Abstract
Pharmacotherapies that increase CNS expression of heme oxygenase-1 (HO-1) and other antioxidant proteins have improved outcome in experimental models of spontaneous intracerebral hemorrhage (ICH). In order to more specifically investigate the relationship between HO-1 and ICH outcome, mice expressing human HO-1 driven by the glial fibrillary acidic protein (GFAP) promoter (GFAP.HMOX1 mice) were tested in a model of in situ parenchymal hemorrhage. Injection of collagenase into the striata of wild-type (WT) mice resulted in a 26.3% mortality rate, with deaths equally distributed between males and females. Mortality was reduced to 4.48% in GFAP.HMOX1 mice. Cell viability in the injected striata of surviving WT mice was reduced by about half at one week and was significantly increased in transgenics; this benefit persisted over a 22 day observation period. Cell counts guided by design-based stereology indicated loss of ~40% of neurons in WT hemorrhagic striata at one week, which was decreased by half in transgenics; no significant differences in microglia or astrocyte numbers were observed. Blood-brain barrier disruption and short-term neurological deficits were also mitigated in GFAP.HMOX1 mice, but long-term outcome did not differ from that of WT survivors. These results suggest that astrocyte HO-1 overexpression provides robust neuroprotection after acute intracerebral hemorrhage. Further investigation of drug or genetic therapies that selectively increase astrocyte HO-1 is warranted.
Keywords: Glia, Intracerebral hemorrhage, Nrf2, Stroke, Subarachnoid hemorrhage
Introduction
Spontaneous intracerebral hemorrhage (ICH) accounts for about 10% of strokes, and continues to have a grim prognosis despite improvements in neurointensive care (Rincon and Mayer, 2013). One month mortality approximates 50%; recovery to independent living status is attained in only 20% of survivors (Broderick et al., 1999), and the vast majority report poor health-related quality of life (Christensen et al., 2009; Delcourt et al., 2016). The mechanisms mediating perihematomal cell loss have not been precisely defined, but considerable experimental evidence supports the participation of oxidative and inflammatory injury cascades that may be amenable to targeted pharmacotherapies. One promising target is heme oxygenase-1 (HO-1), which catalyzes the rate-limiting step of heme breakdown to carbon monoxide, iron and bilirubin. HO-1 robustly protects astrocytes from heme-mediated injury in vitro (Benvenisti-Zarom and Regan, 2007), and may have beneficial effects on heme clearance in vivo (Parfenova et al., 2005; Schallner et al., 2015). Conversely, an increase in the early inflammatory response has also been observed after ICH when expression is primarily localized to microglia and macrophages (Wang and Doré, 2007).
HO-1 is minimally expressed in the CNS under physiological conditions, but it is induced by oxidative stress via Nrf2-regulated transcriptional activation. In rodents, systemic treatment with low molecular weight Nrf2 activators increased CNS HO-1, with expression localized most prominently to perivascular astrocytes (Alfieri et al., 2013; Jazwa et al., 2011; Yamauchi et al., 2004). This treatment was sufficient to attenuate cell loss, blood-brain barrier breakdown, inflammation, and neurological deficits after ICH in wild-type mice, while Nrf2 knockout prevented any benefit and worsened injury (Iniaghe et al., 2015; King et al., 2011; Wang et al., 2007; Zhao et al., 2007). However, since Nrf2 regulates the expression of multiple antioxidant and anti-inflammatory proteins, the relationship between HO-1 induction and protection cannot be precisely defined by these experiments.
The use of transgenic mice provides a more specific method to test the effect of HO-1 per se on injury and outcome after ICH. In a prior study, HO-1 overexpression that was restricted to GFAP+ astrocytes markedly reduced mortality and behavioral deficits after striatal injection of autologous blood (Chen-Roetling et al., 2015). In light of these robust results, determination of the consistency of this effect across ICH models seemed warranted. In the present study, ICH was induced in wild-type and transgenic mice by striatal injection of bacterial collagenase, which disrupts multiple vessels and produces a more severe injury than stereotactic blood injection (MacLellan et al., 2008). In addition to mortality, perihematomal cell viability, neurological deficits, and histological endpoints were quantified.
Materials and Methods
Animals
Mice expressing human HO-1 in GFAP+ cells (GFAP.HMOX1 mice, FVB background) were descended from founding pairs provided by the Schipper lab (Song et al., 2012b). They were bred and housed with control wild-type (WT) mice in the Thomas Jefferson University Laboratory Animal Facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All breeding and experimental protocols were approved by the Institutional Animal Care and Use Committee and were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023). Genotype was verified by PCR using previously-published primers (Chen-Roetling et al., 2015). HO-1 overexpression specifically in astrocytes has previously been confirmed by double immunofluorescence staining (Chen-Roetling et al., 2015). Mice were used for experiments when 3–6 months old.
