Abstract
Purpose
The blood pressure “error signal” represents the difference between an individual’s mean diastolic blood pressure and the diastolic blood pressure at which 50% of cardiac cycles are associated with a muscle sympathetic nerve activity burst (the “T50”). In this study we evaluated whether T50 and the error signal related to the extent of change in blood pressure during autonomic blockade in young and older women, to study potential differences in sympathetic neural mechanisms regulating blood pressure before and after menopause.
Methods
We measured muscle sympathetic nerve activity and blood pressure in 12 premenopausal (25±1 years) and 12 postmenopausal women (61±2 years) before and during complete autonomic blockade with trimethaphan camsylate.
Results
At baseline, young women had a negative error signal (−8±1 versus 2±1 mmHg, p<0.001; respectively) and lower muscle sympathetic nerve activity (15±1 versus 33±3 bursts/min, p<0.001; respectively) than older women. The change in diastolic blood pressure after autonomic blockade was associated with baseline T50 in older women (r=−0.725, p=0.008) but not in young women (r=−0.337, p=0.29). Women with the most negative error signal had the lowest muscle sympathetic nerve activity in both groups (young: r=0.886, p<0.001; older: r=0.870, p<0.001).
Conclusions
Our results suggest that there are differences in baroreflex control of muscle sympathetic nerve activity between young and older women, using the T50 and error signal analysis. This approach provides further information on autonomic control of blood pressure in women.
Keywords: aging, baroreflex function, menopause, sympathetic nerve activity
Introduction
Understanding age-related changes in blood pressure regulation is essential to preventing and treating hypertension, as the risk of hypertension increases with age in both sexes. In women after the onset of menopause, the risk of developing hypertension is greater compared to that in aging men [1]. Additionally, aging is accompanied by an increase in muscle sympathetic nerve activity (MSNA) [2]. This may explain, in part, why blood pressure increases with age, and in particular in older women [2]. Furthermore, a decrease in resting cardiac vagal tone in women has been reported after surgical menopause [3]. These alterations may possibly explain the sharp elevation in the prevalence of hypertension that occurs after menopause [1], which is likely related to the decline in female sex hormones [4]. Estrogen affects central sympathetic outflow in animals [5, 6] and humans [7], and augments sympathetic baroreflex sensitivity. Sympathetic baroreflex sensitivity is greater during the mid-luteal phase of the menstrual cycle when endogenous estrogen levels are relatively high in comparison to the follicular phase when estrogen levels are low [7]. Dramatic decreases in female sex hormones levels after menopause may therefore alter autonomic control of blood pressure and explain why MSNA increases further after menopause. Additionally, the loss of the vasodilator effects of estrogen and/or β-adrenergic mediated vasodilation [8] after menopause, appears to increase vascular resistance [9].
We have recently shown that the autonomic support of blood pressure is greater in postmenopausal women compared with young women [10]. Specifically, Barnes et al. reported that systemic autonomic blockade reduced arterial blood pressure to a greater degree in postmenopausal women than young women. However, there was no relationship between these changes in mean arterial blood pressure and measures of baseline MSNA in either group of women, although the association was significant when the groups were combined. Subsequently, we wanted to gain a better understanding of non-neural factors that may be influencing acute blood pressure regulation in these populations.
Blood pressure threshold analysis can be used to evaluate the occurrence of sympathetic bursts over a range of diastolic blood pressure (DBP) values, thus providing a valid measure of resting spontaneous baroreflex sympathetic function [11, 12]. In the present study, we used this calculation to better understand how resting vasoconstrictor nerve activity affects the blood pressure set point in young premenopausal and older postmenopausal women. The DBP at which there is a 50% likelihood of an MSNA burst occurring is called the T50 [12, 13]. This value provides information about the average setting of the baroreflex over the operating range of DBP [13, 14], although may not related with the prevailing mean blood pressure in an individual. The blood pressure error signal could offer insight into the difference in burst occurrence [13]. Also, the difference (error signal) between the T50 and mean DBP is a useful measure of the influence of both sympathetic neural processes and non-neural factors (e.g., angiotensin II, aldosterone) on blood pressure control [13, 15, 16]. We have previously shown that the blood pressure error signal is more negative in young women compared to young men, indicating that non-neural influences are greater in young women [13]. It is possible that the reduction in female sex hormones levels seen during the menopausal transition may result in changes in T50 and error signal, and these indices could be related to autonomic support in young and older women.
Therefore, the purpose of the present study was to evaluate whether T50 and the error signal relate to the extent of change in blood pressure during autonomic blockade in young premenopausal and older postmenopausal women. We hypothesized that greater T50 values and a more positive error signal would be positively related to the reduction in DBP in both young and older women; however, these relationships would be more pronounced in postmenopausal women.
