Abstract
Gastric cancer is a leading cause of cancer-related mortality worldwide, and options to treat gastric cancer are limited. Fluorouracil (5Fu)-based chemotherapy is frequently used as a neoadjuvant or an adjuvant agent for gastric cancer therapy. Most patients with advanced gastric cancer eventually succumb to the disease despite the fact that some patients respond initially to chemotherapy. Thus, identifying molecular mechanisms responsible for chemotherapy resistance will help design novel strategies to treat gastric cancer. In this study, we discovered that residual cancer cells following 5Fu treatment have elevated expression of hedgehog target genes GLI1 and GLI2, suggestive of hedgehog (Hh) signaling activation. Hh signaling, a pathway essential for embryonic development, is an important regulator for putative cancer stem cells/ residual cancer cells. We found that high GLI1/GLI2 expression is associated with some features of putative cancer stem cells, such as increased side population. We demonstrated that GLI2 knockdown sensitized gastric cancer cells to 5Fu treatment, decreased ABCG2 expression, and reduced side population. Elevated GLI2 expression is also associated with an increase in tumor sphere size, another marker for putative cancer stem cells. We believe that GLI2 regulates putative cancer stem cells through direct regulation of ABCG2. ABCG2 can rescue the Gli2 shRNA effects in 5Fu response, tumor sphere formation and side population changes, suggesting that ABCG2 is an important mediator for GLI2-associated 5Fu resistance. The relevance of our studies to gastric cancer patient care is reflected by our discovery that high GLI1/GLI2/ABCG2 expression is associated with a high incidence of cancer relapse in two cohorts of gastric cancer patients who underwent chemotherapy (containing 5Fu). Taken together, we have identified a molecular mechanism by which gastric cancer cells gain 5Fu resistance.
Keywords: 5Fu, Gastric cancer, ABCG2, Hedgehog, Gli2
1. INTRODUCTION
Gastric cancer is the third leading cause of cancer-related deaths, with nearly 1 million cases a year and about 700,000 deaths worldwide ( McLean and El-Omar, 2014; Razzak, 2014; Ferlay et al., 2015; Torre et al., 2015). The incidence of gastric cancer varies significantly in different geographic regions, with about 70% of the incidence in Eastern Asia. As a major solid tumor, gastric cancer development involves complex genetic and environment interactions.
Chemotherapeutic intervention is commonly used in the neoadjuvant, adjuvant, or primary treatment options of advanced gastric cancer whereas surgical resection is the curable therapy for early cancer (Cunningham et al., 2006). Despite the decline in gastric cancer incidence in the last few decades, the percentage of gastric cancer with metastasis has been on the rising between 1990 and 2011 (Bernards et al., 2013). The median survival of advanced gastric cancer remains 15–17 months (Orditura et al., 2014; Proserpio et al., 2014). While some patients respond initially to chemotherapy, almost all advanced gastric cancer patients eventually develop relapsed disease. Therefore, drug resistance is a major barrier to achieve effective gastric cancer treatment.
The first-line chemotherapy of gastric cancer includes 5Fu, cisplatin, and epirubicin according to NCCN guidelines (Ajani et al., 2013; Yuan et al., 2014). Furthermore, improved chemotherapeutic agents, including taxanes (docetaxel and paclitaxel), oral fluoropyrimidines (capecitabine and S-1), oxaliplatin and irinotecan are emerging.
Fluorouracil (5Fu), an analog of uracil with a fluorine atom substituted at the carbon-5 position of the pyrimidine ring in place of hydrogen, fulfills the expectations of biochemical, pharmacologic, and clinical activities of anticancer drugs. The 5-fluorinated pyrimidines have been widely used in the treatment of breast, gastric, colorectal, pancreatic cancers, and squamous cell carcinomas arising in the head and neck (Papanastasopoulos and Stebbing, 2014). The primary mechanisms of action for 5Fu include 1) incorporation of fluorouridine triphosphate into RNA to interfere with RNA synthesis and function, 2) inhibition of thymidylate synthase, 3) incorporation of fluorodeoxyuridine triphosphate and deoxyuridine triphosphate into DNA, and 4) genotoxic stress to trigger programmed cell death pathways.
Resistance to 5Fu in gastric cancer is a major clinical problem. While there are a number of mechanisms reported to be responsible for drug resistance (Domingo-Domenech et al., 2012; Keysar et al., 2013; Zahreddine et al., 2014; Della Corte et al., 2015; Alonso et al., 2016), activation of hedgehog (Hh), wnt and notch signaling pathways is particularly appealing as it is consistent with the “cancer stem cell (CSC)” theory (Yang et al., 2010; Nozawa et al., 2013; Basset-Seguin et al., 2015). Like wnt and notch signalings, Hh signaling is an important regulator for embryonic development, tissue polarity, cell differentiation and cancer development (Feldmann et al., 2007; Domingo-Domenech et al., 2012; Steg et al., 2012a, b; Zahreddine et al., 2014). Thus, specific inhibitors for these signaling pathways may be used to sensitize cancer cells to 5Fu treatment. However, the significance of Hh signaling (or wnt and notch signalings) for 5Fu resistance in gastric cancer has not been well established.
To elucidate the underlying mechanism for 5Fu resistance in gastric cancer, we evaluated target gene expression of the Hh pathway in the residual cells following 5Fu treatment. We discovered the activation of the Hh-ABCG2 signaling axis in 5Fu-treated cells. We further demonstrated the significance of Hh signaling for regulation of side population. Similar studies were also conducted using acquired 5Fu resistant cell line N87/FR. We demonstrated the significance of GLI2 for 5Fu resistance. This mechanistic study may help develop effective strategies against 5Fu resistance in gastric cancer.