Striatal Collagenase Injection
Collagenase (Sigma-Aldrich C9572) was reconstituted from the lyophilized product (0.1 mg/33μl), aliquoted, and stored at −70°C for up to one month prior to use. Careful handling of collagenase dilutions (0.04 units/μl) was necessary to avoid loss of activity, as previously described in detail (Chen-Roetling et al., 2013). Mice were anesthetized with 1.5% isoflurane in oxygen and were placed into a stereotaxic frame. Temperature was continuously monitored using a rectal probe and maintained at 37±0.5°C with a heating lamp. Using aseptic technique, the skin over the skull was incised and bregma was identified. A burr hole was then made 2.5 mm to the right and 0.5 mm anterior to bregma, and 30 gauge needle with attached glass syringe was inserted to a depth of 3 mm. Collagenase (0.04 units in 1 μl) was then injected at a rate of 0.6 μl per minute. The needle was withdrawn 1 mm 4 minutes later, and was removed completely after another 4 minute pause. Surgical control mice were subjected to anesthesia and needle trauma only. After wound repair, mice recovered in a warm environment.
Hematoma volume
In order to determine if hemorrhage volume after collagenase injection differed in WT and GFAP.HMOX1 mice, hematoma volume was quantified by high frequency ultrasound imaging, using the Vevo 2100 high frequency ultrasound imaging scanner (FujiFilm VisualSonics, Toronto, Ontario) as previously described (Stanczak et al., 2015).
Outcome Assessment
All testing was conducted by observers blinded to mouse genotype.
Methylthiazolyldiphenyl-Tetrazolium (MTT) Assay
Striatal cell viability was quantified by MTT assay, as previously described and validated (Chen-Roetling et al., 2013). At 7 and 22 days after collagenase injection, mice were euthanized under deep isoflurane anesthesia. Injected and contralateral striata were rapidly excised under a dissecting microscope and dissociated by trituration in 1 ml Hanks Balanced Salt Solution supplemented with 27.5 mM glucose, 20.5 mM sucrose and 4.2 mM sodium bicarbonate. MTT solution (0.25 mg in 1 ml) was added and the cell suspension was incubated for 4 min at 37°C. After low speed centrifugation, the supernatant was discarded and formazan was extracted in 2 ml isopropanol. Formazan absorbance (562 nm) was quantified and expressed as a percentage of that in contralateral striata.
Histology
Under isoflurane anesthesia, mice were perfused with 5 ml isotonic saline followed by 50 ml 4% paraformaldehyde, followed by postfixation and cryopreservation in 20% sucrose. Sectioning and immunostaining were conducted by FD Neurotechnologies, Inc., Columbia, MD, USA as previously described (Chen-Roetling et al., 2013), using the following antibodies: anti-NeuN, Millipore, Billerica, MA; Cat. No. MAB377B, 1:1000 dilution; anti-Iba1, Wako Chemicals USA, Richmond, VA, Cat. No. 019-19741, 1:10.000; anti-GFAP, Invitrogen, Carlsbad, CA, Cat. No. 13-0300, 1:30,000. Unbiased counting of immunoreactive cells was guided by the Stereologer system (Stereology Resource Center, Chester, MD) (Long et al., 1998), using the optical fractionator method (Chen-Roetling et al., 2013; West, 1993).
Blood-brain barrier permeability assay
Mice were injected with 2% Evans blue as previously described (Lu et al., 2014). Evans blue binds to plasma albumin and is a marker of protein leakage across the blood-brain barrier. After saline perfusion, striata were excised and Evans blue was extracted following an established protocol (Uyama et al., 1988). Fluorescence (ex:620 nm, em:680 nm) of the solution was then measured.
Neurological Deficit Testing
A neurological deficit score was calculated daily for the first week after hemorrhage and weekly thereafter via the method of Huang et al.(Huang et al., 1994), which minimizes animal disturbance while assigning scores as follows: 0: nl; 1: contralateral flexion when mice lifted by tail; 2: circling right but normal rest posture; 3: leftward leaning at rest; 4: no spontaneous movement. Neurological deficits were also assessed weekly with the adhesive removal and corner tests, as previously described (Chen et al., 2011).
HO-1 Expression Assays
Striata were removed and placed into 500 μl RIPA Lysis and Extraction Buffer (Thermo Fisher Cat. No. 89900) containing protease inhibitor cocktail (Sigma Aldrich P8340). Human and mouse HO-1 expression were quantified using species-specific ELISA kits (Enzo Life Sciences, Cat. No. ADI-960-071 and ADI-EKS-800).
HO Activity Assay
Striata were excised, sonicated in ice-cold potassium phosphate buffer, and centrifuged (13,000 × g, 2 min). Supernatant samples (40 μg protein) were immediately placed into septum-sealed vials containing 25 μM hemin and 1.5 mM NADPH (total volume 120 μl). Vial headspace air was purged and replaced with air that had passed through a catalytic converter to remove ambient CO; reaction was run at 37°C for 15 min and then was quickly stopped by freezing on dry ice. Headspace CO was quantified by gas chromatography (Peak Laboratories, Mountain View, CA, USA).
Statistics
Mortality differences were assessed with Fisher’s exact test. Other differences between two groups were analyzed with an unpaired t-test, and differences between three or more groups with one-way analysis of variance (ANOVA) and the Bonferroni multiple comparisons test. Behavioral outcome data with multiple time point assessments were analyzed using two-way ANOVA.
Results
Mortality
143 mice (72 males and 71 females) were injected with collagenase in this study (Table 1). The overall mortality rate was 16.1% (10 males and 13 females). 20 of 76 WT mice (26.3%) died within 24 hours of collagenase injection; deaths were evenly distributed between males and females. 3 of 67 (4.48%) GFAP.HMOX1 mice died, all females; two of these deaths occurred within 24 hours, and the third occurred at 21 days.
