Abstract
Cardiovascular diseases are approximately three times higher in patients with neurological deficits than in patients without neurological deficits. MicroRNA-126 (MiR-126) facilitates vascular remodeling and decreases fibrosis and is emerging as an important factor in the pathogenesis of cardiovascular diseases and cerebral stroke. In this study, we tested the hypothesis that decreased miR-126 after ischemic stroke may play an important role in regulating cardiac function. Wild-type (WT), specific conditional-knockout endothelial cell miR-126 (miR-126EC−/−), and miR-126 knockout control (miR-126fl/fl) mice were subjected to distal middle cerebral artery occlusion (dMCAo) (n = 10/group). Cardiac hemodynamics and function were measured using transthoracic Doppler echocardiography. Mice were sacrificed at 28 days after dMCAo. WT mice subjected to stroke exhibited significantly decreased cardiac ejection fraction and increased myocyte hypertrophy, fibrosis as well as increased heart inflammation, infiltrating macrophages, and oxidative stress compared to non-stroke animals. Stroke significantly decreased serum and heart miR-126 expression and increased miR-126 target genes, vascular cell adhesion protein-1, and monocyte chemotactic protein-1 gene, and protein expression in the heart compared to non-stroke mice. MiR-126EC−/− mice exhibited significantly decreased cardiac function and increased cardiomyocyte hypertrophy, fibrosis, and inflammatory factor expression after stroke compared to miR-126fl/fl stroke mice. Exosomes derived from endothelial cells of miR-126EC−/− (miR-126EC−/−EC-Exo) mice exhibited significantly decreased miR-126 expression than exosomes derived from miR-126fl/fl (miR-126fl/fl-EC-Exo) mice. Treatment of cardiomyocytes subjected to oxygen glucose deprivation with miR-126fl/fl-EC-Exo exhibited significantly decreased hypertrophy than with miR-126EC−/−EC-Exo treatment. Ischemic stroke directly induces cardiac dysfunction. Decreasing miR-126 expression may contribute to cardiac dysfunction after stroke in mice.
Keywords: Ischemia, Stroke, Cardiac dysfunction, MicroRNA-126, Brain-heart interaction, Cardiomyocyte
Introduction
Stroke is a prominent cause of mortality and long-term disability and is accompanied by unusually high social and medical costs [1, 2]. The major causes of death in stroke-related mortalities are a consequence of neurological damage and/or cardiovascular complications [3]. Comorbidity of stroke with hypertension, diabetes, or cardiac abnormalities aggravates stroke outcome, disability, risk of recurrent stroke, and mortality [4, 5]. However, cardiac dysfunction is encountered frequently among stroke patients, even in the absence of primary heart disease [6, 7]. Post-stroke neurological deficits increase the risk of cardiovascular diseases roughly by three times [3], and the ischemic brain transmits indirect cell death signals to the heart [8]. Necropsy analyses of patients who suffered a fatal cerebral stroke indicate a high prevalence of coronary atherosclerosis and myocardial infarction [9]. Patients can develop myocardial injuries after stroke even when patients do not have pre-existing cardiac diseases [6, 7, 10–12]. However, it is unclear how cerebral ischemic stroke regulates cardiac function, what are the direct effects of stroke on cardiac function, and what are the underlying molecular mechanisms. In this study, using a cerebral ischemic stroke model in mice, we study the “brain and heart interaction“ after stroke.
MicroRNAs (miRs) are small non-coding RNA molecules that regulate several gene expressions, pathways, and complex biological networks at the cellular level acting either exclusively or together with other miRs [13]. MiRs regulate both transcriptional and post-transcriptional gene expression as well as regulate several circuits involved in tissue repair, inflammation, hypoxia-response, and angiogenesis [14]. MicroRNA-126 (MiR-126) is endothelial cell (EC) specific and plays a key role in regulating EC function, controlling angiogenesis, and maintaining vascular integrity [14]. MiR-126 facilitates vascular remodeling, decreases fibrosis in multiple organs, and has been reported to be beneficial in the treatment of atherosclerosis and restenosis [14, 15]. MiR-126 is emerging as a key player in the pathogenesis of cardiovascular diseases and ischemic stroke [16, 17] and is involved in endovascular inflammation and platelet activation [18]. MiR-126 expression in serum is positively correlated with left ventricular ejection fraction (LVEF) [18]. To date, the direct effects and role of miR-126 on the interaction between brain and heart after stroke have not been investigated. In this study, we hypothesize that the decrease of miR-126 expression after stroke may play an important role in regulating cardiac function, and we show that cerebral ischemic stroke in mice decreases heart miR-126 expression and increases cardiac dysfunction.
Materials and Methods
Extraluminal Permanent Distal Middle Cerebral Artery Occlusion (dMCAo) Model
Adult (3–4 months) male wild-type (WT) C57/BJ6L mice were purchased from Jackson Laboratory. Mice were anesthetized with 3.5% isoflurane in a mixture of N2O:O2 (2:1) and maintained at 0.5% ~ 1.5% isoflurane using a facemask and were subjected to sham control or right extraluminal permanent dMCAo, as previously described [19, 20]. Briefly, a midline incision was opened between the orbit and the ear. A small burr hole was produced in the skull. The main branch of the MCA was coagulated with a small heater probe, and the vessel was transected. The same procedures without artery coagulation were performed on sham control mice (n = 10/group).
Generation of Specific Conditional EC MiR-126 Knockout (MiR-126EC−/−) Mice
Briefly, we crossbred PDGFiCreER:miR-126flox/flox with miR-126flox/flox mice [21, 22] (these transgenic mice were generously provided by Dr. Calvin Kuo, Stanford University). Genotyping was performed at 4 weeks after birth. Then, both the PDGFiCreER:miR-126flox/flox and miR-126flox/flox mice were treated with tamoxifen (1 mg/10 g body weight of tamoxifen dissolved in corn oil); 4 doses were administered every other day i.p. The tamoxifen-treated PDGFiCreER:miR-126flox/flox mice have specific EC miR-126 deletion (called miR-126EC−/−). MiR-126fl/fl mice were employed as knockdown control. MiR-126EC−/− and miR-126fl/fl mice were subjected to dMCAo (n = 10/group).
Cardiac Function Measurements
Cardiac function was measured by echocardiography before stroke and at 4 weeks after stroke. Transthoracic Doppler echocardiography was performed on conscious mice using a Doppler echocardiograph (Acuson C516) equipped with a 15-MHz linear transducer (15L-8), as previously reported [23]. Mice were trained for 3 days before echocardiography. Briefly, the mouse was picked up by the nape of the neck and held firmly in the palm of one hand in the supine position. In our experience, most mice develop bradycardia during the first training session; however, with repeated training the bradycardia disappears and mice remain calm. After training, the left hemithorax was shaved and a pre-warmed ultrasound transmission gel was applied to the chest. LVEF was measured using the formula: LVEF = [(LVAd − LVAs) / LVAs × 100], where LVAd is LV diastolic area and LVAs is LV systolic area. All primary measurements were digitized by goal-directed, diagnostically driven software and 3 beats were averaged for each measurement.
