Abstract
NK-92 cells, and their derivative, designated aNK, were obtained from a patient with non-Hodgkin lymphoma. Prior clinical studies employing adoptively transferred irradiated aNK cells have provided evidence of clinical benefit and an acceptable safety profile. aNK cells have now been engineered to express IL-2 and the high affinity (ha) CD16 allele (designated haNK). Avelumab is a human IgG1 anti–PD-L1 monoclonal antibody, which has shown evidence of clinical activity in a range of human tumors. Prior in vitro studies have shown that avelumab has the ability to mediate antibody-dependent cell-mediated cytotoxicity (ADCC) of human tumor cells when combined with NK cells. In the studies reported here, the ability of avelumab to enhance the lysis of a range of human carcinoma cells by irradiated haNK cells via the ADCC mechanism is demonstrated; this ADCC is shown to be inhibited by anti-CD16 blocking antibody and by concanamycin A, indicating the use of the granzyme/perforin pathway in tumor cell lysis. Studies also show that while NK cells have the ability to lyse aNK or haNK cells, the addition of NK cells to irradiated haNK cells does not inhibit haNK-mediated lysis of human tumor cells, with or without the addition of avelumab. Avelumab-mediated lysis of tumor cells by irradiated haNK cells is also shown to be similar to that of NK cells bearing the V/V Fc receptor high affinity allele. These studies thus provide the rationale for the clinical evaluation of the combined use of avelumab with that of irradiated adoptively transferred haNK cells.
Keywords: avelumab, ADCC, haNK cells, NK-92 cells, anti-PD-L1
Introduction
Several checkpoint inhibitor anti–PD-1/PD-L1 monoclonal antibodies (MAbs) have recently been approved by the Food and Drug Administration for indications in various cancer types including melanoma, lung cancer, and bladder cancer.1, 2 Avelumab, an anti-PD-L1 MAb, has demonstrated clinical activity in studies in patients with a range of human cancers;3-11 it has just been approved by the Food and Drug Administration for the treatment of Merkel cell and bladder carcinoma, and is in several Phase III studies in other indications. Despite the clinical success with anti-PD-1/PD-L1 MAbs, the majority of patients with carcinomas do not derive clinical benefit from these agents. Numerous preclinical and clinical studies are ongoing and planned concerning the use of these checkpoint inhibitor antibodies in combination with other forms of therapy, including other immunotherapeutics.12-16 Avelumab and atezolizumab are unique among currently employed anti-PD-L1 MAbs in that they are fully human immunoglobulin IgG1s with a non-mutated Fc, which also renders them capable of mediating antibody-dependent cell-mediated cytotoxicity (ADCC).17, 18
Several other MAbs currently employed in cancer therapy are also of the IgG1 isotype, including cetuximab, trastuzumab, and rituximab. While it is difficult to prove, evidence exists that, in addition to the ability of these antibodies to mediate anti-tumor activity by direct ligand receptor interactions, the ADCC mechanism may also be involved in anti-tumor effects. This is derived from reports that patients receiving cetuximab, trastuzumab, or rituximab, and whose natural killer (NK) cells express the high affinity CD16 valine (V) allele (V/V), experience better clinical outcomes than those with the lower affinity V/phenylalanine (F) and F/F genotypes.19-22 Reports vary as to what percentage of the population is of the V/V genotype, but most studies report approximately 10-20%.23
An allogenic NK cell line engineered to express the high affinity (ha) CD16 allele has recently been reported.24, 25 The haNK cell line is derived from NK-92 cells, which were derived from a patient with non-Hodgkin lymphoma.24 A derivative of NK-92, designated aNK, has been employed in several clinical studies as an irradiated adoptively transferred agent. Up to 1010 irradiated aNK cells repeatedly infused in patients have demonstrated safety and evidence of clinical benefit.24, 26-28 The haNK cells described here have also been engineered to endogenously express IL-2; this property enables haNK cells to propagate in the absence of IL-2 and permits greater NK killing due to the replenishing of granzyme/perforin stocks.29, 30
We report here the ability of the human IgG1 anti-PD-L1 MAb avelumab to enhance the lysis of a range of human carcinoma cells by irradiated haNK cells via the ADCC mechanism. These studies provide the rationale for the potential combined use of adoptively transferred irradiated haNK cells with the checkpoint inhibitor avelumab.
Materials and Methods
Cell lines and cultures
The parental cell line NK-92 was originally established from a male 50-year-old patient with non-Hodgkin lymphoma, whose bone marrow was infiltrated with large granular lymphocytes (LGL).31 The NK-92 and a clone of NK-92 designated aNK are dependent on IL-2 for proliferation. NK-92 cells were genetically modified to produce IL-2 in an autocrine loop, as well as to express a high affinity variant (V158) of the CD16 FcγRIII, and have been designated haNK cells.24 haNK cells and aNK cells were provided by NantBioScience (Culver City, CA) through a Cooperative Research and Development Agreement (CRADA) with the National Cancer Institute, NIH. haNK cells are cultured in phenol free X-Vivo-10 medium (Lonza, Walkersville, MD) supplemented with 5% human heat-inactivated AB serum (Omega Scientific, Tarzana, CA) at a concentration of 5×105/ml. Human tumor cell lines (H441: lung carcinoma; SKOV3: ovarian carcinoma; H460: lung carcinoma; HCC4006: lung carcinoma; SW403: colorectal carcinoma; MDA-MB-231: breast carcinoma; and HTB-4: bladder carcinoma) were purchased from American Type Culture Collection (Manassas, VA). All cultures were free of mycoplasma and maintained in RPMI-1640 supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine (Mediatech, Herndon, VA). Peripheral blood mononuclear cells (PBMCs) from healthy donors were obtained from the NIH Clinical Center Blood Bank (NCT00001846). PBMCs from 3 breast cancer patients were obtained from a clinical trial at the National Cancer Institute (NCT00179309).
Antibodies
The anti-PD-L1 MAb avelumab and matching IgG1 isotype control were obtained from EMD Serono (Rockland, MA) as part of a CRADA with the National Cancer Institute, NIH. Cetuximab (Bristol-Myers Squibb, Princeton, NJ) was obtained from the NIH Pharmacy.
Evaluation of haNK cell cytokine production
Culture supernatants were evaluated for secretion of IFN-γ, IL-10, IL-2, and IL-8 using a multiplex cytokine/chemokine kit (Meso Scale Discovery, Gaithersburg, MD).
