Abstract
The thalamus is an evolutionarily conserved structure with extensive reciprocal connections to cortical regions. While its role in transmitting sensory signals is well-studied, its broader engagement in cognition is unclear. In this review, we discuss evidence that the thalamus regulates functional connectivity within and between cortical regions, determining how a cognitive process is implemented across distributed cortical microcircuits. Within this framework, thalamic circuits do not necessarily determine the categorical content of a cognitive process (e.g. sensory details in feature-based attention), but rather provide a route by which task-relevant cortical representations are sustained and coordinated. Additionally, thalamic control of cortical connectivity bridges general arousal to the specific processing of categorical content, providing an intermediate level of cognitive and circuit description that will facilitate mapping neural computations onto thought and behavior.
Introduction
Arousal is often conceptualized as the general context in which specific cognitive processes such as attention occur [1]. However, in contrast to attention, where enhanced excitability and changes in functional cortical connectivity are observed among neurons encoding related sensorimotor features [2,3] arousal is associated with broad increase in forebrain excitability [1] and functional cortical connectivity [4]. Neural specificity of attentional processing is required for augmenting relevant inputs over distractors, enabling one to have a conversation in a crowded room while filtering out irrelevant but equally loud conversations [5]. Such specificity is not expected for general sensorimotor responsiveness that diminishes during sleep or anesthesia, and one often used as a measure of arousal [6].
While making an explicit categorical distinction between arousal and cognitive processing has driven experimental progress in psychology and behavioral neuroscience over the last few decades, we think that its continued use may limit our ability to map cognition onto underlying neural circuits and computations. For example, while the construct of attention proposes specialized computations that differentially amplify sensory inputs [7–9], such computations occur within a larger context that involves changing behavioral goals, evolving decisions and memory updating [10–13]. This context may not involve the same level of topographic specificity seen for visual cortical response modulation by visuospatial attention [14] but it is far from global or general. Instead, engagement in broadly similar cognitive tasks evokes the same patterns of functional connectivity within and across cortical networks despite differences in the categorical content [15]. We think that defining task-relevant functional cortical connectivity patterns is critical, as it constitutes a missing intermediate level of neural description that flanks global changes in cortical connectivity associated with general arousal on one end and specific patterns of cortical microcircuit activity associated with making categorical perceptual or mnemonic judgments on another. On the cognitive end, this level of circuit and computational organization would translate into distinct directed arousal states, which makes intuitive sense, since an organism is invariably aroused about something (an object), whether it is food, mate or memory. Experimentally, this is equivalent to the idea of task engagement, where the construction of a task set requires changing functional connectivity within and across task-relevant cortical regions [16]. In the next few sections, we will review recent evidence that indicates a central role for the thalamus in regulating cortical connectivity, and therefore, the construction of a directed arousal state. That is, beyond the well-recognized role of certain thalamic circuits in relaying categorical content to and between cortical regions is the control of connectivity within and across task-relevant cortical networks. This newly recognized thalamic role, as a master regulator of functional cortical connectivity, places this subcortical structure closer to the center of cognitive control.
Task-relevant changes in functional cortical connectivity
The combination of transcranial magnetic stimulation (TMS) with electroencephalographic (EEG) recordings has become an extremely powerful approach for probing and manipulating functional connectivity across cortical networks of the human brain [17]. Initial seminal studies have shown that broad changes in cortical connectivity are associated with broad changes in arousal. For example, TMS applied to the premotor cortex results in highly diminished responses in prefrontal or parietal areas during slow wave sleep compared to quiet wakefulness, supporting the general idea that cortical connectivity breaks down during sleep [4].
More recently, this functional approach has been applied to understanding cortical network interactions during different cognitive processes. For example, the superior parietal lobule (SPL) establishes connectivity patterns with the occipital, parietal, and frontal cortices that are enhanced during short term working memory despite differences in the categorical content of items across different trials [18]. Similarly, in feature-based attention, broadly attending to faces or motion differentially enhances connectivity between prefrontal cortex (PFC) and the visual motion sensitive area (MT) or the fusiform face area (FFA), respectively [19]. Importantly, specific patterns of functional connectivity are trainable and can enhance performance on tasks that have not been previously encountered if they broadly utilize similar cortical connections [15].
