Abstract
Background & Aims
Aflatoxin, which causes hepatocellular carcinoma, may also cause gallbladder cancer. We investigated whether patients with gallbladder cancer have higher exposure to aflatoxin than patients with gallstones.
Methods
We measured aflatoxin B1 (AFB1)–lysine adducts in plasma samples from the Shanghai Biliary Tract Cancer case–control study, conducted from 1997 through 2001. We calculated age- and sex-adjusted odds ratios (ORs) and 95% CIs and the population-attributable fraction for 209 patients with gallbladder cancer and gallstones vs 250 patients with gallstones without cancer (controls). In 54 patients with gallbladder cancer, tumor tissue was examined for the R249S mutation in TP53, associated with aflatoxin exposure, through targeted sequencing.
Results
The AFB1–lysine adduct was detected in 67 of 209 patients with gallbladder cancer (32%) and 37 of the 250 controls (15%) (χ2 P <.0001), almost 3-fold more patients with gallbladder cancer than controls (OR, 2.71; 95% CI, 1.70–4.33). Among participants with detectable levels of AFB1–lysine, the median level of AFB1–lysine was 5.4 pg/mg in those with gallbladder cancer, compared to 1.2 pg/mg in controls. For patients in the 4th of quartile of AFB1–lysine level vs the 1st quartile, the OR for gallbladder cancer was 7.61 (95% CI, 2.01–28.84). None of the 54 gallbladder tumors sequenced were found to have the R249S mutation in TP53. The population-attributable fraction for cancer related to aflatoxin was 20% (95% CI, 15%–25%).
Conclusions
In a case–control study of patients with gallbladder cancer and gallstones vs patients with gallstones without cancer, we associated exposure to aflatoxin (based on plasma level of AFB1–lysine) with gallbladder cancer. Gallbladder cancer does not appear associate with the R249S mutation in TP53. If aflatoxin is a cause of gallbladder cancer, it may have accounted for up to 22% of the gallbladder cancers in Shanghai, China during the study period, and could account for an even higher proportion in high-risk areas. If our findings are verified, reducing aflatoxin exposure might reduce the incidence of gallbladder cancer.
Keywords: biliary tract cancer, epidemiology, etiology, carcinogen
Introduction
Gallbladder cancer (GBC) is a highly fatal disease, with a median survival of about six months.1 Geographically, GBC rates are strikingly variable, with the highest rates in south-central Chile and northern India.2 Although GBC is the most common biliary tract cancer.3 knowledge of GBC etiology remains limited. Gallstones are the most important risk factor for GBC, but little is known about other preventable risk factors that contribute to the development of GBC among patients with gallstones.4
Aflatoxin has been hypothesized to increase risk of GBC.5 Exposure to aflatoxin is associated with increased risk of liver cancer 6, 7 and has been shown to increase cellular proliferation in the biliary tract of monkeys 8-11 and humans.12 The excretion of aflatoxin into bile can lead to high concentrations in the biliary tree,13, 14 and aflatoxin exposure has been associated with gallbladder carcinogenesis in primates.15 We recently demonstrated a strong association between circulating aflatoxin B1 (AFB1)–lysine adducts and GBC in humans.16
We sought to verify and extend this novel association among 250 patients with GBC and 250 patients with gallstones without cancer (controls) from the Shanghai Biliary Tract Cancer Study. We tested for AFB1–lysine adducts, which accumulate up to 30-fold higher with chronic versus single exposure,17 are associated with aflatoxin exposure over the previous 2–3 months,18 and are highly correlated with dietary aflatoxin exposure and DNA adduct formation.19, 20 In a subset of patients with GBC, we also assessed targeted sequencing data for the R249S TP53 mutation. Given that the R249S TP53 mutation has been associated with aflatoxin-related hepatocellular cancer,21, we hypothesized that many tumor samples would contain this aflatoxin-associated mutation.
Methods
As previously described, the Shanghai Biliary Tract Cancer Study enrolled 368 patients with GBC without a previous non-skin cancer who were identified through a rapid-reporting system including the Shanghai Cancer Institute and 42 collaborating hospitals in 10 urban districts of Shanghai 22 from June 1997 through May 2001. Patients with biliary stones (including 774 with gallstones) undergoing cholecystectomy or medical treatment at the same hospital as the index patient with GBC and 959 healthy population-based participants were frequency matched by sex and age (within 5 years). Over 90% of eligible patients with GBC, 95% of eligible patients with gallstones, and 83% of population-based individuals participated.22 The Shanghai Cancer Institute and National Cancer Institute (NCI) institutional review boards approved the study. All participants provided written informed consent for biospecimen and questionnaire data collection.