ADCC assay and blocking experiments
Irradiated (10 Gy) haNK cells, irradiated (10 Gy) aNK cells, and NK cells isolated from healthy donors were all used as effectors in 111In-release lysis assays performed for 4 hr and/or 18 hr. NK cells from healthy donors were isolated using the Human NK Cell Isolation (negative selection, Kit 130-092-657, Miltenyi Biotech, San Diego, CA) following the manufacturer's instructions, resulting in > 90% purity, or the EasySep NK cell isolation kit (STEMCELL Technologies, Vancouver, BC, Canada) per the manufacturer's instructions, resulting in >90% purity. Prior to assay, cells were rested overnight in RPMI-1640 medium containing 10% human AB serum, as previously described.17 Target cells were labeled with 20 μCi of 111In-oxyquinoline (GE Healthcare, Chicago, IL) at 37°C for 20 min and used as targets at 2-3,000 cells/well in a 96-well round-bottom culture plate at various effector to target cell (E:T) ratios as indicated. For ADCC experiments, the targets were first incubated for 30 min with the test MAb and isotype control MAb before the haNK cells were added, as previously described.17 The plates were incubated for 4 hr or 18 hr at 37°C in a humidified atmosphere containing 5% CO2, then harvested and counted on a Wizard2 gamma counter (PerkinElmer, Shelton, CT). All samples were tested in triplicate and specific lysis was calculated from the average. Spontaneous release was determined by incubating targets with medium alone; complete lysis was determined by incubating targets with 0.05% Triton X-100 (Sigma-Aldrich, St. Louis, MO). Specific lysis was determined using the following equation: Percent lysis = (experimental - spontaneous)/(complete - spontaneous) × 100. For the blocking experiments, irradiated haNK cells were pre-incubated for 2 hr with either anti-CD16 antibody (50 μg/ml, clone B73.1, eBioscience, San Diego, CA) or concanamycin A (CMA; 200 nM, Sigma) before being used in lysis assays with the H460 human lung carcinoma cell line as a target at a 25:1 E:T ratio. Both NK lysis and ADCC assays mediated by avelumab or cetuximab were performed in 4-hr or 18-hr 111In release assays as indicated.
CD16 (FcγRIIIa) genotyping
DNA was extracted from the PBMCs of healthy donors using a QIAamp DNA Blood Mini kit (Qiagen, Valencia, CA), and stored at -80°C until use. The polymorphism of CD16 at amino acid position 158 that is a valine (V) vs. phenylalanine (F) was determined using allele-specific droplet digital polymerase chain reaction (ddPCR) employing the TaqMan array for CD16 (rs396991; Life Technologies, Waltham, MA).23, 32, 33 A master reaction mix was prepared, and 1 μl of genotyping DNA was added. The PCR reaction was performed on a Bio-Rad T100 thermal cycler (Bio-Rad, Hercules, CA) for 40 cycles at 95°C for 10 min, 94°C for 30 sec, and 60°C for 1 min. The plate was read on a Bio-Rad QX200 droplet reader. Data were analyzed with Bio-Rad QuantaSoft v.1.5 software.
RNA analysis
haNK cells were cultured and untreated, or irradiated with 10 Gy of gamma radiation 24 hr prior to RNA extraction. Total RNA was extracted from 1×106 cells per sample, using an RNeasy Mini kit, from Qiagen. Each sample was adjusted to 330 ng in 10 μl of total RNA. Nanostring analysis of the isolated RNA was completed with the nCounter PanCancer Immune Profiling Panel (NanoString Technologies, Inc., Seattle, WA), run by the Genomics Laboratory, Frederick National Laboratory for Cancer Research, Frederick, MD. Raw data files (RCC) and reporter library files (RLF) were uploaded into nSolver analysis software, version 3.0.22, 2011-2016 (NanoString). The nCounter Advanced Analysis-Quick Analysis (nSolver, NanoString) was used to analyze the raw data. The covariates selected represent the treatment of haNK cells (irradiated and non-irradiated). Non-irradiated samples were used as the categorical reference values. Normalization of raw data was calculated through the geNorm algorithm, which chose the most consistently expressed housekeeping genes for dataset normalization. Differential expression by multivariate linear regression included significant changes by P-value and log2 fold change. Genes with differential expression that had statistical significance of P < 0.05 were selected. All genes with consistent upregulation by treatment, in both of the independent experiments, were included in further analyses. A cutoff of log2 fold change > 0.75 was applied to genes downregulated by treatment. Data output of the top genes, including their log2 fold change in differential expression, was uploaded into Ingenuity Pathway Analysis (IPA), version 31813283 (Qiagen) for further investigation. The IPA - Core analysis revealed the top five relevant Diseases and Biological Functions as well as the top five relevant upstream molecules, by P-value of overlap. Agglomerative clustering of normalized data was used to generate a heat map of the top genes by nSolver Java TreeView, nSolver analysis software, version 3.0.22, 2011-2016 (NanoString). Analysis properties included the z-score transformation of genes and Euclidean distance analysis, which calculated the mean distance between cluster elements. Samples and genes were run as an ordered data set and all samples were included in one analysis allowing for cross comparison between independent experiments.
Statistical analyses
Statistical analyses were performed in GraphPad Prism 7 (GraphPad Software, La Jolla, CA), using multiple T-tests, with a desired false discovery rate of 1.00%.
Results
A prior report described the phenotype of haNK cells prior to a post-lethal irradiation (10 Gy) and demonstrated that irradiated haNK cells express high levels of perforin, granzyme, CD107a, as well as other NK markers, including some expression of PD-1, PD-L1, NKG2D, Tim3, and 4-1BB.25 We have now evaluated the effect of 10 Gy irradiation on RNA expression in haNK (Fig. 1). Total RNA from non-irradiated and irradiated haNK cells from two independent experiments was analyzed with the nCounter PanCancer Immune Profiling Panel (NanoString). The nCounter Advanced Analysis-Quick Analysis (nSolver, NanoString) revealed differentially expressed genes due to 10 Gy irradiation exposure. Ninety-eight genes had a statistically significant change in gene expression due to irradiation, with 76 downregulated and 22 upregulated. The Heat Map Analysis (nSolver, NanoString) (Fig. 1a) depicts the 16 most upregulated genes and the top 35 downregulated genes. The majority of genes upregulated or downregulated with radiation were found to be associated with cellular activation. The top five upstream regulators and the top five diseases or biological functions for upregulated and downregulated gene sets were identified by Ingenuity Pathway Analysis (Qiagen) (Fig. 1b and c). Genes with upregulated expression following irradiation of haNK cells are predicted to be governed by CTLA4, NOTCH1, CFLAR, VIP and PTX3. Upregulated genes are involved in the activation of leukocytes and lymphocytes as well as the migration of lymphatic system cells, the immune response of cells and the proliferation of blood cells.
Figure 1.