Methods
Participants
Data related to this study cohort has been previously analyzed and published [10]. Twelve young premenopausal (age 25±1 years; height 167±2 cm; body mass 66±2 kg) and 12 older postmenopausal (61±2 years; height 164±2 cm; body mass 64±2 kg) women completed the study. Participants were healthy, normotensive, nondiabetic, non-obese (body mass index <30 kg/m2), non-smoking, and free of cardiovascular and other chronic diseases, as determined by a review of their medical histories and a brief physical exam. None of the participants were on any cardiovascular acting medications. Both groups had comparable physical activity levels (young women exercised 3.3±1.7 sessions/week and older women exercised 3.3±2.5 sessions/week). Young women were studied in the early follicular phase of the menstrual cycle or during the low-hormone phase of oral contraceptive use, to minimize the effects of reproductive hormone variation across the menstrual cycle. Pregnant women, those breastfeeding and women who experienced surgically-induced menopause or had used menopausal hormone therapy were excluded. All young women were asked to complete a pregnancy test within 48 h of the study day. Postmenopausal was defined as at least one 1 year since the last menstruation [17], with an average of 9±3 years in this cohort. All procedures were reviewed and approved by the Institutional Review Board at Mayo Clinic and conformed to the ethical principles of the Declaration of Helsinki. All subjects provided written informed consent prior to study participation.
Experimental Protocol
Detailed information on the protocols and experimental procedures used in this study has been described previously [10]. Participants were admitted to the Center for Translational Science Activities’ Clinical Research Unit (CRU) at the Mayo Clinic after an overnight fast and at least 24 hours without caffeine, alcohol, or vigorous exercise. The timeline of the experimental protocol is shown in Figure 1. Participants rested in the supine position during instrumentation and throughout the study. A 5 cm 20-gauge arterial catheter was inserted into the brachial artery of the non-dominant arm using local anesthesia (2% lidocaine) and aseptic technique. A pressure transducer positioned at the level of the heart was connected to the catheter to measure continuous beat-to-beat blood pressure. An intravenous catheter was placed in the contralateral arm for drug administration. Heart rate was recorded continuously using a 3-lead ECG.
Figure 1.
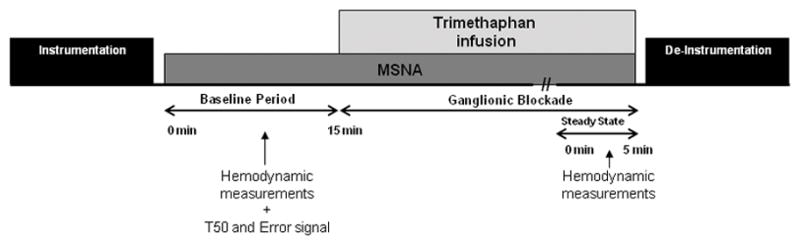
Timeline of experimental protocol. Hemodynamic measurements include arterial pressure and heart rate. MSNA, muscle sympathetic nerve activity.
After the placement of arterial and IV catheters, microneurography was performed while subjects rested in the supine position. Multiunit postganglionic MSNA was recorded from the peroneal nerve posterior to the fibular head using insulated tungsten microelectrodes. A muscle sympathetic fascicle was identified when taps on the muscle belly or passive muscle stretched evoked mechanoreceptive impulses [18]. The recorded signal was amplified 80,000-fold, band pass filtered (700 to 2000 Hz), rectified, and integrated (resistance-capacitance integrator circuit; time constant: 0.1 s) by a nerve traffic analyzer (662C-4 Nerve Traffic Analysis System, University of Iowa, Iowa City, IA). Finally the signal was recorded at 250 Hz (WinDaq, DATAQ Instruments, Akron, OH). Once a good quality electrode site for measurement of MSNA was found, 15 min of baseline data (MSNA, arterial pressure and heart rate) were recorded with the subject resting quietly.
After this baseline period, ganglionic blockade was then achieved using incremental intravenous infusion of trimethaphan camsylate (Cambridge Laboratories, Wallsend, UK) until subjects demonstrated <5 bpm increase in heart rate during phase II of the Valsalva maneuver [19]. The average dose to achieve blockade was 4.3±0.2 and 1.9±0.2 mg/min for young and older women, respectively. Trimethaphan blocks neurotransmission at the autonomic ganglia by competing for acetylcholine on the postsynaptic nicotinic receptors. Once a stable heart rate and blood pressure had been reached, MSNA, arterial pressure and heart rate were again recorded over 5 min. The disappearance of sympathetic bursts was observed during ganglionic blockade in both groups. After discontinuation of the study and de-instrumentation, subjects remained in the CRU for at least 2h for observation.
