Abstract
Hepatoma-derived growth factor (HDGF) is a heparin-binding growth factor which has previously been shown to be expressed in a variety of cancers. HDGF over-expression has also previously been correlated with a poor prognosis in several cancers. The significance of HDGF in prostate cancer, however, has not been investigated. Here, we show that HDGF is over-expressed in both androgen-sensitive LNCaP cells and androgen-insensitive DU145, 22RV1 and PC-3 cells. Forced over-expression enhanced cell viability of RWPE-1 cells, while HDGF knockdown reduced cell proliferation in human prostate cancer cells. We also show that HDGF may serve as a survival related protein as ectopic over-expression of HDGF in RWPE cells up-regulated the expression of anti-apoptosis proteins cyclin E and BCL-2, while simultaneously down-regulating pro-apoptotic protein BAX. Western blot analysis also showed that HDGF over-expression modulated the activity of phospho AKT as well as NF-kB, and these results correlated with in vitro migration and invasion assays. We next assessed the therapeutic potential of HDGF inhibition with a HDGF monoclonal antibody and vitamin k2, showing reduced cell proliferation as well as inhibition of NF-kB expression in HDGF over-expressed RWPE cells treated with a HDGF monoclonal antibody and vitamin K2. Collectively, our results suggest that HDGF is a relevant protein in prostate oncogenesis and may serve as a potential therapeutic target in prostate cancer.
Keywords: HDGF, Vitamin K2, AKT, NF-kB, invasion, migration, proliferation
1. Introduction
Prostate cancer (PCa) is one the most commonly diagnosed cancers and is currently the second leading cause of cancer-related deaths among men in the U.S. [1]. While surgery, androgen ablation and radiation therapy are effective treatments of prostate cancer, cases commonly progress to a hormone independent state. The current standard of treatment in advanced, hormone-refractory prostate cancer is unsatisfactory [2]. New modalities of treatment are, therefore, needed. Deeper understanding of the molecular mechanisms underlying PCa development and progression may lead to the development of novel targeted therapies designed to treat PCa.
Hepatoma-derived growth factor, HDGF, is a heparin-binding growth factor originally identified after being purified from supernatant cultures of human hepatoma cell lines [3]. Previous works have shown HDGF to be a potent mitogen, stimulating the growth of vascular smooth muscle cells, hepatoma cells and endothelial cells [4; 5; 6]. HDGF has also been shown to be highly expressed in a variety of malignancies, including hepatocellular carcinoma (HCC), gastric cancer, non-small cell lung cancer (NSCLC), pancreatic cancer and melanoma [7; 8; 9; 10; 11]. Over-expression of HDGF also appears to correlate with a poor prognosis in patients with HCC, lung and gastric cancers [7; 8; 9]. Although the underlying molecular mechanisms by which HDGF promotes carcinogenesis are not entirely understood, it appears to play a critical role in the development of a variety of malignancies and appears to be involved in a variety of cancer promoting processes, including cancer cell growth, regulation of apoptosis, angiogenesis and invasion [12; 13; 14; 15; 16]. In pre-clinical models, therapeutic targeting of HDGF appears to have anti-cancer properties. Zhao and colleagues showed that anti-HDGF neutralizing antibodies in combination with bevacizumab and chemotherapy prevented relapse of NSCLC in a heterotransplant model, while Kishima and colleagues showed that antisense oligonucleotides of HDGF suppressed growth of hepatoma cells [6; 17].
Despite its known role in the development and pathogenesis of other malignancies, little is known about the potential role HDGF may play in the development of PCa. We, therefore, set out to characterize HDGF in the development of PCa as well as its potential anti-cancer properties by targeted inhibition in PCa cell lines.
2. Materials and Methods
Chemicals and reagents
Fetal calf serum (FCS) and RPMI-1640 were obtained from American Type Cell Culture (ATCC), Manassas, VA, USA. HDGF overexpression plasmid was obtained from OriGene Technologies (Rockville, MD). Cell viability assay kit was purchased from Dojindo Molecular Technologies Inc., Gaithersburg, MD. Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA). Vitamin K2 (VK2) was obtained from Sigma Aldrich (St. Louis, MO). Matrigel was obtained from BD Biosciences (San Jose, CA). Transwell culture inserts were purchased from Corning, USA.
Cell lines and cell culture
RWPE-1, LNCaP, DU-145, 22RV1 and PC-3 cell lines were obtained from American Type Cell Culture (ATCC), Manassas, VA, USA. RWPE-1 cells were cultured in keratinocyte serum free medium (KSFM) supplemented with bovine pituitary extract and epidermal growth factor (Thermo Fisher Scientific). Prostate cancer cells were cultured in RPMI medium supplemented with 10% FCS and 50 mg/mL gentamycin. Cells were maintained at 37°C, 5% CO2 environment
Transient transfection
We selected two sites in the HDGF mRNA sequence as siRNA targets based on principles described previously [18]. The targeted HDGF sequences, based on which the siRNAs were chemically synthesized by IDT Technologies (Coralville, IA), were 5′-AACCGGCAGAAGGAGUACAAA-3′ (siRNA-1) and 5′-AAAUCAACAGCCAACAAAUAC-3′ (siRNA-2). The negative control siRNAs were also purchased from IDT. In vitro transfections were done using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following manufacturer’s protocols.