Histological and Immunohistochemical Assessment
Mice were sacrificed at 28 days after dMCAo. The brain and the heart were isolated and were fixed by 4% paraformaldehyde before being embedded in paraffin. Brain coronal tissue sections (bregma –1 mm to +1 mm) were cut (6 μm thick) and stained with hematoxylin and eosin for calculation of lesion volume [24]. Heart coronal sections (6 μm thick) were cut, and PicroSirius Red (PSR) staining was employed to assess myocyte cross-sectional area (MCSA, identifies cardiomyocyte size) [25] and interstitial collagen fraction (ICF) measurement [26]. ICF is a measurement of cardiac interstitial and perivascular fibrosis, measured by a percent rate of PSR-stained collagen area to total myocardial area. For immunostaining, antibodies against mouse CD68 (ED1, a marker for monocytes/macrophages; 1:30, Bio-Rad); transforming growth factor (TGF-β; 1:500, Santa Cruz); monocyte chemotactic protein-1 (MCP-1; 1:100, Abcam); vascular cell adhesion molecule 1 (VCAM-1, 1:200, Santa Cruz); NADPH oxidase-2 (NOX2; 1:400, BD Bioscience) were employed. Negative controls were processed in a similar fashion but without the primary antibody.
Immunostaining Quantification
Five slides from each heart, with each slide containing 3 fields of view, were digitized under a ×20 objective (Olympus BX40) using a 3-CCD color video camera (Sony DXC-970MD) interfaced with an MCID image analysis system (Imaging Research). Image analysis was performed in a blinded fashion. Positive areas of PSR, TGF-β, NOX2, MCP-1 and positive cell number of ED1 in the fields of view were calculated.
Mouse Neonatal Cardiomyocyte Culture
Mouse neonatal hearts were enzymatically dissociated into a single cell suspension, as previously described [27]. Briefly, the hearts were finely cut and broken up into single cells in 0.1% collagenase/ dispace (Roche). The cells were cultured in DMEM with 10% fetal bovine serum (FBS) and 1% antibiotic/antimycotic (Invitrogen). To test whether the cultured cell are cardiomyocyte, anti-sarcomeric alpha actinin (cardiomyocyte and skeletal muscle cell marker, EA-53, Abcam) immunostaining was performed.
Primary EC Culture and EC-Exosome Isolation
To test whether endothelial cell source miR-126 affects cardiomyocytes, exosomes derived from brain endothelial cells of miR-126fl/fl (miR-126fl/fl-EC-Exo) and miR-126EC−/− (miR-126EC−/−EC-Exo) mice were used to treat ischemic cardiomyocytes in culture. Briefly, brain ECs were isolated from adult male miR-126fl/fl and miR-126EC−/− mice following a previously described protocol [28]. Primary brain ECs were cultured up to 3 passages and then cultured in exosome-depleted FBS media (Systembio) for 3 days. After exosomes were isolated, exosome-depleted FBS was used for all further cell cultures. EC-Exo was isolated using Exo Quick-TC, following standard protocol and suspended in PBS. EC-Exo concentration was quantified using the IZON qNano device (Izon, Christchurch, New Zealand). To decrease the heterogeneity of EC-Exo treatment, we tightly controlled the EC culture conditions, such as EC passage, density, and culture time. Protein concentration was determined for each EC-Exodose employed.
To induce cardiomyocyte ischemia in vitro, oxygen-glucose deprivation (OGD) was performed. Cardiomyocyte culture media was replaced with glucose and serum-free media and placed in a hypoxia chamber (Forma Anaerobic System, Thermo Fisher Scientific) for 2 h of OGD at 37 °C. The experimental groups include (1) cardiomyocyte control; (2) cardiomyocytes +20 ng/ml miR-126fl/fl-EC-Exo; (3) cardiomyocytes +20 ng/ml miR-126EC−/−EC-Exo for 24 h.
Knockdown of MiR-126 in Cultured Mouse Cardiomyocyte
To knockdown miR-126 expression in cultured cardiomyocytes, mmu-miR-126-3p inhibitor (miR126 knockdown), or scrambled negative control inhibitor (Thermo Scientific) was performed using an electroporation transfection method. Briefly, mouse cardiomyocytes were harvested and resuspended in 95 μl Ingenio electroporation solution (Mirus) and in 5 μl of 20 μM miR-126 inhibitor or scrambled control (Dharmacon). The cell suspension was placed in an Ingenio cuvette (Mirus) and electroporated in a Lonza Nucleofector using program Y-01. Then cells were cultured for 2 days and miR-126 expression was measured.
Cardiomyocyte Structure Changes
F-actin staining was performed to identify cell size. Briefly, fixed cells were labeled with anti-F-actin (CytoPainter F-actin Staining Kit-Green Fluorescence, Abcam) and nuclei were labeled with DAPI. For cell surface area measurements, 6 areas were randomly selected from each 10-mm cover-slipped area [29].
Western Blot Assay
Protein was isolated from samples using Trizol (Invitrogen). Protein concentration was measured using the BCA kit (Thermo Fisher Scientific). MCP-1 (1:1000, Abcam), NOX2 (1:1000, BD Bioscience), TGF-β (1:1000, Santa Cruz), or VCAM-1 (1:500, Santa Cruz) primary antibodies were employed. Anti-β-actin (1:10,000; Abcam) antibody was loaded for control measurements.
Real-Time PCR
We isolated the total RNA with TRIzol (Invitrogen) to make cDNA using the M-MLV (Invitrogen) standard protocol. The TaqMan real-time PCR was used and run on a ViiA7 system (Applied Biosystems). Each sample was tested in triplicate, and analysis of relative gene expression data was performed using the 2−ΔΔCT method. The following primers for real-time PCR were designed using Primer Express software (Applied Biosystems):
ED1: FWD: GAAGGAAAGAGCTGAAGAGCAG;
REV: AGGTTTAGGAGAGGGTTTCCAC
MCP-1: FWD: CTGCTACTCATTCACCAGCAAG;
REV: CTCTCTCTTGAGCTTGGTGACA
NOX2: FWD: GAATTGTACGTGGACAGACTGC;
REV: CAAGTCATAGGAGGGTTTCCAG
TGF-β: FWD: GCAACATGTGGAACTCTACCAG;
REV: GTATTCCGTCTCCTTGGTTCAG
VCAM-1: FWD: CAGGTGGAGGTCTACTCATTCC;
REV: CTCCAGATGGTCAAAGGGATAC.