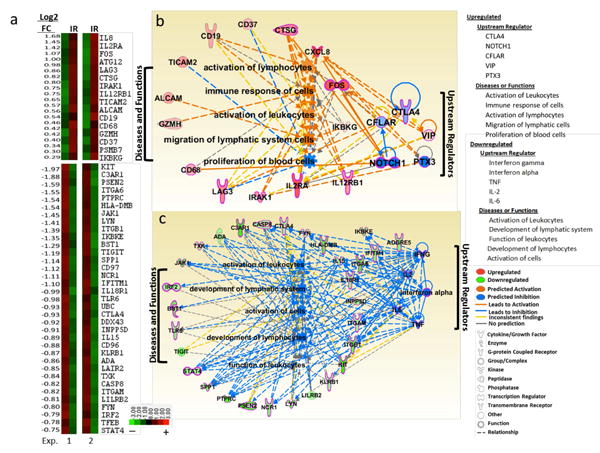
Gene expression of haNK cells ± 10 Gy irradiation. haNK cells were intact or irradiated 24 hr prior to RNA extraction and analysis by nCounter PanCancer Immune Profiling Panel. Heat Map Euclidean Distance Analysis depicts the top differentially expressed genes by irradiation in comparison to non-irradiated haNK cells (a), nSolver analysis software version 3.0.22, (P < 0.05). Upregulated (top panel) and downregulated (bottom panel) genes are shown for two independent experiments (left and right panels). Top Upstream Regulators and Diseases and Biological Functions predicted to be associated with the corresponding upregulated (b) and downregulated (c) genes by Ingenuity Pathway Analysis are shown. Several genes were upregulated by irradiation (CD19, CD37, CTSG, CXCL8, FOS, IKBKG, IL12RB1, IL2RA, IRAK1, LAG3), while others were downregulated (ADA, C3AR1, CASP8, CTLA4, FYN, HLA-DMB, IL15, IL18R1, INPP5D, ITGAM, ITGB1, KIT, KLRB1, LILRB2, LYN, NCR1, PSEN2, PTPRC, SPP1, STAT4, TIGIT, TLR6). The NF-κB complex was predicted to be affected by irradiation as IRAK1 and IKBKG were upregulated and IL18R1 and INPP5D were downregulated. Additionally, FOS, a nuclear phosphoprotein important for the AP-1 transcription factor complex, was upregulated by irradiation. Downregulated genes include those associated with immune cell activation by the Src tyrosine kinases: FYN, LYN, and PTPRC. Downregulated genes involved in immune-regulation include CTLA4, HLA-DMB, and TIGIT, whereas LAG3 was found to be upregulated. NK cell–related genes that were downregulated include KLRB1, TIGIT and NCR1, whereas IL12RB1 was upregulated by irradiation.
Since any clinical application of haNK will involve the use of lethally irradiated cells, all studies reported below were conducted with irradiated haNK cells. Non-irradiated and irradiated haNK cells (10 Gy) were evaluated for cytokine production in supernatant fluids over a 48-hr period (6, 12, 24, 48 hr) (Supplemental Fig. 1). Increased levels of both IFN-γ and IL-8 were produced by irradiated haNK cells vs. non-irradiated haNK cells. haNK cells continued to produce IL-10 and IL-2 at 6, 12, 24, and 48 hr post-irradiation, albeit at lower levels than non-irradiated haNK cells (Supplemental Fig. 1). Levels of TNF-α were below the level of detection of assays in both irradiated and non-irradiated haNK cells. haNK cells were engineered to express IL-2 for two reasons. The first is to negate the need for the use of exogenous IL-2 for cell proliferation. The other reason is that IL-2 has been shown to replenish the granular stock of NK cells and thus enhances the granzyme-mediated lysis of potentially “exhausted” NK cells; it is this phenomenon that led to prior studies showing that NK cells can be “serial killers,” i.e., lysing greater levels of target with time.29, 30 Studies were thus conducted to determine if avelumab-mediated ADCC of haNK cells would enhance with longer exposure to targets. As seen in Figure 2a, haNK alone lysis of H460 human lung carcinoma cells increased from 4 to 18 hr at each E:T ratio (IgG, black squares); avelumab-mediated ADCC of H460, moreover, also increased from 4 to 18 hr at each E:T ratio (blue circles). A human IgG1 was also used as an isotype control in all experiments to define that the ADCC-mediated lysis was indeed due to avelumab. In addition to IgG1 control antibody, assays were performed with no Mab in the wells, with identical lysis as the control antibody. Therefore, only the control IgG1 antibody is shown. Similar results were also seen in lysis in 4 vs. 18 hr assays employing as a target the human cervical cancer CaSki cell line (Fig. 2b). Additional studies also showed similar results employing five other human cell lines (Fig. 2c-g). Studies evaluating ADCC at a range of concentrations of avelumab showed similar tumor lysis results due to antibody saturation.
Figure 2.
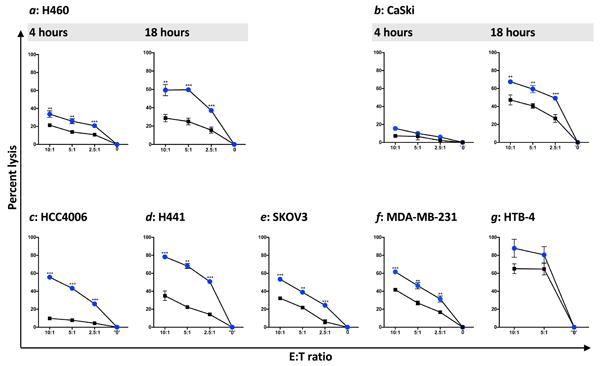
haNK ADCC mediated by avelumab. Tumor cell lysis mediated by irradiated haNK cells and avelumab (ADCC) was evaluated in 111In-release assays at different E:T ratios as indicated. 0 indicates target cell lysis in the absence of effector cells. Both 4-hr and 18-hr assays were performed for (a) H460 lung carcinoma, and (b) CaSki cervical carcinoma, employing avelumab (blue circles) or IgG (black squares) at 1 ng/ml. All other assays were 18 hr, and c-f used avelumab or IgG at 2 ng/ml; HCC4006: lung carcinoma; H441: lung carcinoma; SKOV3: ovarian carcinoma; MDA-MB-231: breast carcinoma. g, HTB-4: bladder carcinoma employed avelumab or IgG at 2 μg/ml. Results shown are the averages (SD) of triplicate measurements from one of at least three comparable repeat experiments. Multiple t-tests were used to compare each avelumab dose with IgG control at all E:T ratios. *** p < 0.001, **p < 0.01, * p < 0.05.
Studies were conducted to determine if the enhanced lysis of tumor cells by the addition of avelumab to haNK cells is indeed classic ADCC. ADCC is mediated by the interaction of CD16 on NK cells with the Fc receptor on IgG1 human antibodies. As seen in Figure 3a, the addition of anti-CD16 blocking Ab inhibited the avelumab-mediated haNK lysis of tumor cells, while not inhibiting the endogenous haNK lysis. The anti-CD16 concentration was determined from titration curves. Lower anti-CD16 concentrations did not block ADCC, possibly due to the high expression of CD16 on haNK cells. CMA is known to inhibit granzyme/perforin–mediated lysis by NK cells. As seen in Figure 3b, lysis of tumor cells by haNK cells, with or without avelumab, is also greatly inhibited by the addition of CMA.
Figure 3.