2. RESULTS
2.1. 5Fu treatment influences Hh signaling and CSC properties in gastric cancer
Based on cancer stem cell theory (Wang and Dick, 2005; Cho and Clarke, 2008), we predicted that residual cancer cells after chemotherapy may contain high levels of drug resistant genes. We first measured the half maximal inhibitory concentration (IC50) for 5Fu in gastric cancer N87 cells based on the cell viability in the presence of different concentrations of 5Fu (Fig. 1A). We then treated N87 cells with 5 µM of 5Fu (about the IC50 dose) for 48 h, and examined expressions of GLI1 and GLI2, two important transcriptional factors for the Hh signaling pathway. We found elevated expressions of GLI1 and GLI2 in 5Fu-treated cells. In particularly, the relative level of GLI2 expression in the treated cells was over 15 times higher than that of the untreated control cells (Fig. 1B), suggesting that cells with activated Hh signaling survive better after 5Fu treatment. The elevated expressions of GLI1 and GLI2 appear to be specific to 5Fu treatment because we did not see significant changes in many other genes including JAG2 and TGFβ2 (data not shown here).
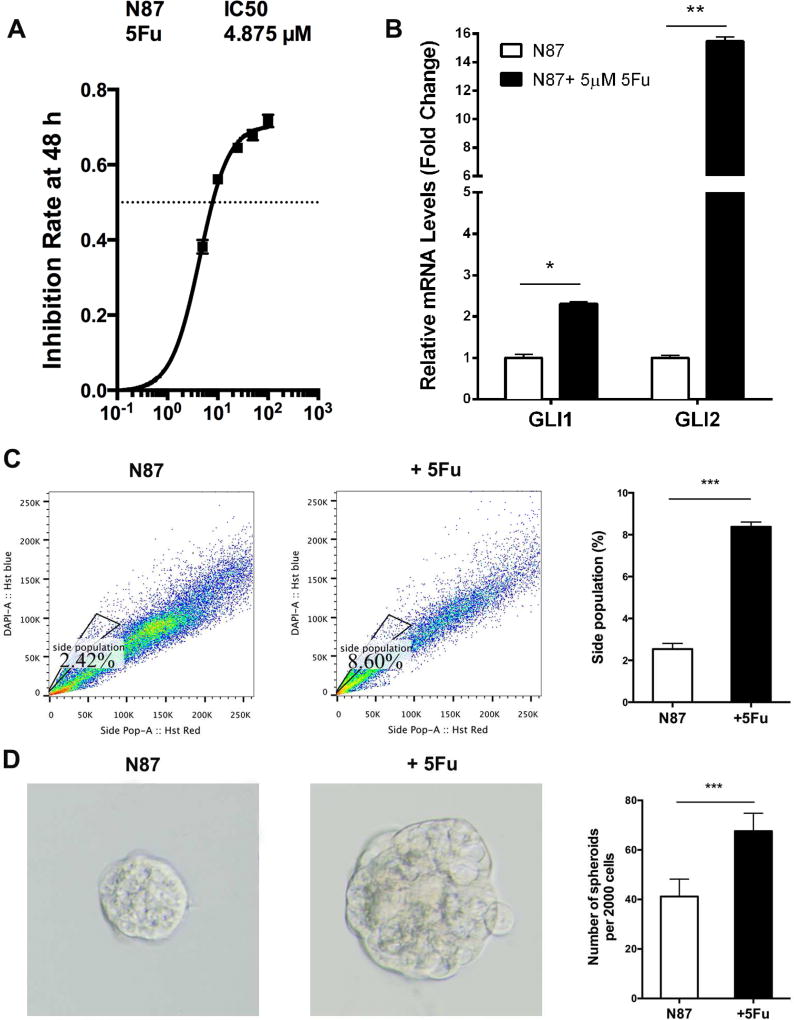
Fig. 1. Intrinsic response of gastric cancer cells to 5Fu.
A: The IC50 dose of 5Fu was calculated from measurement of the inhibition of cell viability by different concentrations of 5Fu (48 h). The X-axis indicates 5Fu concentration (µM), and the Y-axis indicates the reduction of cell viability. B: qPCR analysis of GLI1 and GLI2 in N87 cells with or without 5Fu (5 µM) treatment. C: Side population detected by flow cytometry after staining with Hoechst 33342. The average value from three independent experiments on side population was shown on the right. D: The average diameter of tumor spheres was measured from over 50 tumor spheres, and the typical morphology was shown on the left. Significant difference was indicated by * (P < 0.05), ** (P < 0.005), or *** (P < 0.0005).
Because Hh signaling is critical for the maintenance of putative cancer stem cells or residual cancer cells (Reya et al., 2001; Taipale and Beachy, 2001; Takebe et al., 2015), we first examined several features of putative cancer stem cells (Fukamachi et al., 2011; Ishimoto et al., 2011; Wang et al., 2011; Chen et al., 2012; Jiang et al., 2012a; Chen et al., 2013; Dong et al., 2013; Rassouli et al., 2016) following 5Fu treatment in N87 cells. The side population is often enriched in cancer stem cells, and ABCG2 is the major gene regulating side population (Jiang et al., 2012b). We found that 5Fu treatment for N87 cells caused an increase of side population (2.42% for untreated cells and 8.60% for treated cells) (Fig. 1C), indicating that residual cancer cells (or putative cancer stem cells) are enriched after 5Fu treatment. This result is consistent with that the elevated expressions of GLI1 and GLI2 following 5Fu treatment.
Tumor sphere formation is a known biological readout of cancer stem cells (Weiswald et al., 2015). We found that the tumor spheres (secondary) formed from the 5Fu-treated N87 cells are much bigger than that of the control cells (Fig. 1D), implying the high cancer stem cell activity after 5Fu treatment.
Both side population and tumor sphere size were increased following 5Fu treatment in N87 cells, indicating that elevated Hh signaling may be responsible for the maintenance of residual cancer cells (or putative cancer stem cells) following 5Fu treatment in gastric cancer cells.