Data analysis
Muscle sympathetic nerve activity
Sympathetic bursts were assessed in the integrated neurogram and were identified by a custom-manufactured automated analysis program [20] that associates each sympathetic burst with the appropriate cardiac cycle by compensating for latency. The automated analysis was then reviewed by study personnel blinded to the specifics of the study day and corrected manually. MSNA is reported as burst frequency (bursts/min) and burst incidence (bursts/100 heartbeats).
Hemodynamic measurements
Heart rate was derived from the ECG. Systolic blood pressure (SBP), DBP and mean arterial pressure (MAP) were derived from the arterial pressure waveform.. Briefly, the brachial pressure waveform was downloaded onto a personal computer and analyzed offline using data acquisition software (WinDaq, DATAQ Instruments, Akron, OH). Beat-to-beat stroke volume was then calculated using Modelflow which computes an aortic waveform based on non-linear pressure–volume, pressure–compliance and pressure–characteristic impedance equations, incorporating age, sex, height and body mass [21].
Baroreflex threshold analysis and determination of blood pressure error signal
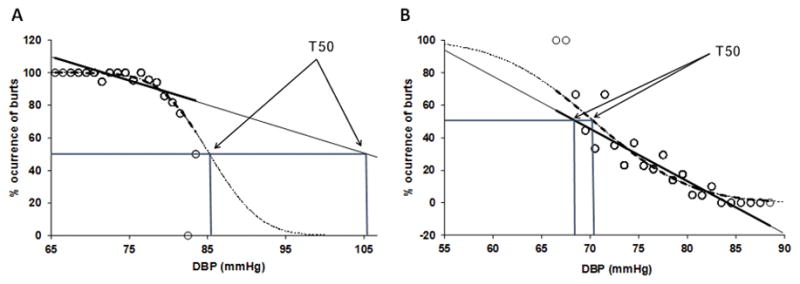
A baroreflex threshold curve was calculated for each subject to examine how DBP relates to the occurrence of a sympathetic burst in a steady-state baseline recording (Figure 2). Diastolic blood pressures are grouped into bins of 1 mmHg and the percentage of heart beats associated with a burst is determined within the bins [13]. For each individual, we calculated a T50, or midpoint value, representing the DBP at which there is a 50% likelihood of a sympathetic burst occurring [11, 13]. Since the threshold diagram is a sigmoid curve [22] a true threshold line can be calculated only in the middle portion. To avoid errors introduced by the sigmoid shape (see appendix) we linearized the relationship using probit transformation [11, 13]. To calculate the blood pressure error signal, we subtracted average DBP during baseline from the T50 pressure value in each subject [13]. Baroreflex sensitivity was assessed using the slope of the linear correlation between MSNA and DBP during steady-state baseline recording.
Figure 2.
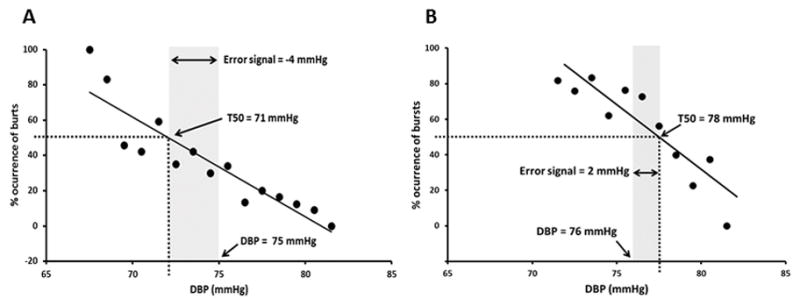
Representative example of a threshold curve in one young woman (A) and one older woman (B) at rest. Lower diastolic blood pressures (DBP) are associated with a higher percent occurrence of sympathetic bursts. The T50 is the DBP at which there is a 50% likelihood of burst occurrence. Shaded area represents the error signal: T50 minus the mean DBP [14,13].
Statistical analysis
All group data are reported as mean ± SEM. For mean data comparison between young and older women an unpaired Student’s t-test was used. The relationships of T50 and error signal (T50-DBP) to MSNA and the magnitude of change during autonomic blockade in cardiovascular variables were measured using linear regression analysis and the Pearson correlation coefficient, in the combined group and separately on each subgroup of young and older women. Data were analyzed statistically using SPSS v.18.0 for Windows (SPSS Inc., Chicago, IL, USA). A significance level of p<0.05 was set a priori to determine statistical significance.
Results
Older women had greater baseline SBP (young vs. older, 129±4 vs. 145±4 mmHg; p=0.006) and pulse pressure (young vs. older, 54±2 vs. 71±4 mmHg; p=0.001) compared with young women. Baseline MAP (young vs. older, 93±2 vs. 98±2 mmHg; p=0.136), DBP (young vs. older, 75±2 vs. 74±2 mmHg; p=0.848) and heart rate (young vs. older, 64±3 vs. 58±2 bpm; p=0.179) were not different.