MiR-126 Measurement
MiR-126 expression was measured by TaqMan miRNA assay (Life Technology), as previously described [30]. Briefly, samples (serum, heart, or cultured cells) were lysed in Qiazol reagents and the total RNA was isolated. PCR amplification was performed with the TaqMan miRNA assay kit according to the manufacturer’s protocols, with U6 snRNA as an internal control.
Statistical Analysis
All data are expressed as mean ± SE. Independent two-sample t-test was used to compare the differences between dMCAo and non-stroke groups. When multiple comparisons were performed, Hochberg’s step-up procedure was used to adjust p-values. The pair-wise comparison was set at 0.05.
Results
Cerebral Ischemic Stroke Induces Cardiac Dysfunction, Cardiomyocyte Hypertrophy, Fibrosis, Inflammation, and Oxidative Stress Compared to Non-stroke Mice
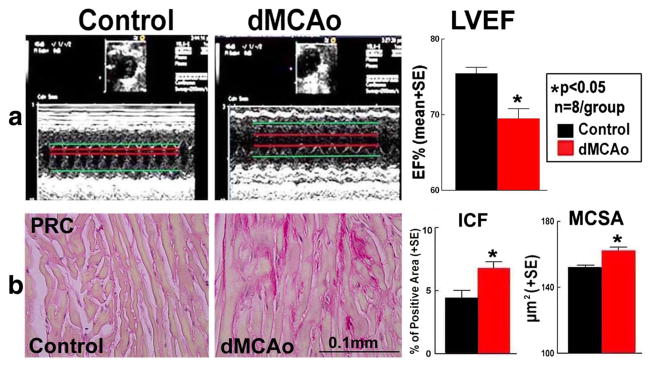
To test whether stroke induces cardiac dysfunction, echocardiography was employed in conscious mice at 28 days after stroke. The ischemic lesion volume in WT-dMCAo mice was 10.79% ± 0.97%. Figure 1a shows that stroke significantly decreased cardiac function identified by decreased LVEF compared to non-stroke mice (p < 0.05). Cardiac fibrosis was evident both in the perivascular and in the myocardial interstitial area in the dMCAo group. Figure 1b shows that stroke significantly increased myocyte cross-sectional area and interstitial fibrosis compared to non-stroke mice. These data indicate that stroke initiates myocyte hypertrophy and interstitial fibrosis.
Fig. 1.
Stroke induces cardiac dysfunction, cardiomyocyte hypertrophy, and fibrosis compared to non-stroke mice a Echocardiography measurements of left ventricular ejection fraction (EF) in conscious mice at 28 days after stroke. b PicroSirius Red (PSR) staining for myocyte cross-sectional area (MCSA) and interstitial collagen fraction (ICF) measurement
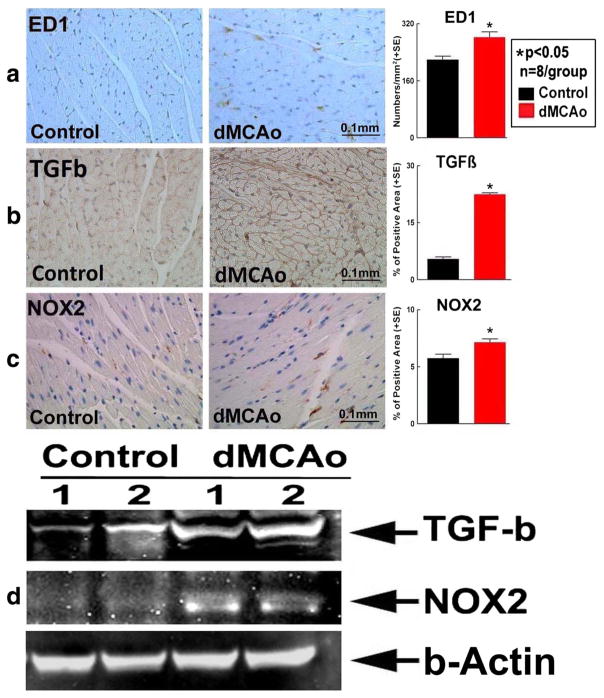
To test the mechanisms of stroke-induced cardiac dysfunction, oxidative stress, inflammatory factor (infiltrating macrophages, ED1), TGF-β and oxidative stress (NOX2) expression were measured in the heart. Figure 2 shows that stroke significantly increased ED1, TGF-β, and NOX2 expression in the heart compared to WT control mice, as measured by immunostaining and Western blot.
Fig. 2.
Stroke significantly induces cardiac inflammation and oxidative stress compared to non-stroke mice. a ED1, b TGF-β, c NOX2 immunostaining and quantitative data, d Western blot assay
Cerebral Ischemic Stroke Significantly Decreases Heart and Serum MiR-126 and Increases MiR-126 Targets MCP-1 and VCAM-1 Expression in Heart Tissue
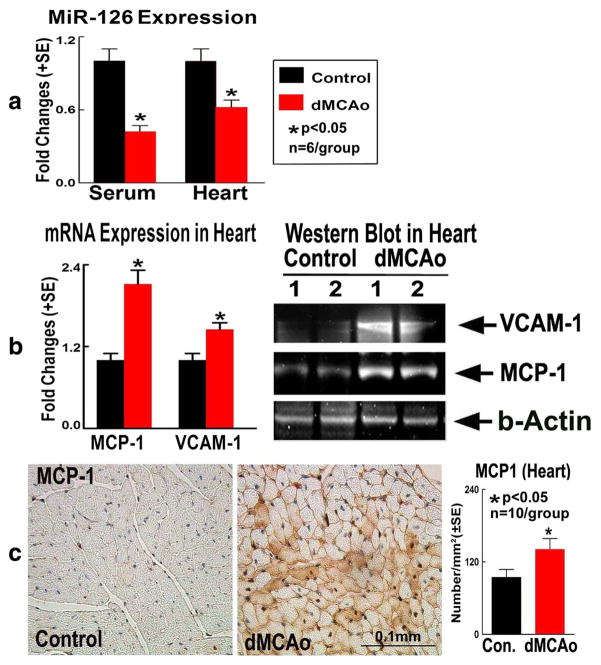
To test possible mechanisms of stroke-induced cardiac dysfunction, miR-126 expression was measured in blood serum and heart tissue. Figure 3a shows that dMCAo significantly decreased serum and heart miR-126 expression compared to non-stroke mice.
Fig. 3.
