Abstract
Purpose
We sought to identify autoantigens recognized by antibodies in breast cancer patient sera with potential diagnostic or prognostic significance.
Experimental Design
Serum from a female breast cancer patient exhibiting a high titer antinuclear antibody was used to screen a HeLa cDNA expression library, leading to the cloning of a cDNA for the Mr 32,000 subunit of replication protein A (RPA32). RPA32 expression and localization were assayed in autologous tumor by monoclonal antibody staining. A specific ELISA using recombinant protein was used to screen sera from 801 breast cancer patients and 65 controls.
Results
A relationship between anti-replication protein A (RPA) antibodies and the ductal breast carcinoma of the proband was suggested by overexpression and aberrant localization of RPA32 in tumor cells as compared with surrounding normal ductal tissue and by the presence of anti-RPA32 antibodies before the diagnosis. The prevalence of anti-RPA32 antibodies was significantly higher (P < 0.01) among breast cancer patients (87 of 801 patients) than among noncancer controls (0 of 65 controls). Similarly, anti-RPA32 antibodies were present in 4 of 39 patients with intraductal in situ carcinoma. No associations were found between anti-RPA antibodies and survival, occurrence of a second tumor, metastases, or antibodies to p53. Reactivity to RPA32 also was detected in sera from 3 of 47 patients with other cancers.
Conclusions
In view of the central role of RPA in DNA replication, recombination, and repair, we suggest that autoimmunity to RPA32 may reflect molecular changes involved in the process of tumorigenesis. The finding of antibodies to RPA32 before diagnosis and their prevalence in in situ carcinoma suggest that they are potentially useful markers of early disease.
INTRODUCTION
Autoantibodies are frequently observed in sera of patients with malignancies and generally have been thought to be nonspecific and a reflection of cancer-related general immune system dysfunction (1, 2). Accumulating evidence, however, suggests that changes in the immune response during malignant transformation are antigen driven and that some cancer-associated autoantigens might be involved in cellular functions related to tumorigenesis (3–6). Autoimmune responses in cancer patients to cell cycle-regulatory proteins, including p53 (7–9), cyclin B1 (10), cyclin-dependent kinase 4 (11), cdc27 (12), and p73, (13), may reflect molecular events that lead to tumorigenesis. Consistent with this hypothesis, cancer-related autoimmunity often appears to be directed against mutant forms of proteins (4, 8, 11, 14) or is associated with overexpression of the autoantigens in autologous tumor cells (4, 9).
Associations between the presence of certain ANAs4 and cancer cell type, diagnosis, and patient outcome suggest that recognition of specific autoantigens by cancer patient sera might have potential diagnostic and prognostic value (3, 9, 15, 16). We hypothesize that ANAs present in cancer patients’ sera may be related to the process of tumorigenesis, and we have begun to test this hypothesis by cloning nuclear antigens recognized by breast cancer patient sera.
Here we report that autoantibodies found in serum from a patient with a ductal breast carcinoma recognize RPA32 as an autoantigen. RPA is a highly conserved, single-stranded DNA-binding multisubunit protein complex involved in eukaryotic DNA replication, recombination, and repair (17–22). RPA has been reported to be rarely recognized as an autoantigen by sera from patients with systemic autoimmune diseases (23, 24), but there are no reports of autoimmunity to this protein in any other human disease. Here we examine the relationship of anti-RPA antibodies to cancer and investigate the potential of these autoantibodies as diagnostic and prognostic markers.
MATERIALS AND METHODS
Patients
In January 1997, JH, a 61-year-old woman who had seronegative RA since 1992 and Raynaud’s phenomenon since 1995, was found by routine mammography to have a 0.5-cm moderately differentiated infiltrating ductal carcinoma of the left breast. At the time of diagnosis of breast cancer, the RA was well controlled on weekly methotrexate and daily minocycline and analgesics. The rheumatoid factor was negative, and the ANA by indirect immunofluorescence was reactive at a titer of 1:2560 with a speckled pattern. JH was treated with lumpectomy and radiation therapy. The estrogen receptor was positive, and the axillary lymph nodes were free of cancer. Tamoxifen treatment was started in June 1997, and follow-up examinations to August of 2001 showed no evidence of recurrence or metastatic disease. The clinical impression that the Raynaud’s phenomenon as well as the high titer ANA might have been paraneoplastic led us to select JH’s serum for the cloning experiments.
Sera from JH were collected multiple times from 1995 on and stored in the serum bank of the Division of Rheumatology of WSU until use. Sera from 65 patients with a diagnosis of fibromyalgia and osteoarthritis were obtained from the Rheumatology Clinic of WSU. This control group included 46 females (mean age, 58 years) and 19 males (mean age, 59.9 years). Sera and outcome measures from 801 female breast cancer patients (mean age, 57.8 years) were collected by the WSU Breast Cancer Prognostic Study between 1980 and 1990. These were made available by the Karmanos Cancer Institute. Sera from 22 lung cancer patients and 35 head and neck cancer patients were collected at the time of diagnosis in the Detroit Medical Center Hematology/Oncology Clinic at WSU. The lung cancer patients included five patients with adenocarcinoma, six patients with squamous cell carcinoma, seven patients with small cell carcinoma, and four patients with large cell carcinoma. These patients have been described previously (15). All sera were stored at −70°C until use.