The change in DBP with ganglionic blockade, T50 and MSNA, expressed as burst frequency and burst incidence, were higher in older women (Table 1). Sensitivity slope was not different between groups (Table 1). The change in DBP during autonomic blockade was inversely associated with T50 (r=−0.739, p<0.001); however, these results were age-specific (Figure 3A). A greater reduction in DBP was associated with higher T50 values in older women (r=−0.725, p=0.008) but not in young women (r=−0.337, p=0.29). The T50 was also associated with the reduction in SBP (r=−0.677, p<0.001), pulse pressure (r=−0.561, p=0.004) and MAP (r=−0.722, p<0.001) in the combined group. These relationships no longer existed when the group was split into young and older women. Similarly, T50 was significantly associated with MSNA burst frequency (Figure 3B) and burst incidence (r=0.632, p=0.001) in the combined group, although these results were not age-specific.
Table 1.
Change in DBP during ganglionic blockade and T50, error signal, sensitivity slope and baseline MSNA for young and older women
| Variables | Young Women (n=12) | Older Women (n=12) |
|---|---|---|
| Change in DBP after autonomic blockade, mmHg | −5 ± 6 (−16–4) | −19 ± 6* (−31–−9) |
| T50, mmHg | 66 ± 1 (57–75) | 74 ± 2* (62–87) |
| Error signal (T50-DBP), mmHg | −8 ± 1 (−17–−3) | 2 ± 1* (−5–10) |
| Sensitivity slope (bursts/100hb·mmHg) | −1.12 ± 0.28 (−3.53–0.02) | −0.87 ± 0.16 (−1.98–−0.09) |
| MSNA burst frequency, bursts/min | 15 ± 1 (8–23) | 33 ± 3* (14–46) |
| MSNA burst incidence, bursts/100 hb | 25 ± 2 (10–36) | 57 ± 5* (29–80) |
Data are presented as mean±SEM (minimum-maximum). DBP, diastolic blood pressure; hb, heartbeats; MSNA, muscle sympathetic nerve activity.
P<0.05.
Figure 3.
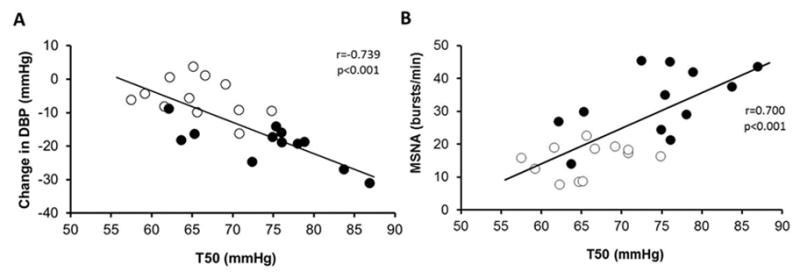
Linear regression analysis of the correlation between T50 at baseline and change in diastolic blood pressure (DBP) during autonomic blockade (A) and baseline muscle sympathetic nervous activity (MSNA) burst frequency (B). Closed symbols indicate older women and open symbols indicate young women.
Young women had a more negative error signal that older women (Table 1). There was also an inverse relationship between the error signal and the change in DBP in the combined group of women (Figure 4), suggesting that women who operate at a higher tonic blood pressure than their T50 (i.e., a negative error signal) have a smaller change in DBP when autonomic tone is removed. In contrast, in the subgroups of young and older women there were no associations between the error signal and the change in DBP (r=−0.542, p=0.82; r=−0.166, p=0.61; young vs. older, respectively). Error signal was associated with the change in SBP (r=−0.758, p<0.001), pulse pressure (r=−0.710, p<0.001) and MAP (r=−0.754, p<0.001) in the combined group; however, these results were not age-specific.
Figure 4.
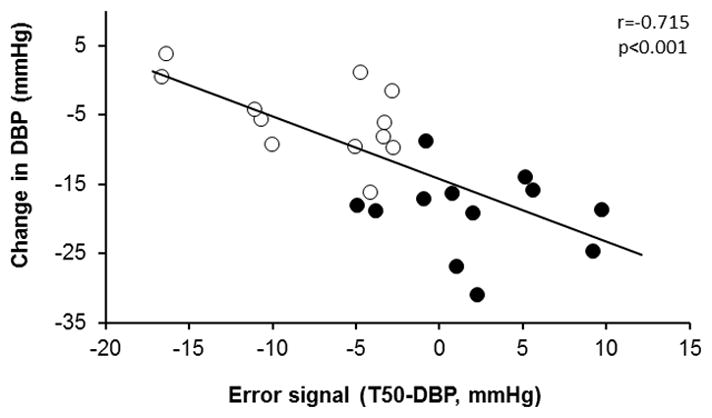
Linear regression analysis of the correlation between the error signal (T50-DBP [diastolic blood pressure]) at baseline and change in DBP during autonomic blockade. Closed symbols indicate older women and open symbols indicate young women.
