed p53 mutation in respiratory epithelium: a novel mechanism for field carcinogenesis. J Clin Invest. 1997;100:2133–7. doi: 10.1172/JCI119748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomkiel JE, Alansari H, Tang N, et al. Autoimmunity to the Mr 32,000 subunit of replication protein a in breast cancer. Clin Cancer Res. 2002;8:752–8. [PMC free article] [PubMed] [Google Scholar]
- 51.Fernández Madrid F, Tang N, Alansari H, et al. Autoantibodies to annexin XI and other autoantigens in the diagnosis of breast cancer. Cancer Res. 2004;64:5089–96. doi: 10.1158/0008-5472.CAN-03-0932. [DOI] [PubMed] [Google Scholar]