In addition to task-relevant enhancement of connectivity across cortical regions, functional connectivity within a particular region appears to be relevant for sustained representation of categorical content. This can be inferred from a recent study by Rose et al. [20] where TMS-evoked activation of a parietal region encoding an unattended categorical item held in working memory resulted in performance deficits attributed to memory trace interference. This finding was particularly surprising because that item was undecodable compared to the attended one, and provides support to the idea that working memory traces can be stored in a latent state through recurrent local synaptic connections [21–23]. The notion that local synaptic interactions can maintain a categorical representation in time is supported by multiple animal studies [24,25] the most compelling of which is that of the songbird premotor system (nucleus HVC), where a synaptic chain holds a song representation over time [26].
Thalamic regulation of functional cortical connectivity
The idea that the thalamus serves as an information relay is derived from the known function of the lateral geniculate nucleus (LGN), which receives topographical inputs from the retina and projects topographically to primary visual cortex (V1). The classical Hubel and Wiesel model [27] predicts a transformation from a concentric, center/surround representation to orientation tuning through spatially offset LGN inputs on individual V1 neurons (Figure 1A). Key to this transformation is that thalamic inputs control visual-evoked spike timing of cortical neurons and thereby dictates the structure of their receptive fields (the content of their sensory representation) [28]. Given the theoretical and experimental success in understanding this early visual transformation, it became increasingly attractive to think of all thalamic function being a variation on this theme. As such, influential conceptual frameworks have been introduced to extend the idea of information relay to thalamic circuits that do not receive inputs from sensory organs as the LGN does [29]. Instead of relaying information from the periphery to the cortex, the idea is that these ‘higher order’ circuits relay information across cortical regions.
Figure 1.
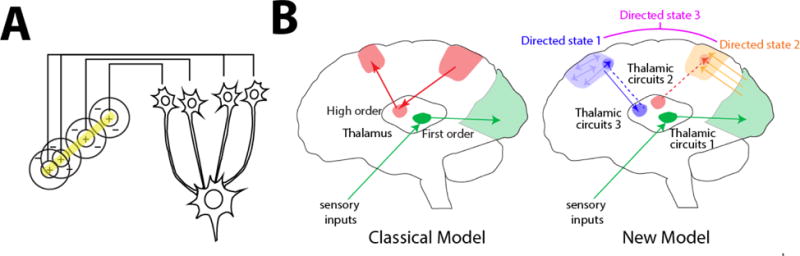
Thalamic control of functional cortical connectivity. (A) The classical transformation of visual representation from center/surround to orientation tuning involves drive from the lateral geniculate nucleus (LGN) to primary visual cortex, where the resulting cortical representation is dependent on thalamic input (information transmission). (B) The classical model reflects the idea of information relay from the LGN, which is shared by other sensory systems (green), and extended to account for thalamic nuclei that receive driving inputs from the cortex rather than subcortical or sensory inputs. The New model suggests that many of these circuits operate to enhance connectivity within a cortical region (e.g. Blue), or across different cortical areas (e.g. Orange). In these scenarios thalamic inputs do not necessarily drive spiking (do not dictate cortical spike times), but rather enhance how spike times are generated in response to specific inputs (either local, or from area A, B, C, etc…). A particular connectivity pattern reflects a directed arousal state, as defined in this review. Such states can encompass multiple cortical nodes and utilize multiple thalamic amplifiers (e.g. Directed state 3), depending on the complexity of the associated cognitive process.
Multiple lines of evidence suggest that this idea is incomplete and misses a fundamental thalamic function; the control of functional connectivity within and across cortical regions. Anatomically, thalamic output to the cortex is quite variable, with only a minority of thalamic circuits exhibiting a topographic and spatially restricted innervation of a single cortical target [30,31]. Additionally, many thalamic circuits receive convergent inputs from a single or multiple cortical regions that do not appear to be compatible with the idea of topographic relay [32].