Table 1.
Astrocyte HO-1 overexpression reduces mortality after collagenase-induced ICH. Mortality rates in wild-type (WT) and transgenic mice expressing human HO-1 in astrocytes (GFAP.HMOX1) after striatal injection of 0.04 units collagenase.
| WT | GFAP.HMOX1 | ||||
|---|---|---|---|---|---|
| Mortality | N | Mortality | N | P value | |
| Male | 25.0% | 40 | 0 | 32 | 0.0017 |
| Female | 27.8% | 36 | 8.57% | 35 | 0.0632 |
| Both | 26.3% | 76 | 4.48% | 67 | 0.0004 |
Astrocyte HO-1 increases striatal cell viability
Collagenase injection reduced striatal cell viability in surviving WT mice to 51.4±4.5% of that in the contralateral striatum at 7 days, with little subsequent change through 22 days (Fig. 1). Viability increased to 64.2±4.3% in GFAP.HMOX1 mice, which was also stable over the following 15 days.
Figure 1.
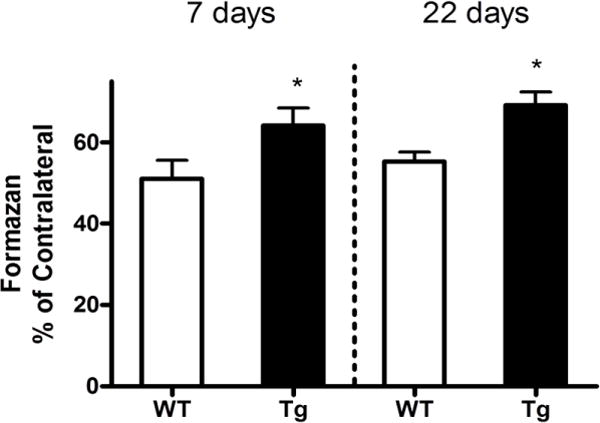
Increased striatal cell viability after collagenase-induced ICH in GFAP.HMOX1 mice. Mean MTT reduction to formazan by striatal cell suspensions at 7 and 22 days after collagenase injection was normalized to that in contralateral striata. *P < 0.05 v. WT group, ±S.E.M., n = 8–13/condition.
Effect of HO-1 overexpression on neuron and glial cell number
At 7 days after sham surgery, stereological cell counts indicated 2.87±0.06 × 106 neurons in the ipsilateral striatum (Fig. 2). This value was decreased by about 40% by collagenase injection in WT mice, and was significantly higher in GFAP.HMOX1 mice. Consistent with prior observations (Nedergaard et al., 2003; Sturrock, 1980), GFAP-positive astrocytes and microglia were much less abundant than neurons in sham-operated mice. Their numbers increased after collagenase injection, but no significant difference was observed between WT and GFAP.HMOX1 groups.
Figure 2.
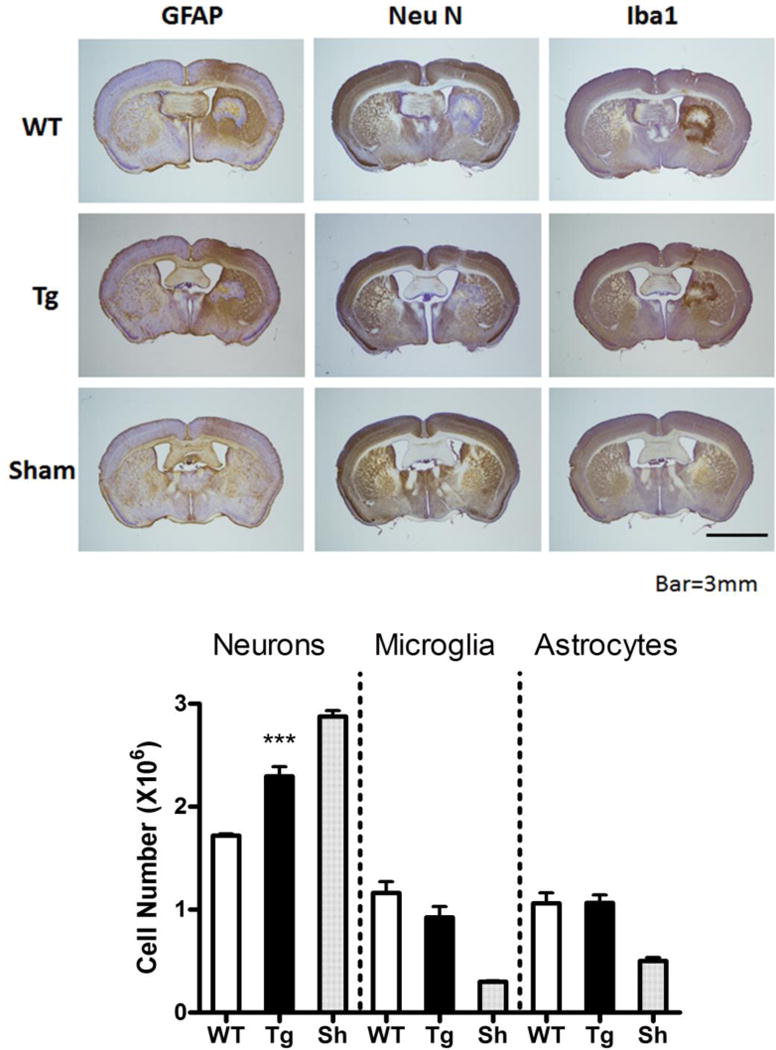
Astrocyte HO-1 overexpression protects neurons after ICH. Representative sections though the hematoma at 7 days after striatal collagenase injection in wild-type (WT) or transgenic (Tg) GFAP.HMOX1 mice, or after sham surgery (WT mouse), stained with antibodies to NeuN, GFAP, and Ibal, with 3,3′-diaminobenzidine chromagen and cresyl-violet counterstain. Bars represent mean numbers (±S.E.M.) of striatal neurons, microglia, and astrocytes calculated from stereologic cell counts. Sham (Sh) group contained equal numbers of wild-type (WT) and transgenic GFAP. HMOX1 mice. ***P < 0.001 v. WT, n = 4/condition.