2.2. 5-Fu resistant N87 cell keeps high level of Hh signaling and CSC properties
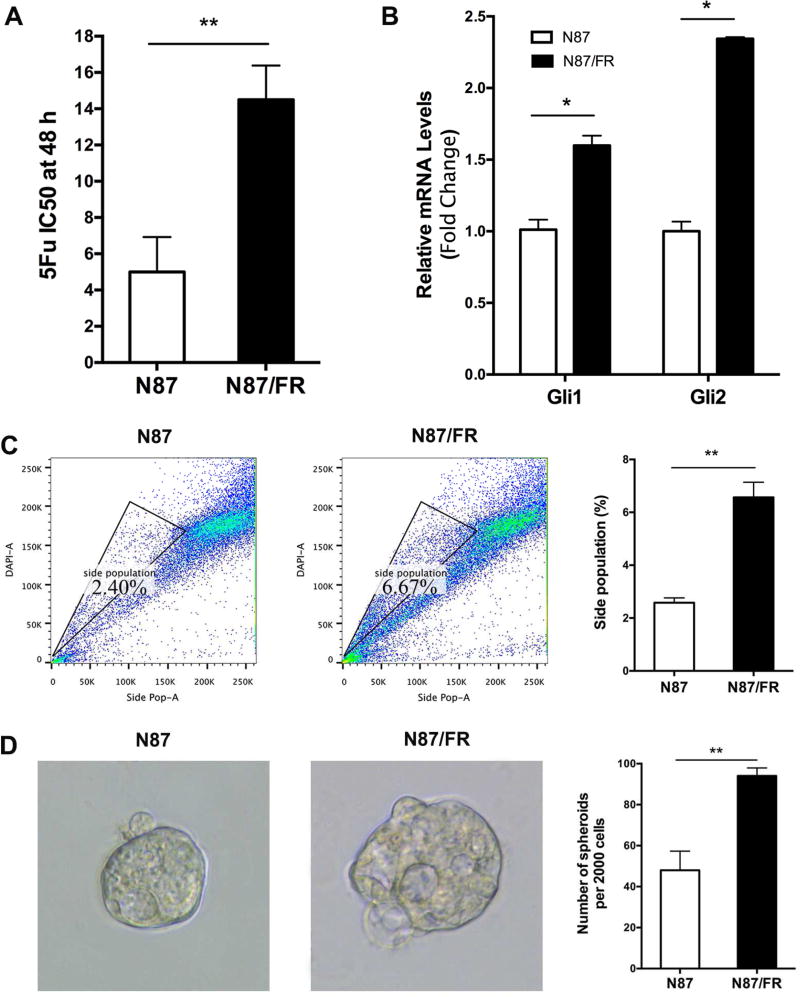
Elevated Hh signaling in cells after 5Fu treatment indicates the role of Hh signaling for intrinsic drug resistance in N87 cells. In the meantime, we established an acquired 5Fu resistant cell line, N87/FR, from the parental N87 cells through many rounds of 5Fu treatment with increased concentrations of 5Fu. N87/FR has an IC50 of more than 15 µM, whereas the parental cells have an IC50 of about 5 µM (Fig. 2A). We also examined gene expressions of GLI1 and GLI2, and found elevated levels of GLI1 and GLI2 in N87/FR in comparison with the parental N87 cells, indicating activated Hh signaling in 5Fu resistant cells (Fig. 2B). Similarly, we found that N87/FR has a higher percentage of side population, and significantly larger tumor spheres in comparison with N87 cells (Fig. 2C and D).
Fig. 2. Analysis of acquired 5Fu resistant cells.
A: The IC50 of 5Fu in parental and acquired resistant N87/FR cells was measured following 5Fu treatment at different concentrations. B: GLI1 and GLI2 expressions were measured by qPCR. C: Side population was detected after flow cytometry, and the average value was shown on the right (from three independent experiments). D: Tumor sphere morphology was shown on the left, and the diameter were calculated from over 50 tumor spheres formed from the corresponding cells. Significant difference was indicated by * (P < 0.05), ** (P < 0.005), or *** (P < 0.0005).
Thus, elevated Hh signaling appears to be associated with an increased side population and bigger tumor spheres in both situations: after 5Fu treatment or in acquired resistant cells. The former represents intrinsic resistance to 5Fu, whereas the latter represents acquired resistance to 5Fu.
2.3. Knockdown of GLI2 affects the CSC properties
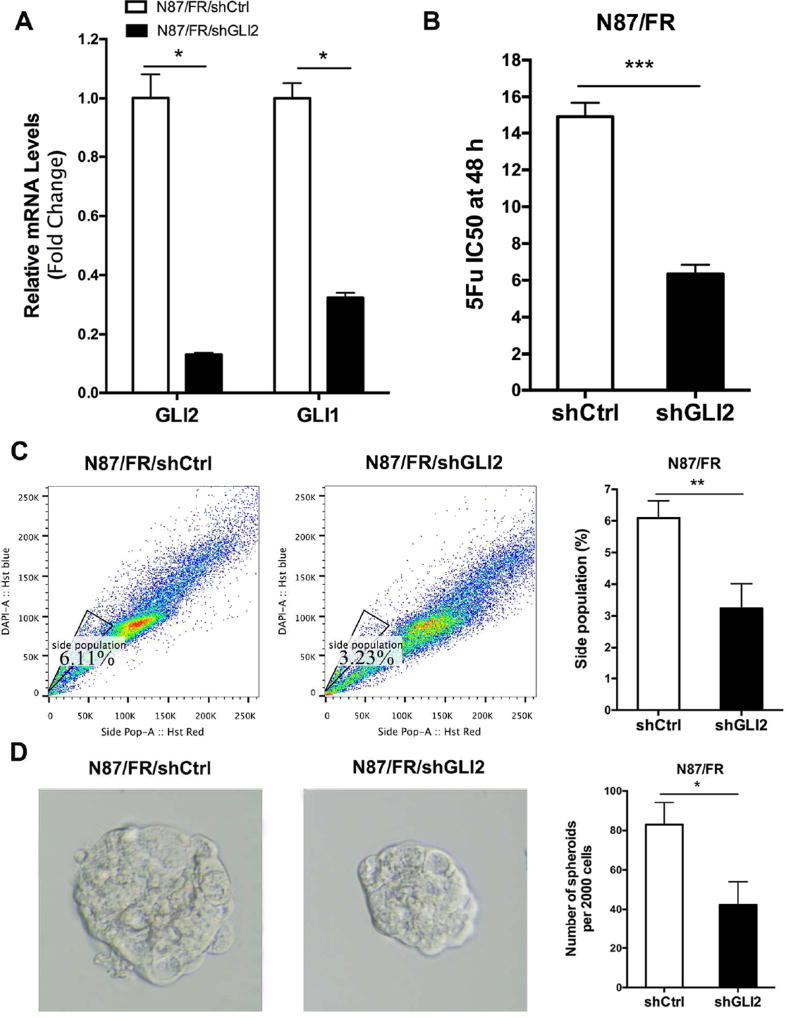
To evaluate the functional relevance of Hh signaling for drug resistance in N87 cells, we first knocked down GLI2 expression by expressing GLI2 shRNAs (shGLI2) in the 5Fu resistant N87/FR cells. We discovered that knocking down GLI2 not only reduced GLI2 expression by 80%, but also decreased GLI1 transcript (>70%) (Fig. 3A), which is consistent with the notion that GLI2 regulates GLI1 expression in a number of biological systems (Motoyama et al., 1998; Ikram et al., 2004; Li et al., 2012). Down-regulation of GLI2 in N87/FR cells reduced the IC50 from 15 µM to about 6 µM (Fig. 3B), which is similar to the IC50 of the parental N87 cells.