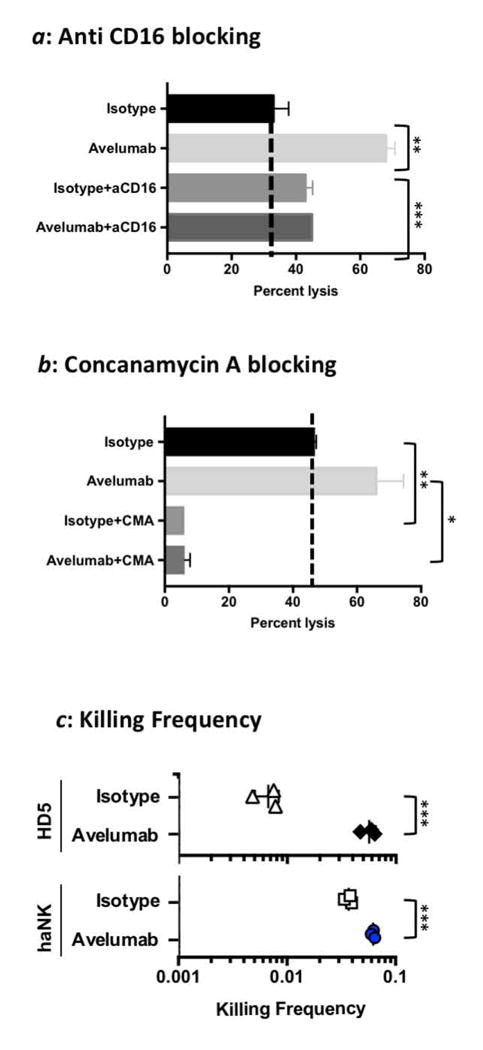
Characterization of haNK lysis and ADCC induced by avelumab. a: The H460 human lung carcinoma cell line was used as a target in an 18-hr assay to evaluate if haNK ADCC mediated by avelumab (1 ng/ml) could be blocked using anti-CD16 antibody (50 μg/ml). (b) The H460 cell line was used as a target in an 18-hr assay to evaluate if haNK ADCC mediated by avelumab (1 ng/ml) could be blocked using CMA. Irradiated haNK cells were used at a 25:1 E:T ratio. Results are the mean (SEM) lysis of triplicate measurements in one of four repeat experiments. Dotted lines show the percent endogenous haNK lysis. T-tests were employed to compare the treatments. (c) Irradiated haNK cells and non-irradiated NK cells from a healthy donor (HD) were evaluated for killing frequency. NK cells were co-incubated with 111In-labeled H460 target cells at low E:T ratios for 18 h. % specific lysis was used to calculate killing frequency by dividing the number of target cells killed by the number of effector cells used for the 0.625:1 ratio. *** P< 0.001, ** P< 0.01, * P< 0.05.
To determine the number of targets killed per effector cell, we used an 111In release assay at low E:T ratios in order for each effector cell to have access to excess target cells. As seen in Figure 3c, there was low killing frequency of NK cells from HD5 (on average 149 NK cells were required to kill one tumor target cell). In contrast, only 27 haNK cells were required to kill one tumor target cell (P = 0.007). It should be noted that NK cells from other healthy donors may have higher killing frequencies. Avelumab increased killing capacity of HD5 8-fold by ADCC (P = 0.002), while avelumab increased haNK cell killing frequency by 1.7-fold (P = 0.009).
Future studies are planned to adoptively transfer irradiated haNK cells (with or without ADCC mediating IgG1 human antibodies such as avelumab); experiments were therefore conducted to determine if healthy donor NK cells would lyse irradiated haNK cells as targets. In these experiments, aNK cells, from which haNK cells were derived, were also used as targets. Irradiated aNK cells have been adoptively transferred in several clinical studies with evidence of clinical benefit to some cancer patients, and with minimal/acceptable toxicity with multiple infusions of up to 1010 cells. As seen in Table 1a, NK cells from a healthy donor were able to lyse both irradiated aNK and irradiated haNK cells as targets with lysis increasing with time. NK cells from three additional donors gave similar but slightly variable results. As seen in Table 1b, the addition of avelumab or cetuximab did not increase the aNK or haNK lysis seen at 18 hr. Similar results were seen using NK cells from four additional donors.
Table 1. Healthy donor NK cells can lyse aNK and haNK cells.
| a | |||
|---|---|---|---|
| Effector | Target | Time (h) | % Lysis ± SD |
| NK (HD1) | |||
| aNK | 4 | 12.3 ± 1.8 | |
| 12 | 90 ± 3 | ||
| 18 | 86 ± 2.8 | ||
| haNK | 4 | 22 ± 1.6 | |
| 8 | 44.6 ± 1.6 | ||
| 12 | 54.3 ± 1.5 | ||
| 18 | 71.2 ± 4 | ||
| b | |||
|---|---|---|---|
| Effector | Target | huIgG1 | % Lysis ± SD |
| NK (HD2) | |||
| aNK | control | 76 ± 12 | |
| haNK | control | 69 ± 1.5 | |
| aNK | avelumab | 52 ± 6 | |
| haNK | avelumab | 51 ± 7 | |
| aNK | cetuximab | 71 ± 4 | |
| haNK | cetuximab | 77 ± 5 | |
(a) Healthy donor (HD) NK cells were utilized as effector cells in a killing assay with either 111In-labeled aNK or haNK cells as targets at a 10:1 E:T ratio for the indicated times.
(b) Healthy donor (HD) NK cells were utilized as effector cells in a killing assay with either 111In-labeled aNK or haNK cells as targets at a 10:1 E:T ratio for 18 hr. The assay was done in the presence of control IgG1, avelumab (2 ng/ml), or cetuximab (1 μg/ml).
In light of the previous findings, experiments were conducted using purified NK cells from four healthy donors and three cancer patients to determine if the presence of NK cells would inhibit the lysis of tumor cells by haNK cells, or by avelumab plus haNK cells (ADCC) in an 18 hour assay. Figure 4a shows representative results employing NK cells from one healthy donor with the CD16 FF allele. MDA-MB-231 breast carcinoma cells were employed as targets. In these studies, the concentration of viable NK cells used is based on the concentration of NK cells in the periphery (1-6%). Irradiated haNK cells were used at a concentration that would be equivalent to infusing 108 irradiated haNK cells in a 70 kg patient. Thus the ratio of viable NK to irradiated haNK in these experiments was 10:1. The E:T ratio was five haNK cells to one tumor target (MDA-MD-231). In wells with both haNK cells and NK cells, the total E:T ratio was therefore 55:1. While the percentage of lysis seen with the admixture of irradiated haNK and NK cells was not quite additive to the sum of NK and haNK lysis, the endogenous lysis of tumor cells by irradiated haNK cells was not inhibited by the presence of viable NK cells (Fig. 4a). Moreover, the addition of avelumab to haNK-mediated ADCC of tumor cells was not inhibited by the presence of viable NK cells (Fig. 4a). Since EGFR is also expressed on MDA-MB-231 breast carcinoma cells, we conducted similar experiments using cetuximab to mediate ADCC with haNK cells in the presence of NK cells (Fig. 4b), with results similar to those seen with the use of avelumab. We then conducted similar studies in which the ratio of viable NK cells to irradiated haNK cells was 1:1. This would simulate the ratios of NK and haNK cells if 109 irradiated haNK cells were inoculated into a 70 kg patient. As seen in Figure 4c and d, similar results were obtained at the 1:1 ratio of NK to haNK cells as with the 10:1 ratio. Results employing avelumab to mediate ADCC are shown in Figure 4c, and cetuximab to mediate ADCC with haNK cells in Figure 4d, both in the presence of NK cells. The results shown are representative of results using NK cells from three additional donors (the results from one of these are shown in Supplemental Fig. 2).