In the combined group, women with the most negative error signal () had the lowest average sympathetic nervous system activity, measured as burst frequency (r=0.910, p<0.001) and burst incidence (r=0.931, p<0.001). Significant associations between the error signal and MSNA burst frequency persisted in both young women and older women when the groups were analyzed separately (Figure 5). Similar correlations were observed between the error signal and MSNA burst incidence (data not shown).
Figure 5.
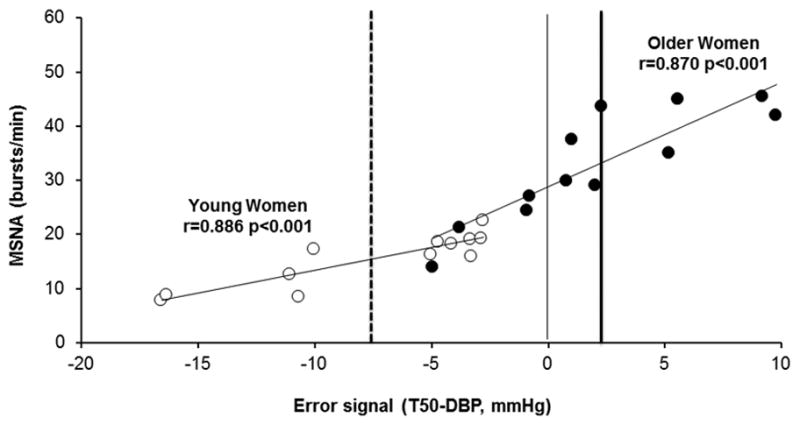
Linear regression analysis of the correlation between the error signal (T50-DBP [diastolic blood pressure]) and muscle sympathetic nerve activity (MSNA) burst frequency. Young women (open symbols) have a negative mean error signal (dashed vertical line) while older women (closed symbols) have a positive mean error signal (thick solid vertical line).
Discussion
The main finding of this study is that there are important differences in baroreflex control of MSNA between young and older women using T50 and error signal analysis. Older women had a higher T50 value and a more positive error signal in comparison to young women. Our results show that a greater reduction in blood pressure during autonomic blockade is related to a larger T50 and error signal. Additionally, the relationship between these indices is different between young and older women, suggesting that aging and/or the decreased levels of endogenous female sex hormones associated with menopause may influence sympathetic and non-neural mechanisms that control blood pressure in women. This approach provides further information on the relationship between blood pressure and autonomic support both in young and older women, in addition to our previous findings [10].
In the present study, we used the midpoint of the baroreflex curve, the T50, to determine if the sympathetic baroreflex control of blood pressure was different between young and older postmenopausal women. The T50 value gives information about the average setting of the baroreflex over a range of arterial pressures based on the fact that sympathetic outflow is not constant [14]. We saw a positive relationship between MSNA and T50, such that individuals with a higher T50 showed the greatest decreases in blood pressure during autonomic blockade. This association was more prominent in older postmenopausal women. This novel finding indicates how greater T50 values are associated with higher levels of MSNA at a given diastolic blood pressure. This explains the greater reduction in DBP observed in older women when abolishing sympathetic nerve activity via autonomic blockade. Previously, we have shown that the baroreflex is functioning in both groups [10], but that it is operating at different set points (i.e. at higher levels of MSNA for a given diastolic blood pressure in older women). The T50 value calculated in the current study is a way to quantify this, helping to clarify the differences between groups and increasing the understanding of the neural control of blood pressure. The brainstem nuclei involved in regulation autonomic function receive a number of peripheral afferent and higher subcortical/cortical inputs [23], which contribute to variability in the blood pressure threshold for sympathetic disinhibition, resulting in a burst of sympathetic activity. Accordingly, heartbeats with identical blood pressure values are not consistently associated with sympathetic bursts [14]. The T50 value is probably set by many factors, including central and peripheral (e.g., renal) neural and non-neural factors [15, 16], as well as the central effect of estrogen on modulating autonomic function [13, 23]. Experiments in rats have shown that an acute injection of estrogen decreases sympathetic nerve activity and increases parasympathetic output [23].