For the current analysis, we focused on patients with gallstones as the comparison group rather than population-based participants so that we could distinguish between an association with GBC and an association with gallstones since gallstones are the strongest risk factor for GBC (i.e., an association between aflatoxin and GBC compared to population-based participants might reflect an association with gallstones rather than a direct association with GBC). Comparing GBC to gallstone controls allowed us to evaluate whether exposure to aflatoxin was associated with risk of GBC in the context of gallstones. We included plasma samples from 250 patients with GBC and 250 patients with gallstones, which were estimated to provide 80% power to detect an OR greater than or equal to 1.9. These patients were randomly selected from participants with plasma available (267 of the 368 original patients with GBC and 730 of the 774 patients with gallstones). We also included 30 blinded duplicate quality control (QC) plasma samples, 15 from patients with GBC and 15 from patients with gallstones. The laboratory was blinded to case status.
We measured AFB1–lysine adducts in plasma samples using isotope dilution mass spectrometry, as previously described.17 AFB1–lysine adducts levels have been shown to be stable in blood samples stored at -80 degrees C for many years.17 Forty patients with GBC did not have gallstones indicated through questionnaire, medical record abstraction, ultrasound, specimen collection or histology, or a combination of these, and one GBC patient was missing gallstone status. We restricted the main analyses to the 209 patients with GBC who had evidence of gallstones to facilitate comparison to the 250 patients with gallstones but not cancer (controls).
We compared characteristics between patients with GBC and controls using the Kruskal-Wallis test for difference in medians for continuous variables and the χ2 test (Fisher's exact test for variables with fewer than 5 observations in a cell) for categorical variables. We used unconditional logistic regression to estimate age- and sex-adjusted odds ratios (ORs) and 95% CIs for the association of GBC versus gallstones with 1) AFB1–lysine adduct detection [i.e., levels above the limit of detection (≥0.5 pg aflatoxin–lysine/mg albumin) were categorized as 1 and levels below the limit of detection were categorized as 0] and 2) among individuals with detectable AFB1–lysine adducts, AFB1–lysine adduct level (categorized according to quartiles among controls and detectable AFB1–lysine adducts). Agreement for the 30 QC samples (both blinded duplicates detectable or both undetectable) was 77% (coefficient of variation, 32%). We also conducted analyses stratified by sex and investigated modification of the association between aflatoxin exposure and GBC by sex and continuous age using likelihood ratio tests (LRTs) for interaction. We evaluated variables in Table 1 as potential confounders, but none changed the magnitude of the OR for AFB1–lysine adduct detection by >10%. We assessed statistical significance at P < .05 using two-sided tests. Analyses were conducted in SAS version 9.3 (SAS Institute Inc).
Table 1. Characteristics of Patients with Gallbladder Cancer Compared with Patients with Gallstones.
| Gallbladder Cancer (n = 209) | Gallstone patient controls (n = 250) | P Valuea | |
|---|---|---|---|
| Age, median (range), y | 68 (37-74) | 64 (34-74) | <.0001 |
| Female sex, No. (%) | 155 (74.2) | 174 (69.6) | 0.3 |
| Self-reported past body mass index 5 years prior to interview | 0.1 | ||
| Underweight: <18.5 | 18 (8.6) | 17 (6.8) | |
| Normal: 18.5–24.9 | 111 (53.1) | 156 (62.4) | |
| Overweight: 25-29.9 | 69 (33.0) | 68 (27.2) | |
| Obese: >=30.0 | 11 (5.3) | 9 (3.6) | |
| Education, No. (%) | <.0001 | ||
| None | 117 (56.0) | 76 (30.4) | |
| Primary or Junior Middle | 45 (21.5) | 67 (26.8) | |
| Senior Middle | 28 (13.4) | 69 (27.6) | |
| Community College or University | 19 (9.1) | 38 (15.2) | |
| Ever smoking, No. (%)b | 52 (25.0) | 58 (23.2) | 0.7 |
| Ever drinking, No. (%) | 33 (15.8) | 34 (13.6) | 0.5 |
| Acute or chronic cholecystitis, No. (%) | 0.03 | ||
| No | 4 (1.9) | 9 (3.6) | |
| Yes | 85 (40.7) | 127 (50.8) | |
| Missing | 120 (57.4) | 114 (45.6) | |
| Coronary heart disease, No. (%) | 22 (10.5) | 37 (14.8) | 0.2 |
| Tuberculosis, No. (%) | 28 (13.4) | 34 (13.6) | 0.9 |
| Hypertension, No. (%) | 77 (36.8) | 93 (37.2) | 0.9 |
| Diabetes, No. (%) | 30 (14.4) | 40 (16.0) | 0.6 |
| Kidney or bladder infection, No. (%) | 47 (22.5) | 74 (29.6) | 0.1 |
| Chronic gastritis, No. (%)b | 38 (18.2) | 73 (29.3) | 0.006 |
| Gastric ulcer, No. (%)b | 21 (10.1) | 20 (8.0) | 0.5 |
| Duodenal ulcer, No. (%)b | 15 (7.2) | 33 (13.3) | 0.03 |
| Appendicitis, No. (%) | 28 (13.4) | 62 (24.8) | 0.002 |
Calculated with the Kruskal-Wallis test for difference in medians for continuous variables and the χ2 test for categorical variables using Fisher's exact test for variables with fewer than 5 observations in a cell.