Figure 4.
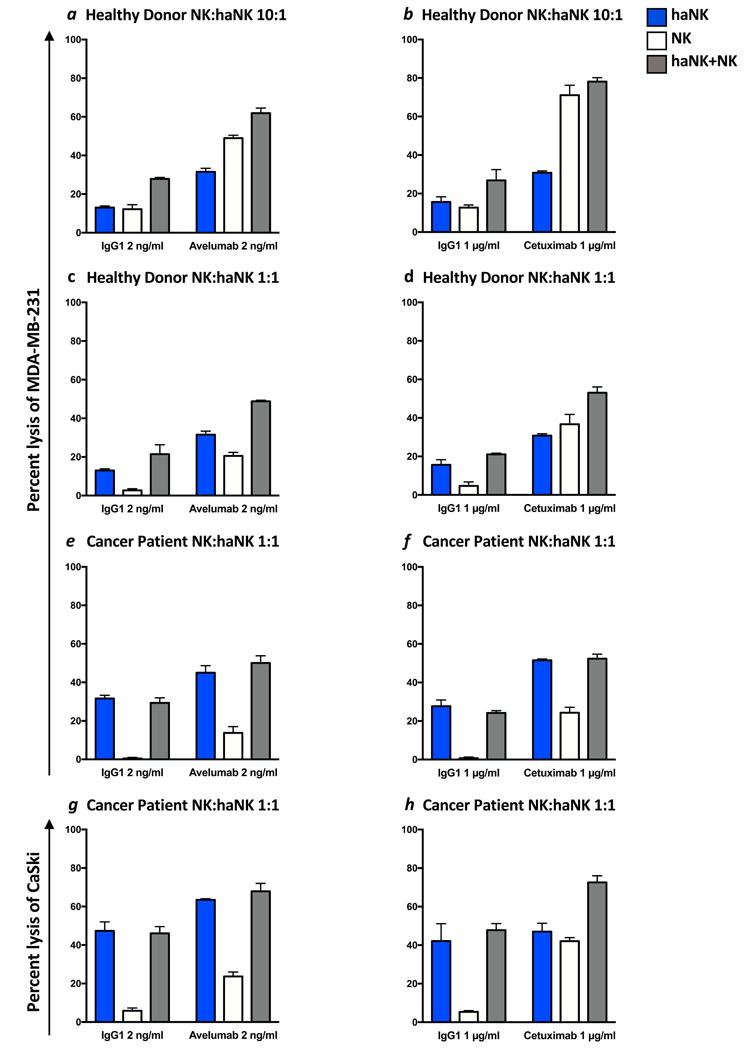
MDA-MB-231 breast carcinoma cells (a-f), and CaSki cervical carcinoma cells (g-h) were 111In-labeled as targets and incubated with haNK cells alone, NK cells alone, or haNK and NK cells together at ratios mimicking those seen in the blood after infusion of 108 haNK cells (a-b) or 109 haNK cells (c-h). haNK lysis (with IgG1 control antibody) and ADCC induced by avelumab (2 ng/ml) or cetuximab (1 μg/ml) were evaluated. Bars show the mean (SD) lysis of triplicate wells. NK cells from four different healthy donors and three cancer patients were tested with similar results, and the results from one healthy donor and one cancer patient are shown. Additional results are shown in Supplemental Figure 2. (a-b) Using MDA-MB-231 cells as targets, haNK cells were used at a 5:1 E:T ratio (blue), NK cells were used at a 50:1 E:T ratio (white), and NK cells and haNK cells were combined at a 10:1 ratio, resulting in an overall E:T ratio of 55:1 (grey) to mimic NK:haNK ratios in the blood after infusion of 108 haNK cells to a 70 kg patient. (c-f) Using MDA-MB-231 cells as targets, haNK cells were used at a 5:1 E:T ratio (blue), NK cells were used at a5:1 E:T ratio (white), and NK cells and haNK cells were combined at a 1:1 ratio, resulting in an overall E:T ratio of 10:1 (grey) to mimic NK:haNK ratios in the blood after infusion of 109 haNK cells to a 70 kg patient, showing the results for the healthy donor in c and d, and the results for the cancer patient in e and f. (g-h) An additional tumor cell line, CaSki, was used as a target with NK cells from the cancer patient as effectors. haNK cells were used at a 5:1 E:T ratio (blue), NK cells were used at a 5:1 E:T ratio (white), and NK cells and haNK cells were combined at a 1:1 ratio, resulting in an overall E:T ratio of 10:1 (grey) to mimic NK:haNK ratios in the blood after infusion of 109 haNK cells to a 70 kg patient.
Similar studies were also conducted with NK cells isolated from three patients with metastatic breast cancer, using the ratio of NK:haNK cells if 109 haNK cells were infused into a 70 kg patient thus, as mentioned above, giving an NK:haNK ratio of 1:1 in the presence of tumor target cells. The genotype for CD16 was not known for these patients. For these studies, both MDA-MB-231 breast carcinoma cells (Fig. 4e and f) and CaSki cervical carcinoma cells (Fig. 4g and h) were used as targets. As described above using NK cells from healthy donors, the percentage of lysis seen with the admixture of irradiated haNK and NK cells was not additive to the sum of NK and haNK lysis, but the endogenous lysis of tumor cells by irradiated haNK cells was not inhibited by the presence of viable NK cells. Moreover, the addition of avelumab to haNK-mediated ADCC of tumor cells was not inhibited by the presence of viable NK cells (Fig. 4e and g). Since EGFR is also expressed on both MDA-MB-231 and CaSki cells, we used cetuximab to mediate ADCC with haNK cells in the presence of NK cells (Fig. 4f and h), with results similar to those seen with the use of avelumab. These results are representative of results using NK cells from two additional cancer patients (the results from one of these are shown in Supplemental Fig. 2).