Stroke mice exhibit significantly decreased heart and serum miR-126 and increased miR-126 targets MCP-1 and VCAM-1 expression in heart tissue. a MiR-126 measurement in serum and heart tissue. b MCP-1 and VCAM-1 real-time PCR and Western blot assays. c MCP-1 immunostaining and quantitative data
To test whether stroke regulates miR-126 target gene and protein expression, selected specific miR-126 target genes and proteins (MCP-1 and VCAM-1 [31, 32]) were measured. Figure 3b, c shows that stroke increased heart tissue MCP-1 and VCAM-1 gene and protein expression compared to non-stroke control. The data indicate that stroke decreased miR-126 and increased its targets, MCP-1 and VCAM-1 gene and protein expression in the heart tissue.
Cerebral Ischemic Stroke in MiR-126EC−/− Mice Results in Increased Cardiac Dysfunction, Cardiac Hypertrophy, Interstitial Fibrosis, Inflammation and Oxidative Stress Compared to WT MiR-126fl/fl Stroke Mice
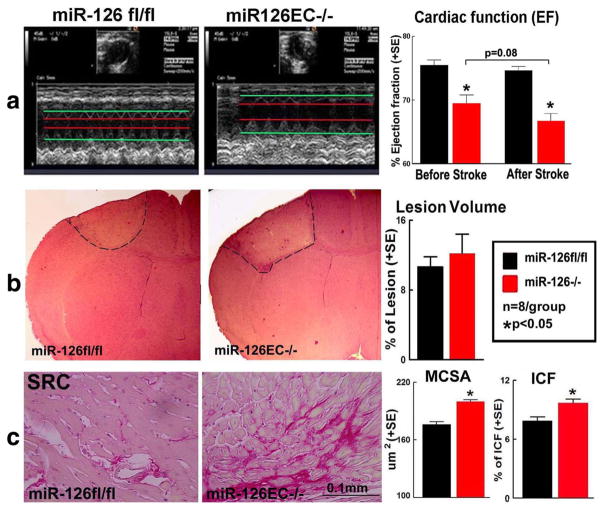
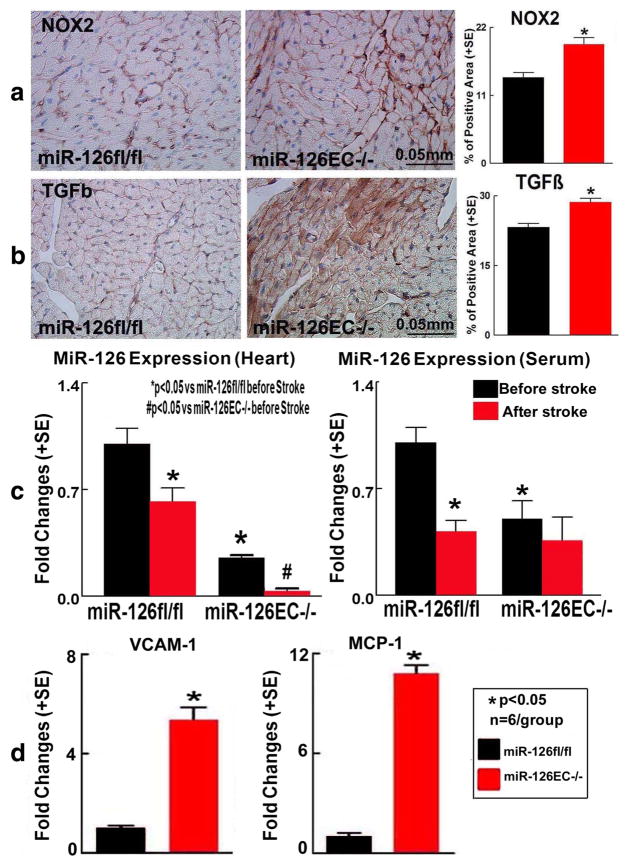
To test if miR-126 affects cardiac function, echocardiography was performed in miR-126EC−/− and miR-126fl/fl mice before and after stroke. Figure 4a shows that miR-126EC−/− mice exhibit significantly decreased cardiac function, evidenced by decreased LVEF before stroke compared to miR-126fl/fl mice. Stroke in miR-126EC−/− mice induced a trend of worse cardiac function measured by decreased LVEF compared to non-stroke miR-126EC−/− mice (p = 0.08) and miR-126fl/fl-stroke mice (p < 0.05), respectively. These data indicate that stroke exacerbates cardiac dysfunction in miR-126EC−/− mice compared with stroke in miR-126fl/fl mice.
Fig. 4.
Stroke in miR-126EC−/− mice significantly increases cardiac dysfunction, hypertrophy, and interstitial fibrosis compared to WT miR-126fl/fl stroke mice. a Echocardiography measurement in miR-126fl/fl and miR-126EC−/− mice after stroke. b H&E staining from brain tissue and brain lesion volume measurement. c PSR staining for MCSA and ICF measurement
We then tested whether there were any differences in the ischemic lesion in the miR-126EC−/− and miR-126fl/fl mice. Figure 4b shows that there was no significant difference in lesion volume between miR-126fl/fl stroke and miR-126EC−/− stroke mice. Ischemic lesions in both sets of mice were located in the frontal and parietal cortex and the underlying corpus callosum without damage to the cardiovascular regions of the brain (insular cortex) and hypothalamus. Thus, ischemic lesion size and location are not responsible for the differential cardiac response to stroke in the miR-126EC−/− and miR-126fl/fl mice.
To test whether miR-126 deficiency exacerbates cardiac hypertrophy and fibrosis, MCSA and ICF were measured. Figure 4c shows that stroke in miR-126EC−/− stroke mice significantly increased cardiomyocyte hypertrophy and cardiac fibrosis compared to miR-126fl/fl stroke mice.
Figure 5a, b shows that stroke in miR-126EC−/− mice significantly increased heart TGF-β and NOX2 expression compared to stroke in miR-126fl/fl mice. MiR-126EC−/− stroke mice also exhibit significantly increased VCAM-1 and MCP-1 gene expression in the heart tissue compared to miR-126fl/fl stroke mice (Fig. 5d).
Fig. 5.
Stroke in miR-126EC−/− mice significantly increases inflammation and oxidative stress in the heart compared to WT miR-126fl/fl stroke mice. a NOX2 and b TGF-β immunostaining and quantitative data. c MiR-126 expression in serum and heart tissue. d Heart tissue VCAM-1 and MCP-1 gene expression measured by real-time PCR
To test whether stroke regulates miR-126 expression in miR-126EC−/− mice, miR-126 expression in serum and heart were measured. Figure 5c shows that miR-126EC−/− mice had lower levels of miR-126 expression in serum and heart than miR-126fl/fl mice (p < 0.05). Stroke exacerbates the decrease of miR-126 expression in heart in both miR-126EC−/− and miR-126fl/fl mice.