Fig. 3. The effect of GLI2 knockdown on 5Fu response in N87/FR cells.
A: The detection of GLI1 and GLI2 expressions after shGLI2 knockdown by qPCR. B: The effect of shGLI2 on the IC50 of 5Fu was measured using the same method shown in Fig.1B. C: Change of side population by shGLI2 after 5Fu treatment, and the average change was shown on the right. D: Change of tumor sphere size by shGLI2. The average value was shown on the right (from three independent experiments). Significant difference was indicated by * (P < 0.05), ** (P < 0.005), or *** (P < 0.0005).
In consistent with reduced expressions of GLI1 and GLI2 after GLI2 shRNA expression, we found that GLI2 shRNA also reduced the side population (6.11% in N87/FR and 3.23% in N87/FR/shGLI2) (Fig. 3C). We also found that GLI1 knockdown significantly reduced the size of tumor spheres (Fig. 3D).
These data indicate that GLI2 is a major trigger for the 5Fu resistance in gastric cancer cells, probably through regulation of residual cancer cells/cancer stem cells.
2.4. Regulation of ABCG2 by Hh signaling in 5Fu resistance cells in gastric cancer
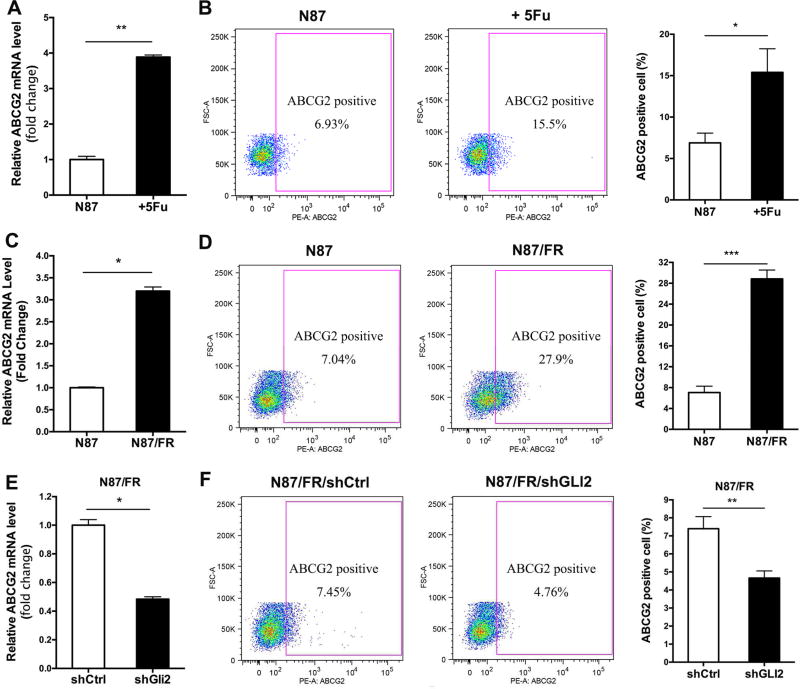
It is known that ABCG2 is responsible for exclusion of Hoechst 33342 dye and the subsequent formation of side population. We examined ABCG2 expression following 5Fu treatment, and found that 5Fu treatment caused an increase of ABCG2 expression in parental N87 cells (both transcript and protein) (Fig. 4A and B). Similarly, acquired resistant N87/FR cells have elevated ABCG2 expression (Fig. 4C and D).
Fig. 4. The effect of shGLI2 and 5Fu on ABCG2 expression.
A: qPCR analysis of ABCG2 after 5Fu treatment in N87 cells. B: Flow cytometry analysis of surface ABCG2 protein expression. The average positivity from there independent experiments was shown on the right. C and D: Comparison of 5Fu resistant N87/FR cells with the parental N87 cells on ABCG2 expression (transcript level shown on the left; and cell surface expression shown on the right). E and F: The effect of GLI2 knockdown on ABCG2 transcript (left) and on cell surface expression (right). Significant difference was indicated by * (P < 0.05), ** ( P < 0.005), or *** (P < 0.0005).
Conversely, shGLI2 significantly reduced ABCG2 expression, at both the RNA level and the protein level in N87/FR cells (Fig. 4E and F), indicating that ABCG2 is regulated by GLI2 in gastric cancer cells.
We believe that GLI1/2 transcription factors can direct ABCG2 expression through transcriptional regulation. First, a GLI-binding consensus site (Yang et al., 2010) appears in the ABCG2 promoter (data not shown), suggesting that ABCG2 may be a transcriptional target of GLI molecules. Furthermore, previous studies in lymphomas showed that GLI proteins may directly regulate ABCG2 expression by transcriptional regulation (Singh et al., 2011). We have obtained similar results in gastric cancer cells (unpublished data).
From these data, we conclude that GLI2 can directly regulate ABCG2 expression in gastric cancer cells, leading to an increase in side population.