Reduced blood-brain barrier disruption in GFAP.HMOX1 mice
As previously reported (Lu et al., 2014), striatal Evans blue signal was increased 24 hours after collagenase injection. Most of this leakage was prevented by astrocyte HO-1 overexpression (Fig. 3).
Figure 3.
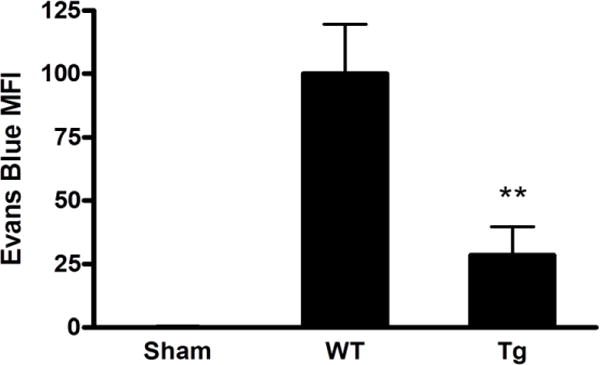
Reduced blood-brain barrier breakdown after collagenase injection in GFAP.HMOX1 mice. Mean Evans blue fluorescence intensity (MFI±S.E.M.) of striatal extracts from WT and transgenic GFAP.HMOX1 mice at 24 hours after collagenase injection. **P < 0.01 v. WT, n = 6 (WT), 7 (GFAP.HMOX1) or 4 (sham, 2 WT and 2 GFAP.HMOX1).
Astrocyte HO-1 reduces neurological deficits
Mean neurological deficit scores indicated severe impairment in wild type mice in the first four days after hemorrhage that was attenuated in GFAP.HMOX1 mice (Fig. 4). Recovery was observed in both groups over the first week and scores overlapped on days 14 and 21. A severe early sensorimotor deficit was also demonstrated with the adhesive removal test, which was robustly reduced in transgenics. However, differences in the corner test did not reach statistical significance at any time point.
Figure 4.
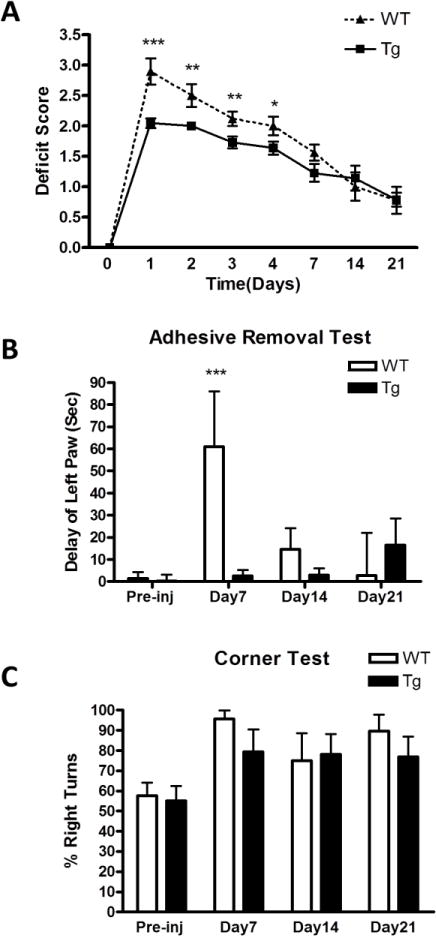
Astrocyte HO-1 reduces neurological deficits. A. Mean neurological deficit scores at indicated time points after ICH in WT and GFAP.HMOX1 mice, n = 9–22/condition, P < 0.001, two-way ANOVA. B, C: Pre-injury and weekly testing results for adhesive removal and corner tests, 8–13/condition. *P < 0.05, **P < 0.01, ***P < 0.001 v. transgenic GFAP.HMOX1 group.
HO-1 expression and HO activity
Consistent with prior observations (Chen-Roetling et al., 2015), striatal ICH increased native HO-1, as measured by mouse-specific ELISA. Human HO-1 in GFAP.HMOX1 striata was 4–5-fold greater than mouse HO-1, and was not altered by collagenase injection (Fig. 5). HO activity in transgenic mice was significantly increased over wild-type in collagenase-injected striata, but the magnitude was less than predicted by HO-1 protein assay (Fig. 6).
Figure 5.