MiR-126 Regulates Cardiomyocyte Hypertrophy In vitro
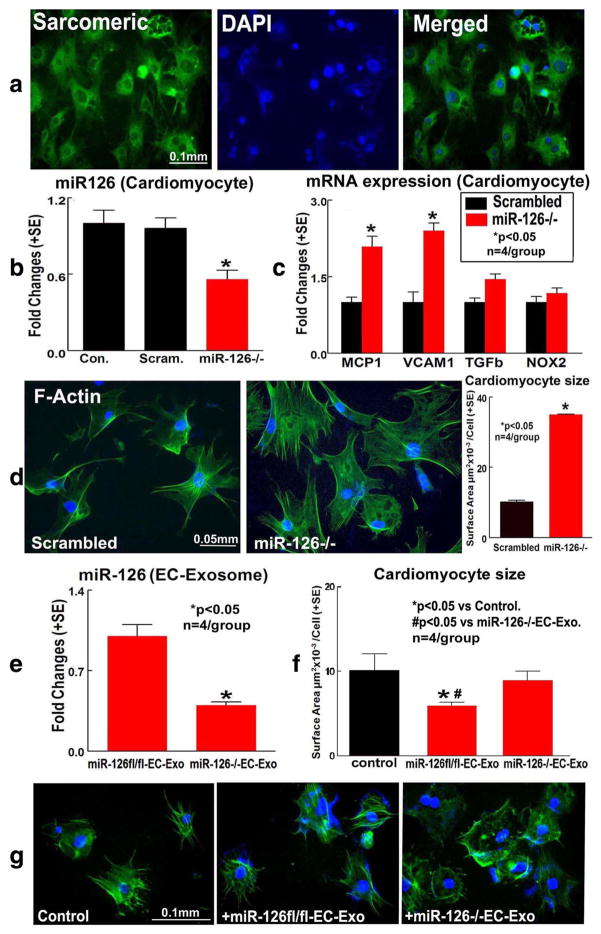
To confirm whether miR-126 directly regulates cardiomyocyte hypertrophy, cell size was measured by surface area calculation using fluorescence microscopy after anti-F-actinin staining in cultured cardiomyocytes. Using sarcomeric a-actinin staining, we found that 95% of cultured cells are cardiomyocytes (Fig. 6a). In addition, knockdown of miR-126 in cardiomyocytes significantly decreased cardiomyocyte miR-126 expression (Fig. 6b) and increased MCP-1 and VCAM-1 expression (Fig. 6c) compared to scrambled control. MiR-126 knockdown in cardiomyocyte also significantly increased cardiomyocyte size compared to scrambled knockdown control (Fig. 6d). However, knockdown of miR-126 in cardiomyocytes did not affect cardiomyocyte cell death compared to scrambled cardiomyocyte knockdown control (data not shown). The data indicate that miR-126 regulates cardio-myocyte hypertrophy.
Fig. 6.
MiR-126 regulates cardiomyocyte MCP-1 and VCAM-1 expression as well as regulates cardiomyocyte hypertrophy in primary cultured cardiomyocyte cell: a Sarcomeric a-actinin (a cardiomyocyte maker) shows that more than 95% cultured cells are cardiomyocyte. b MiR-126 expression in scrambled and miR-126 knockdown cardiomyocyte. c MCP-1, VCAM-1 and TGF-β, and NOX2 gene expression measured by real-time PCR. d Cardiomyocyte surface area measurements. Knockdown of miR-126 expression in cardiomyocyte increases cardiomyocyte size. e MiR-126EC−/− EC-Exo significantly decreases miR-126 expression compared to miR-126fl/fl-EC-Exo. f, g MiR-126fl/fl-EC-Exo treatment decreases, but miR-126EC−/−EC-Exo treatment fails to decrease cardiomyocyte hypertrophy compared to non-treatment control
MiR-126 is a secreted miR and is primarily expressed in endothelial cells [33]. We first measured miR-126 expression in exosomes derived from brain endothelial cells of miR-126fl/ fl (miR-126fl/fl-EC-Exo) and miR-126EC−/− (miR-126EC−/− EC-Exo) mice. Figure 6e shows that miR-126EC−/−EC-Exo exhibits significantly decreased miR-126 expression compared to miR-126fl/fl-EC-Exo. Second, we tested whether miR-126 in endothelial cell-derived exosomes affects cardiomyocytes. Exosomes derived from brain endothelial cells of miR-126fl/fl (miR-126fl/fl-EC-Exo) and miR-126EC−/− (miR-126EC−/−EC-Exo) were added to cultured cardiomyocytes subjected to OGD. Figure 6f, g shows that the addition of miR-126fl/fl-EC-Exo significantly decreased cardiomyocyte size compared to non-treatment OGD cardio-myocyte control or miR-126EC−/−EC-Exo-treated group, respectively. In addition, miR-126EC−/−EC-Exo treatment failed to decrease OGD cardiomyocyte hypertrophy compared to non-treatment cardiomyocyte control (p > 0.05). These data indicate that depletion of EC source miR-126 may fail to protect cardiomyocyte from ischemic hypertrophy.
Discussion
In this study, we found that stroke induces cardiac dysfunction and increases inflammatory and oxidative stress in the heart compared to non-stroke mice. Stroke decreases serum and heart miR-126 and increases miR-126 target gene and protein expression. Using specific conditional miR-126EC−/− mice, we are the first to demonstrate that miR-126EC−/− stroke mice have more severe cardiac dysfunction and hypertrophy compared to miR-126fl/fl stroke mice, suggesting the importance of miR-126 as a mediator of brain and heart interaction after stroke.
Brain and Heart Interaction after Stroke
Acute cerebrovascular disease, acute brain injury, intracranial inflammation and intracranial hypertension can all cause cardiac injury [3]. During the first 3 months following acute ischemic stroke, 19.0% of patients encounter at least one major adverse cardiac episode [3]; approximately 28.5% of stroke patients have an LVEF less than 50% [3]; 13–29% of stroke patients have systolic dysfunction [34]; and 35–74% have ischemic changes on electrocardiography [3]. Therefore, there may be a causal relationship between brain damage and heart dysfunction. In this study, we demonstrated that stroke leads to heart dysfunction at 28 days after stroke, demonstrated by decreased LVEF, as well as increased cardiac interstitial fibrosis and hypertrophy compared to non-stroke mice. Thus, there is a compelling need to investigate mechanisms of stroke-induced heart dysfunction, which may help to develop therapeutic approaches specifically designed to reduce neurological deficits and also to prevent or reduce cardiac dysfunction after stroke.