Cell viability assay
RWPE-1, LNCaP and PC-3 cells (2×103 cells/ml) were seeded in 96-well tissue culture plates and incubated until cells attached to wells. LNCaP and PC-3 cells were then transfected with a final concentration of 100 nM HDGF siRNA or control siRNA for 24, 48 and 72 hours, while RWPE cells were transfected with a final concentration of 100nM of HDGF-pcDNA3.1 or pcDNA3.1 for 24, 48 or 72 hours. Cell viabilities were determined using a cell counting kit-8 (CCK-8) from Dojindo Molecular Technologies. Optical density was measured at 450 nm using a BIO-RAD microplate reader model 680.
Cell Cycle analysis
HDGF-pcDNA3.1 or pcDNA3.1 transfected RWPE-1 cells seeded in 6-well plates were incubated for 48 h. Following this, cells were harvested and washed twice with phosphate-buffered saline (PBS). Cell pellets were fixed in 70% ethanol, treated with RNase A (Sigma-Aldrich) and stained with propidium iodide (Sigma-Aldrich). DNA content data were acquired using CELLQuest software on a flow cytometer (FACSCalibur; Becton Dickinson, Mountain View, CA).
Western blot analysis
HDGF-pcDNA3.1 transfected RWPE cells were lysed with sample solubilizing buffer and subjected to SDS-PAGE, transferred to nitrocellulose membrane for Western blot analysis. The following antibodies were used for immunoblotting: anti-HDGF (Santa Cruz Biotechnology Inc), anti-NF-kB (MBL International Inc.), anti-BCL2 (Cell Signaling Technologies), anti-BAX (Cell Signaling Technologies), anti-cyclin E (Cell Signaling Technologies), anti-AKT (Cell Signaling Technologies), anti-phosphorylated AKT (pAKT) (Ser473)(Cell Signaling Technologies) and anti beta-Actin-peroxidase (Sigma Aldrich) antibodies were used with vendor’s recommended dilutions. Cells transfected with empty vector were used as controls.
Real Time PCR analysis
Expression levels of HDGF in RWPE-1 and PCa cells (LNCaP, 22Rv1, PC-3 and DU145) were analyzed by the quantitative Real-Time PCR method. High-capacity cDNA reverse transcription kit (Applied Biosystem, CA, USA) was used to synthesize the cDNA from mRNA in Mastercycler PCR machine (Eppendorf, USA). 100ng of cDNA was used to quantify the expression of HDGF using SYBR Green quantification method (Thermo Scientific, USA). Premade HDGF and Actin primers were obtained from Sigma-Aldrich. The real time PCR was performed using Applied Biosystems (7300 RT PCR) Thermocycler two step cycling protocol set by 40 cycles with 10 minutes initial denaturation at 95°C, further denaturation at 95°C for 15 seconds and followed by annealing/extension at 60°C for 60 minutes. The Ct values were extracted using the SDS-software (Applied Biosystems, CA, USA).
Confocal Immunofluorescence Analysis
LNCaP cells were cultured in 8-well chamber tissue culture slides. At 80–90% confluence, cells were washed with phosphate-buffered saline (PBS) and fixed in 4% formaldehyde for 15 min at room temperature and followed by three washings with PBS. Cells were blocked for 1 h in 5% Goat normal serum/phosphate-buffered saline (Invitrogen) and incubated with a mouse monoclonal IgG anti-HDGF antibody (1:500) (Santa Cruz Biotechnology, Santa Cruz, CA) for overnight at 4°C temperature. Goat anti-mouse IgG secondary antibody conjugated with FITC (Invitrogen) (1:50) along with 30 nM DAPI were used for visualization. The wells were subsequently washed with PBS and layered with Fluorogel (Electron Microscopy Sciences, Hatfield, PA, USA) for visual analysis using Olympus Fluoview confocal microscope.
Boyden chamber assay
Control RWPE-1 and RWPE-1 cells transfected with HDGF for 48h were collected and subsequently seeded in a Transwell (Corning) chamber and incubated for 24 h. Cells were then removed from the top of the membrane using a pipette and any remaining cells were removed using a Q-tip. A HEMA 3 staining set from Fisher Scientific was used to fix and stain the cells. Following this, each membrane was rinsed with water and any remaining stain was removed from the top of each membrane using a Q-tip. Membranes were analyzed for cell migration using a light microscope (Nikon). Invasion assays were performed in Transwell chambers coated with matrigel.
Statistical Analysis
Statistical analysis was performed with Graph Pad Prism 5 software. Data were compared using Student’s t-test. P < 0.05 was considered statistically significant.