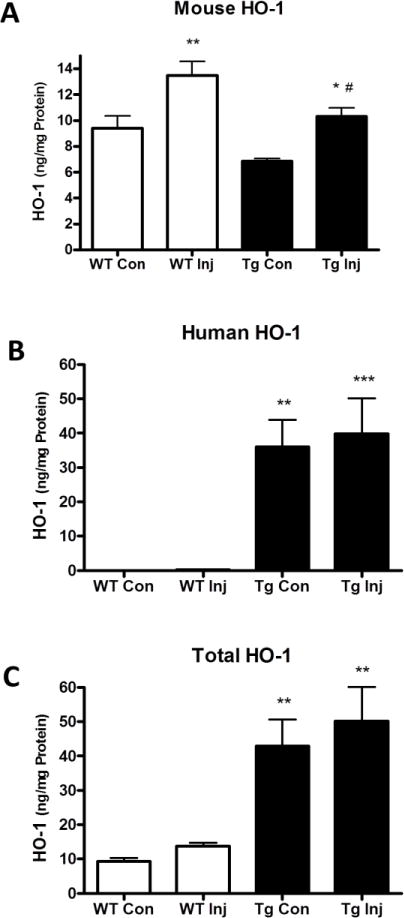
Striatal HO-1 expression after collagenase injection. Mouse (A), human (B), and mouse + human (C) HO-1 expression in injected (Inj) and contralateral (Con) striatal lysates of WT and transgenic GFAP.HMOX1 (Tg) mice at 7 hours after collagenase injection. *P < 0.05, **P < 0.01 v. contralateral, #P < 0.05 v. WT Inj in A, **P < 0.01. ***P < 0.001 v. WT in B and C, n = 6/condition.
Figure 6.
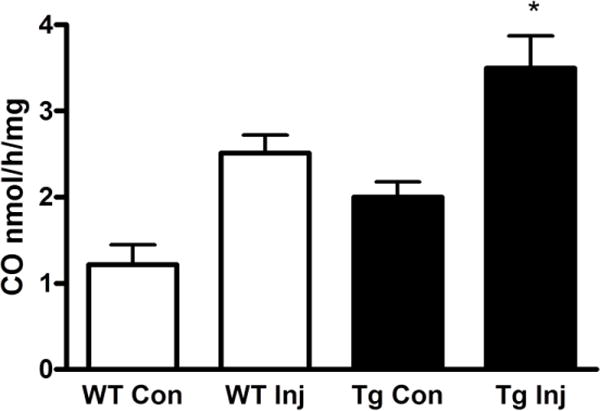
Striatal HO activity is increased in GFAP.HMOX1 mice after ICH. Mean HO activity (±S.E.M., n = 7/condition) in WT and transgenic (Tg) collagenase-injected (Inj) and contralateral (Con) striatal lysates, determined by gas chromatography-based carbon monoxide assay at 2 days. *P < 0.05 v. mean value of injected WT lysates.
Hematoma volume
At 24 hours after collagenase injection, hematoma volumes as quantified by high-frequency ultrasound imaging were 9.23±0.94 μl in WT and 10.34±2.48 μl in GFAP.HMOX1 mice (P = 0.67, n = 8/condition).
Discussion
These results demonstrate that astrocyte HO-1 has a robust neuroprotective effect after ICH. In agreement with a prior report (MacLellan et al., 2008), collagenase injection produced a severe injury in WT mice, manifested by a 26% mortality rate and a ~50% reduction in perihematomal cell viability after one week in survivors. Mortality rate was reduced by over 80% in GFAP.HMOX1 mice. Divergent mortality rates in WT and transgenic groups may have introduced bias in the quantification of other parameters, since the most severely injured animals in the WT group were more likely to die at early time points and be unavailable for further testing. Despite this limitation, surviving WT mice sustained more perihematomal cell loss, blood-brain barrier disruption and neurological deficits than their transgenic counterparts. Considered along with prior results demonstrating reduced mortality of GFAP.HMOX1 mice in the autologous blood injection ICH model (Chen-Roetling et al., 2015), astrocyte HO-1 overexpression is one of the most effective interventions reported to date after experimental hemorrhagic stroke.
Histological analysis guided by design-based stereology indicated that the cell loss produced by collagenase was selective for neurons, by far the most abundant population in the mouse striatum (Sturrock, 1980), while astrocytes and microglia increased in number. Consistent with the effect on mortality, neuron loss was decreased by about half in GFAP.HMOX1 mice. Neurons have weak antioxidant defenses and are particularly vulnerable to blood lysate, hemoglobin and iron (Jaremko et al., 2010; Kress et al., 2002), so their death after ICH is not surprising. Although the specific neuroprotective mechanisms of astrocyte HO-1 have not been precisely defined, trophic support provided to neurons by astrocytes may be particularly relevant after hemorrhagic CNS injuries, and may be augmented by HO-1 activity. Astrocytes are resistant to heme-mediated injury due to their ability to rapidly upregulate HO-1 and the iron-binding protein ferritin (Regan et al., 2002; Teng et al., 2004). They also take up hemin and hemoglobin, at least in vitro, and may play a key role in their degradation to less toxic moieties (Chen-Roetling and Regan, 2016a; Dang et al., 2011). HO-catalyzed heme breakdown in astrocytes yields iron, which is sequestered (Hoepken et al., 2004), bilirubin, which is exported via multidrug resistance protein 1 (Gennuso et al., 2004), and CO, which is freely diffusible. The latter two products have antioxidant, anti-inflammatory, and vasodilatory effects that combined with heme elimination likely contribute to the neuroprotection observed in GFAP.HMOX1 mice (Leffler et al., 2011; Otterbein et al., 2016; Stocker et al., 1987).