SDS-PAGE and Immunoblotting of HeLa Nuclear Extracts
HeLa cells were obtained from the American Type Culture Collection (Manassas, VA). Nuclear extracts were prepared by the method of Wood and Earnshaw (25), resuspended in SB [10 mm PIPES (pH 7.4), 80 mm KCl, 20 mm NaCl, 250 mm sucrose, 5 mm EGTA, 1 mm DTT, 50% glycerol, 0.01% bromphenol blue, and 5% β-mercaptoethanol] at a concentration of 5 × 106 nuclei/ml, and sonicated. Extracts were boiled for 5 min and then separated by SDS-PAGE as described by Laemmli (26). Proteins were transferred to nitrocellulose, and immunoblotting was performed as described previously by Towbin et al. (27). All sera were diluted 1:100 or as otherwise specified in PTX plus 4% BSA and incubated with membranes at room temperature for 1 h. Mouse monoclonal antibodies to RPA32 (NA18; Oncogene Research Products, Cambridge, MA) were used at a dilution of 1:1000. Three washes were performed in GB [50 mm triethanolamine-HCl (pH 7.4), 100 mm NaCl, 2 mm K-EDTA, 0.5% Triton X-100, and 0.1% SDS], followed by incubation for 1 h in sheep antihuman IgG or goat antimouse IgG horseradish peroxidase-linked secondary antibodies (Amersham, Arlington, IL) diluted 1:1500. Bound antibodies were detected by enhanced chemiluminescence (Amersham).
cDNA Library Screening and Sequence Determination
The JH prototype serum was used to screen a Uni-Zap XR λgt11 HeLa cell cDNA expression library (Stratagene, La Jolla, CA). Phage were grown on Escherichia coli XL Blue and induced overnight by overlaying with nitrocellulose filters soaked in 1 mm isopropyl-1-thio-β-d-galactopyranoside. Filters were hybridized at room temperature for 2 h with sera diluted 1:500 in PTX plus 4% BSA. After washing three times at room temperature in GB, filters were incubated with 125I-labeled protein A (Amersham) diluted at 1:2000 in PTX/4% BSA. Washes were repeated, and the filters were exposed to X-ray film at −70°C with an intensifying screen. Hybridization-positive phage were purified by repeated rounds of screening. The cDNA inserts were isolated by in vivo excision as per library instructions. Sequences were determined from plasmid DNA using an ABI Prism 377 sequencer by the Core DNA Sequencing Facility, Center for Molecular Medicine and Genetics, WSU. A BLAST search of GenBank5 was performed to determine sequence identity of the cloned cDNA.
Immunodepletion
Phage-encoded RPA32 was induced as indicated above, and filters with bound protein were rinsed in 2× SSC and air dried. JH serum, diluted 1:100 in PTX, was incubated with a protein-bound filter for 2 h. Depleted serum was recovered, incubated twice more with fresh filters, and used immediately to probe immunoblots.
Immunohistochemistry
Paraffin sections were deparaffinized and microwaved for 15 min in sodium citrate buffer and then incubated an additional 30 min in hot buffer. After blocking for 10 min in horse serum, sections were incubated for 2 h at room temperature with the mouse monoclonal antibody specific to RPA32 at a 1:10 dilution. This antibody reacts with a single band of Mr 32,000 on immunoblots of whole HeLa cell extracts. Sections were then incubated with biotinylated antimouse antibody (Vector Laboratories, Burlingame, CA) diluted 1:200 for 20 min, followed by incubation with avidin-biotin peroxidase reagent (Vector Laboratories) for 20 min and development with 3-amino-9-ethylcarbazole.
RPA32 Expression and Purification
A cDNA encoding full-length human RPA32 (17) was subcloned into the EcoRI and SmaI sites downstream from the T7 promoter in a modified pCYB vector (New England Biolabs, Beverly, MA). Expression from this construct results in a fusion protein containing RPA32 fused at its COOH terminus to the protein splicing element (intein) encoded by the Saccharomyces cerevisiae VMA1 (Mr 50,000) and a chitin-binding domain of Bacillus circulans (Mr 5,000). The fusion protein was expressed in E. coli BL21λDE3. Bacteria were grown to an A600 nm of 0.4 and induced with 1 mm isopropyl-1-thio-β-d-galactopyranoside for 4 h; harvested by centrifugation at 5,000 × g; resuspended in 20 mm HEPES (pH 8.0), 500 mm NaCl, and 0.1 mm EDTA; and sonicated. The lysate was centrifuged for 10 min at 12,000 × g, and the resulting supernatant was loaded onto a chitin column. The column was washed with 10 column volumes of 500 mm NaCl, 20 mm HEPES (pH 8.0), 0.1 mm EDTA, and 0.1% Triton X-100, and purified RPA32 was eluted after intein cleavage for 48 h at 4°C in 50 mm β-mercaptoethanol. The yield was approximately 2 mg purified protein/liter induced culture.
RPA32 ELISA
One µg of purified RPA32 was incubated overnight in each well of 96-well ELISA plates (Corning, Corning, NY). This amount of protein per well was determined empirically using JH serum and the monoclonal RPA32 antibody as positive controls and several normal sera known not to react with RPA32 on immunoblots as negative controls. Bound protein was washed twice with PBST and blocked for 1 h in 5% powdered milk in PBST. Test sera were added at a dilution of 1:100 and incubated for 1 h at room temperature. Each plate included JH serum at a dilution of 1:500 as a positive control as well as negative controls without either antigen or primary antiserum. All sera were tested in duplicate. After incubation, plates were washed three times (5 min each) with PBST and incubated with alkaline phosphatase-conjugated goat antihuman secondary antibodies (Life Technologies, Inc., Gaithersburg, MD) diluted 1:1000 in PBST plus 5% dry milk. Washes were repeated and followed by the addition of the alkaline phosphatase substrate p-nitrophenyl phosphate (Sigma, St. Louis, MO). Absorbance at 405 nm was recorded after a 1-h incubation at room temperature. Under these conditions, the absorbance readings for JH serum were >0.5 at a 1:500 dilution and >1.2 at a 1:100 dilution. Normal serum negative controls had average absorbance readings of >0.2 at a 1:100 dilution.