Percentages exclude individuals with missing data.
In a subset of 54 patients with GBC with fresh-frozen tumor tissue and sufficient intact double-stranded DNA, we searched for the presence of the somatic aflatoxin-related R249S mutation in TP53 through targeted sequencing of TP53 and additional cancer-related genes (PTEN, HRAS, KRAS, STK11, CDKN2A, and CTNNB1) using a previously validated targeted ion torrent sequencing panel.23 Sequencing was conducted at the NCI Cancer Genomics Research Laboratory. Sequence reads were aligned to the human genome and variants were called using GATK and TVC pipelines. Predicted variants were manually examined in IGV, and the R249S site in TP53 was examined in all tumors. The average depth of the targeted sequencing was greater than 1000 reads.
In addition, we calculated the population attributable fraction (PAF) using the formula PAF = Pe (RRe-1) / [1 + Pe (RRe-1)], where Pe is the prevalence of aflatoxin exposure (as measured by detectable AFB1–lysine adducts among gallstone controls) and RRe is the relative risk (as estimated by the OR) of gallstone-related GBC due to aflatoxin exposure. For the sake of comparison, we also calculated the PAF using the crude OR from the previously reported study of 36 patients with GBC and 29 patients with gallstones (and 47 community controls) from Chile using the Pe among gallstone controls and the crude OR for gallbladder cancer compared to gallstone patient controls.16 Samples from the Chilean study were tested for AFB1–lysine adducts in the same laboratory as the current analysis. We used STATA version 13 (StataCorp LP) to obtain 95% CIs for the PAFs and to explore the impact of using adjusted estimates. We present the crude PAFs since PAFs calculated using adjusted estimates (age and sex for Shanghai and at least weekly ají rojo paste consumption for Chile) were not substantively different (data not shown).
Results
Patients with GBC and evidence of gallstones were slightly older and less educated than controls with gallstones (Table 1). Patients with GBC and controls were generally similar with respect to sex, body mass index, ever smoking, and ever drinking (Table 1).
AFB1–lysine adducts were detectable (i.e., ≥0.5 pg aflatoxin–lysine/mg albumin) in 32% of patients with GBC (67/209) and 15% of controls (37/250) (Table 2). AFB1–lysine adduct detection was nearly three times more likely in patients with GBC compared to controls (OR, 2.71; 95% CI, 1.70–4.33). Among individuals with detectable AFB1–lysine adduct levels, patients with GBC had higher levels [median = 5.4 pg/mg, interquartile range (IQR) = 13.1] than controls (median = 1.2 pg/mg, IQR = 1.4) (Figure 1, Supplemental Figure 1), and the association between aflatoxin and GBC increased notably as AFB1–lysine adduct levels increased, from 1.23 (95% CI, 0.26–5.76) for quartile 2 versus quartile 1 to 7.61 (95% CI, 2.01–28.84) for quartile 4 versus quartile 1 (P-trend = .0006). Results were similar when all 250 patients with GBC were included (OR for detection, 2.96; 95% CI, 1.90–4.61 and OR for quartile 4 versus quartile 1, 9.97; 95% CI, 2.73–36.44, P-trend < .0001). Among the patients with GBC who had detectable AFB1–lysine adducts, the levels similar in those with gallstones (N = 67, median = 5.4) compared to those without gallstones (N = 17, median = 4.2) (Kruskal-Wallis, P = .8).