3. Results
3.1 HDGF expression in human prostate cancer cells
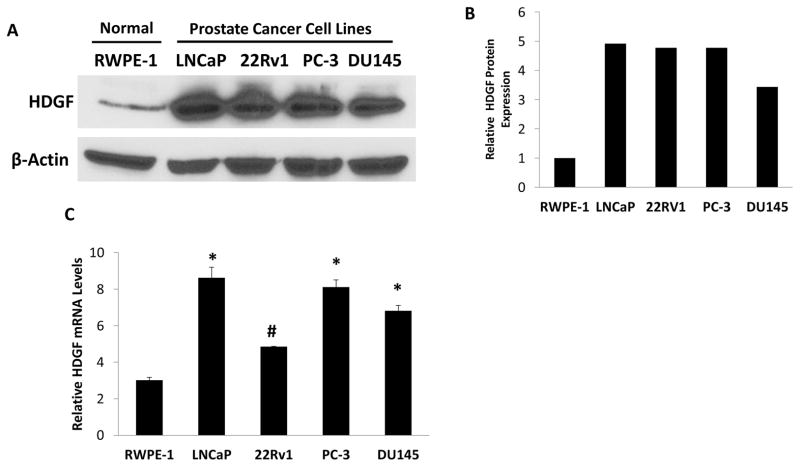
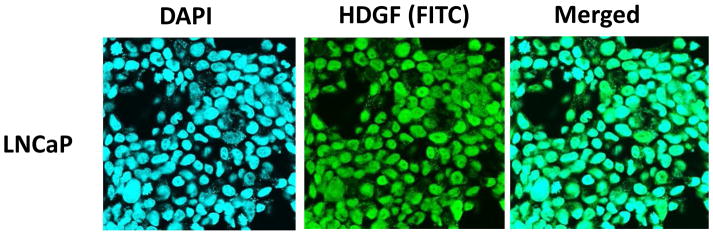
To investigate the possible role of HDGF in prostate oncogenesis, first we examined the expression levels of HDGF by Western blot analysis in a benign prostate cell line, RWPE-1, and in 4 different PCa cell lines, LNCaP, 22RV1, DU145 and PC-3. As shown in Figure 1a, HDGF appears to be over-expressed in all 4 PCa cell lines, while being minimally expressed in RWPE-1 cells. While the highest relative expression levels were found in the androgen sensitive LNCaP cell line, markedly increased expression levels were also found in the 3 androgen independent cell lines, 22RV1, DU145 and PC-3. Further, we also analyzed the HDGF mRNA levels in the above indicated cell lines by real time PCR analysis (Figure 1c). Results of this analysis also showed that HDGF is highly expressed in PCa cell lines compared to benign RWPE-1 cells. As LNCaP cells relatively express higher levels of HDGF, we used this cell line to determine the expression by confocal immunofluorescence analysis. Interestingly these results showed that HDGF is predominantly localized in nucleus (Figure 2).
Figure 1.
HDGF is over expressed in PCa cells. A). Western Blot results showed that HDGF is over expressed in several PCa cells but minimally expressed in RWPE-1 cells. Briefly, protein samples were resolved on 10% SDS-PAGE, transferred to NCP and probed with anti-HDGF antibody to detect HDGF expression. B. Semi-quantitative results of HDGF expression in PCa cells as determined by ImageJ analysis. These results suggest that HDGF is over expressed in multiple PCa cells. C. Real time PCR analysis of HDGF transcript levels. The cDNA prepared from RWPE-1, LNCaP, 22Rv1, PC-3 and DU145 cells were used as a template to quantitate the transcript levels using SYBR Green Q-PCR assay. HDGF transcript levels were normalized using β-actin transcript levels. Results indicate that increased HDGF transcript levels are present in PCa cell lines compared to benign RWPE-1 cells. *p<0.001 and #p<0.01 compared to control RWPE-1 cells.
Figure 2.
Analysis of HDGF expression by confocal immunofluorescence microscopy. Briefly, LNCaP cells cultured in tissue culture slides were probed with mouse anti-HDGF mAb antibody. HDGF expression was detected using goat anti-mouse FITC and counterstained with DAPI. Confocal image analysis suggests that HDGF is predominantly localized in nucleus as shown in the merged images of DAPI and HDGF (FITC).
3.2 Effects of HDGF over-expression and gene silencing on cell viability and proliferation of RWPE-1, LNCaP and PC-3 cells
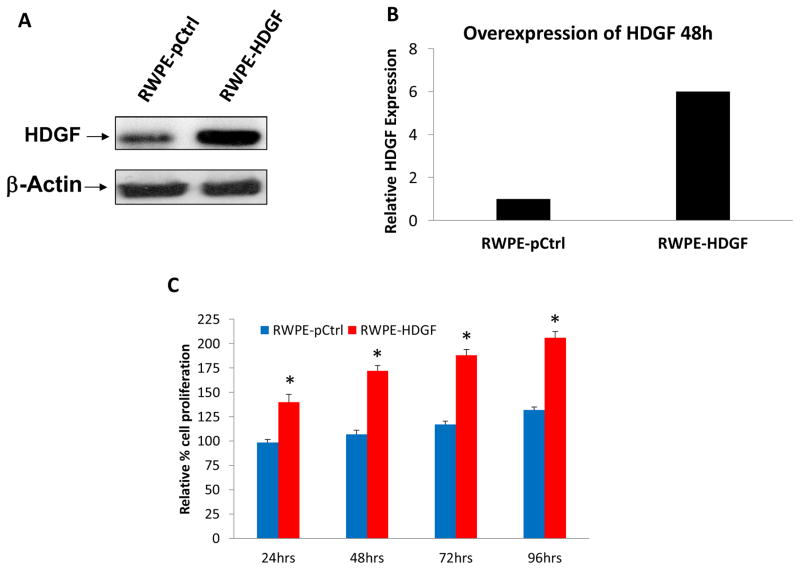
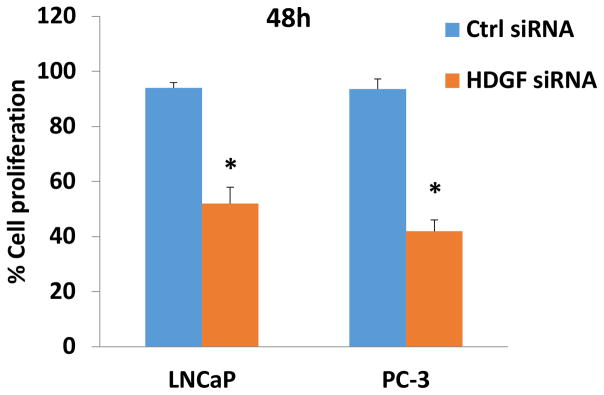
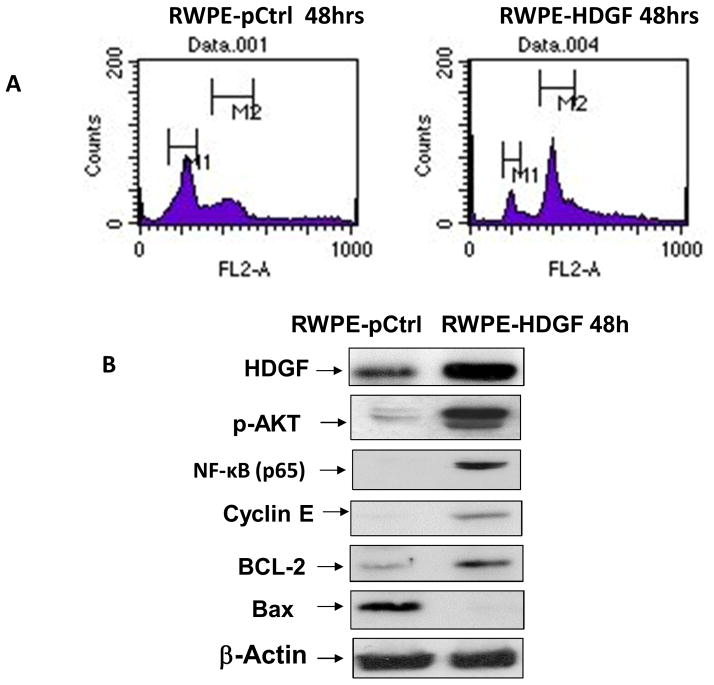
To further explore the potential role of HDGF in prostate cancer, we examined the effects of HDGF over-expression in RWPE-1. As shown in Figure 3c, ectopically over-expressed HDGF in RWPE-1 cells resulted in a 1.5 fold increased proliferative rate 48 hours post-transfection. Since increased proliferation is a hallmark of PCa, our cell proliferation results suggest that HDGF may have an important role in the development of PCa. In complementary to these findings, down regulation of HDGF by siRNA significantly