Studies were also conducted to rule out lysis of the tumor cells by avelumab alone in these experiments. As seen in Figure 5a, no lysis of breast carcinoma tumor cells by avelumab alone is seen, but avelumab does enhance irradiated haNK-mediated tumor cell lysis. As haNK cells express PD-L1,25 studies were also conducted to determine if irradiated haNK cells could lyse as targets viable 111In-labeled irradiated haNK cells. As shown in Figure 5b, no haNK fratricide was observed with or without the addition of avelumab. haNK-mediated lysis, with or without avelumab, of MDA-MB-231 human breast carcinoma cells was employed as a positive control.
Figure 5.
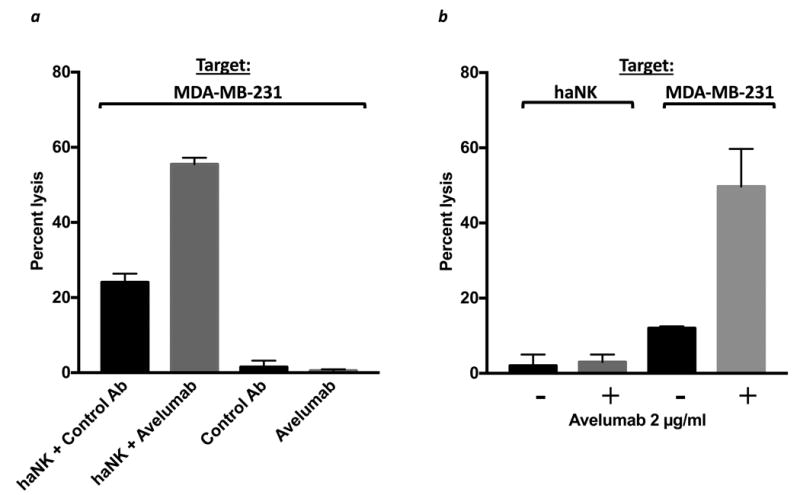
Avelumab alone does not lyse tumor cells, and haNK cells have no cytotoxic activity against other haNK cells. (a) Irradiated haNK cells were used in an 18-hr assay, using MDA-MB-231 (human breast carcinoma) as a target at an E:T ratio of 7.5:1. Target cells were also incubated with avelumab (2 ng/ml) or isotype control IgG1 Ab (2 ng/ml) alone, without effector cells. haNK killing (black bars) and ADCC mediated by avelumab (grey bars) are shown. (b) Irradiated haNK cells were used in a 4-hr assay, using irradiated haNK cells or MDA-MB-231 cells as targets at an E:T ratio of 20:1. Endogenous haNK lysis (black bars) and ADCC mediated by avelumab (2 μg/ml) (grey bars) are shown.
Discussion
The studies reported here take advantage of the unique properties of two potential immunotherapeutics for use in combination. The anti-PD-L1 fully human IgG1 avelumab mediates ADCC17 and the haNK cells express the high affinity CD16, which enhances ADCC activity.25 Avelumab has demonstrated clinical benefit in a range of human tumor types. 3-11 Since PD-L1 is also expressed on some normal immune cell subsets, a careful evaluation of 123 different immune cell subsets in patients' peripheral blood was carried out prior to and post one, three, and nine cycles of avelumab, and no statistically significant changes in any subset were observed, including those subsets expressing PD-L1.34 Moreover, no toxicity above that observed with other anti-PD-L1 MAbs was seen.35, 36 Studies37 also showed that the addition of avelumab to in vitro assays also enhanced antigen-specific T-cell responses.
Since the NK-92 cells, from which haNK and aNK cells were derived, were originally obtained from a patient with non-Hodgkin lymphoma,31 all aNK clinical studies were carried out with lethally irradiated cells prior to adoptive transfer; all potential clinical studies with haNK cells will also be carried out with lethally irradiated cells. Thus, all the experiments reported here with haNK or aNK cells were carried out with lethally irradiated cells.
The effects of irradiation on gene expression governing haNK cell function revealed genes that were differentially expressed post vs. pre-irradiation that may have implications for haNK cell function. Multiple differentially expressed genes identified here have previously been associated with irradiation. For example, irradiation has been shown to increase oxidative stress, which activates inflammatory functions such as those governed by NF-kB signaling and the AP-1 complex.38, 39 Genes involved in the transcription factor regulation of immune components, such as those associated with NF-kB, were identified as differentially expressed in haNK cells by irradiation exposure. It has been shown that irradiation of the parent NK-92 cells halted their proliferation while both cytotoxicity and immune signaling functions remained intact.24, 31 In the studies reported here, performed 24 hr post-irradiation of haNK cells, the genes that were differentially expressed may have implications for therapeutic use. Downregulation of killer cell lectin like receptor B1 (KLRB1) may be beneficial as this receptor plays a role in the inhibition of NK cell cytotoxicity.40 Natural cytotoxicity triggering receptor 1 (NCR1), however, supports NK cell cytotoxicity41, 42 and is also downregulated by irradiation. The upregulation of interleukin 12 receptor, beta 1 (IL12RB1) may indicate an enhanced response to pro-inflammatory cytokines such as IL12, supporting an effective antitumor response. Irradiation of haNK cells also reduced expression of three immunoregulatory genes, CTLA4, HLA-DMB, TIGIT, and the increased expression of one, LAG3. These changes, taken together, may result in greater lytic activity of haNK cells. The studies reported here also show the continued production of both IL-2 and IL-10 for 48 hr post-irradiation, but at lower levels than non-irradiated haNK cells. Levels of production of IL-8 and IFN-γ, on the other hand, were greater for up to 24 hr in irradiated haNK cells vs. non-irradiated haNK cells.
It is not surprising that NK cells derived from healthy donors or cancer patients would lyse irradiated aNK or haNK cells since the parent NK-92 cells were derived from a cancer patient. The experiments shown in Table 1 demonstrate that neither avelumab nor cetuximab enhanced this NK-mediated lysis. This was important to show because haNK cells do express PD-L1. As a consequence of this finding, studies were conducted to determine whether the NK lysis of haNK cells would inhibit lysis of the tumor cells by haNK cells, with or without avelumab. Multiple studies toward this end were carried out using concentrations of NK cells found in the periphery and concentrations of haNK cells in the periphery if 108 or 109 irradiated haNK cells would be adoptively transferred. Representative results are shown in Figure 4 (and Supplemental Fig. 2), where it can be seen that the presence of viable NK cells from either healthy donors or cancer patients displayed little if any inhibition of tumor cell lysis by irradiated haNK cells. It should also be noted that several clinical studies have been carried out with these amounts (108 and 109) of aNK cells adoptively transferred multiple times with minimal toxicity and clinical responses observed.24, 26-28 Experiments described here (Fig. 5b) showed no haNK fratricide, i.e., the ability of haNK cells to lyse haNK cells. Prior studies25 have shown that the IgG1 MAbs trastuzumab, pertuzumab, and cetuximab can enhance haNK cell lysis of tumor cells via the ADCC mechanism.