Table 2. Association of Aflatoxin B1-Lysine Adducts in Patients with Gallbladder Cancer Compared with Patients with Gallstones.
| Aflatoxin B1–Lysine Adduct | No. (%) Gallbladder Cancer | No. (%) Gallstones | OR (95% CI)a |
|---|---|---|---|
| Detection | |||
| No | 142 (68) | 213 (85) | 1.0 |
| Yes | 67 (32) | 37 (15) | 2.71 (1.70-4.33) |
| Among participants with detectable levels | |||
| Quartile 1 | 5 (7) | 10 (27) | 1.0 |
| Quartile 2 | 6 (9) | 9 (24) | 1.23 (0.26-5.76) |
| Quartile 3 | 13 (19) | 8 (22) | 2.86 (0.69-11.85) |
| Quartile 4 | 43 (64) | 10 (27) | 7.61 (2.01-28.84) |
Adjusted for age and sex.
Figure 1.
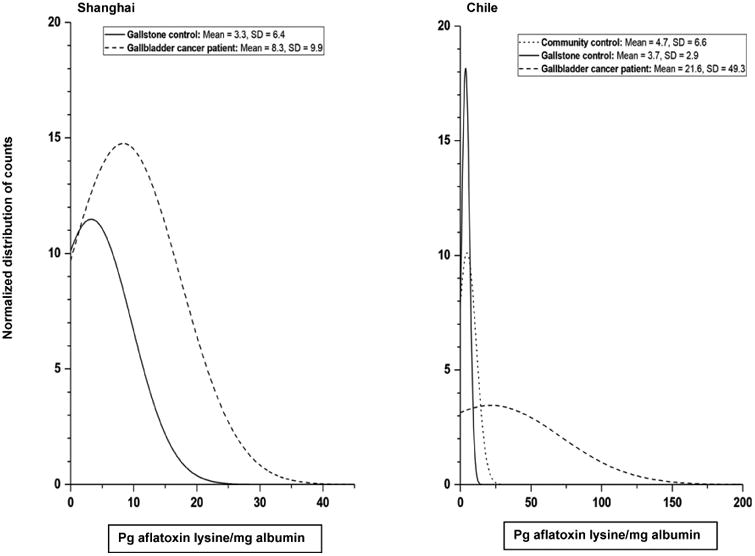
Normalized distribution of detectable (≥0.5 pg aflatoxin–lysine/mg albumin) aflatoxin B1-lysine adduct levels in the A) Shanghai Biliary Tract Cancer Case–control Study and the B) previously-published gallbladder cancer study in Chile.16
Of the 54 male patients with GBC, 12 had detectable levels of AFB1–lysine adducts. The OR for AFB1–lysine adduct detection was 1.64 (95% CI: 0.66–4.08) in males and 3.20 (95% CI: 1.84–5.57) in females, and the OR for quartile 4 versus quartile 1 was 17.19 (95% CI: 0.66–451.09) in men (P-trend = .04) and 7.28 (95% CI: 1.54–34.56) in women (P-trend = .006). In formal testing, sex did not modify the association between aflatoxin and GBC (LRT P = .3 for detection and quartiles). Similarly, age did not modify the association (LRT P = 1.0).
Of the 54 GBC samples evaluated for somatic mutations by targeted sequencing of TP53, PTEN, HRAS, KRAS, STK11, CDKN2A, and CTNNB1, none exhibited mutant R249S reads above background. However, 17 mutations at other locations in TP53 were identified in 16 different tumor samples (29.6%). Eight of these 16 tumors had mutations in at least one of the other genes (Supplemental Figure 2). Three additional tumors had mutations in CDKN2A, CTNNB1, and/or STK11. Mutations did not appear to be related to AFB1–lysine adduct status (Supplemental Figure 2). A previous analysis in the Shanghai Biliary Tract Cancer Study tested a subset of patients with GBC for chronic hepatitis B virus (HBV) infection as measured by the presence of both HBV surface antigen (HBsAg) and IgG antibody to HBV core antigen (anti-HBc), including 44 of the 54 patients with GBC who had sequencing data.24 Of those 44, only 4 (9.1%) had chronic HBV infections.