inhibited the cell proliferation of both androgen-dependent LNCaP cells and androgen independent PC-3 cells (Figure 4). Taken together these results assert that HDGF is a survival related molecule important for prostate oncogenesis. We next examined the effects of HDGF over-expression on cell cycle progression (Figure 5a). DNA content analysis of RWPE-1 cells expressing HDGF by flow cytometry showed that the majority of the cells were in the G2/M phase (55%) of the cell cycle while only 28% of the control RWPE-1 cells were at G2/M phase. The percentage of RWPE- HDGF cells in G0/G1 phase were significantly lesser (12%) compared to control RWPE-1 cells (49%). These data imply that HDGF enforces transition of cells from G0/G1 to G2/M phase to promote mitotic activity in prostate cells.
Figure 3.
Ectopic expression of HDGF in RWPE-1 cells promotes cell proliferation. Over expression of HDGF is confirmed in the transfected cells by Western blot and quantification by ImageJ analysis (A &B). (C) Mock transfected (pCtrl) and RWPE-1 cells transfected with HDGF were seeded in a 96 well plate and the viability of the cells were determined at different time points. Results show that the RWPE-1 HDGF cells proliferate at approximately 180% of the rate of control RWPE-1 after 72 hours. *p<0.01 compared to control transfected cells.
Figure 4.
Knock down of HDGF expression by siRNA transfection inhibits LNCaP and PC-3 cell proliferation. To determine the gene silencing effects of HDGF in PCa, LNCaP and PC-3 cells were transfected with HDGF siRNA for 48h using Lipofectamine reagent. Following this, the viability of HDGF siRNA transfected cells were determined using CCK-8 cell viability assay. Control siRNA transfected cells served as controls. Data showed that HDGF siRNA significantly inhibited the cell proliferation of both LNCaP and PC-3 cells suggesting that HDGF is an important survival related molecule in PCa cells. *p<0.01 compared to respective control groups.
Figure 5.
Transfection of HDGF confers malignant characteristics to normal RWPE-1 cells. A) Cell cycle analysis results show that overexpression of HDGF in RPWE-1 cells results in the alteration of cell cycle profile. To assess the overexpression effect of HDGF on cell cycle progression of benign prostate cells, RWPE-1 was transfected with HDGF for 48h. Mock transfected cells were used as control. After 48h transfection, these cells were subjected to cell cycle analysis. Data show that majority of cells (55%) transfected with HDGF were in G2/M phase while only 28% of control RWPE-1 cells were in G2/M phase. No significant changes in S phase of RWPE-HDGF and control RWPE-1 were observed. On the other hand 12% of RWPE-1 cells transfected with HDGF were in G0/G1 phase in comparison to G0/G1 (49%) of control RWPE-1 cells. These data thus suggests HDGF promotes mitosis in transfected RWPE-1 cells. B) RWPE-1 cells transfected with HDGF for 48h was utilized to determine the modulatory effects of HDGF expression on pro-survival signaling molecules. Western blot results show that HDGF expression activates AKT and NF-kB resulting in the up regulation of proliferation associated molecules (Cyclin E and BCL-2) and down regulation of pro-apoptotic molecule, Bax suggesting HDGF is critical for the survival of prostate cells.
