Abstract
The Ah receptor (AHR) is capable of binding a structurally diverse group of compounds that can be found in the diet, produced by bacteria in the gut and through endogenous metabolism. The gastrointestinal tract is a rich source of AHR ligands, which have been shown to protect the gut upon challenge with either pathogenic bacteria or toxic chemicals. The human AHR can be activated by a broader range of ligands compared to the mouse AHR, suggesting that studies in mice may underestimate the impact of AHR ligands in the human gut. The protective effect of AHR activation appears to be due to modulating the immune system within the gut. While several mechanisms have been established, due to the increasingly pleotropic nature of the AHR, other mechanisms of action likely exist that remain to be identified. The major contributors to AHR function in the gut and the most appropriate level of receptor activation that maintains intestinal homeostasis warrants further investigation.
Keywords: Ah receptor, AHR, indole, IL22, intestine, gastrointestinal
Graphical abstract
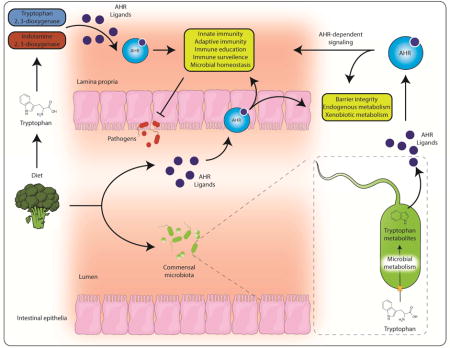
Introduction
Evidence suggests the predominant biological activities of the AHR are evoked through ligand binding. Thus, one key aspect of AHR research has centered upon the identification of the endogenous ligand for this receptor. However, the characterization of multiple, structurally diverse physiological AHR ligands have led to a complex story likely to be context specific. It is now apparent that a biological ‘cocktail’ of endogenous, pseudo-endogenous1 and dietary AHR ligands exist and that spatiotemporal availability, generation (or metabolic elimination) and competition between disparate ligands represent the major factors dictating the physiological activities and functions of the AHR. Importantly, emerging evidence highlights the physiological relevance of intrinsic AHR ligand activation beyond xenobiotic metabolism.
Dietary AHR ligands
In essence, an organisms’ diet comprises a complex mixture of xenobiotics, some of which provide nutritional and/or other physiological value while others may be toxins. Barring environmental, occupational or lifestyle (smoking) exposure to dioxins etc., the diet is now recognized as a major source of AHR ligands. The AHR has a promiscuous capacity to bind and respond to raw and cooked dietary components; including those derived from plants e.g. fruits and cruciferous vegetables (Figure 1) [1–7]. AHR ligation by dietary components likely serves to prime phase I cytochrome P450 metabolism within the gastrointestinal tract and through hepatic first-pass metabolism at the onset of ingestion to detoxify/eliminate potentially harmful dietary constituents [8]. In addition, the emergence of AHR as a pleiotropic factor with activities beyond metabolism suggests that dietary AHR ligands likely influence a wide range of physiological processes such as immune surveillance and microbiota/host interactions [9–11].
Figure 1.
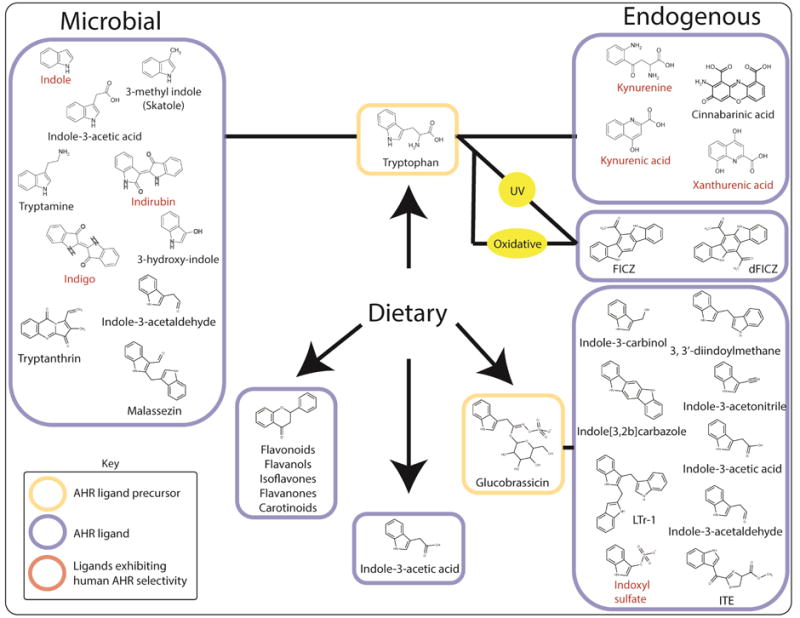
Sources and examples of known dietary, endogenous and microbiota-derived AHR ligands.