Finally, we calculated the PAF for aflatoxin using patients with gallstones as a representation of the general population given the similarity of aflatoxin levels that we observed in patients with gallstones and community controls previously.16 Based on the Pe of 15% among gallstone-patient controls and the crude OR for detectable AFB1–lysine adducts of 2.72, the PAF for cancer related to aflatoxin was 20% (95% CI: 15%–25%). In the previous Chilean study, the Pe for gallstone-patient controls was 24% and the crude OR for AFB1–lysine adduct detection and GBC versus gallstone-patient controls was 5.56.16 The PAF was 52% (95% CI: 38%–63%) in the Chile study.
Discussion
In this study, we showed that patients with GBC were three times more likely to have detectable levels of AFB1–lysine adducts than gallstone-patient controls, and the risk increased with increasing AFB1–lysine adduct levels. This finding, from a region with low to moderate rates of GBC, reinforces a previous report from Chile, a high-risk area for GBC, suggesting that aflatoxin exposure may contribute to this disease in both low- and high-risk regions. However, the mechanism by which aflatoxin might cause GBC is unknown. We did not identify any patients with GBC with the R249S mutation in TP53, suggesting that this mutation may not be a major mechanism of aflatoxin-related carcinogenesis in the gallbladder. If future studies demonstrate a causal relationship between aflatoxin and GBC, then the results of this study suggest that 20% of GBC tumors that developed during the time the Shanghai Biliary Tract Cancer Study was conducted (June 1997—May 2001) may have been prevented if aflatoxin exposure had been completely eliminated.
Aflatoxin has been long established as a risk factor for hepatocellular carcinoma.6 Previous studies support the biologically plausibility of an association between aflatoxin and GBC as well.5 In a primate study, six of 35 (17%) monkeys that received AFB1 for ≥ 2 months developed gallbladder or bile duct tumors over the course of 13 years.15 In addition, exposure to aflatoxin has been shown to increase cellular proliferation in the biliary tract in monkeys 8-11 and humans.12 Furthermore, a Danish occupational exposure study found that livestock feed production workers, with possible work exposures of 170ng AFB1/day, had notably elevated risk of gallbladder and bile duct cancer compared to all employees in Denmark (standardized proportional incidence ratio: 219, 95% CI: 89–455).25 Recently, the hypothesis was directly evaluated for the first time in a small case–control study in Chile,16 which found strong associations between aflatoxin and GBC compared to patients with gallstones and population-based controls.
Although we hypothesized that patients with GBC exposed to aflatoxin would have the R249S mutation in TP53, we did not find any R249S mutations in the subset of patients with GBC evaluated through sequencing. Even in the liver, however, the mechanism by which the R249S p53 protein may cause liver cancer are unclear,21 and some evidence suggests that the R249S mutation is not sufficient for carcinogenesis without biologic interaction with other risk factors, particularly the hepatitis B virus HBx protein.21, 26-29 In our study, less than 10% of the patients with GBC had chronic HBV infection. However, 17 somatic mutations in TP53 were identified in 16 different tumor samples, consistent with previous studies finding TP53 as the most commonly mutated gene in GBC.30
If aflatoxin causes GBC, this novel association with GBC may have important implications. First, aflatoxin abatement programs may decrease the burden of this disease in high-risk areas, as has been observed for hepatocellular carcinoma.31 Interestingly, gallbladder and biliary tract cancer mortality in China decreased by 22.5% from 1990 to 2013.32 This decline parallels the reduction in exposure to aflatoxin observed in China with the advent of aflatoxin abatement programs; in areas with high aflatoxin exposure, the median aflatoxin–albumin adduct level decreased from 19.3 pg/mg albumin in 1989, to 2.3 in 1999, to undetectable (i.e., <0.5 pg/mg) in 2009-2012.31 Samples from the Shanghai Biliary Tract Cancer Study were collected from June 1997 through May 2001 during the middle of the time period when aflatoxin exposure was declining, and our PAF estimate of 20% is consistent with the 22.5% decrease in gallbladder and biliary tract cancer mortality in China from 1990 to 2013.
In Chile, however, concern over aflatoxin exposure has only recently developed. The AFB1–lysine adduct levels were somewhat higher in the previously published study in Chile than in Shanghai (Figure 1); among those with detectable levels in Chile, the median was 7.6 pg/mg for patients with GBC and 3.5 pg/mg for controls with gallstones,16 as opposed to 5.4 pg/mg and 1.2 pg/mg in Shanghai. Thus, the proportion of gallbladder cancers that could potentially be due to aflatoxin exposure might be even higher in Chile. If a causal role of aflatoxin in gallbladder carcinogenesis is verified, an aflatoxin abatement program could help reduce the incidence of GBC in Chile. Given that the PAF for gallstones has been estimated at 80%,22 reducing the prevalence of gallstones would also have a major impact. Although much work remains to be done to establish the role of aflatoxin in gallbladder carcinogenesis, if aflatoxin causes GBC, the situation may be analogous to that of HBV and aflatoxin in China, where the greatest reduction in liver cancer risk came from programs that targeted both HBV and aflatoxin exposure.31 The potential role of aflatoxin in gallbladder carcinogenesis remains to be investigated for other high-risk areas, such as Northern India.