In a recent study, we investigated the role of the mediodorsal thalamus (MD) in sustaining distinct categorical representations in the PFC, and ones that are dependent on recurrent lateral interactions between PFC subnetworks [33]. These observations were made in the context of an attentional control task, where mice selected between conflicting visual and auditory targets based on one of two task rules that were randomly presented on a trial-by-trial basis. While distinct PFC populations represented the task rules through lateral connections (synaptic chains analogous to the ones seen in the songbird HVC [26]) making rule decoding straightforward, MD neurons showed qualitatively similar temporal tuning but did not distinguish between the two rules. Bidirectional optogenetic manipulations along with multi-site recordings in the context of this task showed that MD inputs were critical for enhancing lateral connectivity between PFC neurons but neither dictated their baseline excitability (measured through changes in spike rates) or their categorical tuning. As such, and in contrast to orientation tuning in V1 whose structure is dependent on LGN inputs, PFC receptive fields in this task are dependent on lateral inputs from other tuned PFC neurons, with MD enhancing the relevant functional connections. This is a clear example of the thalamus enhancing cortical connectivity without influencing categorical content directly (Figure 1B).
While enhancing local cortical connectivity without relaying information has not been explicitly described previously, its ubiquity may be inferred from studies of the primate Pulvinar, a region with structural parallels to MD. Enhanced local connectivity would be consistent with Pulvinar inactivation diminishing neural responses in primary visual cortex [34] and shifting higher visual cortical regions to a sleep-like state [35]. Similar thalamic input is also a reasonable candidate for enhanced lateral connectivity in parietal cortex that is hypothesized to account for prospective visual receptive field expansion during a saccade [36]. Lack of categorical representation is consistent with Pulvinar neurons reflecting confidence, not stimulus category during perceptual decision making [37].
Beyond enhanced local cortical connectivity, thalamic circuits may also enhance connectivity across cortical areas. For example, during the delay period of sustained visuospatial attention, enhanced connectivity between Inferior temporal area TEO and higher order visual cortex V4 appears to be mediated through the Pulvinar and correlates with appropriate task performance [38]. Given that an individual cortical region receives inputs from multiple thalamic structures, we suggest a model in which different thalamic inputs shift the connectivity weights within a cortical region allowing it to shift between processing and sustaining locally generated categorical representation and participating in distributed categorical processing across multiple cortical nodes (Figure 1B). Testing this framework will undoubtedly expand our understanding not only of thalamic function, but of cognition more broadly.
Conclusion
The idea that cognitive constructs such as attention, working memory and decision making will ultimately be mapped onto distinct circuits and computations may end up being inaccurate. Instead, these processes are now known to utilize overlapping neural substrates (e.g. area LIP in the primate brain is known to be involved in visuospatial attention [10–13] as well as decision making). With better understanding of brain function at the computational level [39], these constructs may be replaced with a set of algorithms whose deployment will vary according to behavioral demands, and together give rise to varying degrees of overlap among what we currently recognize as individual constructs. Because these algorithms will be distributed across cortical regions (sensory, motor, mnemonic, abstract, etc…), functional connectivity across individual cortical nodes will reflect engagement in specific cognitive tasks. In this review, we introduced the idea that a specific pattern of functional cortical connectivity defines a ‘directed arousal’ state, to emphasize a level of cognitive and neural description that connects general arousal to specific categorical processing. This level of organization may also be completely dissociable from general arousal in that such states could still occur during sleep; with ones predominantly reflecting local processing during non-rapid eye movement sleep, and others reflecting inter-regional interactions during rapid eye movement sleep [40–42]. In humans, the combination of TMS and neuroimaging approaches is providing us with a wealth of data on how these states relate to cognitive processing. Animal studies provide much needed mechanistic understanding, and based on recent data we described here, we think the thalamus operates as a master regulator of functional cortical connectivity. These findings expand our understanding of cognitive function, and set the stage for exploring this role of thalamus as a diagnostic and correction target in the context of human cognitive disorders.
Highlights.