Striatal cell viability, as quantified by MTT assay, was also significantly increased in GFAP.HMOX1 mice, although the observed effect was rather modest. Cell viability per se may underestimate the overall benefit of HO-1, as cells may be dysfunctional but still alive. The unequal mortality rates in WT and Tg groups should also be considered when interpreting these data. In the 22 day cell viability experiment, 5 of 13 WT mice died, while all GFAP.HMOX1 mice survived. It is probable that non-surviving WT mice had severely reduced striatal cell viability prior to death, but were excluded from the cell viability analysis according to the a priori study design. The net effect was loss of data from the most severely injured mice in the WT group, likely narrowing the difference with the Tg group.
Total HO-1 expression in the striata of GFAP.HMOX1 mice was increased approximately fourfold compared with WT mice, but activity as measured by carbon monoxide assay was increased by only ~40%, in agreement with a recent observation after pharmacological HO-1 induction (Iwamori et al., 2015). This discrepancy is likely due at least in part to constitutive expression of HO-2, which is abundantly expressed in the mammalian brain and is the predominant neuronal isoform (Parfenova and Leffler, 2008). The protection observed in GFAP.HMOX1 mice despite this rather modest increase in overall HO activity may reflect a profound benefit of astrocyte-localized expression after acute parenchymal hemorrhage. However, an effect that is independent of enzymatic activity cannot be completely excluded. Lin et al. have reported that a catalytically inert HO-1 mutant, a truncated HO-1 lacking its C-terminus, or intact HO-1 protein enter the nuclei of NIH 3T3 cells and positively regulate the transcription of antioxidant genes, including HO-1 itself (Lin et al., 2007; Lin et al., 2008b). In the present study, native mouse HO-1 protein was not increased in GFAP.HMOX1 mice, as would be expected if this non-canonical pathway were active. Conversely, it was modestly but significantly reduced, consistent with the protective effect of human HO-1 (Muñoz et al., 2005).
The benefit of astrocyte HO-1 overexpression on perihematomal cell viability persisted through the 22 day observation period in this study, which exclusively used young adult mice. It remains to be determined if this effect will be consistent in aged animals. Continuous transgenic expression of human HO-1 in astrocytes for 48 weeks results in iron deposition, accompanied by oxidative mitochondrial injury in subcortical tissues and a hyperdopaminergic state (Song et al., 2012a; Song et al., 2012b). Tyrosine hydroxylase, dopamine transporter, dopamine, and 5-hydroxytryptamine are upregulated in the basal ganglia, D1 dopamine receptor binding is reduced, and mice develop schizophreniform behavioral changes. These observations suggest that despite the relative resistance of astrocytes to iron (Kress et al., 2002), a sustained increase in HO-1 may ultimately lead to iron-dependent cytotoxicity. Any therapeutic strategies aimed at upregulating astrocyte HO-1 should be of limited duration, and may be detrimental in a neurodegenerative disease context.
A transient and selective increase in astrocyte HO-1 may be feasible after an acute injury such as ICH for two reasons. First, the half-life of HO-1 mRNA and protein (~3 and 8 hours, respectively) suggest that the effect of any pharmacologic inducer would terminate shortly after drug elimination, targeting the interval of greatest neuronal risk while limiting toxicity (Lin et al., 2008a; Takahashi et al., 1997). Second, most low molecular weight HO-1 inducers activate the Keap1-Nrf2 regulatory pathway, which is particularly active in astrocytes and repressed in neurons, consistent with the latter’s weak antioxidant defenses (Baxter and Hardingham, 2016; Bell et al., 2015). Studies to date indicate that peripheral administration of Nrf2 activators induces HO-1 in the CNS, with selectivity for astrocytes. Jazwa et al reported that sulforaphane 50 mg/kg i.p. increased HO-1 only in astrocytes in the mouse striatum and ventral midbrain, without any effect on expression in neurons, microglia or endothelial cells (Jazwa et al., 2011). Similar results have been reported after sulforaphane injection in rats (Alfieri et al., 2013) and hemin injection in mice (Chen-Roetling and Regan, 2016b).
The translational significance of these observations remains to be defined. The relationship between astrocytes and neurons varies considerably in rodents and humans, and may be an important consideration when investigating astrocyte-mediated effects in neuronal injury models. The astrocyte: neuron ratio increases with phylogeny and is approximately 1:3 in the rodent CNS, compared with 1:1 to 1.4:1 in humans (Nedergaard et al., 2003; Weise et al., 2015). Human astrocytes are also larger and more complex, with domains incorporating ~2 million synapses compared with only ~120,000 for the mouse (Herculano-Houzel, 2014; Oberheim et al., 2009), associated with significant differences in gene expression and cellular physiology (Zhang et al., 2016). Extrapolation of the results of this or related studies to clinical ICH should therefore proceed with caution. Assessment of the effect of therapies that increase astrocyte HO-1 in large animal ICH models is an appropriate next step.