3.3 HDGF expression activates pro-survival mediated pathways in RWPE-1 cells
We next sought to determine the molecular basis for the enhanced viability and proliferation seen with over-expression of HDGF in RWPE-1 cells. AKT activation has previously been shown to promote cell survival via decreasing the activity of pro-apoptotic proteins or increasing the activity of anti-apoptotic proteins [19; 20]. Previous studies have shown NF-kB to promote a variety of processes including PCa cell growth, proliferation, angiogenesis and invasion [21; 22].
We, therefore, sought to determine if these two pathways were activated by HDGF over-expression. As shown in Figure 5b, AKT (phosphorylated form) and NF-kB (p65 domain) were found to be activated in RWPE-1 cells expressing HDGF compared to control RWPE-1 cells. Given the known importance of both the NF-kB and AKT pathways in promoting cell survival, we next assessed whether HDGF expression regulates apoptosis in RWPE-1 cells. As presented in Figure 5b, HDGF over-expression in RWPE-1 cells resulted in increased activation of anti-apoptotic molecules Cyclin-E and BCL-2 and simultaneous decrease in the pro-apoptotic molecule Bax. Taken together, these results suggest that HDGF over-expression may play a role in regulating survival-mediated pathways via activation of the AKT and NF-kB pathways in PCa development.
3.4 Effects of HDGF inhibition on HDGF over-expressed RWPE-1 cells
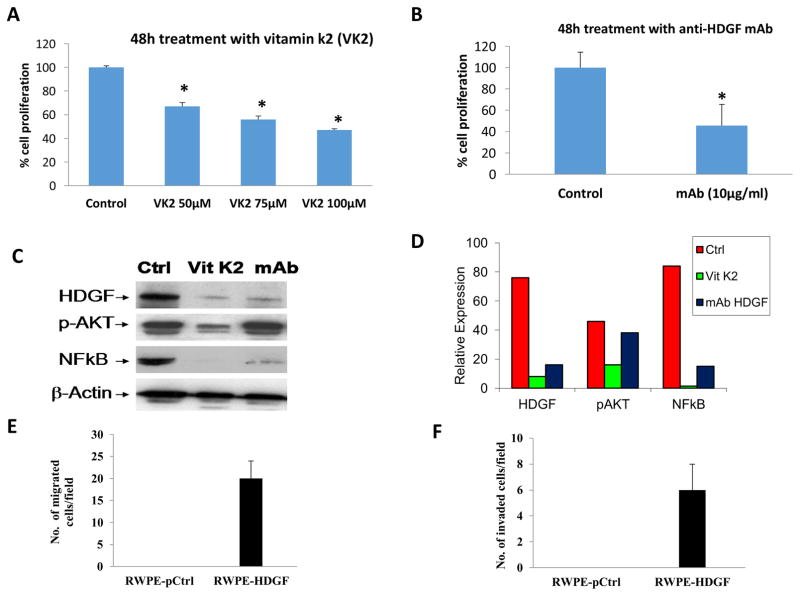
We next investigated the therapeutic potential of HDGF inhibition in HDGF over-expressing RWPE-1 cells. Previous studies have shown that vitamin K2 (VK2) suppressed the growth of hepatocellular carcinoma (HCC) cell lines by targeting the expression of HDGF in vitro [23]. In the above study, the authors showed that VK2 reduced the expression of HDGF in three different HCC-derived cell lines, HepG2, HuH-7, and SK-Hep-1. Monoclonal antibody against HDGF was also shown to target HDGF in lung cancer cells [24]. We, therefore, sought to determine the therapeutic relevance of VK2 as well as a monoclonal antibody to HDGF in RWPE cells over-expressing HDGF. Figure 6a shows the results of VK2 treatment. Compared to control untreated HDGF over-expressing RWPE-1 cells, treatment with VK2 significantly reduced the growth of these cells in a dose-dependent manner. Figure 6b shows the results of monoclonal antibody treatment. Compared to untreated control cells, a 60% reduction in cell viability was noted with HDGF monoclonal antibody therapy in HDGF over-expressing RWPE-1 cells. Figure 6c shows the results of Western blot analysis of RWPE-HDGF following treatment with VK2 and mAb for 48h. Expression of HDGF, AKT (phosphorylated form) and NF-kB (p65 domain) were all substantially reduced with VK2 treatment. On the other hand, HDGF monoclonal antibody treatment decreased the levels of HDGF and NF-kB without affecting pAKT. Although mAb downregulated the expression of HDGF, the underlying mechanism of differential effects of mAb on the signaling molecules are not known at this time and needs further investigation.
Figure 6.