2.5. Overexpression of ABCG2 rescues the CSC properties
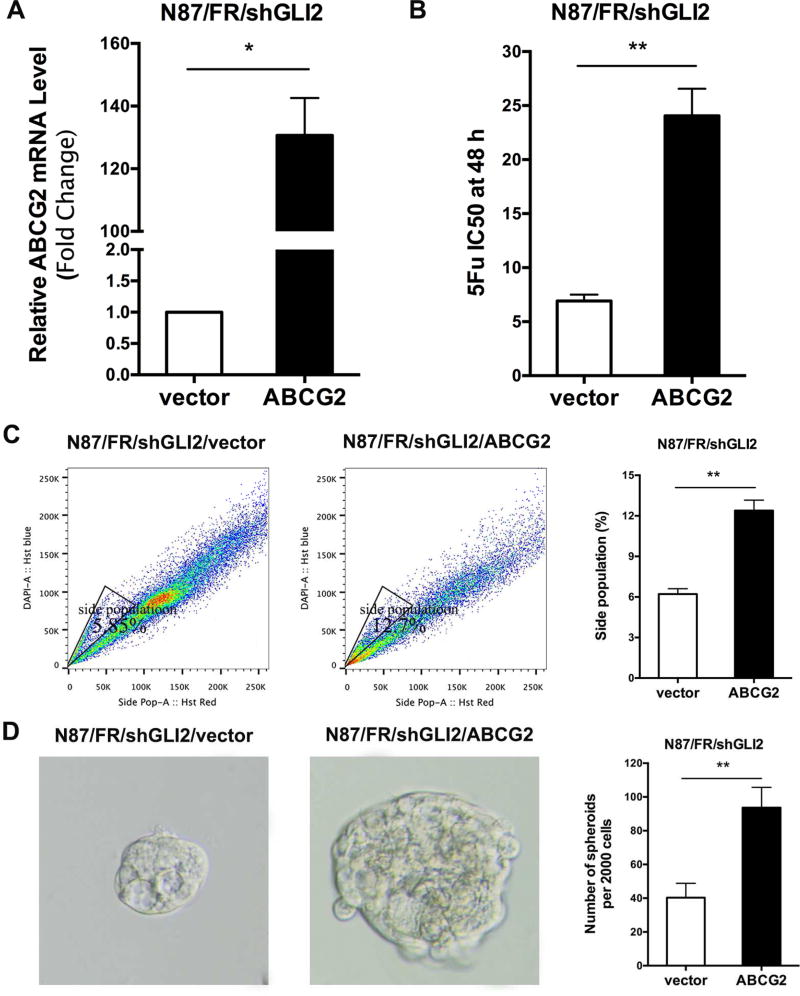
We predict that if ABCG2 is the major mediator for GLI2-associated 5Fu resistance, ectopic expression of ABCG2 in shGLI2-expressing N87/FR cells should increase drug resistance. As shown in Fig. 5, overexpression of ABCG2 (Fig. 5A) increased the IC50 of 5Fu by 4 folds (Fig. 5B), which is associated with an increase in side population (Fig. 5C) and sphere formation (Fig. 5D).
Fig. 5. Rescuing effects of ectopic expression of ABCG2 on shGLI2.
A: ABCG2 was ectopically expressed in acquired resistant N87/FR cells with shGLI2. B: IC50 detection after ABCG2 overexpression in N87/FR/shGLI2 cells. C: Comparison of the side population in ABCG2 ectopic expressed cells and the parental N87/FR/shGLI2 cells. D: Tumor sphere formation after ABCG2 overexpression. Significant difference was indicated by * (P < 0.05), ** (P < 0.005), or *** (P < 0.0005).
The effect of ectopic ABCG2 expression on the IC50 of 5Fu was very similar to that from elevated GLI2 expression, suggesting that ABCG2 appears to be the major mediator for regulating drug sensitivity in gastric cancer. In consistent with our hypothesis, ectopic expression of ABCG2 had no significant effects on expression of GLI1, GLI2 and PTCH1 in N87 cells (unpublished data), further confirming that Hh signaling is upstream of ABCG2 in our experiment system.
2.6 Relevance of the GLI2-ABCG2 signaling axis in human gastric cancer
The relevance of our data in N87 and N87/FR cells to gastric cancer patients was reflected by analysis of cancer relapse in two cohorts of patients with gastric cancer (http://www.cbioportal.org). We correlated the high or low expressions of GLI1/GLI2/ABCG2 in the tumor with cancer relapse in patients with chemotherapy (all patients had 5Fu treatment), which is the standard care for gastric cancer patients. We found that the patients with high GLI1/GLI2/ABCG2 levels in the tumor had 40.5% tumor recurrence. In contrast, those patients with low GLI1/GLI2/ABCG2 expressions had only 32.9% with relapse (Table 1). The odd ratio for cancer relapse in high GLI1/GLI2/ABCG2 expression group is 1.7308. In another cohort, we found that those patients with high GLI1/GLI2/ABCG2 had 30.8% with cancer relapse, while the patients with low expression of GLI1/GLI2/ABCG2 had 17.8% with relapsed cancer. The odd ratio of cancer relapse with high GLI1/GLI2/ABCG2 group in the second cohort is 1.2319. These data indicate that high expressions of GLI1/GLI2/ABCG2 increase the risk of cancer relapse in gastric cancer patients with chemotherapy.
Table 1.
Cancer relapse in patients with chemotherapy in high and low expression of GLI1/GLI2/ABCG2
| Total patient number |
Patient number with high GLI1/GLI2/ABCG2 |
Patient number with low GLI1/GLI2/ABCG2 |
Odd ratio for cancer relapse in patients with high GLI1/GLI2/ABCG2 |
||
|---|---|---|---|---|---|
| Relapse | No relapse | Relapse | No relapse |
||
| 295 | 4 | 13 | 24 | 135 | 1.2319 |
| 478 | 17 | 42 | 92 | 280 | 1.7308 |
Taken all the data together, our study reveals a new mechanism responsible for 5Fu resistance in gastric cancer. We found the activation of the GLI2-ABCG2 signaling axis in residual cancer cells following 5Fu treatment and in 5Fu resistant cell line N87/FR. Down-regulation of GLI2 sensitized cancer cells to 5Fu treatment. We believe that GLI2 mediates cancer cell resistance to 5Fu through direct regulation of ABCG2, resulting in changes in side population and tumor sphere formation. The relevance of our data to gastric cancer patients is confirmed further by the increasing risk of cancer relapse in patients with high GLI1/GLI2/ABCG2 expressions in the primary tumor.