The most widely investigated dietary AHR ligands are those generated from the plant glucosinolates such as glucobrassicin a component of cruciferous vegetables. Mechanical breakdown facilitates myrosinase-dependent hydrolysis of glucobrassicin to a number of products, including indole-3-carbinol (I3C) and indole-3-acetonitrile (I3ACN), both of which exhibit AHR agonist activity [7,12–15]. Within the acidic environment of the stomach, I3C is susceptible to acid condensation yielding indolo[3, 2b]carbazole (ICZ), and 3, 3′-diindoylmethane (DIM), both of which exhibit AHR binding potential [13,14,16,17]. These acid condensation products may represent the dominant AHR ligand binding activity in vivo when compared to I3C [18]. Pharmacokinetic data points to rapid absorption but low systemic retention of I3C, whereas ICZ, and DIM are present at steady-state levels due to a combination of higher chemical stability and longer plasma half-life relative to I3C [19,20].
In addition, numerous other dietary constituents including flavones, isoflavones, flavanones, and carotenoids have been shown to elicit AHR activity and are either known or inferred AHR ligands [21–24]. It is clear that the diet represents a rich source of AHR ligands, however this generates a degree of complexity, which makes it difficult to determine the relative contribution of individual AHR ligands with regard to overall physiology. Although ICZ exhibits high-affinity (low nM) AHR binding similar to that exhibited by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the majority of the dietary AHR ligands identified to date are weak ligands by comparison. Additional complexity due to the fact that, despite being AHR ligands, many are characterized as AHR antagonists with regard to canonical AHR signaling. In addition, those that exhibit weak agonist activity may, if present at sufficient concentrations, act as competitive antagonists of more potent ligands e.g. flavonoids [25]. Although manifesting as AHR ligands, it is likely that many dietary components influence signaling through AHR-independent means, thus their contribution to physiology through AHR binding is difficult to interpret, particularly in vivo.
Tryptophan, a precursor to AHR ligands
A theme has emerged, which highlights a central role for the essential amino acid tryptophan as a major physiological reservoir for the synthesis of AHR ligands. A large body of evidence has demonstrated that tryptophan can undergo a range of spontaneous and enzyme-catalyzed conversions provided by the host and its associated microbiota to yield numerous physiologically relevant AHR ligands. Early observations indicating enhanced in vitro and in vivo AHR activity following exposure to ultra-violet radiation implicated light as a factor associated with endogenous AHR ligand production [26–28]. A series of studies identified a number of UV-dependent tryptophan photo-oxidation products, including 6-formyl and 6, 12-diformylindolo[3, 2b]carbazole (FICZ and dFICZ, respectively) as specific, high-affinity AHR ligands (Figure 1) [27,29–32]. Recently, evidence demonstrating UV-independent conversion of tryptophan or its metabolites to FICZ under conditions of oxidative stress implicates a route for extra-dermal AHR ligand generation in the absence of light [33]. In contrast to the spontaneous and therefore unregulated generation of UV-mediated tryptophan photo-oxidation products, studies have identified the major physiological route of tryptophan metabolism as a source of AHR ligands. Processing through the kynurenine pathway accounts for > 90% of tryptophan metabolism and is initially catalyzed by isoforms of the functionally homologous tryptophan-2, 3-dioxygenase (TDO1/2) and indolamine-2, 3-dioxygenase (IDO1/2) enzymes [34,35]. Induction of TDO or IDO within the liver or periphery respectively, leads to tryptophan localized depletion and the production of kynurenine, kynurenic-, xanthurenic- and cinnabarinic acids, each of which displays AHR ligand mediated activity (Figure 1 and 2) [36–39]. Studies have revealed the physiological link between tryptophan and the AHR to be more complex than previously envisioned, with a bidirectional axis existing in which tryptophan metabolites modulate AHR activity and the AHR modulates tryptophan metabolism. The expression of IDO, particularly during inflammatory stress through Toll-like or IFNγ receptor ligation, is elevated through the combinatorial action of STAT1, NFκB and the AHR, thus generating a kynurenine pathway-AHR-IDO-AHR autocrine loop [40–42].