As mentioned above, there are reports showing clinical benefit in patients undergoing therapy with other IgG1 MAbs such as cetuximab,19, 22 trastuzumab,21 or rituximab20 whose NK cells express the high affinity CD16 V/V allele. On the other hand, there are also reports that no such benefit exists.23, 43 These conflicting results may be due to multiple factors, including (a) the assays used to detect the V/V and the other CD 16 alleles, (b) the amount of ligand seen by the ADCC-mediating antibody on tumor cells, and (c) other factors of tumor cell phenotype, such as MHC class I and MICA. We have previously evaluated the influence of levels of MICA, MHC class I, and PD-L1 on tumor cells and if this influenced haNK lysis or avelumab/haNK–mediated ADCC, and no apparent strong correlations were seen. It has been previously shown that high levels of MHC on tumor cells can inhibit NK-mediated lysis via interaction with the inhibitor killer immunoglobulin-like receptor (KIR) on NK cells. Since NK-92, aNK, and haNK cells express extremely low levels of KIR, lysis of MHC class I positive tumor cells was observed. Future studies will involve a more comprehensive analysis of tumor cell phenotype employing RNA sequencing and immunome studies of both tumor targets and irradiated haNK cells to help define which tumor cell phenotype is more amenable to haNK or haNK plus avelumab-mediated lysis.
Prior studies have shown that separate mechanisms are involved in tumor cell lysis mediated by NK cells vs. T cells. Phase I/II studies with irradiated haNK cells will be required prior to combination therapy with avelumab. The potential combined use of avelumab with adoptively transferred irradiated haNK cells may provide these multiple mechanisms of tumor cell lysis, i.e., the ability of avelumab to act as a checkpoint inhibitor and allow T-cell–mediated tumor cell lysis, and the interaction of the Fc region of avelumab with the high affinity CD16 allele on haNK cells to enhance “NK” tumor cell lysis. The studies reported here demonstrate that avelumab can enhance haNK-mediated lysis of numerous carcinoma cell types and help to provide the rationale for future combination therapies employing these two immunotherapeutics.
Supplementary Material
What's New.
NK 92 (aNK) cells have been engineered to express IL-2 and the high affinity CD16 allele (designated haNK). Avelumab is a human IgG1 anti–PD-L1, which has shown evidence of clinical activity. The ability of avelumab to enhance the lysis of a range of human carcinoma cells by irradiated haNK cells via the ADCC mechanism is demonstrated. These studies thus provide the rationale for the clinical evaluation of the combined use of these two novel immunotherapeutics.
Acknowledgments
The authors thank Sarah Tritsch and Michelle Padget for their technical assistance. We also thank Debra Weingarten for her editorial assistance in the preparation of this manuscript.
Funding: This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health (NIH), as well as through Cooperative Research and Development Agreements (CRADAs) between NantBioScience and the NCI, and EMD Serono and the NCI.
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- CMA
concanamycin A
- CRADA
Cooperative Research and Development Agreement
- ddPCR
droplet digital polymerase chain reaction
- E:T
effector to target cell
- F
phenylalanine
- ha
high affinity
- HD
healthy donor
- IL12RB1
interleukin 12 receptor, beta 1
- IPA
Ingenuity Pathway Analysis
- KIR
killer immunoglobulin-like receptor
- KLRB1
killer cell lectin like receptor B1
- LGL
large granular lymphocytes
- MAb
monoclonal antibodies
- MHC
major histocompatibility complex
- MICA
MHC class I chain-related protein A
- NK
natural killer
- NCR1
natural cytotoxicity triggering receptor 1
- PBMC
peripheral blood mononuclear cell
- RCC
raw data files
- RLF
reporter library files
- V
valine
Footnotes
Conflict of Interest: The authors reported no potential conflicts of interest.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384–91. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apolo AB, Infante JR, Hamid O, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with metastatic urothelial carcinoma from the JAVELIN solid tumor phase 1b trial: analysis of safety, clinical activity, and PD-L1 expression. J Clin Oncol; American Society of Clinical Oncology, Annual Meeting; Chicago, IL. June 4-6, 2016; 2016. (suppl; abstr 4514) [Google Scholar]
- 4.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–85. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung HC, Arkenau HT, Wyrwicz L, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced gastric or gastroesophageal junction cancer from JAVELIN solid tumor phase Ib trial: analysis of safety and clinical activity. J Clin Oncol; American Society of Clinical Oncology, Annual Meeting; Chicago, IL. June 4-6, 2016; 2016. (suppl; abstr 4009) [Google Scholar]
- 6.Dirix LY, Takacs I, Nikolinakos P, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase Ib JAVELIN solid tumor trial (abstr S1-04). 38th Annual San Antonio Breast Cancer Symposium; Texas. Dec 8-12, 2015; 2015. [Google Scholar]
- 7.Disis ML, Patel MR, Pant S, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase Ib trial: safety and clinical activity (abstr 5533). J Clin Oncol; American Society of Clinical Oncology, Annual Meeting; Chicago, IL. June 4-6, 2016; 2016. (suppl; abstr 5533) [Google Scholar]
- 8.Gulley JL, Rajan A, Spigel DR, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in in patients with metastatic or recurrent non-small-cell lung cancer progressing after platinum-based chemotherapy: a phase Ib trial (abstr 3090). The European Cancer Congress 2015; Vienna, Austria. Sept. 25-29, 2015. [Google Scholar]
- 9.Hassan R, Thomas A, Patel MR, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced unresectable mesothelioma from the JAVELIN solid tumor phase Ib trial: safety, clinical activity, and PD-L1 expression. J Clin Oncol; American Society of Clinical Oncology, Annual Meeting; Chicago, IL. June 4-6, 2016; 2016. (suppl; abstr 8503) [Google Scholar]
- 10.Le Tourneau C, Hoimes CJ, Zarwan C, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced adrenocortical carcinoma from the JAVELIN solid tumor phase Ib trial: safety and clinical activity. J Clin Oncol; American Society of Clinical Oncology, Annual Meeting; Chicago, IL. June 4-6, 2016; 2016. (suppl; abstr 4516) [Google Scholar]
- 11.Rajan A, Heery CR, Perry S, et al. Safety and clinical activity of anti-programmed death-ligand 1 (PD-L1) antibody (ab), avelumab (MSB0010718C) in advanced thymic epithelial tumors (TETs). J Clin Oncol; American Society of Clinical Oncology, Annual Meeting; Chicago, IL. June 4-6, 2016; 2016. (suppl; abstr e20106) [Google Scholar]
- 12.Karaki S, Anson M, Tran T, et al. Is there still room for cancer vaccines at the era of checkpoint inhibitors. Vaccines (Basel) 2016;4:E37. doi: 10.3390/vaccines4040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bang YJ, Wyrwicz L, Park YI, et al. Avelumab (MSB0010718C; anti-PD-L1) + best supportive care (BSC) vs BSC ± chemotherapy as third-line treatment for patients with unresectable, recurrent, or metastatic gastric cancer: the phase 3 JAVELIN Gastric 300 trial. J Clin Oncol; American Society of Clinical Oncology, Annual Meeting; Chicago, IL. June 4-6, 2016; 2016. (suppl; abstr TPS4135) [Google Scholar]