For each sera tested, an adjusted absorbance value was calculated by averaging readings from duplicate wells and subtracting the average value of the “no primary antiserum” control. A final ELISA value was obtained by dividing each adjusted value by that of the JH control from the same plate. To determine positive reactivity, we chose as our cutoff value a final ELISA value of 0.425. This value represents 3 SDs above the average for the 65 noncancer control sera and allowed clear differentiation between positive and negative sera. The average final ELISA values for positive and negative sera were 0.689 and 0.175, respectively. All positive sera were tested on immunoblots of purified RPA32 to verify that the reactivity observed by ELISA was not because of recognition of a minor contaminant antigen in the purified RPA32 preparation.
p53 ELISA
Sera were assayed at a 1:100 dilution using a commercially available p53 ELISA assay kit (Oncogene Research, Cambridge, MA). The positive cutoff absorbance value was determined by comparison with a known p53-positive human serum, as per the manufacturer’s instructions.
Statistical Methods
Prevalences or frequencies of occurrence and associations were determined using 2 × 2 χ2 analyses and, where appropriate, χ2 with Yate’s correction. Differences in mean survival were analyzed using t test and ANOVA.
RESULTS
Identification of RPA32 as an Autoantigen Recognized by a Breast Cancer Patient Serum
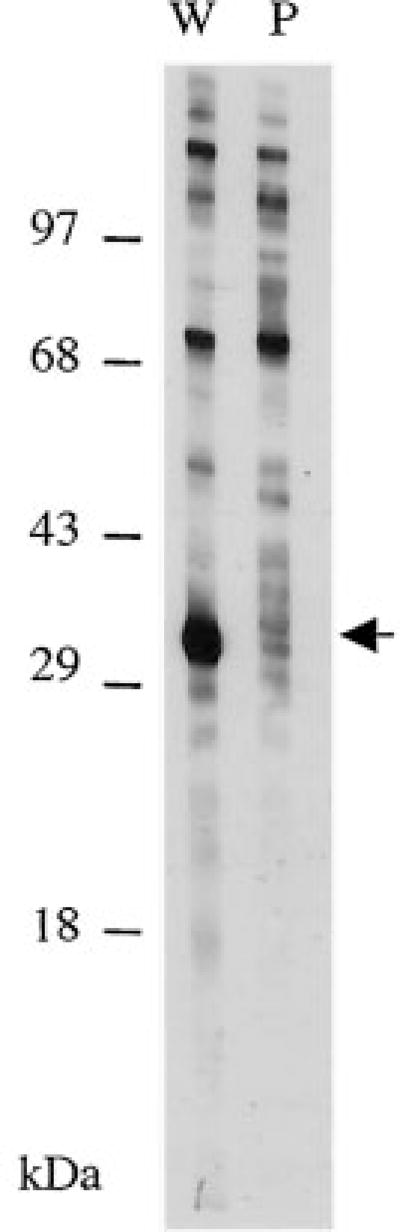
Serum from breast cancer patient JH was used to probe an immunoblot of a HeLa cell nuclear extract and showed strong reactivity of an IgG antibody to an antigen of Mr 32,000 (Fig. 1). By indirect immunofluorescence with HEp-2 cells as substrate, JH serum gave a fine speckled pattern in the nucleus and cytoplasm (data not shown), in a pattern similar to that described previously for RPA (23).
Fig. 1.
The cloned breast cancer autoantigen corresponds to RPA32. Immunoblots of HeLa nuclear antigens probed with JH serum. Lane W, whole serum; Lane P, serum after preabsorption with immobilized plaque protein corresponding to RPA32.
This same patient serum then was used as a probe to screen a HeLa cDNA expression library to identify autoantigens. Six positive clones were identified by screening 600,000 plaques. Three plaques were purified to homogeneity by successive rounds of screening. Sequencing of the cDNA inserts revealed that two were identical to each other. A BLAST sequence homology search of the conceptual translation of this cDNA revealed identity to amino acids 38–270 of RPA32 (17).
Recombinant plaque protein was immobilized onto nitrocellulose filters and used to deplete reactive antibodies from JH serum, which was then used to probe immunoblots of HeLa nuclear protein. Reactivity to the Mr 32,000 protein was greatly reduced by preabsorption with plaque protein, yet no other reactivity was noticeably affected (Fig. 1). This result shows that the RPA32 epitope(s) recognized by the autoantibodies was not shared by other nuclear proteins. Furthermore, it indicates that the region of RPA32 encoded by the phage includes most of the epitopes recognized by the autoimmune response. Because we were able to deplete the reactivity using bacterially produced protein, these data also show that the autoimmune response to RPA32 in JH is largely directed against primary amino acid sequence epitopes rather than against posttranslational modifications.