Conclusions
Mice overexpressing human HO-1 specifically in astrocytes are protected from collagenase-induced ICH, consistent with their resistance to injury in the blood injection model. Further investigation of pharmacological and genetic therapies that increase astrocyte HO-1 in experimental ICH models is warranted.
Highlights.
Astrocyte heme oxygenase-1 (HO-1) overexpression reduced mortality after ICH.
HO-1 transgenic mice had less neuron loss and blood-brain barrier disruption.
Early neurological deficits were also reduced in the HO-1 mice.
Therapies that increase astrocyte HO-1 may be beneficial after ICH.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (Grant No. RO1NS079500 and R01NS095205 to RFR) and the Canadian Institutes for Health Research (to HMS).
Hyman Schipper has served as consultant to Osta Biotechnologies, Immunotec Inc., Molecular Biometrics Inc., TEVA Neurosciences, and Caprion Pharmaceuticals. Raymond Regan has received research support from CSL Behring.
Abbreviations
- GFAP
glial fibrillary acidic protein
- HO
heme oxygenase
- ICH
intracerebral hemorrhage
- MTT
Methylthiazolyldiphenyl-tetrazolium
- Nrf2
nuclear factor erythroid 2-related factor 2
- Tg
transgenic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have declared no conflicts of interest.
References
- Alfieri A, et al. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radic Biol Med. 2013;65:1012–22. doi: 10.1016/j.freeradbiomed.2013.08.190. [DOI] [PubMed] [Google Scholar]
- Baxter PS, Hardingham GE. Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes. Free Radic Biol Med. 2016;100:147–152. doi: 10.1016/j.freeradbiomed.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KF, et al. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat Commun. 2015;6:7066. doi: 10.1038/ncomms8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenisti-Zarom L, Regan RF. Astrocyte-specific heme oxygenase-1 hyperexpression attenuates heme-mediated oxidative injury. Neurobiol Dis. 2007;26:688–95. doi: 10.1016/j.nbd.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick JP, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Statement for Healthcare Professionals From a Special Writing Group of the Stroke Council, American Heart Association. Stroke. 1999;30:905–915. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- Chen-Roetling J, et al. A rapid fluorescent method to quantify neuronal loss after experimental intracerebral hemorrhage. J Neurosci Methods. 2013;216:128–36. doi: 10.1016/j.jneumeth.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Roetling J, Regan RF. Haptoglobin increases the vulnerability of CD163-expressing neurons to hemoglobin. J Neurochem. 2016a;139:586–595. doi: 10.1111/jnc.13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Roetling J, Regan RF. Targeting the Nrf2-Heme Oxygenase-1 Axis after Intracerebral Hemorrhage. Curr Pharm Des. 2016b doi: 10.2174/1381612822666161027150616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Roetling J, et al. Astrocyte overexpression of heme oxygenase-1 improves outcome after intracerebral hemorrhage. Stroke. 2015;46:1093–8. doi: 10.1161/STROKEAHA.115.008686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, et al. Increased striatal injury and behavioral deficits after intracerebral hemorrhage in hemopexin knockout mice. J Neurosurg. 2011;114:1159–67. doi: 10.3171/2010.10.JNS10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MC, et al. Quality of life after intracerebral hemorrhage: results of the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke. 2009;40:1677–82. doi: 10.1161/STROKEAHA.108.538967. [DOI] [PubMed] [Google Scholar]
- Dang TN, et al. Uptake, metabolism and toxicity of hemin in cultured neurons. Neurochem Int. 2011 doi: 10.1016/j.neuint.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Delcourt C, et al. Associations with health-related quality of life after intracerebral haemorrhage: pooled analysis of INTERACT studies. J Neurol Neurosurg Psychiatry. 2016 doi: 10.1136/jnnp-2016-314414. [DOI] [PubMed] [Google Scholar]
- Gennuso F, et al. Bilirubin protects astrocytes from its own toxicity by inducing up-regulation and translocation of multidrug resistance-associated protein 1 (Mrp1) Proc Natl Acad Sci U S A. 2004;101:2470–5. doi: 10.1073/pnas.0308452100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62:1377–91. doi: 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- Hoepken HH, et al. Iron accumulation, iron-mediated toxicity and altered levels of ferritin and transferrin receptor in cultured astrocytes during incubation with ferric ammonium citrate. J Neurochem. 2004;88:1194–202. doi: 10.1046/j.1471-4159.2003.02236.x. [DOI] [PubMed] [Google Scholar]
- Huang Z, et al. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–5. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- Iniaghe LO, et al. Dimethyl fumarate confers neuroprotection by casein kinase 2 phosphorylation of Nrf2 in murine intracerebral hemorrhage. Neurobiol Dis. 2015;82:349–58. doi: 10.1016/j.nbd.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamori S, et al. A novel and sensitive assay for heme oxygenase activity. Am J Physiol Renal Physiol. 2015;309:F667–71. doi: 10.1152/ajprenal.00210.2015. [DOI] [PubMed] [Google Scholar]
- Jaremko KM, et al. Accelerated Hemolysis and Neurotoxicity in Neuron-Glia-Blood Clot Co-cultures. J Neurochem. 2010;114:1063–73. doi: 10.1111/j.1471-4159.2010.06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwa A, et al. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal. 2011;14:2347–60. doi: 10.1089/ars.2010.3731. [DOI] [PubMed] [Google Scholar]