Error signal analysis offers insight into MSNA burst occurrence and is a tool that can be used to understand the range of the baroreflex threshold curve on which an individual is operating [13]. In our study, older women had a positive error signal (a mean DBP below the T50) and showed higher nerve activity in contrast to young women, who had a negative error signal (a mean DBP above the T50) and lower nerve activity. These results are supportive of previous findings suggesting greater autonomic support of blood pressure in older women [10]. Thus, older women operate on a part of the threshold curve associated with >50% likelihood of burst occurrence. This positive error signal could be related with the tendency of older women to have higher resting MSNA than young women and show augmented autonomic support of their blood pressure, as evidenced by older women having a greater reduction in blood pressure during ganglionic blockade than young women. Other studies have reported a lower autonomic support of blood pressure in young women compared with young men [24], further supporting our hypothesis that female sex hormones influence autonomic regulation of blood pressure. Therefore, it appears that blood pressure regulation in women after menopause is more similar to that observed in men.
Premenopausal women have less effective sympathetic baroreflex buffering (the ability of the baroreflex to buffer acute changes in blood pressure under certain conditions [e.g., when moving from a sitting to standing position]) compared to men of similar age [24]. Cardiac baroreflex sensitivity is associated with the magnitude of decrease in blood pressure during a vasoactive drug bolus in young men and older women, whereas this relationship is absent in young women [25]. Our results suggest that young women may have less ability to buffer decreases in blood pressure via the baroreflex, while older postmenopausal have the greatest decrease in DBP related with the highest values of T50, and therefore, they may show more robust baroreflex sensitivity. These results are consistent with other evidence that young women have a lower capacity to regulate blood pressure and maintain orthostatic tolerance compared with men [26, 27].
We have shown previously that postmenopausal women have higher vascular resistance compared with young women, which could be explained by aging, the loss of estrogen and decrease in vasodilator mechanisms [4, 10]. Enhanced β-adrenergic vasodilator responsiveness in young women is important in offsetting the vasoconstrictor effects of resistance when MSNA increases, and this mechanism appears to be lost in postmenopausal women [4]. The increased incidence of hypertension in older women [1] could be explained, in part, by this lack of ability of the β-adrenergic receptors to balance vasoconstrictor tone [4]. Previous results suggest that reduced sex hormones levels after menopause can contribute to this lower vasodilator capacity in aging women [8] because female sex hormones may enhance β-adrenergic mediated dilation by increasing nitric oxide availability [28].
Our study has some limitations. First, trimethaphan is no longer produced and there are only small quantities remaining for research. Therefore, we did not study men or assess α-adrenergic sensitivity during autonomic blockade. Secondly, we could have used another method, such as the modified Oxford technique, to assess baroreflex function in these women. Finally, we only studied healthy women and our results can only be applied to normotensive and lean individuals. Autonomic control of blood pressure and the relationships between T50 and the error signal remains to be studied in hypertensive women.
In summary, we have found important differences in baroreflex control of MSNA between young and older women using T50 and error signal analysis. In older women the baroreflex operates at higher levels of MSNA. This also explains why abolishing MSNA in these women via autonomic blockade has a greater effect on blood pressure. Greater T50 values and a more positive error signal were positively related with the reduction in DBP; furthermore, these relationships were more pronounced in older postmenopausal women. Finally, point out that T50 is at higher DBP for older women, mean DBP is similar between young and older women and therefore older women have a positive error signal and young women have a negative error signal (DBP much higher than T50), and MSNA is higher in older women compared to young women at their respective mean DBP. Our results support recent findings about a greater autonomic control of blood pressure in older women compared with young women.
Acknowledgments
We thank Shelly Roberts, Sarah Wolhart, Luke Matzek, Alexander Allen, Casey Hines, Pamela Engrav, Nancy Meyer, and Christopher Johnson for their continued assistance throughout the project.
Grants
This work was supported by National Institutes of Health grants RR024150 (Center for Translational Science Activities), AG038067 (Jill N. Barnes), HL083947 (B. Gunnar Wallin, Nisha Charkoudian, Michael J. Joyner), American Heart Association grant 2170087 (Emma C. Hart) and Mobility Grant Abroad “José Castillejo” for Young PhD CAS14/00239 (Ana B. Peinado).
Appendix
In the original description of baroreflex threshold diagrams, Wallin et al. [22] found that in subjects with cardiac arrhythmias the wide ranges of blood pressure variations resulted in S-shaped relationship between occurrence of sympathetic bursts and DBP. To define the relationship statistically probit transformation was used, and then the data points fitted a straight line which was characterized in terms of the T50 point and the slope of the probit line. In contrast, when variations of blood pressure are small (as in sinus rhythm) and data points fall within the linear part of the threshold diagram, linear regression has generally been assumed to provide valid measures of T50 and slope. This approach has been used in several studies. If, however, subjects have high or low resting burst incidence the data points may belong to the curved parts of the S curve. In such cases linear regressions may lead to values for T50 and/or slope that differ from those obtained with the probit method (Figure 6). Linear and probit analyses of baroreflex threshold curves have not been compared previously in a systematic way. Therefore, to clarify at which burst incidence levels discrepancies between the two methods may occur, we made such comparisons in a group of healthy subjects with burst incidences ranging from low to very high values.