Expression of RPA32 in Autologous Proband Tumor
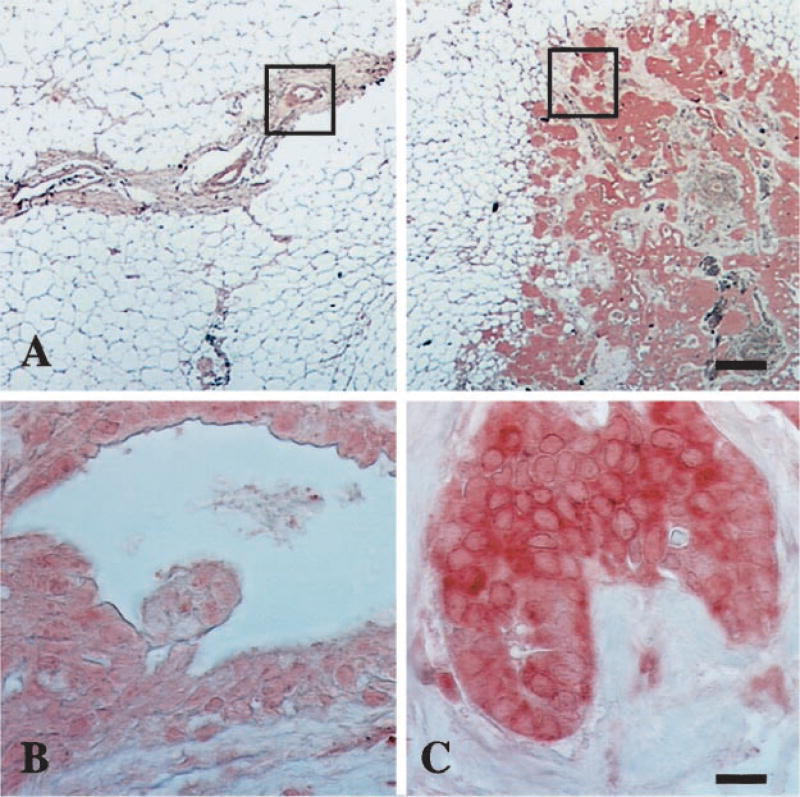
Because studies suggest that autoimmune responses to tumor antigens may be directed against overexpressed protein (4, 9), we asked whether RPA32 was overexpressed in the tumor of the proband. Staining of paraffin-embedded JH breast tissue with a monoclonal antibody specific to RPA32 revealed significantly more of the protein in tumor cells than in adjacent benign ductal epithelium (Fig. 2). Moreover, the distribution of RPA32 was altered in the tumor cells. Cell fractionation studies indicate that RPA is almost exclusively nuclear (28). In agreement with this study, in patient JH tissue, we could detect RPA only weakly in the nuclei of normal breast tissue. In contrast, RPA staining in tumor cells was very abundant in both nuclei and cytoplasm. We do not know whether this distribution of the protein results from a mutation in RPA or from overexpression and saturation of the nuclear import mechanism (29). Nonetheless, it suggests that autoimmunity to RPA in JH might be related to molecular changes in the tumor cells.
Fig. 2.
Immunohistochemical staining of JH breast tissue with anti-RPA32. A, representative adjacent areas of normal duct epithelium (left panel) and infiltrating ductal carcinoma (right panel); bar, 1 mm. Magnification of normal duct epithelium (B) and carcinoma (C); magnified areas are boxed in A. Bar, 20 µm.
Anti-RPA32 Antibodies Were Present in the Proband Both before Diagnosis of Breast Cancer and after Treatment
Because serum samples had been collected from JH both before diagnosis and after treatment, we were able to assay for changes in RPA32 autoantibodies relative to disease status. We reasoned that if the anti-RPA response developed during early stages of tumor growth, it might be detectable before clinical diagnosis. To test this, we developed a specific ELISA using bacterially expressed recombinant human RPA32. Expression, purification, and validation of the antigen are shown in Fig. 3. Anti-RPA32 antibodies were assayed by ELISA in sera from JH that had been collected 18, 6, and 3 months before the diagnosis of cancer. At a serum dilution of 1:500, we obtained absorbance readings of 1.50, 1.74, and 1.99, respectively, comparable with a reading of 1.45 obtained for a serum sample taken at the time of diagnosis. All of these values are well above the empirically determined ELISA positive cutoff value of 0.425 (see “Materials and Methods”). These results suggest that the anti-RPA response may develop during the early stages of tumor growth, and thus these antibodies are potentially useful as a diagnostic tool in some patients.
Fig. 3.
Production and purification of RPA32. Coomassie Blue-stained SDS-PAGE gels of total lysates of uninduced (Lane U) and induced (Lane I) cultures of bacteria showing the induced Mr 87,000 RPA32/intein/chitin-binding domain fusion protein (top arrow) and purified RPA32 recovered after intein cleavage (Lane P, bottom arrow). Lane M, immunoblot of the purified protein probed with monoclonal anti-RPA32 antibodies.
The high titer of anti-RPA antibodies remained relatively unchanged through the latest JH serum sample taken March 1, 2001, almost 4 years after tumor removal. An absorbance reading of 1.71 was obtained for this sample. The persistence of RPA32 autoantibodies in JH after tumor removal suggests that these antibodies may not be useful markers of disease status or recurrence. A similar persistence of autoantibodies to p53 after tumor removal has been reported in breast cancer patients (30).
Prevalence of Anti-RPA32 Antibodies in Breast Cancer
To determine whether an autoantibody response to RPA32 was common in breast cancer, we assayed 801 sera that had been collected from breast cancer patients and 65 sera from normal subjects who did not have cancer. A positive ELISA value was obtained for 87 (10.9%) of the sera from breast cancer patients, but none of the normal controls. The lack of anti-RPA antibodies in our control group is consistent with previous reports that anti-RPA antibodies could not be detected in a total of 62 healthy individuals and 159 patients with autoimmune diseases (23, 24). The significant difference in prevalence of anti-RPA32 antibodies between control and breast cancer groups (P < 0.01) strongly suggests an association between the development of anti-RPA32 antibodies and breast cancer. Furthermore, of 39 breast cancer patients who were diagnosed at an early stage with intraductal in situ carcinoma, 4 (10.3%) had anti-RPA antibodies. This finding suggests that the anti-RPA response may develop very early with respect to tumor formation.