MiR-126 May Contribute to Brain-Heart Interaction after Stroke
Select miRs are altered by stroke and cardiovascular dysfunction [35]. Circulating miRs mediate intercellular communication [36], and some circulating miRs are correlated with brain miR changes after stroke [37]. Previous studies have reported that knockout of miR-126 induces cardiac dysfunction [38, 39]. MiR-126 expression level in circulation is an indicator of systemic inflammatory and angiogenic status [40, 41]. MiR-126 concentration in the circulation is significantly decreased in ischemic stroke [17] and acute myocardial infarction patients [16]. In the plasma, miR-126 expression level correlates inversely with cardiac troponin-I concentration, which is a biomarker of myocardial damage [42]. Administration of miR-126 increases EC proliferation and angiogenesis and improves cardiac neovascularization and cardiac function [15]. MiR-126 upregulation by intravenous injection of a miR-126 mimic increases vascular density in the heart and improves cardiac function in an animal model of pulmonary arterial hypertension [43]. Our data show that stroke significantly decreases circulating and heart miR-126 expression compared to non-stroke mice. Knockdown of miR-126 in miR-126EC−/− mice subjected to stroke exhibits significantly increased cardiac dysfunction compared to miR-126fl/fl with stroke mice. MiR-126EC−/− stroke mice exhibit significantly increased cardiac fibrosis and hypertrophy compared to miR-126fl/fl stroke mice. In vitro, we also found that ECs secrete miR-126. MiR-126fl/fl-EC-Exo treatment of cardiomyocytes subjected to OGD exhibits significantly reduced hypertrophy than OGD cardiomyocytes treated with miR-126EC−/−EC-Exo, suggesting that miR-126 ameliorates OGD-induced cardiomyocyte damage.
Many other factors may also regulate brain-heart interaction. Previous studies found that brain control of the heart is mediated through the sympathetic and parasympathetic branches of the autonomic nervous system [44]. Cardiovascular regions of the brain also regulate brain-heart interaction after stroke [45]; for example, the right insular cortex has been implicated in cardiovascular sympathetic control [12]. In this study, to avoid the effects of direct brain lesion location of cardiac damage, we used a dMCAo model which induces ischemic lesions in the frontal and parietal cortex while the cardiovascular regions of the brain and hypothalamus are not affected [19, 20]. In addition, there was no significant difference in brain ischemic lesion volume between miR-126fl/fl and miR-126EC−/− stroke mice. Thus, miR-126 may facilitate brain and heart interaction, and decreasing miR-126 after stroke may contribute to induction of cardiac dysfunction.
MiR-126 May Have Inhibitory Effects on Heart Inflammation and Oxidative Stress after Stroke
In our study, using in vitro and in vivo experiments, we found that miR-126EC−/− stroke mice exhibit significantly increased cardiac dysfunction compared to miR-126fl/fl stroke mice. However, how miR-126 regulates cardiomyocyte function is not clear.
Following acute brain injury or ischemia, neuroinflammatory response that impacts recovery includes microglial activation, leukocytes infiltration into the brain, stimulation and secretion of pro-inflammatory factors, and lymphocytes [46, 47]. MiRs control cellular expression machinery acting through “single miR/ multiple targets” or “multiple miRNAs/single targets” mechanisms. In our study, miR-126EC−/− mice have significantly increased inflammatory factors expression in the heart tissue. MCP1, VCAM-1, and Spred-1 are targets of miR-126 [38]; therefore, it is likely that stroke-induced decrease of miR-126 subsequently increased miR-126’s target gene expression. Reduced miR-126 expression may induce vascular inflammation by increasing the levels of VCAM-1, fibrinogen, and leukocyte counts [32]. MiR-126 binds directly to the 3′-untranslated region of MCP-1 mRNA and controls MCP-1 production in a human monocyte/macrophage cell line [31]. Increased expression of MCP-1 in ischemic brain tissue after stroke worsens injury and is associated with the recruitment of inflammatory cells [48, 49]. Monocytes are a major source of pro-inflammatory cytokines following myocardial tissue injury [50] and are key inflammatory mediators of fibrosis in several pathological processes [48]. In this study, we found that stroke significantly increases MCP-1 and VCAM-1 expression as well as increases ED1 positive inflammatory cell numbers in the heart compared to non-stroke mice. Stroke in miR-126EC−/− mice increased inflammatory factors and inflammatory cell numbers compared to stroke in miR-126fl/fl mice. Therefore, decreasing miR-126 and consequently increasing MCP-1 and VCAM-1 expression in the heart may promote infiltration of inflammatory cells into the heart after stroke.
Inflammation and Cardiac Disease
Chronic inflammation has been implicated to play a critical role in the development and progression of ischemic heart failure and drives persistent myofibroblast activity and cardiac fibrosis [51]. Monocyte-released TGF-β increases cardiac hypertrophy and fibrosis [51]. TGF-β is a potent stimulator of cardiac myofibroblast activation and contributes to extracellular matrix deposition in the infarct by upregulating collagen and fibronectin synthesis as well as by decreasing matrix degradation [52]. NOX2, a potent source of reactive oxygen species, promotes the transition of fibroblasts to myofibroblasts and mediates cardiac inflammation and fibrosis [53]. TGF-β stimulation also increases the expression level of NOX2 [53]. Thus, NOX2 oxidative stress mediates cardiac fibrosis through TGF-β-dependent manner [53]. We found that stroke increases heart TGF-β and NOX2 expression in the heart. Stroke in miR-126EC−/− mice enhances TGF-β and NOX2 expression compared to stroke in miR-126fl/ fl mice. Our data indicate that miR-126 regulates inflammatory effects and monocyte infiltration as well as regulates TGF-β and NOX2 expression. Therefore, miR-126/inflammation/oxidative stress may be involved in stroke-induced cardiac fibrosis and cardiac dysfunction.
Limitations of the Study
Stroke regulates many miRs. We are not excluding the possibility that other factors may also contribute to brain-heart interaction after stroke. Our data establishes a direct mechanistic link between ”brain and heart interaction” and suggests that the miR-126 signaling pathway may be involved in brain-heart interaction after stroke. Other factors, including other miRs and other targets of miR-126, may also impact brain-heart interaction and warrant further investigation.
Conclusions
In this study, we found that the miR-126 signaling pathway may be involved in stroke-induced cardiac dysfunction. Regulation of miR-126 may be an important target for the development of novel therapeutic strategies to prevent heart dysfunction and progressive myocardial fibrosis after stroke.
Acknowledgments
The authors wish to thank Cynthia Roberts, Qinge Lu, and Sutapa Santra for the technical assistance. This work was supported by National Institute of Neurological Disorders and Stroke R01 NS083078-01A1 (JC) and R01 NS099030-01 (JC).