- King MD, et al. Attenuation of hematoma size and neurological injury with curcumin following intracerebral hemorrhage in mice. J Neurosurg. 2011;115:116–23. doi: 10.3171/2011.2.JNS10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress GJ, et al. The relationship between intracellular free iron and cell injury in cultured neurons, astrocytes, and oligodendrocytes. J Neurosci. 2002;22:5848–55. doi: 10.1523/JNEUROSCI.22-14-05848.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler CW, et al. Carbon monoxide as an endogenous vascular modulator. American Journal of Physiology - Heart and Circulatory Physiology. 2011;301:H1–H11. doi: 10.1152/ajpheart.00230.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PH, et al. Ubiquitin-proteasome system mediates heme oxygenase-1 degradation through endoplasmic reticulum-associated degradation pathway. Biochim Biophys Acta. 2008a;1783:1826–34. doi: 10.1016/j.bbamcr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Lin Q, et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007;282:20621–33. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- Lin QS, et al. Catalytic inactive heme oxygenase-1 protein regulates its own expression in oxidative stress. Free Radic Biol Med. 2008b;44:847–55. doi: 10.1016/j.freeradbiomed.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JM, et al. Stereological estimation of total microglia number in mouse hippocampus. J Neurosci Methods. 1998;84:101–8. doi: 10.1016/s0165-0270(98)00100-9. [DOI] [PubMed] [Google Scholar]
- Lu X, et al. Systemic hemin therapy attenuates blood-brain barrier disruption after intracerebral hemorrhage. Neurobiol Dis. 2014;70C:245–251. doi: 10.1016/j.nbd.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLellan CL, et al. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab. 2008;28:516–25. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- Muñoz AM, et al. Glial overexpression of heme oxygenase-1: a histochemical marker for early stages of striatal damage. J Chem Neuroanat. 2005;29:113–26. doi: 10.1016/j.jchemneu.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, et al. New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–87. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein LE, et al. Heme Oxygenase-1 and Carbon Monoxide in the Heart: The Balancing Act Between Danger Signaling and Pro-Survival. Circ Res. 2016;118:1940–59. doi: 10.1161/CIRCRESAHA.116.306588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenova H, et al. Epileptic seizures cause extended postictal cerebral vascular dysfunction that is prevented by HO-1 overexpression. Am J Physiol Heart Circ Physiol. 2005;288:H2843–50. doi: 10.1152/ajpheart.01274.2004. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Leffler CW. Cerebroprotective functions of HO-2. Curr Pharm Des. 2008;14:443–53. doi: 10.2174/138161208783597380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan RF, et al. Ferritin induction protects cortical astrocytes from heme-mediated oxidative injury. Neuroscience. 2002;113:985–994. doi: 10.1016/s0306-4522(02)00243-9. [DOI] [PubMed] [Google Scholar]
- Rincon F, Mayer SA. The Epidemiology of Intracerebral Hemorrhage in the United States from 1979 to 2008. Neurocrit Care. 2013;19:95–102. doi: 10.1007/s12028-012-9793-y. [DOI] [PubMed] [Google Scholar]
- Schallner N, et al. Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J Clin Invest. 2015;125:2609–25. doi: 10.1172/JCI78443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, et al. Schizophrenia-like features in transgenic mice overexpressing human HO-1 in the astrocytic compartment. J Neurosci. 2012a;32:10841–53. doi: 10.1523/JNEUROSCI.6469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, et al. Unregulated brain iron deposition in transgenic mice over-expressing HMOX1 in the astrocytic compartment. J Neurochem. 2012b;123:325–36. doi: 10.1111/j.1471-4159.2012.07914.x. [DOI] [PubMed] [Google Scholar]
- Stanczak M, et al. High frequency US evaluation of intracerebral hemorrhage in a small animal model. RSNA 2015. 2015 H276-SD-THA3. [Google Scholar]
- Stocker R, et al. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–6. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Sturrock RR. A comparative quantitative and morphological study of ageing in the mouse neostriatum, indusium griseum and anterior commissure. Neuropathol Appl Neurobiol. 1980;6:51–68. doi: 10.1111/j.1365-2990.1980.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Takahashi K, et al. Possible implications of the induction of human heme oxygenase-1 by nitric oxide donors. J Biochem. 1997;121:1162–8. doi: 10.1093/oxfordjournals.jbchem.a021710. [DOI] [PubMed] [Google Scholar]
- Teng ZP, et al. Adenoviral transfer of the heme oxygenase-1 gene protects cortical astrocytes from heme-mediated oxidative injury. Neurobiol Dis. 2004;17:179–187. doi: 10.1016/j.nbd.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Uyama O, et al. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab. 1988;8:282–4. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- Wang J, Doré S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130:1643–52. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med. 2007;43:408–14. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise CM, et al. A post-mortem stereological study of striatal cell number in human obesity. Obesity (Silver Spring) 2015;23:100–4. doi: 10.1002/oby.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–85. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, et al. Hemin induces heme oxygenase-1 in spinal cord vasculature and attenuates barrier disruption and neutrophil infiltration in the injured murine spinal cord. J Neurotrauma. 2004;21:1017–30. doi: 10.1089/0897715041651042. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, et al. Transcription Factor Nrf2 Protects the Brain From Damage Produced by Intracerebral Hemorrhage. Stroke. 2007;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]