3. DISCUSSION
As a major contributor for cancer-related mortality, most patients are diagnosed with advanced disease that the five-year survival rate is very low (<5%) (Ferlay et al., 2015). Chemotherapy with cisplatin and 5-FU has been the first-line treatment option for advanced gastric cancer, but most patients develop cancer relapse after initial treatment. Although the regulatory mechanisms for chemotherapy resistance in gastric cancer have been reported in the last ten years (Yu and Xie, 2016), little data have been linked with the mechanisms to cancer relapse. Our results indicate that the GLI2-ABCG2 signaling axis is an important mechanism regulating side population and 5Fu resistance in gastric cancer cells. Our data show that knocking down GLI2 will sensitize cancer cells to 5Fu treatment. More importantly, we have shown that high GLI1/GLI2/ABCG2 expressions are associated with an increasing risk of developing cancer relapse in gastric cancer patients who underwent 5Fu based chemotherapy (Table 1). In addition, we have investigated the expression of ABCG2 in patients who underwent chemotherapy (with cisplatin and 5Fu), and found that high ABCG2 expression is associated with poor survival of the patients (data not shown). Since inhibitors for GLI1 and ABCG2 are already available, we predict that these novel reagents, together with chemotherapy, will improve the overall survival of gastric cancer patients.
As a critical regulator for embryonic development and cancer cell stemness maintenance in a number of cancer types (Reya et al., 2001; Taipale and Beachy, 2001; Yang et al., 2010; Liu et al., 2014; Takebe et al., 2015), Hh signaling has been associated with drug resistance in previous studies (Liu et al., 2014; Della Corte et al., 2015; Xu et al., 2015) although the underlying molecular mechanisms are not known. Our studies link GLI2 to ABCG2 to side population, and thus further promote our understanding of GLI2-mediated drug resistance. We found that the ligands, Shh and Ihh, were not significantly altered by 5Fu in N87 cells (data not shown), suggesting that up-regulation of GLI1/2 was not caused by canonical Hh signaling. This implies that the SMO antagonists, such as vismodegib (Rimkus et al., 2016), will not be effective in sensitizing gastric cancer cells to chemotherapy. Targeting ABCG2, on the other hand, will be a more feasible strategy to improve the sensitivity of gastric cancer cells to 5Fu. Although ABCG2 functions to transport several types of small molecules, such as dyes and some chemotherapeutical drugs, 5Fu transport is not affected by ABCG2 (Sarkadi et al., 2004; Yoon et al., 2016), confirming that high ABCG2 expression may represent cancer stem cell population (side population).
4. MATERIALS AND METHODS
4.1. Cell lines
The human gastric cancer cell line NCI-N87 (subsequently referred to as N87) was purchased from the American Type Culture Collection (ATCC, USA) and cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum with 100 U/mL penicillin and 100 µg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2. N87 cells were then maintained in 20 µM 5-Fluorouracil (5Fu, Sigma, USA) for approximately three months to obtain the stable resistant cell line named N87/FR.
4.2. Cell viability
Cells (4000/well) were seeded into 96-well plates the day before, culture medium was replaced by fresh medium with or without various concentrations of 5Fu (5, 10, 25, 50, 100 µM) for incubation 48 h, then stained using alamar Blue (Thermo scientific, USA) as an indicator of cell viability according to the manufacturer’s instructions. Six wells were counted for each drug concentration and at least three independent experiments were done. The IC50 value was defined as the concentration that resulted in a 50% reduction in cell growth compared with growth of the control.
4.3. RNA extraction, RT-PCR and quantitative PCR (qPCR)
Total RNA isolation from cells was extracted using Trizol reagent (Invitrogen, USA) and reverse transcribed into cDNA using the First-Strand Synthesis Kit (Roche, USA) according to the manufacturer’s instructions. qPCR analyses were conducted according to 40 cycles of PCR amplification (95°C for 10 s, 55°C for 15 s and 72°C for 20 s) in an ABI7500 detection system (Applied Biosystems, USA) using Master mix (Roche) with the following probes ordered from Applied Biosystems: GLI1 (Hs01110766), GLI2 (Hs00257977), ABCG2 (Hs01053790) and GAPDH (Hs03929097).
4.4. Lentiviral infection
GLI2 knockdown plasmid of pLKO.1/shGLI2 was stored at our lab, and ABCG2 overexpression plasmid pSIN4/ABCG2 was purchased from Addgene (#25983, USA). For viral package, plasmids PRRE, RSV/REV, CMVG and pLKO.1/shGLI2 or pSIN4/ABCG2 were cotransfected into HEK293T cells using Lipofectamine 3000 (Invitrogen). After 48 h, lentiviral supernatant was filtered through a 0.45-µm filter, supplemented with 10 µg/mL polybrene, and used to infect N87/FR or N87/FR shGLI2 cells. Twenty-four hours after infection, cells were selected with 1 µg/mL puromycin (Sigma) for two weeks.
4.5. Flow cytometry analyses
Single cells were trypsined using Accutase (Gibco, USA), re-suspended in PBS containing 10% FBS, incubated with PE-conjugated ABCG2 (1:100 dilution, Biolegend, USA) for 30 min at 4°C and analyzed on a FACS Canto II (Beckton Dickinson, USA) using Flowjo software after washes.
4.6. Side population assay
For side population, single cells were re-suspended at a concentration of 1 × 106/mL in RPMI-1640 with 2% FBS and 10 mM HEPES, stained with 5 µg/mL Hoechst 33342 (Invitrogen) and incubated at 37°C for 90 min with shaking. After washing with ice-cold HBSS containing 2% FBS and 10 mM HEPES, cells were dispersed in ice-cold HBSS containing 2 µg/mL propidium iodide (PI, Invitrogen). Negative control was conducted by adding ABC transporter inhibitor fumitremorgin C (FTC, 10 µM, Calbiochem, USA) before addition of Hoechst 33342.
4.7. Sphere formation
Cells were dissociated using Accutase, re-suspended in sphere formation medium which was made of Neural Basal medium with 1× B-27, 20 ng/mL of EGF, 10 ng/mL of bFGF and 5 µg/mL of heparin, and seeded on ultralow attachment 24-well plates in a concentration of 2000 cells each well (Corning, USA). Sphere formation medium was changed every other day and the cells in suspension culture were observed and counted under microscope. The sphere forming efficiency was calculated by counting the number of spheres formed from 2000 cells.