Footnotes
Compliance with Ethical Standards
Conflict of Interest Jieli Chen declares that she has no conflict of interest. Chengcheng Cui declares that she has no conflict of interest. Xiaoping Yang declares that she has no conflict of interest. Jiang Xu declares that he has no conflict of interest. Poornima Venkat declares that she has no conflict of interest. Alex Zacharek declares that he has no conflict of interest. Peng Yu declares that he has no conflict of interest. Michael Chopp declares that he has no conflict of interest.
References
- 1.Koh SH, Park HH. Neurogenesis in stroke recovery. Transl Stroke Res. 2016 doi: 10.1007/s12975-016-0460-z. [DOI] [PubMed] [Google Scholar]
- 2.Cassidy JM, Cramer SC. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl Stroke Res. 2016 doi: 10.1007/s12975-016-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wira CR, 3rd, Rivers E, Martinez-Capolino C, Silver B, Iyer G, Sherwin R, et al. Cardiac complications in acute ischemic stroke. The Western Journal of Emergency Medicine. 2011;12(4):414–20. doi: 10.5811/westjem.2011.2.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imam YZ, D’Souza A, Malik RA, Shuaib A. Secondary stroke prevention: improving diagnosis and management with newer technologies. Transl Stroke Res. 2016;7(6):458–77. doi: 10.1007/s12975-016-0494-2. [DOI] [PubMed] [Google Scholar]
- 5.Ergul A, Hafez S, Fouda A, Fagan SC. Impact of comorbidities on acute injury and recovery in preclinical stroke research: focus on hypertension and diabetes. Transl Stroke Res. 2016;7(4):248–60. doi: 10.1007/s12975-016-0464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ay H, Koroshetz WJ, Benner T, Vangel MG, Melinosky C, Arsava EM, et al. Neuroanatomic correlates of stroke-related myocardial injury. Neurology. 2006;66(9):1325–9. doi: 10.1212/01.wnl.0000206077.13705.6d. [DOI] [PubMed] [Google Scholar]
- 7.Oppenheimer SM. Neurogenic cardiac effects of cerebrovascular disease. Curr Opin Neurol. 1994;7(1):20–4. doi: 10.1097/00019052-199402000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa H, Tajiri N, Vasconcellos J, Kaneko Y, Mimura O, Dezawa M, et al. Ischemic stroke brain sends indirect cell death signals to the heart. Stroke. 2013;44(11):3175–82. doi: 10.1161/STROKEAHA.113.001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gongora-Rivera F, Labreuche J, Jaramillo A, Steg PG, Hauw JJ, Amarenco P. Autopsy prevalence of coronary atherosclerosis in patients with fatal stroke. Stroke. 2007;38(4):1203–10. doi: 10.1161/01.str.0000260091.13729.96. [DOI] [PubMed] [Google Scholar]
- 10.Tokgozoglu SL, Batur MK, Topcuoglu MA, Saribas O, Kes S, Oto A. Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke. 1999;30(7):1307–11. doi: 10.1161/01.str.30.7.1307. [DOI] [PubMed] [Google Scholar]
- 11.Swerdel JN, Janevic TM, Kostis WJ, Faiz A, Cosgrove NM, Kostis JB. Association between dehydration and short-term risk of ischemic stroke in patients with atrial fibrillation. Transl Stroke Res. 2016 doi: 10.1007/s12975-016-0471-9. [DOI] [PubMed] [Google Scholar]
- 12.Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42(9):1727–32. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 13.Rosenzweig S, Carmichael ST. Age-dependent exacerbation of white matter stroke outcomes: a role for oxidative damage and inflammatory mediators. Stroke. 2013;44(9):2579–86. doi: 10.1161/STROKEAHA.113.001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra PK, Tyagi N, Kumar M, Tyagi SC. MicroRNAs as a therapeutic target for cardiovascular diseases. J Cell Mol Med. 2009;13(4):778–89. doi: 10.1111/j.1582-4934.2009.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20(4):368–76. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu A, Chen SJ, Chang YS, Chen HC, Chu PH. Systemic approach to identify serum microRNAs as potential biomarkers for acute myocardial infarction. Biomed Res Int. 2014;2014:418628. doi: 10.1155/2014/418628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long G, Wang F, Li H, Yin Z, Sandip C, Lou Y, et al. Circulating miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurol. 2013;13:178. doi: 10.1186/1471-2377-13-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei XJ, Han M, Yang FY, Wei GC, Liang ZG, Yao H, et al. Biological significance of miR-126 expression in atrial fibrillation and heart failure. Braz J Med Biol Res. 2015;48(11):983–9. doi: 10.1590/1414-431X20154590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuraoka M, Furuta T, Matsuwaki T, Omatsu T, Ishii Y, Kyuwa S, et al. Direct experimental occlusion of the distal middle cerebral artery induces high reproducibility of brain ischemia in mice. Experimental animals/Japanese Association for Laboratory Animal Science. 2009;58(1):19–29. doi: 10.1538/expanim.58.19. [DOI] [PubMed] [Google Scholar]
- 20.Rosell A, Agin V, Rahman M, Morancho A, Ali C, Koistinaho J, et al. Distal occlusion of the middle cerebral artery in mice: are we ready to assess long-term functional outcome? Transl Stroke Res. 2013;4(3):297–307. doi: 10.1007/s12975-012-0234-1. [DOI] [PubMed] [Google Scholar]
- 21.Claxton S, Kostourou V, Jadeja S, Chambon P, Hodivala-Dilke K, Fruttiger M. Efficient, inducible Cre-recombinase activation in vascular endothelium. Genesis. 2008;46(2):74–80. doi: 10.1002/dvg.20367. [DOI] [PubMed] [Google Scholar]
- 22.Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, et al. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135(24):3989–93. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- 23.Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Phys. 1999;277(5 Pt 2):H1967–74. doi: 10.1152/ajpheart.1999.277.5.H1967. [DOI] [PubMed] [Google Scholar]
- 24.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10(2):290–3. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Carretero OA, Liao TD, Peng H, Shesely EG, Xu J, et al. Local angiotensin II aggravates cardiac remodeling in hypertension. Am J Physiol Heart Circ Physiol. 2010;299(5):H1328–38. doi: 10.1152/ajpheart.00538.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mito S, Ozono R, Oshima T, Yano Y, Watari Y, Yamamoto Y, et al. Myocardial protection against pressure overload in mice lacking Bach1, a transcriptional repressor of heme oxygenase-1. Hypertension. 2008;51(6):1570–7. doi: 10.1161/HYPERTENSIONAHA.107.102566. [DOI] [PubMed] [Google Scholar]