HDGF inhibitors decrease the proliferation of RWPE-1 cells transformed with HDGF. RWPE-1 cells expressing HDGF (RWPE-HDGF) were seeded in 96 well plates. At 50% confluency, cells were treated with either Vitamin K2 (50–100 μM) or a monoclonal antibody specific to HDGF (10 μg/ml) for 48h. Untreated cells served as a control. At the end of treatment, the viability of the cells was determined by cell viability assay as described in materials and methods. Results show that both Vitamin K2 and the monoclonal antibody to HDGF significantly decreased the proliferation of RWPE-HDGF cells. *p<0.05 compared to control groups. Down regulation of HDGF expression in RWPE-HDGF cells by vitamin k2 and mAb results in the inhibition of NFκB (p65). RWPE-HDGF cells treated with Vitamin K2 or mAb for 48h were subjected to Western blot analysis. Results presented in C and D show that both Vitamin K2 and mAb treatment inhibited the expression of HDGF leading to down regulation of its target molecule NFκB (p65) in the RWPE-HDGF cells. Notably downregulation of pAKT was observed with vitamin k2 treatment but not with mAb treatment. HDGF expression increases migration and invasion of RWPE-1 cells. E) To determine the overexpression effects of HDGF on migration and invasion of RWPE-1, cells were transfected with HDGF plasmid for 48h and subjected to in vitro migration and invasion assays. Briefly, RWPE-1 cells overexpressing HDGF (RWPE-HDGF) were seeded on Transwell chambers for 24h and studied for its ability to migrate. F) RWPE-HDGF cells were also seeded on Transwell chambers coated with matrigel for 24h to monitor their invasion potential. Results show that HDGF expression conferred the migration and invasion properties to normal RWPE-1 cells.
3.5 Effect of HDGF expression on cell migration and invasion in vitro
Cell migration is one of the first steps in cancer metastasis and invasion process. Tumor cells utilize several lytic enzymes, degrading the surrounding extracellular matrix, allowing them to migrate [25]. Both the AKT and NF-kB have been shown to be important mediators of PCa invasion [22; 26]. Given the effects of HDGF expression on the PI3K and NF-kB pathways, we sought to assess the impact of HDGF expression on cell migration and invasion in vitro. The results obtained in our study show that HDGF over-expression in RWPE-1 cells resulted in increased number of cells which migrated and invaded the Transwell filter (Figure 6d and 6e). This data suggests that in addition to the cell survival function, HDGF may also have a role in metastatic progression of PCa.
4. Discussion
Previous studies have shown HDGF to play an important role in the carcinogenesis of a variety of malignancies. The potential role of HDGF in the development of PCa, however, remains unknown. We, therefore, sought to study the carcinogenic potential of HDGF. In the present study, we show that HDGF may be a critical component in the development and progression of PCa. Enhanced proliferative capacity, activation of invasion and metastasis and evasion of apoptosis have all been shown to be hallmarks of cancer [27]. Our expression analysis of HDGF by Western blot and Q-PCR analysis suggests that this molecule is endogenously overexpressed in multiple PCa cells compared to benign prostate cells. Furthermore, immunocytochemical analysis of HDGF in LNCaP cells suggests HDGF to be predominantly a nuclear protein. However whether HDGF is secreted from prostate cells and whether there is any specific receptor for HDGF in PCa cells are not known at this time. In this study, ectopic overexpression of HDGF resulted in enhanced cell proliferation, upregulation of anti-apoptotic proteins and invasion in the benign prostatic epithelial cell line, RWPE-1, while therapeutic targeting of HDGF resulted in reduced cell growth and viability in both androgen sensitive and androgen insensitive human prostate cancer cells. These results are in agreement with studies of HDGF in other cancers, including hepatocellular carcinoma, melanoma, lung cancer, pancreatic cancer and gastric cancer [7; 8; 11; 18; 28]. HDGF has also been shown to be a key regulator of apoptosis. Tsang and colleagues showed that HDGF downregulation activates apoptosis via Bad-mediated mechanisms in hepatocellular carcinoma cells [29]. Targeted inhibition of HDGF has also been shown to reduce the invasive potential of other malignant cells. For example, Meng and colleagues showed that shRNA of HDGF suppressed the invasive capacity of non-small cell lung cancer cells [30]. Our results highlight the potential importance of HDGF in PCa development.
We also sought to understand the underlying molecular mechanisms of HDGF in the carcinogenesis of PCa. Our results show that HDGF overexpression activates AKT in benign prostate cells, while targeting HDGF expression by vitamin k2 results in inhibition of AKT. Overall, these results were consistent with the effects of HDGF expression on cell proliferation, apoptosis and invasion. Collectively, our data suggest that the therapeutic benefits of HDGF based inhibition is, at least in part, mediated by the concomitant inhibition of the AKT pathway.
Studies examining the mechanisms underlying the effects of HDGF on the AKT pathway are currently underway. We also show here that HDGF overexpression upregulates the expression of NF-kB, while HDGF inhibition down-regulates NF-kB expression, suggesting that HDGF regulates the expression of NF-kB. Previous studies have shown the NF-kB is critical in the progression of PCa to castration-resistance [31; 32]. Collectively, our results suggest that the role of HDGF in PCa is, at least in part, modulated by activation of both the NF-kB and AKT pathways.