4.8. Statistics
Experimental data were expressed as the mean ± SD. Unpaired t test was used to determine the differences between two groups. All statistical analyses were performed using the SPSS 15.0 software. A two-tailed value of P less than 0.05 was considered to be statistically significant. Odd ratio was calculated according to a previously described formula (Persoskie and Ferrer, 2017).
Acknowledgments
This work was supported by National Cancer Institute (R01CA155086), The Wells Center for Pediatric Research, Riley Children Foundation, Jeff Gordon Children’s Foundation and IU Simon Cancer Center. This work was also funded by grants from the National Natural Science foundation of China (Nos. 91529302 and 81472641), Key Projects in the National Science & Technology Pillar Program of China (No. 2014BAI09B03).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajani JA, Bentrem DJ, Besh S, D'Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, Hayman JA, Hofstetter WL, Ilson DH, Keswani RN, Kleinberg LR, Korn WM, Lockhart AC, Meredith K, Mulcahy MF, Orringer MB, Posey JA, Sasson AR, Scott WJ, Strong VE, Varghese TK, Jr, Warren G, Washington MK, Willett C, Wright CD, McMillian NR, Sundar H National Comprehensive Cancer, Network. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J. Natl. Compr. Canc. Netw. 2013;11:531–546. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- Alonso S, Hernandez D, Chang YT, Gocke CD, McCray M, Varadhan R, Matsui WH, Jones RJ, Ghiaur G. Hedgehog and retinoid signaling alters multiple myeloma microenvironment and generates bortezomib resistance. J. Clin. Invest. 2016;126:4460–4468. doi: 10.1172/JCI88152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of Hedgehog pathway inhibitors in Basal cell carcinoma. Mol. Cancer Ther. 2015;14:633–641. doi: 10.1158/1535-7163.MCT-14-0703. [DOI] [PubMed] [Google Scholar]
- Bernards N, Creemers GJ, Nieuwenhuijzen GA, Bosscha K, Pruijt JF, Lemmens VE. No improvement in median survival for patients with metastatic gastric cancer despite increased use of chemotherapy. Ann. Oncol. 2013;24:3056–3060. doi: 10.1093/annonc/mdt401. [DOI] [PubMed] [Google Scholar]
- Chen T, Yang K, Yu J, Meng W, Yuan D, Bi F, Liu F, Liu J, Dai B, Chen X, Wang F, Zeng F, Xu H, Hu J, Mo X. Identification and expansion of cancer stem cells in tumor tissues and peripheral blood derived from gastric adenocarcinoma patients. Cell Res. 2012;22:248–258. doi: 10.1038/cr.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhang X, Chu C, Cheung WL, Ng L, Lam S, Chow A, Lau T, Chen M, Li Y, Nie Y, Wong BC, Pang R. Identification of CD44+ cancer stem cells in human gastric cancer. Hepatogastroenterology. 2013;60:949–954. doi: 10.5754/hge12881. [DOI] [PubMed] [Google Scholar]
- Cho RW, Clarke MF. Recent advances in cancer stem cells. Curr. Opin. Genet. Dev. 2008;18:48–53. doi: 10.1016/j.gde.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, Participants MT. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- Della Corte CM, Bellevicine C, Vicidomini G, Vitagliano D, Malapelle U, Accardo M, Fabozzi A, Fiorelli A, Fasano M, Papaccio F, Martinelli E, Troiani T, Troncone G, Santini M, Bianco R, Ciardiello F, Morgillo F. SMO gene amplification and activation of the Hedgehog pathway as novel mechanisms of resistance to anti-epidermal growth factor receptor drugs in human lung cancer. Clin. Cancer Res. 2015;21:4686–4697. doi: 10.1158/1078-0432.CCR-14-3319. [DOI] [PubMed] [Google Scholar]
- Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, Bonal DM, Charytonowicz E, Gladoun N, de la Iglesia-Vicente J, Petrylak DP, Benson MC, Silva JM, Cordon-Cardo C. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012;22:373–388. doi: 10.1016/j.ccr.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Li J, Liu SM, Feng XY, Chen S, Chen YB, Zhang XS. CD33(+)/p-STAT1(+) double-positive cell as a prognostic factor for stage IIIa gastric cancer. Med. Oncol. 2013;30:442. doi: 10.1007/s12032-012-0442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C, Jimeno A, Gabrielson KL, Matsui W, Maitra A. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Fukamachi H, Shimada S, Ito K, Ito Y, Yuasa Y. CD133 is a marker of gland-forming cells in gastric tumors and Sox17 is involved in its regulation. Cancer Sci. 2011;102:1313–1321. doi: 10.1111/j.1349-7006.2011.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikram MS, Neill GW, Regl G, Eichberger T, Frischauf AM, Aberger F, Quinn A, Philpott M. GLI2 is 17 expressed in normal human epidermis and BCC and induces GLI1 expression by binding to its promoter. J. Invest. Dermatol. 2004;122:1503–1509. doi: 10.1111/j.0022-202X.2004.22612.x. [DOI] [PubMed] [Google Scholar]
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- Jiang J, Zhang Y, Chuai S, Wang Z, Zheng D, Xu F, Zhang Y, Li C, Liang Y, Chen Z. Trastuzumab (herceptin) targets gastric cancer stem cells characterized by CD90 phenotype. Oncogene. 2012a;31:671–682. doi: 10.1038/onc.2011.282. [DOI] [PubMed] [Google Scholar]
- Jiang Y, He Y, Li H, Li HN, Zhang L, Hu W, Sun YM, Chen FL, Jin XM. Expressions of putative cancer stem cell markers ABCB1, ABCG2, and CD133 are correlated with the degree of differentiation of gastric cancer. Gastric Cancer. 2012b;15:440–450. doi: 10.1007/s10120-012-0140-y. [DOI] [PubMed] [Google Scholar]