Because we had 10-year follow-up data for these patients, we could ask if there were any associations between the presence of anti-RPA antibodies and a number of disease parameters. In a comparison of RPA32 ELISA-positive and -negative patients, we found no significant differences in the mean survival, the frequency of occurrence of a second primary tumor, or in the frequency of metastasis at time of diagnosis or at a later date.
Tests for an Association with p53 Antibodies
There are numerous reports of the detection of anti-p53 antibodies in breast cancer patients (30–36). Because RPA32 has been reported to form a complex with the tumor suppressor p53 (37), and protein complexes are often targets of the autoimmune response in systemic autoimmune diseases (2, 38, 39), it seemed possible that a p53/RPA32 complex might be a common antigen. To examine this possibility, we asked if patients with anti-RPA32 antibodies also had antibodies to p53. We tested 84 anti-RPA32-positive sera and 59 anti-RPA32-negative sera for anti-p53 antibodies using a commercially available p53 ELISA (Oncogene Research Products). The overall frequency of sera with anti-p53 antibodies was 7%, which is consistent with previous reports of anti-p53 antibodies found in 5–30% of breast cancer patients (30–33, 35, 36). We found no evidence of an association between anti-p53 and anti-RPA32 antibodies. Members of the anti-RPA-positive group were no more frequently positive for anti-p53 antibodies (3 of 84 members) than members of the anti-RPA-negative group (6 of 59 members). Too few patients were positive (n = 3) for both anti-RPA and anti-p53 antibodies to allow competent tests for associations between disease prognosis and autoimmunity to both antigens.
RPA32 Antibodies in Other Cancers
To determine whether autoimmunity to RPA32 was unique to breast cancer or was also present in other cancers, we tested sera from 22 patients with lung cancer and 35 patients with cancer of the head and neck. Two squamous cell lung cancer patients and one head and neck cancer patients were positive. This suggests that anti-RPA antibodies can develop in response to a variety of tumor types and may reflect a commonality in the molecular changes that occur in such tumors.
DISCUSSION
In this work we show for the first time that autoantibodies from patients with breast cancer recognize RPA32 as an autoantigen. The initial observation was made in a woman with RA, Raynaud’s phenomenon, and a high titer ANA who developed a ductal breast carcinoma. The prototype serum led to the cloning of a protein found to be identical to RPA32 described by Erdile et al. (17).
It is notable that the only previous reports of autoantibodies to RPA were made on three patients with systemic lupus erythematosus, two of whom had secondary Sjögren’s syndrome (23, 24). Both systemic lupus erythematosus and Sjögren’s syndrome are known to be associated with a tendency to develop lymphoid malignancies (40–43). One of the patients with systemic lupus erythematosus and Sjögren’s syndrome previously reported with anti-RPA antibodies had a history of lymphoma (24). Thus, it is possible that antibodies to RPA32 in the sera of patients with systemic autoimmune diseases may be early markers of malignancy. Several autoantibodies have also been reported in both autoimmune and cancer patients (44–47). The addition of RPA32 to this group of shared antigens suggests that a more thorough examination of the relationship between the development of autoimmunity in systemic autoimmune diseases and in cancer is warranted.
Our discovery that RPA is an antigenic target of autoimmunity in cancer patients is intriguing because of its essential roles in multiple aspects of DNA metabolism, including replication initiation and elongation (48–50), nucleotide excision repair (20, 51), homologous recombination (19), and transcriptional activation of DNA repair genes (22). Perturbations of any of these activities might be expected to have mutagenic consequences. Indeed, in the budding yeast S. cerevisiae, a specific allele of RFA-2, the RPA32 homologue, has been found to cause chromosomal instability (52), a phenotype commonly associated with cancer and argued to be causative (53). It is plausible that similar mutant forms of human RPA32 might be both tumorigenic and immunogenic. In this regard, it will be important to determine whether the RPA32 gene is mutated in tumors of patients with anti-RPA32 antibodies.
Our observation that RPA32 is overexpressed in tumor cells from JH suggests an additional mechanism by which changes in RPA32 may be tumorigenic. RPA32 is capable of complex formation with the tumor-suppressor protein p53 (37, 54) and can prevent sequence-specific DNA binding by p53 in vitro (37). The RPA32-p53 interaction has been proposed to modulate the p53-mediated DNA damage checkpoint response by sequestering p53 and preventing the transcriptional activation of genes involved in DNA repair (37). Thus, overexpression of RPA32 might effectively inactivate p53 and allow cells with damaged DNA to proceed through the cell cycle. Considering that autoantigens are frequently overexpressed in autologous tumors (4, 6, 9), it is conceivable that overexpression of RPA32 might act in promoting both tumorigenesis and autoimmunity.
Identification of RPA as an autoantigen adds another member to the growing list of cancer autoantigens known to play important roles in cell proliferation (3–6, 11–13). We propose that there may be two general classes of such cancer autoantigens: (a) those associated with specific types of cancers, such as the HCC1 antigen associated with hepatocellular carcinoma (3); and (b) those common to a wide spectrum of cancers, such as p53 (7–9) and RPA32. Whereas the first class may be of particular use as markers for tumor progression and prognosis, the latter class may be more revealing of the underlying molecular changes related to earlier steps in the process of tumorigenesis and may serve as diagnostic markers. Although we failed to find a relationship between anti-RPA32 antibody positivity and disease outcome in breast cancer, other reports have shown a relationship between autoantibody positivity to other autoantigens and outcome in both breast and renal carcinomas (55, 56).