- 14.Larkin JMG, Gordon MS, Thistlethwaite F, et al. Avelumab (MSB0010718C; anti-PD-L1) in combination with axitinib as first-line treatment for patients with advanced renal cell carcinoma. J Clin Oncol; American Society of Clinical Oncology, Annual Meeting; Chicago, IL. June 4-6, 2016; 2016. (suppl; abstr TPS4580) [Google Scholar]
- 15.Pujade-Lauraine E, Colombo N, Disis ML, et al. Avelumab (MSB0010718C; anti-PD-L1) ± pegylated liposomal doxorubicin vs pegylated liposomal doxorubicin alone in patients with platinum-resistant/refractory ovarian cancer: the phase III JAVELIN Ovarian 200 trial. J Clin Oncol; American Society of Clinical Oncology, Annual Meeting; Chicago, IL. June 4-6, 2016; 2016. (suppl; abstr TPS5600) [Google Scholar]
- 16.Ribas A, Chow LQ, Boyd JK, et al. Avelumab (MSB0010718C; anti-PD-L1) in combination with other cancer immunotherapies in patients with advanced malignancies: the phase 1b/2 JAVELIN Medley study. J Clin Oncol; American Society of Clinical Oncology, Annual Meeting; Chicago, IL. June 4-6, 2016; 2016. (suppl; abstr TPS3106) [Google Scholar]
- 17.Boyerinas B, Jochems C, Fantini M, et al. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti-PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol Res. 2015;3:1148–57. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna S, Thomas A, Abate-Daga D, et al. Malignant Mesothelioma Effusions Are Infiltrated by CD3+ T Cells Highly Expressing PD-L1 and the PD-L1+ Tumor Cells within These Effusions Are Susceptible to ADCC by the Anti-PD-L1 Antibody Avelumab. J Thorac Oncol. 2016;11:1993–2005. doi: 10.1016/j.jtho.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bibeau F, Lopez-Crapez E, Di Fiore F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27:1122–9. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 20.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 21.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–96. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–8. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 23.Mellor JD, Brown MP, Irving HR, et al. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol. 2013;6:1. doi: 10.1186/1756-8722-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klingemann H, Boissel L, Toneguzzo F. Natural killer cells for immunotherapy–advantages of the NK-92 cell line over blood NK cells. Front Immunol. 2016;7 doi: 10.3389/fimmu.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jochems C, Hodge JW, Fantini M, et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget. 2016 Nov 6; doi: 10.18632/oncotarget.13411. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arai S, Meagher R, Swearingen M, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–32. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- 27.Tonn T, Schwabe D, Klingemann HG, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15:1563–70. doi: 10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Bhatia S, Burgess M, Zhang H, et al. Adoptive cellular therapy (ACT) with allogeneic activated natural killer (aNK) cells in patients with advanced Merkel cell carcinoma (MCC): preliminary results of a phase II trial (abstr 45). Society for Immunotherapy of Cancer, 31st Annual Meeting; National Harbor, MD. Nov. 9-13, 2016. [Google Scholar]
- 29.Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells--enhancement by therapeutic antibodies. PLoS One. 2007;2:e326. doi: 10.1371/journal.pone.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanherberghen B, Olofsson PE, Forslund E, et al. Classification of human natural killer cells based on migration behavior and cytotoxic response. Blood. 2013;121:1326–34. doi: 10.1182/blood-2012-06-439851. [DOI] [PubMed] [Google Scholar]
- 31.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–8. [PubMed] [Google Scholar]
- 32.Matlawska-Wasowska K, Gale JM, Nickl CK, et al. Pyrosequencing for classification of human FcgammaRIIIA allotypes: a comparison with PCR-based techniques. Mol Diagn Ther. 2014;18:665–73. doi: 10.1007/s40291-014-0120-5. [DOI] [PubMed] [Google Scholar]
- 33.Ravetch JV, Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170:481–97. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donahue RN, Lepone LM, Grenga I, et al. Analyses of the peripheral immunome following multiple administrations of avelumab, a human IgG1 anti-PD-L1 monoclonal antibody. J ImmunoTher Cancer. 2017;5:20. doi: 10.1186/s40425-017-0220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly K, Heery CR, Patel MR, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced cancer: safety data from 1300 patients enrolled in the phase 1b JAVELIN solid tumor trial. J Clin Oncol; American Society of Clinical Oncology, Annual Meeting; Chicago, IL. June 4-6, 2016; 2016. (suppl; abstr 3055) [Google Scholar]
- 36.Pillai RN, Behera M, Owonikoko TK, et al. Evaluation of toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer (NSCLC). J Clin Oncol; American Society of Clinical Oncology, Annual Meeting; Chicago, IL. June 4-6, 2016; 2016. (suppl; abstr 9035) [Google Scholar]
- 37.Grenga I, Donahue RN, Lepone LM, et al. A fully human IgG1 anti-PD-L1 MAb in an in vitro assay enhances antigen-specific T-cell responses. Clin Transl Immunol. 2016;5:e83. doi: 10.1038/cti.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ha YM, Chung SW, Kim JM, et al. Molecular activation of NF-kappaB, pro-inflammatory mediators, and signal pathways in gamma-irradiated mice. Biotechnol Lett. 2010;32:373–8. doi: 10.1007/s10529-009-0165-4. [DOI] [PubMed] [Google Scholar]
- 39.Hallahan DE, Gius D, Kuchibhotla J, et al. Radiation signaling mediated by Jun activation following dissociation from a cell type-specific repressor. J Biol Chem. 1993;268:4903–7. [PubMed] [Google Scholar]
- 40.Konjevic G, Mirjacic Martinovic K, Vuletic A, et al. In-vitro IL-2 or IFN-alpha-induced NKG2D and CD161 NK cell receptor expression indicates novel aspects of NK cell activation in metastatic melanoma patients. Melanoma Res. 2010;20:459–67. doi: 10.1097/CMR.0b013e32833e3286. [DOI] [PubMed] [Google Scholar]
- 41.Crouse J, Bedenikovic G, Wiesel M, et al. Type I interferons protect T cells against NK cell attack mediated by the activating receptor NCR1. Immunity. 2014;40:961–73. doi: 10.1016/j.immuni.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Crouse J, Xu HC, Lang PA, et al. NK cells regulating T cell responses: mechanisms and outcome. Trends Immunol. 2015;36:49–58. doi: 10.1016/j.it.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Weng WK, Levy R. Genetic polymorphism of the inhibitory IgG Fc receptor FcgammaRIIb is not associated with clinical outcome in patients with follicular lymphoma treated with rituximab. Leuk Lymphoma. 2009;50:723–7. doi: 10.1080/10428190902829441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