- Keysar SB, Le PN, Anderson RT, Morton JJ, Bowles DW, Paylor JJ, Vogler BW, Thorburn J, Fernandez P, Glogowska MJ, Takimoto SM, Sehrt DB, Gan GN, Eagles-Soukup JR, Serracino H, Hirsch FR, Lucia MS, Thorburn A, Song JI, Wang XJ, Jimeno A. Hedgehog signaling alters reliance on EGF receptor signaling and mediates anti-EGFR therapeutic resistance in head and neck cancer. Cancer Res. 2013;73:3381–3392. doi: 10.1158/0008-5472.CAN-12-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZJ, Nieuwenhuis E, Nien W, Zhang X, Zhang J, Puviindran V, Wainwright BJ, Kim PC, Hui CC. Kif7 regulates Gli2 through Sufu-dependent and -independent functions during skin development and tumorigenesis. Development. 2012;139:4152–4161. doi: 10.1242/dev.081190. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xu J, He J, Zheng Y, Li H, Lu Y, Qian J, Lin P, Weber DM, Yang J, Yi Q. A critical role of autocrine sonic hedgehog signaling in human CD138+ myeloma cell survival and drug resistance. Blood. 2014;124:2061–2071. doi: 10.1182/blood-2014-03-557298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean MH, El-Omar EM. Genetics of gastric cancer. Nat. Rev. Gastroenterol. Hepatol. 2014;11:664–674. doi: 10.1038/nrgastro.2014.143. [DOI] [PubMed] [Google Scholar]
- Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat. Genet. 1998;20:54–57. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- Nozawa YI, Lin C, Chuang PT. Hedgehog signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction. Curr. Opin. Genet. Dev. 2013;23:429–437. doi: 10.1016/j.gde.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J, Savastano B, Mabilia A, Lieto E, Ciardiello F, De Vita F. Treatment of gastric cancer. World J. Gastroenterol. 2014;20:1635–1649. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanastasopoulos P, Stebbing J. Molecular basis of 5-fluorouracil-related toxicity: lessons from clinical practice. Anticancer Res. 2014;34:1531–1535. [PubMed] [Google Scholar]
- Persoskie A, Ferrer RA. A most odd ratio::interpreting and describing odds ratios. Am. J. Prev. Med. 2017;52:224–228. doi: 10.1016/j.amepre.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proserpio I, Rausei S, Barzaghi S, Frattini F, Galli F, Iovino D, Rovera F, Boni L, Dionigi G, Pinotti G. Multimodal treatment of gastric cancer. World J. Gastrointest. Surg. 2014;6:55–58. doi: 10.4240/wjgs.v6.i4.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassouli FB, Matin MM, Saeinasab M. Cancer stem cells in human digestive tract malignancies. Tumour Biol. 2016;37:7–21. doi: 10.1007/s13277-015-4155-y. [DOI] [PubMed] [Google Scholar]
- Razzak M. Genetics: new molecular classification of gastric adenocarcinoma proposed by The Cancer Genome Atlas. Nat. Rev. Clin. Oncol. 2014;11:499. doi: 10.1038/nrclinonc.2014.138. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo HW. Targeting the sonic Hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers (Basel) 2016;8:pii, E22. doi: 10.3390/cancers8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B, Ozvegy-Laczka C, Nemet K, Varadi A. ABCG2 -- a transporter for all seasons. FEBS Lett. 2004;567:116–120. doi: 10.1016/j.febslet.2004.03.123. [DOI] [PubMed] [Google Scholar]
- Singh RR, Kunkalla K, Qu C, Schlette E, Neelapu SS, Samaniego F, Vega F. ABCG2 is a direct transcriptional target of hedgehog signaling and involved in stroma-induced drug tolerance in diffuse large B-cell lymphoma. Oncogene. 2011;30:4874–4886. doi: 10.1038/onc.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steg AD, Bevis KS, Katre AA, Ziebarth A, Dobbin ZC, Alvarez RD, Zhang K, Conner M, Landen CN. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin. Cancer Res. 2012a;18:869–881. doi: 10.1158/1078-0432.CCR-11-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steg AD, Katre AA, Bevis KS, Ziebarth A, Dobbin ZC, Shah MM, Alvarez RD, Landen CN. Smoothened antagonists reverse taxane resistance in ovarian cancer. Mol. Cancer Ther. 2012b;11:1587–1597. doi: 10.1158/1535-7163.MCT-11-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat. Rev. Clin. Oncol. 2015;12:445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Wang T, Ong CW, Shi J, Srivastava S, Yan B, Cheng CL, Yong WP, Chan SL, Yeoh KG, Iacopetta B, Salto-Tellez M. Sequential expression of putative stem cell markers in gastric carcinogenesis. Br. J. Cancer. 2011;105:658–665. doi: 10.1038/bjc.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiswald LB, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia. 2015;17:1–15. doi: 10.1016/j.neo.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Gong A, Yang H, George SK, Jiao Z, Huang H, Jiang X, Zhang Y. Sonic hedgehog-glioma associated oncogene homolog 1 signaling enhances drug resistance in CD44(+)/Musashi-1(+) gastric cancer stem cells. Cancer Lett. 2015;369:124–133. doi: 10.1016/j.canlet.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–481. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- Yoon C, Cho SJ, Aksoy BA, Park do J, Schultz N, Ryeom SW, Yoon SS. Chemotherapy resistance in diffuse-type gastric adenocarcinoma is mediated by RhoA activation in cancer stem-like cells. Clin. Cancer Res. 2016;22:971–983. doi: 10.1158/1078-0432.CCR-15-1356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yu B, Xie J. Identifying therapeutic targets in gastric cancer: the current status and future direction. Acta Biochim. Biophys. Sin. (Shanghai) 2016;48:90–96. doi: 10.1093/abbs/gmv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Yang Y, Lv W, Song Z, Zhong H. Paclitaxel combined with capecitabine as first-line chemotherapy for advanced or recurrent gastric cancer. Oncol. Lett. 2014;8:351–354. doi: 10.3892/ol.2014.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahreddine HA, Culjkovic-Kraljacic B, Assouline S, Gendron P, Romeo AA, Morris SJ, Cormack G, Jaquith JB, Cerchietti L, Cocolakis E, Amri A, Bergeron J, Leber B, Becker MW, Pei S, Jordan CT, Miller WH, Borden KL. The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature. 2014;511:90–93. doi: 10.1038/nature13283. [DOI] [PMC free article] [PubMed] [Google Scholar]