Our observations suggest that it will be important to test whether there are changes in the RPA32 gene or gene product in patients with anti-RPA32 autoimmunity and to determine whether such changes can promote aspects of tumorigenesis in in vivo assays. In addition, it will be clinically relevant to determine the temporal relationship between cancer and the anti-RPA32 response. Our findings of anti-RPA antibodies before the clinical detection of breast cancer in the proband suggest that this autoimmune response may develop during early events of tumorigenesis. This is supported by our finding that more than 10% of patients with intraductal in situ carcinoma had anti-RPA antibodies. If anti-RPA32 antibodies could consistently be detected before the clinical appearance of tumor, the autoimmune response could potentially be used as a screening assay for occult tumor.
Acknowledgments
We are grateful to Dr. Thomas J. Kelly for the expression constructs of RPA32. We thank Pam Tabaczka for performing the immunohistochemistry and Dr. Michael Hagen for DNA sequencing.
Footnotes
Supported by NIH Grant R21 AR-99-128 (CA–87759–01) and grants from the Flora Temple Fund, Mrs. Mary Webber Parker, and the Karmanos Cancer Institute.
The abbreviations used are: ANA, antinuclear antibody; RPA, replication protein A; RPA32, the Mr 32,000 subunit of RPA; RA, rheumatoid arthritis; WSU, Wayne State University; PTX, 10 mm NaPO4 (pH 7.5), 0.2% Triton X-100, 0.15 m NaCl, 1 mm EGTA, and 1 mm NaN3; PBST, 10 mm NaPO4 (pH 7.5), 0.2% Triton X-100, 0.15 m NaCl, 1 mm EGTA, and 0.1% Tween 20.
References
- 1.Swissa M, Amital-Teplizki H, Haim N, Cohen Y, Shoenfeld Y. Autoantibodies in neoplasia. An unresolved enigma. Cancer (Phila.) 1990;65:2554–2558. doi: 10.1002/1097-0142(19900601)65:11<2554::aid-cncr2820651126>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.Burek CL, Rose NR. Autoantibodies. In: Colvin RB, Bhan AK, McCluskey RT, editors. Diagnostic Immunopathology. 2. New York: Raven Press; 1995. pp. 207–230. [Google Scholar]
- 3.Imai H, Nakano Y, Kiyosawa K, Tan EM. Increasing titers and changing specificities of antinuclear antibodies in patients with chronic liver disease who develop hepatocellular carcinoma. Cancer (Phila.) 1993;71:26–35. doi: 10.1002/1097-0142(19930101)71:1<26::aid-cncr2820710106>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc. Natl. Acad. Sci. USA. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon T, Old LJ. Cancer tumor antigens. Curr. Opin. Immunol. 1997;9:681–683. doi: 10.1016/s0952-7915(97)80049-0. [DOI] [PubMed] [Google Scholar]
- 6.Old LJ, Chen YT. New paths in human cancer serology. J. Exp. Med. 1998;187:1163–1167. doi: 10.1084/jem.187.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caron de Fromentel C, May-Levin F, Mouriesse H, Lemerle J, Chandrasekaran K, May P. Presence of circulating antibodies against cellular protein p53 in a notable proportion of children with B-cell lymphoma. Int. J. Cancer. 1987;39:185–189. doi: 10.1002/ijc.2910390211. [DOI] [PubMed] [Google Scholar]
- 8.Winter SF, Minna JD, Johnson BE, Takahashi T, Gazdar AF, Carbone DP. Development of antibodies against p53 in lung cancer patients appears to be dependent on the type of p53 mutation. Cancer Res. 1992;52:4168–4174. [PubMed] [Google Scholar]
- 9.Ralhan R, Nath N, Agarwal S, Mathur M, Wasylyk B, Shukla NK. Circulating p53 antibodies as early markers of oral cancer: correlation with p53 alterations. Clin. Cancer Res. 1998;4:2147–2152. [PubMed] [Google Scholar]
- 10.Covini G, Chan EK, Nishioka M, Morshed SA, Reed SI, Tan EM. Immune response to cyclin B1 in hepatocellular carcinoma. Hepatology. 1997;25:75–80. doi: 10.1002/hep.510250114. [DOI] [PubMed] [Google Scholar]
- 11.Wolfel T, Hauer M, Schneider J, Serrano M, Wolfel C, Klehmann-Hieb E, De Plaen E, Hankeln T, Meyer zum Buschenfelde KH, Beach D. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science (Wash. DC) 1995;269:1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- 12.Wang RF, Wang X, Atwood AC, Topalian SL, Rosenberg SA. Cloning genes encoding MHC class II-restricted antigens: mutated CDC27 as a tumor antigen. Science (Wash. DC) 1999;284:1351–1354. doi: 10.1126/science.284.5418.1351. [DOI] [PubMed] [Google Scholar]
- 13.Tominaga O, Unsal K, Zalcman G, Soussi T. Detection of p73 antibodies in patients with various types of cancer: immunological characterization. Br. J. Cancer. 2001;84:57–63. doi: 10.1054/bjoc.2000.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins PF, El-Gamil M, Li YF, Kawakami Y, Loftus D, Appella E, Rosenberg SA. A mutated β-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J. Exp. Med. 1996;183:1185–1192. doi: 10.1084/jem.183.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Madrid F, VandeVord PJ, Yang X, Karvonen RL, Simpson PM, Kraut MJ, Granda JL, Tomkiel JE. Antinuclear antibodies as potential markers of lung cancer. Clin. Cancer Res. 1999;5:1393–1400. [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández-Madrid F, Tomkiel JE. Antinuclear antibodies as potential markers in the diagnosis and prognosis of lung cancer. In: Shoenfeld Y, Gershwin ME, editors. Cancer and Autoimmunity. Elsevier Science; 2000. pp. 151–158. [Google Scholar]