-
-
We introduce a framework in which the thalamus controls functional connectivity within and across cortical areas
-
-
Thalamic control of cortical connectivity can occur without relaying or specifying categorical content
-
-
Thalamic construction of a functional cortical network underlies the generation of a directed arousal state
-
-
Directed arousal is an intermediate level of cognitive description that bridges general arousal to precise control of sensorimotor transformations
Acknowledgments
M.N. is supported by a JSPS fellowship. M.M.H. is supported by grants from NIMH, NINDS, Brain and Behavior, Sloan and Klingenstein Foundations as well as the Human Frontiers Science Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 2.Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324(5931):1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421(6921):370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- 4.Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309(5744):2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 5.Kerlin JR, Shahin AJ, Miller LM. Attentional gain control of ongoing cortical speech representations in a “cocktail party”. J Neurosci. 2010;30(2):620–628. doi: 10.1523/JNEUROSCI.3631-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6(8):e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61(2):168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci. 2011;13(1):51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 10.Luckmann HC, Jacobs HI, Sack AT. The cross-functional role of frontoparietal regions in cognition: internal attention as the overarching mechanism. Prog Neurobiol. 2014;116:66–86. doi: 10.1016/j.pneurobio.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Ganguli S, Bisley JW, Roitman JD, Shadlen MN, Goldberg ME, Miller KD. One-dimensional dynamics of attention and decision making in LIP. Neuron. 2008;58(1):15–25. doi: 10.1016/j.neuron.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith PL, Ratcliff R. An integrated theory of attention and decision making in visual signal detection. Psychol Rev. 2009;116(2):283–317. doi: 10.1037/a0015156. [DOI] [PubMed] [Google Scholar]
- 13.Gottlieb J, Balan P. Attention as a decision in information space. Trends Cogn Sci. 2010;14(6):240–248. doi: 10.1016/j.tics.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verhoef BE, Maunsell JH. Attention operates uniformly throughout the classical receptive field and the surround. Elife. 2016;5:e17256. doi: 10.7554/eLife.17256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kundu B, Sutterer DW, Emrich SM, Postle BR. Strengthened effective connectivity underlies transfer of working memory training to tests of short-term memory and attention. J Neurosci. 2013;33(20):8705–8715. doi: 10.1523/JNEUROSCI.5565-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakai K. Task set and prefrontal cortex. Annu Rev Neurosci. 2008;31:219–245. doi: 10.1146/annurev.neuro.31.060407.125642. [DOI] [PubMed] [Google Scholar]
- 17.Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience–virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol. 2000;10(2):232–237. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JS, Kundu B, Casali AG, Postle BR. Task-dependent changes in cortical excitability and effective connectivity: a combined TMS-EEG study. J Neurophysiol. 2012;107(9):2383–2392. doi: 10.1152/jn.00707.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morishima Y, Akaishi R, Yamada Y, Okuda J, Toma K, Sakai K. Task-specific signal transmission from prefrontal cortex in visual selective attention. Nat Neurosci. 2009;12(1):85–91. doi: 10.1038/nn.2237. [DOI] [PubMed] [Google Scholar]
- 20**.Rose NS, LaRocque JJ, Riggall AC, Gosseries O, Starrett MJ, Meyering EE, Postle BR. Reactivation of latent working memories with transcranial magnetic stimulation. Science. 2016;354(6316):1136–1139. doi: 10.1126/science.aah7011. This elegant study deploying multistep working memory tasks and TMS showed that undecodible working memory for unattended item can be reactivaed by TMS or cue redirecting attention to this item. This supports the idea that working memory is stored in recurrent local synaptic connections and can be revived through exogenous stimulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science. 2008;319(5869):1543–1546. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- 22.Barak O, Tsodyks M. Working models of working memory. Curr Opin Neurobiol. 2014;25:20–24. doi: 10.1016/j.conb.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Stokes MG. ‘Activity-silent’ working memory in prefrontal cortex: a dynamic coding framework. Trends Cogn Sci. 2015;19(7):394–405. doi: 10.1016/j.tics.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature. 2012;484(7392):62–68. doi: 10.1038/nature10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Ito HT, Zhang SJ, Witter MP, Moser EI, Moser MB. A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nature. 2015;522(7554):50–55. doi: 10.1038/nature14396. This paper is the first causal interrogation of nucleus reuniens function in spatial working memory. The authors show behavioral effects of this thalamic inactivation on hippocampal and prefrontal spatial tuning that are consistent with a role in modulation functional connectivity. [DOI] [PubMed] [Google Scholar]