This study has limitations. Since aflatoxin contamination has declined in China 31 and aflatoxin-albumin levels capture exposure over the previous few months,18 individuals who were exposed prior to the decline but not recently exposed would have been classified as unexposed but may have had aflatoxin-induced carcinogenic changes from prior exposures. Such misclassification would bias risk estimates toward the null. Correspondingly, the magnitude of the association for aflatoxin and GBC versus gallstones was 2.7 in Shanghai but more than twice as high in Chile,16 which may reflect bias toward the null. The lower magnitude of effect may also reflect lower levels of exposure since these samples were collected during the reduction in aflatoxin exposure in China. Furthermore, the coefficient of variation was relatively high (32%), which could have led to inaccurate exposure assessment that may have biased estimates toward the null. Exposure misclassification may explain we did not see any obvious relationship between somatic mutations and AFB1–lysine adducts. Genomic sequencing in a sufficient number of patients with GBC is an important next step to identify potential molecular subtypes that may be related to aflatoxin. In addition, we used patients with gallstones to represent the general population for the PAF calculations. Although data are limited, the levels of aflatoxin exposure were similar between patients with gallstones and population-based controls in the previous study conducted in Chile.16 Thus, we feel that the aflatoxin exposure in the gallstone controls is reasonably representative of that in the general population and that the PAF is therefore appropriate. Recall bias may have affected self-reported variables but not measurement of AFB1–lysine adducts. Given the cross-sectional nature of these data, we cannot rule out the possibility that the presence of the cancer may have somehow affected AFB1–lysine adduct detection. Future studies may provide additional support by evaluating AFB1–DNA adducts. Ultimately, longitudinal efforts are needed.
This study also has a number of strengths. It was designed specifically to evaluate the association between aflatoxin and GBC, and since it was well-powered, it clearly demonstrated the emerging role of aflatoxin in gallbladder carcinogenesis. In addition, the study had high GBC and gallstone patient ascertainment and participation, minimizing the potential for selection bias. By comparing patients with GBC to patients with gallstones, we were also able to focus on the key transition in gallbladder carcinogenesis from gallbladder inflammation, usually precipitated by gallstones,30 to the development of cancer.
These findings of a strong association between aflatoxin and GBC suggest that aflatoxin may be an important risk factor for GBC, which may have important public health implications. This study is replicated the initial report from Chile in an independent study population from an entirely different part of the world. The lack of R249S mutations suggests that more work is needed to understand the mechanisms by which aflatoxin may cause cancer, particularly outside of the context of HBV infection. If aflatoxin is causally related to GBC, aflatoxin abatement programs could help reduce the public health burden of GBC in high-risk areas, such as Chile, just as it has with liver cancer.
Supplementary Material
Supplemental Figure 1. Histogram of detectable (≥0.5 pg aflatoxin–lysine/mg albumin) aflatoxin B1-lysine adduct levels in A) the Shanghai Biliary Tract Cancer Case–control Study and B) the previously-published gallbladder cancer study in Chile.16
Supplemental Figure 2. Oncoprinta of gene mutations and aflatoxin B1-lysine adduct detection status (≥0.5 pg aflatoxin–lysine/mg albumin) in 54 gallbladder cancer cases with targeted sequencing from the Shanghai Biliary Tract Cancer Case–control Study.
Acknowledgments
The authors wish to thank the collaborating surgeons and pathologists in Shanghai for assistance in patient recruitment and pathology review; Chia–Rong Cheng, Lu Sun, and Kai Wu of the Shanghai Cancer Institute for coordinating data and specimen collection; and Shelley Niwa and Karen Pettit of Westat for support with study and data management. In addition, the authors thank David Check for creating the figures. This work was supported by general funds from the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics and the Office of Research on Women's Health, National Institutes of Health.