While more preclinical studies need to be undertaken, HDGF may be a promising therapeutic target in both androgen sensitive and castration-resistant prostate cancer (CRPC). As shown in our study, the LNCaP and PC-3 cell lines were both equally sensitive to siRNA-based HDGF targeting. Another potential strategy, given the sensitivity found in our work for androgen-dependent LNCaP cells to HDGF inhibition, would be to combine HDGF based targeted treatments with currently approved treatment approaches, such as androgen deprivation therapy. Preclinical studies examining these approaches are currently being studied.
In conclusion, HDGF appears to be an important protein in the pathogenesis of PCa. The results of our study suggest that HDGF plays an important role in cell growth, the regulation of apoptosis and invasion of human prostate cancer cells and this appears to, at least in part, be mediated by the AKT and NF-kB pathways. We also show that HDGF may serve as a therapeutic target in PCa. Based upon these findings, further studies are clearly warranted to assess the underlying molecular mechanisms of HDGF in prostate carcinogenesis as well as the therapeutic potential of HDGF-based targeted treatments for PCa.
Highlights.
Hepatoma-derived growth factor (HDGF) is overexpressed in both androgen dependent and androgen independent prostate cancer
Forced over expression of HDGF promotes cell proliferation of benign prostate epithelial cells
Gene silencing of HDGF reduced cell proliferation in human prostate cancer cells
HDGF upregulates the expression of anti-apoptotic proteins cyclin E and BCL-2, while simultaneously down-regulating Bax expression
Targeting HDGF by vitamin k2 and HDGF monoclonal antibody inhibited the cell proliferation of HDGF overexpressing prostate cells by inhibiting phospho-AKT and NF-kB expression
Our results suggest that HDGF may play a vital role in prostate carcinogenesis and may serve as a therapeutic target in prostate cancer
Acknowledgments
This study was supported by University of Illinois College of Medicine at Rockford and funding received from NIH (R21 CA184646-01A1).
Abbreviations used
- PCa
Prostate Cancer
- HDGF
Hepatoma-derived growth factor
- HCC
hepatocellular carcinoma
- NSCLC
non-small cell lung cancer
- DAPI
4, 6-diamido-2-phenylindole hydrochloride
- VK2
vitamin K2
Footnotes
5. Conflict of interest
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Shetty AV, Thirugnanam S, Dakshinamoorthy G, Samykutty A, Zheng G, Chen A, Bosland MC, Kajdacsy-Balla A, Gnanasekar M. 18alpha-glycyrrhetinic acid targets prostate cancer cells by down-regulating inflammation-related genes. Int J Oncol. 2011;39:635–640. doi: 10.3892/ijo.2011.1061. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura H, Izumoto Y, Kambe H, Kuroda T, Mori T, Kawamura K, Yamamoto H, Kishimoto T. Molecular cloning of complementary DNA for a novel human hepatoma-derived growth factor. Its homology with high mobility group-1 protein. J Biol Chem. 1994;269:25143–25149. [PubMed] [Google Scholar]
- 4.Everett AD, Lobe DR, Matsumura ME, Nakamura H, McNamara CA. Hepatoma-derived growth factor stimulates smooth muscle cell growth and is expressed in vascular development. J Clin Invest. 2000;105:567–575. doi: 10.1172/JCI7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver JA, Al-Awqati Q. An endothelial growth factor involved in rat renal development. J Clin Invest. 1998;102:1208–1219. doi: 10.1172/JCI785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kishima Y, Yoshida K, Enomoto H, Yamamoto M, Kuroda T, Okuda Y, Uyama H, Nakamura H. Antisense oligonucleotides of hepatoma-derived growth factor (HDGF) suppress the proliferation of hepatoma cells. Hepatogastroenterology. 2002;49:1639–1644. [PubMed] [Google Scholar]
- 7.Hu TH, Huang CC, Liu LF, Lin PR, Liu SY, Chang HW, Changchien CS, Lee CM, Chuang JH, Tai MH. Expression of hepatoma-derived growth factor in hepatocellular carcinoma. Cancer. 2003;98:1444–1456. doi: 10.1002/cncr.11653. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto S, Tomita Y, Hoshida Y, Takiguchi S, Fujiwara Y, Yasuda T, Doki Y, Yoshida K, Aozasa K, Nakamura H, Monden M. Expression of hepatoma-derived growth factor is correlated with lymph node metastasis and prognosis of gastric carcinoma. Clin Cancer Res. 2006;12:117–122. doi: 10.1158/1078-0432.CCR-05-1347. [DOI] [PubMed] [Google Scholar]
- 9.Ren H, Tang X, Lee JJ, Feng L, Everett AD, Hong WK, Khuri FR, Mao L. Expression of hepatoma-derived growth factor is a strong prognostic predictor for patients with early-stage non-small-cell lung cancer. J Clin Oncol. 2004;22:3230–3237. doi: 10.1200/JCO.2004.02.080. [DOI] [PubMed] [Google Scholar]