Figure 6.
Data points indicating percentage heart beats associated with a sympathetic burst at different diastolic blood pressures (DBP) in subjects with resting burst incidences of 96 (A) and 22 (B). Baroreflex threshold curves based on linear (solid line) and probit (dashed line) analysis. Corresponding T50 values indicated by arrows.
The material consists of 51 healthy normotensive subjects (33 men, 18 women), aged 21–71 years, MSNA burst incidence ranged between 16 and 97 bursts/100 heartbeats and burst frequency between 11 and 60 bursts/min. The differences between the results obtained with the two methods are summarized in Figure 7. For T50 the results are more or less identical at burst incidence between 30 and 70 (Figure 7A). At lower or higher burst incidences, however, differences start to occur and for burst incidences above 80 or below 20 the differences are sometimes large. Also for the regression lines (Figure 7B) differences between slopes are mostly small when burst incidences are between 30 and 70 but occasional exceptions do occur. Outside this range the differences increase and at high or low burst incidences they may be quite large.
Figure 7.
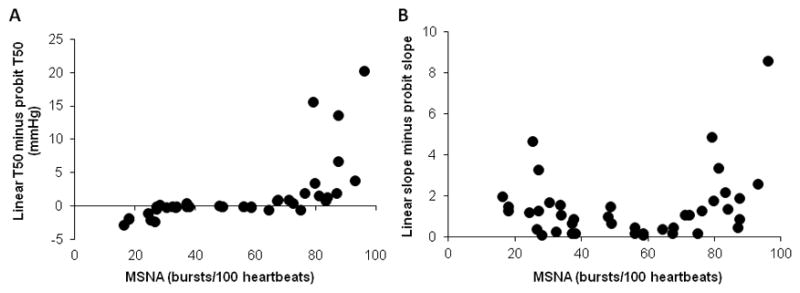
Difference between T50-values calculated with linear and probit methodology in subjects with different levels of resting muscle sympathetic nerve activity (MSNA) (A). For three subjects with burst incidences >80 the difference was >25 and therefore data points are not shown. Difference between slopes of threshold diagrams calculated with linear and probit methodology in subjects with different levels of resting MSNA (B).
The results confirm the supposition that for subjects with burst incidences in the medium range, linear and probit analyses of baroreflex threshold curves give similar results. If the subjects have a burst incidence range outside 30 to 70 bursts/100 heartbeats, however, linear analysis may result in erroneous values of T50 and slope (i.e baroreflex sensitivity); only probit analysis provides reliable measures. Since MSNA burst incidence is known to increase with age [29], probit analysis should be the preferred method in most studies of age related baroreflex control (like the present one). Special care is important when investigating patient groups with pathological increases of MSNA. A recent example is a study of patients with Takotsubo Cardiomyopathy [30] in which linear regression analysis was used at burst incidences above 90; obviously in that case probit analysis would have been the adequate method. In addition, at burst incidences >90 all data points may fall on the flat part of the S curve and if so, baroreflex analysis of threshold curves becomes highly unreliable even with probit methodology.
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25(3):305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 2.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45(4):522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 3.Mercuro G, Podda A, Pitzalis L, Zoncu S, Mascia M, Melis GB, Rosano GM. Evidence of a role of endogenous estrogen in the modulation of autonomic nervous system. Am J Cardiol. 2000;85(6):787–789. doi: 10.1016/s0002-9149(99)00865-6. [DOI] [PubMed] [Google Scholar]
- 4.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the beta-adrenergic receptors. J Physiol. 2011;589(Pt 21):5285–5297. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saleh MC, Connell BJ, Saleh TM. Medullary and intrathecal injections of 17beta-estradiol in male rats. Brain Res. 2000;867(1–2):200–209. doi: 10.1016/s0006-8993(00)02313-1. [DOI] [PubMed] [Google Scholar]
- 6.Saleh TM, Connell BJ. 17beta-estradiol modulates baroreflex sensitivity and autonomic tone of female rats. J Auton Nerv Syst. 2000;80(3):148–161. doi: 10.1016/s0165-1838(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 7.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101(8):862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 8.Harvey RE, Barnes JN, Charkoudian N, Curry TB, Eisenach JH, Hart EC, Joyner MJ. Forearm vasodilator responses to a beta-adrenergic receptor agonist in premenopausal and postmenopausal women. Physiol Rep. 2014;2(6):1–6. doi: 10.14814/phy2.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreau KL, Donato AJ, Tanaka H, Jones PP, Gates PE, Seals DR. Basal leg blood flow in healthy women is related to age and hormone replacement therapy status. J Physiol. 