- 26.Long MA, Jin DZ, Fee MS. Support for a synaptic chain model of neuronal sequence generation. Nature. 2010;468(7322):394–399. doi: 10.1038/nature09514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chariker L, Shapley R, Young LS. Orientation Selectivity from Very Sparse LGN Inputs in a Comprehensive Model of Macaque V1 Cortex. J Neurosci. 2016;36(49):12368–12384. doi: 10.1523/JNEUROSCI.2603-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherman SM. Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci. 2016;19(4):533–541. doi: 10.1038/nn.4269. [DOI] [PubMed] [Google Scholar]
- 30*.Kuramoto E, Pan S, Furuta T, Tanaka YR, Iwai H, Yamanaka A, Ohno S, Kaneko T, Goto T, Hioki H. Individual mediodorsal thalamic neurons project to multiple areas of the rat prefrontal cortex: A single neuron-tracing study using virus vectors. J Comp Neurol. 2017;525(1):166–185. doi: 10.1002/cne.24054. This paper characterized the axonal arbors of single MD neurons projecting to the frontal cortex. An individual MD neuron can project to multiple frontal cortices with different projection patterns (diffuse or dense) across multiple layers. These findings are consistent with connectivity schemes across thalamus that are ill equipped for point-to-point information transmission. [DOI] [PubMed] [Google Scholar]
- 31.Clasca F, Rubio-Garrido P, Jabaudon D. Unveiling the diversity of thalamocortical neuron subtypes. Eur J Neurosci. 2012;35(10):1524–1532. doi: 10.1111/j.1460-9568.2012.08033.x. [DOI] [PubMed] [Google Scholar]
- 32.Rovo Z, Ulbert I, Acsady L. Drivers of the primate thalamus. J Neurosci. 2012;32(49):17894–17908. doi: 10.1523/JNEUROSCI.2815-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Schmitt LI, Wimmer RD, Nakajima M, Happ M, Mofakham S, Halassa MM. Thalamic amplification of cortical connectivity sustains attentional control. Nature. 2017 doi: 10.1038/nature22073. This is the first paper providing direct evidence that thalamus can enhance cortical connectivity without necessarily specifying categorical content. Rule represenation in PFC is supported by weak local connectivity in PFC and MD inputs enhance PFC connections to sustain categorical rule representations required for sustained attentional control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purushothaman G, Marion R, Li K, Casagrande VA. Gating and control of primary visual cortex by pulvinar. Nat Neurosci. 2012;15(6):905–912. doi: 10.1038/nn.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Zhou H, Schafer RJ, Desimone R. Pulvinar-Cortex Interactions in Vision and Attention. Neuron. 2016;89(1):209–220. doi: 10.1016/j.neuron.2015.11.034. This paper characterized the pulvinar’s interaction with V4 during a visual spatial attention task in the macaque. Pulvinar inactivation diminished performance and caused V4 to transition to sleep-like state, suggesting that the pulvinar is critical to maintaining an active state in V4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Wang X, Fung CC, Guan S, Wu S, Goldberg ME, Zhang M. Perisaccadic Receptive Field Expansion in the Lateral Intraparietal Area. Neuron. 2016;90(2):400–409. doi: 10.1016/j.neuron.2016.02.035. Visual receptive field of LIP neurons show perisaccadic expansion towards the future eye position, a process explained by a computational model that involves a synaptic chain in LIP reflecting a saccadic wave. Modeling this network shows that a non-driving input reflecting the direction of movement is required for both the enhaced connectivity among LIP neurons as well as their receptive field expansion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Komura Y, Nikkuni A, Hirashima N, Uetake T, Miyamoto A. Responses of pulvinar neurons reflect a subject’s confidence in visual categorization. Nat Neurosci. 2013;16(6):749–755. doi: 10.1038/nn.3393. This very interesting study showed that pulvinar neural spiking encodes perceptual confidence during a decision making task rather than stimulus category. This is consistent with the idea that under certain conditions, some pulvinar circuits may encode a neural context, rather than categorical content information that would otherwise be cortically encoded. [DOI] [PubMed] [Google Scholar]
- 38.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337(6095):753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jazayeri M, Afraz A. Navigating the Neural Space in Search of the Neural Code. Neuron. 2017;93(5):1003–1014. doi: 10.1016/j.neuron.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 40.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36(6):1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 41.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 42.Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nat Neurosci. 2013;16(2):139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]