- 10.Tsai HE, Wu JC, Kung ML, Liu LF, Kuo LH, Kuo HM, Chen SC, Chan EC, Wu CS, Tai MH, Liu GS. Up-regulation of hepatoma-derived growth factor facilitates tumor progression in malignant melanoma [corrected] PLoS One. 2013;8:e59345. doi: 10.1371/journal.pone.0059345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uyama H, Tomita Y, Nakamura H, Nakamori S, Zhang B, Hoshida Y, Enomoto H, Okuda Y, Sakon M, Aozasa K, Kawase I, Hayashi N, Monden M. Hepatoma-derived growth factor is a novel prognostic factor for patients with pancreatic cancer. Clin Cancer Res. 2006;12:6043–6048. doi: 10.1158/1078-0432.CCR-06-1064. [DOI] [PubMed] [Google Scholar]
- 12.Lee KH, Choi EY, Kim MK, Lee SH, Jang BI, Kim TN, Kim SW, Kim SW, Song SK, Kim JR, Jung BC. Hepatoma-derived growth factor regulates the bad-mediated apoptotic pathway and induction of vascular endothelial growth factor in stomach cancer cells. Oncol Res. 2010;19:67–76. doi: 10.3727/096504010x12864748215043. [DOI] [PubMed] [Google Scholar]
- 13.Chen B, Huang T, Jiang J, Lv L, Li H, Xia S. miR-141 suppresses proliferation and motility of gastric cancer cells by targeting HDGF. Mol Cell Biochem. 2014;388:211–218. doi: 10.1007/s11010-013-1912-3. [DOI] [PubMed] [Google Scholar]
- 14.Guo H, Li W, Zheng T, Liu Z. MiR-195 targets HDGF to inhibit proliferation and invasion of NSCLC cells. Tumour Biol. 2014;35:8861–8866. doi: 10.1007/s13277-014-2153-0. [DOI] [PubMed] [Google Scholar]
- 15.Thirant C, Galan-Moya EM, Dubois LG, Pinte S, Chafey P, Broussard C, Varlet P, Devaux B, Soncin F, Gavard J, Junier MP, Chneiweiss H. Differential proteomic analysis of human glioblastoma and neural stem cells reveals HDGF as a novel angiogenic secreted factor. Stem Cells. 2012;30:845–853. doi: 10.1002/stem.1062. [DOI] [PubMed] [Google Scholar]
- 16.Shih TC, Tien YJ, Wen CJ, Yeh TS, Yu MC, Huang CH, Lee YS, Yen TC, Hsieh SY. MicroRNA-214 downregulation contributes to tumor angiogenesis by inducing secretion of the hepatoma-derived growth factor in human hepatoma. J Hepatol. 2012;57:584–591. doi: 10.1016/j.jhep.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J, Ma MZ, Ren H, Liu Z, Edelman MJ, Pan H, Mao L. Anti-HDGF targets cancer and cancer stromal stem cells resistant to chemotherapy. Clin Cancer Res. 2013;19:3567–3576. doi: 10.1158/1078-0432.CCR-12-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Ren H, Yuan P, Lang W, Zhang L, Mao L. Down-regulation of hepatoma-derived growth factor inhibits anchorage-independent growth and invasion of non-small cell lung cancer cells. Cancer Res. 2006;66:18–23. doi: 10.1158/0008-5472.CAN-04-3905. [DOI] [PubMed] [Google Scholar]
- 19.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 20.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 21.Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J Cell Biochem. 2004;91:100–117. doi: 10.1002/jcb.10729. [DOI] [PubMed] [Google Scholar]
- 22.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Nakamura H, Liu W, Cao K, Yoshikawa S, Enomoto H, Iwata Y, Koh N, Saito M, Imanishi H, Shimomura S, Iijima H, Hada T, Nishiguchi S. Involvement of hepatoma-derived growth factor in the growth inhibition of hepatocellular carcinoma cells by vitamin K(2) J Gastroenterol. 2009;44:228–235. doi: 10.1007/s00535-008-2304-4. [DOI] [PubMed] [Google Scholar]
- 24.Ren H, Chu Z, Mao L. Antibodies targeting hepatoma-derived growth factor as a novel strategy in treating lung cancer. Mol Cancer Ther. 2009;8:1106–1112. doi: 10.1158/1535-7163.MCT-08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCready J, Sims JD, Chan D, Jay DG. Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer. 2010;10:294. doi: 10.1186/1471-2407-10-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukla S, Maclennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer. 2007;121:1424–1432. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Bernard K, Litman E, Fitzpatrick JL, Shellman YG, Argast G, Polvinen K, Everett AD, Fukasawa K, Norris DA, Ahn NG, Resing KA. Functional proteomic analysis of melanoma progression. Cancer Res. 2003;63:6716–6725. [PubMed] [Google Scholar]
- 29.Tsang TY, Tang WY, Tsang WP, Co NN, Kong SK, Kwok TT. Downregulation of hepatoma-derived growth factor activates the Bad-mediated apoptotic pathway in human cancer cells. Apoptosis. 2008;13:1135–1147. doi: 10.1007/s10495-008-0241-6. [DOI] [PubMed] [Google Scholar]
- 30.Meng J, Xie W, Cao L, Hu C, Zhe Z. shRNA targeting HDGF suppressed cell growth and invasion of squamous cell lung cancer. Acta Biochim Biophys Sin (Shanghai) 2010;42:52–57. doi: 10.1093/abbs/gmp102. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Altuwaijri S, Deng F, Chen L, Lal P, Bhanot UK, Korets R, Wenske S, Lilja HG, Chang C, Scher HI, Gerald WL. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175:489–499. doi: 10.2353/ajpath.2009.080727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bitting RL, Armstrong AJ. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr Relat Cancer. 2013;20:R83–99. doi: 10.1530/ERC-12-0394. [DOI] [PubMed] [Google Scholar]