- 27.Belostotskaya GB, Golovanova TA. Characterization of contracting cardiomyocyte colonies in the primary culture of neonatal rat myocardial cells: a model of in vitro cardiomyogenesis. Cell Cycle. 2014;13(6):910–8. doi: 10.4161/cc.27768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui X, Chopp M, Zacharek A, Ye X, Roberts C, Chen J. Angiopoietin-Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiol Dis. 2011;43(1):285–92. doi: 10.1016/j.nbd.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Sun Y, Carretero OA, Zhu L, Harding P, Shesely EG, et al. Effects of cardiac overexpression of the angiotensin II type 2 receptor on remodeling and dysfunction in mice post-myocardial infarction. Hypertension. 2014;63(6):1251–9. doi: 10.1161/HYPERTENSIONAHA.114.03247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Ning R, Zacharek A, Cui C, Cui X, Yan T, et al. MiR-126 contributes to human umbilical cord blood cell induced Neurorestorative effects after stroke in type-2 diabetic mice. Stem Cells. 2015 doi: 10.1002/stem.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arner E, Mejhert N, Kulyte A, Balwierz PJ, Pachkov M, Cormont M, et al. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes. 2012;61(8):1986–93. doi: 10.2337/db11-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witkowski M, Weithauser A, Tabaraie T, Steffens D, Krankel N, Witkowski M, et al. Micro-RNA-126 reduces the blood thrombogenicity in diabetes mellitus via targeting of tissue factor. Arterioscler Thromb Vasc Biol. 2016 doi: 10.1161/ATVBAHA.115.306094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105(5):1516–21. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rauh R, Fischereder M, Spengel FA. Transesophageal echocardiography in patients with focal cerebral ischemia of unknown cause. Stroke. 1996;27(4):691–4. doi: 10.1161/01.str.27.4.691. [DOI] [PubMed] [Google Scholar]
- 35.Kim JM, Jung KH, Chu K, Lee ST, Ban J, Moon J, et al. Atherosclerosis-related circulating MicroRNAs as a predictor of stroke recurrence. Transl Stroke Res. 2015;6(3):191–7. doi: 10.1007/s12975-015-0390-1. [DOI] [PubMed] [Google Scholar]
- 36.Turchinovich A, Samatov TR, Tonevitsky AG, Burwinkel B. Circulating miRNAs: cell-cell communication function? Front Genet. 2013;4:119. doi: 10.3389/fgene.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng G, Yuan Y, Wu S, He F, Hu Y, Luo B. MicroRNA let-7e is a potential circulating biomarker of acute stage ischemic stroke. Transl Stroke Res. 2015;6(6):437–45. doi: 10.1007/s12975-015-0422-x. [DOI] [PubMed] [Google Scholar]
- 38.Small EM, Frost RJA, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121(8):1022–32. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fei L, Zhang J, Niu H, Yuan C, Ma X. Effects of rosuvastatin and MiR-126 on myocardial injury induced by acute myocardial infarction in rats: role of vascular endothelial growth factor a (VEGF-A) Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2016;22:2324–34. doi: 10.12659/MSM.896983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olivieri F, Spazzafumo L, Bonafe M, Recchioni R, Prattichizzo F, Marcheselli F, et al. MiR-21-5p and miR-126a-3p levels in plasma and circulating angiogenic cells: relationship with type 2 diabetes complications. Oncotarget. 2015;6(34):35372–82. doi: 10.18632/oncotarget.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen F, Du Y, Esposito E, Liu Y, Guo S, Wang X, et al. Effects of focal cerebral ischemia on exosomal versus serum miR126. Transl Stroke Res. 2015;6(6):478–84. doi: 10.1007/s12975-015-0429-3. [DOI] [PubMed] [Google Scholar]
- 42.Long G, Wang F, Duan Q, Chen F, Yang S, Gong W, et al. Human circulating microRNA-1 and microRNA-126 as potential novel indicators for acute myocardial infarction. Int J Biol Sci. 2012;8(6):811–8. doi: 10.7150/ijbs.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potus F, Ruffenach G, Dahou A, Thebault C, Breuils-Bonnet S, Tremblay E, et al. Downregulation of microRNA-126 contributes to the failing right ventricle in pulmonary arterial hypertension. Circulation. 2015;132(10):932–43. doi: 10.1161/CIRCULATIONAHA.115.016382. [DOI] [PubMed] [Google Scholar]
- 44.Silvani A, Calandra-Buonaura G, Dampney RA, Cortelli P. Brain-heart interactions: physiology and clinical implications. Philos Trans A Math Phys Eng Sci. 2016;374(2067) doi: 10.1098/rsta.2015.0181. [DOI] [PubMed] [Google Scholar]
- 45.Daniele O, Caravaglios G, Fierro B, Natale E. Stroke and cardiac arrhythmias. J Stroke Cerebrovasc Dis. 2002;11(1):28–33. doi: 10.1053/jscd.2002.123972. [DOI] [PubMed] [Google Scholar]
- 46.Liesz A, Kleinschnitz C. Regulatory T cells in post-stroke immune homeostasis. Transl Stroke Res. 2016;7(4):313–21. doi: 10.1007/s12975-016-0465-7. [DOI] [PubMed] [Google Scholar]
- 47.Atangana E, Schneider UC, Blecharz K, Magrini S, Wagner J, Nieminen-Kelha M, et al. Intravascular inflammation triggers intracerebral activated microglia and contributes to secondary brain injury after experimental subarachnoid hemorrhage (eSAH) Transl Stroke Res. 2016 doi: 10.1007/s12975-016-0485-3. [DOI] [PubMed] [Google Scholar]
- 48.Ahmad M, Graham SH. Inflammation after stroke: mechanisms and therapeutic approaches. Transl Stroke Res. 2010;1(2):74–84. doi: 10.1007/s12975-010-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao Y, Tsirka SE. Chemokines and their receptors in intracerebral hemorrhage. Transl Stroke Res. 2012;3(1):70–9. doi: 10.1007/s12975-012-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130(2):147–58. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mewhort HE, Lipon BD, Svystonyuk DA, Teng G, Guzzardi DG, Silva C, et al. Monocytes increase human cardiac myofibroblast-mediated extracellular matrix remodeling through TGF-beta1. Am J Physiol Heart Circ Physiol. 2016;310(6):H716–24. doi: 10.1152/ajpheart.00309.2015. [DOI] [PubMed] [Google Scholar]
- 52.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74(2):184–95. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y, Zhang J. Nox2 contributes to cardiac fibrosis in diabetic cardiomyopathy in a transforming growth factor-beta dependent manner. Int J Clin Exp Pathol. 2015;8(9):10908–14. [PMC free article] [PubMed] [Google Scholar]