- 17.Erdile LF, Wold MS, Kelly TJ. The primary structure of the 32-kDa subunit of human replication protein A. J. Biol. Chem. 1990;265:3177–3182. [PubMed] [Google Scholar]
- 18.Erdile LF, Heyer WD, Kolodner R, Kelly TJ. Characterization of a cDNA encoding the 70-kDa single-stranded DNA-binding subunit of human replication protein A and the role of the protein in DNA replication. J. Biol. Chem. 1991;266:12090–12098. [PubMed] [Google Scholar]
- 19.Moore SP, Erdile L, Kelly T, Fishel R. The human homologous pairing protein HPP-1 is specifically stimulated by the cognate single-stranded binding protein hRP-A. Proc. Natl. Acad. Sci. USA. 1991;88:9067–9071. doi: 10.1073/pnas.88.20.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Z, Henricksen LA, Wold MS, Ingles CJ. RPA involvement in the damage-recognition and incision steps of nucleotide excision repair. Nature (Lond.) 1995;374:566–569. doi: 10.1038/374566a0. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Lu X, Peterson CA, Legerski RJ. An interaction between the DNA repair factor XPA and replication protein A appears essential for nucleotide excision repair. Mol. Cell. Biol. 1995;15:5396–5402. doi: 10.1128/mcb.15.10.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh KK, Samson L. Replication protein A binds to regulatory elements in yeast DNA repair and DNA metabolism genes. Proc. Natl. Acad. Sci. USA. 1995;92:4907–4911. doi: 10.1073/pnas.92.11.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Lozano R, Gonzalez-Escribano F, Sanchez-Roman J, Wichmann I, Nunez-Roldan A. Presence of antibodies to different subunits of replication protein A in autoimmune sera. Proc. Natl. Acad. Sci. USA. 1995;92:5116–5120. doi: 10.1073/pnas.92.11.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Lozano R, Wichmann I, Garcia A, Sanchez-Roman J, Gonzalez-Escribano F, Nunez-Roldan A. Presence of antibodies to replication protein A in some patients with systemic lupus erythematosus (SLE) Clin. Exp. Immunol. 1996;103:74–76. doi: 10.1046/j.1365-2249.1996.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood ER, Earnshaw WC. Mitotic chromatin condensation in vitro using somatic cell extracts and nuclei with variable levels of endogenous topoisomerase II. J. Cell Biol. 1990;111:2839–2850. doi: 10.1083/jcb.111.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond.) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Biotechnology. 1992;24:145–149. [PubMed] [Google Scholar]
- 28.Treuner K, Eckerich C, Knippers R. Chromatin association of replication protein A. J. Biol. Chem. 1998;273:31744–31750. doi: 10.1074/jbc.273.48.31744. [DOI] [PubMed] [Google Scholar]
- 29.Jullien D, Gorlich D, Laemmli UK, Adachi Y. Nuclear import of RPA in Xenopus egg extracts requires a novel protein XRIPα but not importin α. EMBO J. 1999;18:4348–4358. doi: 10.1093/emboj/18.15.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metcalfe S, Wheeler TK, Picken S, Negus S, Jo Milner A. p53 autoantibodies in 1006 patients followed up for breast cancer. Breast Cancer Res. Treat. 2000;2:438–443. doi: 10.1186/bcr91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davidoff AM, Iglehart JD, Marks JR. Immune response to p53 is dependent upon p53/HSP70 complexes in breast cancers. Proc. Natl. Acad. Sci. USA. 1992;89:3439–3442. doi: 10.1073/pnas.89.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlichtholz B, Legros Y, Gillet D, Gaillard C, Marty M, Lane D, Calvo F, Soussi T. The immune response to p53 in breast cancer patients is directed against immunodominant epitopes unrelated to the mutational hot spot. Cancer Res. 1992;52:6380–6384. [PubMed] [Google Scholar]
- 33.Labrecque S, Naor N, Thomson D, Matlashewski G. Analysis of the anti-p53 antibody response in cancer patients. Cancer Res. 1993;53:3468–3471. [PubMed] [Google Scholar]
- 34.Coomber D, Hawkins NJ, Clark M, Meagher A, Ward RL. Characterisation and clinicopathological correlates of serum antip53 antibodies in breast and colon cancer. J. Cancer Res. Clin. Oncol. 1996;122:757–762. doi: 10.1007/BF01209124. [DOI] [PubMed] [Google Scholar]
- 35.Huober J, Sprenger H, Costa SD, Zentgraf H, Schmid H, Kaufmann M, Bastert G. Prognostic significance of p53 autoantibodies in serum of patients with breast carcinoma. Zentbl. Gynaekol. 1996;118:560–564. [PubMed] [Google Scholar]
- 36.Angelopoulou K, Yu H, Bharaj B, Giai M, Diamandis EP. p53 gene mutation, tumor p53 protein overexpression, and serum p53 autoantibody generation in patients with breast cancer. Clin. Biochem. 2000;33:53–62. doi: 10.1016/s0009-9120(99)00084-3. [DOI] [PubMed] [Google Scholar]