2003;547(Pt 1):309–316. doi: 10.1113/jphysiol.2002.032524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes JN, Hart EC, Curry TB, Nicholson WT, Eisenach JH, Wallin BG, Charkoudian N, Joyner MJ. Aging enhances autonomic support of blood pressure in women. Hypertension. 2014;63(2):303–308. doi: 10.1161/HYPERTENSIONAHA.113.02393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart EC, Joyner MJ, Wallin BG, Karlsson T, Curry TB, Charkoudian N. Baroreflex control of muscle sympathetic nerve activity: a nonpharmacological measure of baroreflex sensitivity. Am J Physiol Heart Circ Physiol. 2010;298(3):H816–822. doi: 10.1152/ajpheart.00924.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Querido JS, Wehrwein EA, Hart EC, Charkoudian N, Henderson WR, Sheel AW. Baroreflex control of muscle sympathetic nerve activity as a mechanism for persistent sympathoexcitation following acute hypoxia in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301(6):R1779–1785. doi: 10.1152/ajpregu.00182.2011. [DOI] [PubMed] [Google Scholar]
- 13.Wehrwein EA, Joyner MJ, Hart EC, Wallin BG, Karlsson T, Charkoudian N. Blood pressure regulation in humans: calculation of an “error signal” in control of sympathetic nerve activity. Hypertension. 2010;55(2):264–269. doi: 10.1161/HYPERTENSIONAHA.109.141739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568(Pt 1):315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osborn JW, Jacob F, Guzman P. A neural set point for the long-term control of arterial pressure: beyond the arterial baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol. 2005;288(4):R846–855. doi: 10.1152/ajpregu.00474.2004. [DOI] [PubMed] [Google Scholar]
- 16.Osborn JW. Hypothesis: set-points and long-term control of arterial pressure. A theoretical argument for a long-term arterial pressure control system in the brain rather than the kidney. Clin Exp Pharmacol Physiol. 2005;32(5–6):384–393. doi: 10.1111/j.1440-1681.2005.04200.x. [DOI] [PubMed] [Google Scholar]
- 17.Gracia CR, Sammel MD, Freeman EW, Lin H, Langan E, Kapoor S, Nelson DB. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause. 2005;12(2):128–135. doi: 10.1097/00042192-200512020-00005. [DOI] [PubMed] [Google Scholar]
- 18.Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272(2):383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkins BW, Hesse C, Sviggum HP, Nicholson WT, Moyer TP, Joyner MJ, Eisenach JH. Alternative to ganglionic blockade with anticholinergic and alpha-2 receptor agents. Clin Auton Res. 2007;17(2):77–84. doi: 10.1007/s10286-006-0387-7. [DOI] [PubMed] [Google Scholar]
- 20.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531(Pt 3):861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol. 1993;74(5):2566–2573. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- 22.Wallin BG, Delius W, Sundlof G. Human muscle nerve sympathetic activity in cardiac arrhythmias. Scand J Clin Lab Invest. 1974;34(4):293–300. doi: 10.3109/00365517409049883. [DOI] [PubMed] [Google Scholar]
- 23.Saleh TM, Connell BJ. Role of oestrogen in the central regulation of autonomic function. Clin Exp Pharmacol Physiol. 2007;34(9):827–832. doi: 10.1111/j.1440-1681.2007.04663.x. [DOI] [PubMed] [Google Scholar]
- 24.Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111(4):494–498. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- 25.Barnes JN, Matzek LJ, Charkoudian N, Joyner MJ, Curry TB, Hart EC. Association of cardiac baroreflex sensitivity with blood pressure transients: influence of sex and menopausal status. Front Physiol. 2012;3:1–6. doi: 10.3389/fphys.2012.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol. 1998;275(6 Pt 2):R1909–1920. doi: 10.1152/ajpregu.1998.275.6.R1909. [DOI] [PubMed] [Google Scholar]
- 27.Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol. 2001;281(5):H2028–2035. doi: 10.1152/ajpheart.2001.281.5.H2028. [DOI] [PubMed] [Google Scholar]
- 28.Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol. 2012;590(Pt 9):2069–2079. doi: 10.1113/jphysiol.2011.224642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaccaro A, Despas F, Delmas C, Lairez O, Lambert E, Lambert G, Labrunee M, Guiraud T, Esler M, Galinier M, Senard JM, Pathak A. Direct evidences for sympathetic hyperactivity and baroreflex impairment in Tako Tsubo cardiopathy. PLoS One. 2014;9(3):e93278. doi: 10.1371/journal.pone.0093278. [DOI] [PMC free article] [PubMed] [Google Scholar]