Footnotes
Author Involvement: Study concept and design (Koshiol, Gao, Wang, Ferreccio, Shen, Hildesheim, Hsing); acquisition of data (Koshiol, Gao, Dean, Egner, Jones, Wang, Rashid, Luo, Ferreccio, Malasky, Shen, Hsing, Groopman); statistical analysis (Koshiol, Van Dyke, Zhu); interpretation of data (Koshiol, Gao, Dean, Egner, Nepal, Rashid, Van Dyke, Ferreccio, Zhu, Andersen, Hildesheim, Hsing, Groopman); drafting of manuscript (Koshiol); critical review and revision of manuscript (Koshiol, Gao, Dean, Egner, Nepal, Jones, Wang, Rashid, Luo, Van Dyke, Ferreccio, Malasky, Shen, Zhu, Andersen, Hildesheim, Hsing, Groopman).
Conflicts of Interest: No authors have any relevant conflicts of interest.
Author names in bold designate shared co-first authorship.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Key C, Meisner ALW. Cancers of the Liver and Biliary Tract. In: Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner MJ, editors. SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988-2001, Patient and Tumor Characteristics. 07-6215. Bethesda: NIH; 2007. [Google Scholar]
- 2.Koshiol J, Ferreccio C, Devesa SS, et al. Biliary Tract Cancers. In: Thun MJ, Linet MS, Cerhan JR, Haiman C, editors. Cancer Epidemiology and Prevention. 4th. Oxford University Press; 2017. in press. [Google Scholar]
- 3.Castro FA, Koshiol J, Hsing AW, et al. Biliary tract cancer incidence in the United States-Demographic and temporal variations by anatomic site. Int J Cancer. 2013;133:1664–71. doi: 10.1002/ijc.28161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wistuba II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer. 2004;4:695–706. doi: 10.1038/nrc1429. [DOI] [PubMed] [Google Scholar]
- 5.Foerster C, Koshiol J, Guerrero AR, et al. The case for aflatoxins in the causal chain of gallbladder cancer. Med Hypotheses. 2016;86:47–52. doi: 10.1016/j.mehy.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aflatoxins A review of human carcinogens Part F: Chemical agents and related occupations. 100F. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; Lyon, France: IARC; 2012. pp. 225–244. [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect. 2010;118:818–24. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuthbertson WF, Laursen AC, Pratt DA. Effect of groundnut meal containing aflatoxin on Cynomolgus monkeys. Br J Nutr. 1967;21:893–908. doi: 10.1079/bjn19670089. [DOI] [PubMed] [Google Scholar]
- 9.Deo MG, Dayal Y, Ramalingaswami V. Aflatoxins and liver injury in the rhesus monkey. J Pathol. 1970;101:47–56. doi: 10.1002/path.1711010106. [DOI] [PubMed] [Google Scholar]
- 10.Madhavan TV, Tulpule PG, Gopalan C. Aflatoxin-Induced Hepatic Fibrosis in Rhesus Monkeys. Pathological Features. Arch Pathol. 1965;79:466–9. [PubMed] [Google Scholar]
- 11.Wogan GN. Chemical nature and biological effects of the aflatoxins. Bacteriol Rev. 1966;30:460–70. doi: 10.1128/br.30.2.460-470.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnamachari KA, Bhat RV, Nagarajan V, et al. Hepatitis due to aflatoxicosis. An outbreak in Western India. Lancet. 1975;1:1061–3. doi: 10.1016/s0140-6736(75)91829-2. [DOI] [PubMed] [Google Scholar]
- 13.Emerole GO. Excretion of aflatoxin B1 as a glutathione conjugate. Eur J Drug Metab Pharmacokinet. 1981;6:265–8. doi: 10.1007/BF03189524. [DOI] [PubMed] [Google Scholar]
- 14.Harland EC, Cardeilhac PT. Excretion of carbon-14-labeled aflatoxin B1 via bile, urine, and intestinal contents of the chicken. Am J Vet Res. 1975;36:909–12. [PubMed] [Google Scholar]
- 15.Sieber SM, Correa P, Dalgard DW, et al. Induction of osteogenic sarcomas and tumors of the hepatobiliary system in nonhuman primates with aflatoxin B1. Cancer Res. 1979;39:4545–54. [PubMed] [Google Scholar]
- 16.Nogueira L, Foerster C, Groopman J, et al. Association of aflatoxin with gallbladder cancer in Chile. JAMA. 2015;313:2075–7. doi: 10.1001/jama.2015.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholl PF, Groopman JD. Long-term stability of human aflatoxin B1 albumin adducts assessed by isotope dilution mass spectrometry and high-performance liquid chromatography-fluorescence. Cancer Epidemiol Biomarkers Prev. 2008;17:1436–9. [Google Scholar]