- 37.Miller SD, Moses K, Jayaraman L, Prives C. Complex formation between p53 and replication protein A inhibits the sequence-specific DNA binding of p53 and is regulated by single-stranded DNA. Mol. Cell. Biol. 1997;17:2194–2201. doi: 10.1128/mcb.17.4.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan EM. Autoantibodies: what do they recognize? Verh. Dtsch. Ges. Pathol. 1996;80:1–11. [PubMed] [Google Scholar]
- 39.Tan EM. Autoantibodies and autoimmunity: a three-decade perspective. A tribute to Henry G. Kunkel. Ann. N. Y. Acad. Sci. 1997;815:1–14. doi: 10.1111/j.1749-6632.1997.tb52040.x. [DOI] [PubMed] [Google Scholar]
- 40.Talal N. Benign and malignant lymphoid proliferation in autoimmunity. Recent Results Cancer Res. 1978;64:288–291. doi: 10.1007/978-3-642-81246-0_35. [DOI] [PubMed] [Google Scholar]
- 41.Fye KH, Daniels TE, Zulman J, Michalski JP, Jaffe R, Talal N. Aplastic anemia and lymphoma in Sjogren’s syndrome. Arthritis Rheum. 1980;23:1321–1325. doi: 10.1002/art.1780231117. [DOI] [PubMed] [Google Scholar]
- 42.Pavlidis NA, Drosos AA, Papadimitriou C, Talal N, Moutsopoulos HM. Lymphoma in Sjogren’s syndrome. Med. Pediatr. Oncol. 1992;20:279–283. doi: 10.1002/mpo.2950200403. [DOI] [PubMed] [Google Scholar]
- 43.Anaya JM, McGuff HS, Banks PM, Talal N. Clinicopathological factors relating malignant lymphoma with Sjogren’s syndrome. Semin. Arthritis Rheum. 1996;25:337–346. doi: 10.1016/s0049-0172(96)80019-9. [DOI] [PubMed] [Google Scholar]
- 44.Clark G, Reichlin M, Tomasi TB., Jr Characterization of a soluble cytoplasmic antigen reactive with sera from patients with systemic lupus erythmatosus. J. Immunol. 1969;102:117–122. [PubMed] [Google Scholar]
- 45.Mattioli M, Reichlin M. Heterogeneity of RNA protein antigens reactive with sera of patients with systemic lupus erythematosus. Description of a cytoplasmic nonribosomal antigen. Arthritis Rheum. 1974;17:421–429. doi: 10.1002/art.1780170413. [DOI] [PubMed] [Google Scholar]
- 46.Alspaugh MA, Talal N, Tan EM. Differentiation and characterization of autoantibodies and their antigens in Sjogren’s syndrome. Arthritis Rheum. 1976;19:216–222. doi: 10.1002/art.1780190214. [DOI] [PubMed] [Google Scholar]
- 47.Scanlan MJ, Gout I, Gordon M, Williamson B, Stockert E, Gure AO, Jäger D, Chen YT, Mackay A, O’Hare MJ, Old LJ. Humoral immunity to human breast cancer: antigen definition and quantitative analysis of mRNA expression. Cancer Immun. 2001;1:4. [PubMed] [Google Scholar]
- 48.Melendy T, Stillman B. An interaction between replication protein A and SV40 T antigen appears essential for primosome assembly during SV40 DNA replication. J. Biol. Chem. 1993;268:3389–3395. [PubMed] [Google Scholar]
- 49.Waga S, Stillman B. Anatomy of a DNA replication fork revealed by reconstitution of SV40 DNA replication in vitro. Nature (Lond.) 1994;369:207–212. doi: 10.1038/369207a0. [DOI] [PubMed] [Google Scholar]
- 50.Fang F, Newport JW. Distinct roles of cdk2 and cdc2 in RP-A phosphorylation during the cell cycle. J. Cell Sci. 1993;106:983–994. doi: 10.1242/jcs.106.3.983. [DOI] [PubMed] [Google Scholar]
- 51.Nagelhus TA, Haug T, Singh KK, Keshav KF, Skorpen F, Otterlei M, Bharati S, Lindmo T, Benichou S, Benarous R, Krokan HE. A sequence in the N-terminal region of human uracil-DNA glycosylase with homology to XPA interacts with the C-terminal part of the 34-kDa subunit of replication protein A. J. Biol. Chem. 1997;272:6561–6566. doi: 10.1074/jbc.272.10.6561. [DOI] [PubMed] [Google Scholar]
- 52.Chen C, Kolodner RD. Gross chromosomal rearrangements in Saccharomyces cerevisiae replication and recombination defective mutants. Nat. Genet. 1999;23:81–85. doi: 10.1038/12687. [DOI] [PubMed] [Google Scholar]
- 53.Duesberg P, Rausch C, Rasnick D, Hehlmann R. Genetic instability of cancer cells is proportional to their degree of aneuploidy. Proc. Natl. Acad. Sci. USA. 1998;95:13692–13697. doi: 10.1073/pnas.95.23.13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li R, Botchan MR. The acidic transcriptional activation domains of VP16 and p53 bind the cellular replication protein A and stimulate in vitro BPV-1 DNA replication. Cell. 1993;73:1207–1221. doi: 10.1016/0092-8674(93)90649-b. [DOI] [PubMed] [Google Scholar]
- 55.Smyth PP, Shering SG, Kilbane MT, Murray MJ, McDermott EW, Smith DF, O’Higgins NJ. Serum thyroid peroxidase autoantibodies, thyroid volume, and outcome in breast carcinoma. J. Clin. Endocrinol. Metab. 1998;83:2711–2716. doi: 10.1210/jcem.83.8.5049. [DOI] [PubMed] [Google Scholar]
- 56.Franzke A, Peest D, Probst-Kepper M, Buer J, Kirchner GI, Brabant G, Kirchner H, Ganser A, Atzpodien J. Autoimmunity resulting from cytokine treatment predicts long-term survival in patients with metastatic renal cell cancer. J. Clin. Oncol. 1999;17:529–533. doi: 10.1200/JCO.1999.17.2.529. [DOI] [PubMed] [Google Scholar]