- 18.Sabbioni G, Ambs S, Wogan GN, et al. The aflatoxin-lysine adduct quantified by high-performance liquid chromatography from human serum albumin samples. Carcinogenesis. 1990;11:2063–6. doi: 10.1093/carcin/11.11.2063. [DOI] [PubMed] [Google Scholar]
- 19.Wild CP, Hasegawa R, Barraud L, et al. Aflatoxin-albumin adducts: a basis for comparative carcinogenesis between animals and humans. Cancer Epidemiol Biomarkers Prev. 1996;5:179–89. [PubMed] [Google Scholar]
- 20.Wild CP, Hudson GJ, Sabbioni G, et al. Dietary intake of aflatoxins and the level of albumin-bound aflatoxin in peripheral blood in The Gambia, West Africa. Cancer Epidemiol Biomarkers Prev. 1992;1:229–34. [PubMed] [Google Scholar]
- 21.Gouas D, Shi H, Hainaut P. The aflatoxin-induced TP53 mutation at codon 249 (R249S): biomarker of exposure, early detection and target for therapy. Cancer Lett. 2009;286:29–37. doi: 10.1016/j.canlet.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 22.Hsing AW, Gao YT, Han TQ, et al. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer. 2007;97:1577–82. doi: 10.1038/sj.bjc.6604047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lou H, Villagran G, Boland JF, et al. Genome Analysis of Latin American Cervical Cancer: Frequent Activation of the PIK3CA Pathway. Clin Cancer Res. 2015;21:5360–70. doi: 10.1158/1078-0432.CCR-14-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsing AW, Zhang M, Rashid A, et al. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. Int J Cancer. 2008;122:1849–53. doi: 10.1002/ijc.23251. [DOI] [PubMed] [Google Scholar]
- 25.Olsen JH, Dragsted L, Autrup H. Cancer risk and occupational exposure to aflatoxins in Denmark. Br J Cancer. 1988;58:392–6. doi: 10.1038/bjc.1988.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denissenko MF, Koudriakova TB, Smith L, et al. The p53 codon 249 mutational hotspot in hepatocellular carcinoma is not related to selective formation or persistence of aflatoxin B1 adducts. Oncogene. 1998;17:3007–14. doi: 10.1038/sj.onc.1202214. [DOI] [PubMed] [Google Scholar]
- 27.Gouas DA, Shi H, Hautefeuille AH, et al. Effects of the TP53 p.R249S mutant on proliferation and clonogenic properties in human hepatocellular carcinoma cell lines: interaction with hepatitis B virus X protein. Carcinogenesis. 2010;31:1475–82. doi: 10.1093/carcin/bgq118. [DOI] [PubMed] [Google Scholar]
- 28.Jiang W, Wang XW, Unger T, et al. Cooperation of tumor-derived HBx mutants and p53-249(ser) mutant in regulating cell proliferation, anchorage-independent growth and aneuploidy in a telomerase-immortalized normal human hepatocyte-derived cell line. Int J Cancer. 2010;127:1011–20. doi: 10.1002/ijc.25118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villar S, Ortiz-Cuaran S, Abedi-Ardekani B, et al. Aflatoxin-induced TP53 R249S mutation in hepatocellular carcinoma in Thailand: association with tumors developing in the absence of liver cirrhosis. PLoS One. 2012;7:e37707. doi: 10.1371/journal.pone.0037707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espinoza JA, Bizama C, Garcia P, et al. The inflammatory inception of gallbladder cancer. Biochim Biophys Acta. 2016;1865:245–54. doi: 10.1016/j.bbcan.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen JG, Egner PA, Ng D, et al. Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prev Res (Phila) 2013;6:1038–45. doi: 10.1158/1940-6207.CAPR-13-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387:251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Histogram of detectable (≥0.5 pg aflatoxin–lysine/mg albumin) aflatoxin B1-lysine adduct levels in A) the Shanghai Biliary Tract Cancer Case–control Study and B) the previously-published gallbladder cancer study in Chile.16
Supplemental Figure 2. Oncoprinta of gene mutations and aflatoxin B1-lysine adduct detection status (≥0.5 pg aflatoxin–lysine/mg albumin) in 54 gallbladder cancer cases with targeted sequencing from the Shanghai Biliary Tract Cancer Case–control Study.