Figure 2. The kynurenine pathway as a source of endogenous AHR ligands.
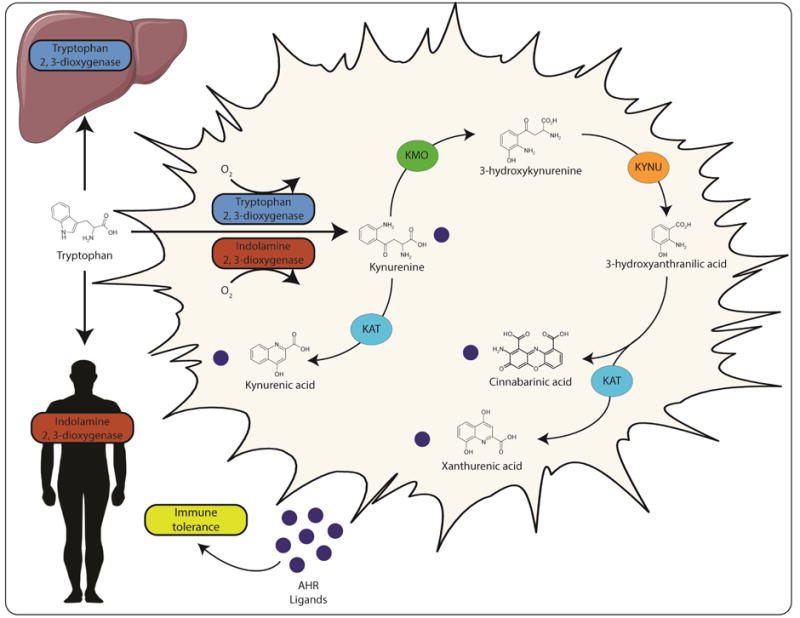
Host tryptophan metabolism through the activity of hepatic tryptophan 2, 3-dioxygenase (TDO) or peripheral indolamine 2, 3-dioxygenase (IDO) yields kynurenine, an AHR ligand. Subsequent metabolism of kynurenine by kynurenine monooxygenase (KMO), kynureninase (KYNU) and kynurenine aminotransferase (KAT) provides additional AHR ligands. These AHR ligands have the capacity to further enhance expression of IDO in an autocrine loop. Activation of the kynurenine pathway is associated with increased immune tolerance and may contribute to gastrointestinal homeostasis.
Pseudo-endogenous AHR ligands
Barrier tissues provide a niche for complex microbial ecosystems. These site-specific microbial populations cooperate and compete with the host organism to generate a dynamic metabolic landscape rich in metabolites, the physiological relevance of which is only now being appreciated and investigated. Evidence supports microbial tryptophan metabolism, particularly within the gastrointestinal tract, as a site for in vivo AHR ligand generation (Figure 1 and 3) [15,43,44]. Bacterial tryptophan uptake, facilitated by transporters, initiates catabolism through tryptophanase and downstream enzymes to generate a succession of tryptophan metabolites that may ultimately modulate AHR function in vivo. Many of the microbiota-derived tryptophan metabolites, including indole, indole-3-acetic acid, indole-3-aldehyde have been demonstrated to be AHR ligands [11,45,46]. Given the abundance and extensive metabolic capacity of bacteria, particularly within the gastrointestinal tract, such ligands are likely present at sufficient concentrations to stimulate AHR activity [43]. Additional tryptophan metabolites including malassezin, tryptanthrin, tryptamine, and 3-methyl indoles are apparent AHR ligands and influence AHR activity, typically assessed through induction of AHR transcriptional targets [43–45,47–50]. Downstream metabolism of microbiota-derived tryptophan metabolites can generate additional AHR ligands e.g. indole can undergo hepatic CYP2E1-mediated conversion to indoxyl, a precursor to indoxyl sulfate, indirubin and indigo, each of which exhibits AHR ligand activity [51–53]. Due to its capacity to bind and respond to bacteria-derived tryptophan metabolites, it is suggested that the AHR may contribute to the surveillance of the microbiota. Although not examined in the context of microbial AHR ligands, studies identify a reduced microbial burden within the gastrointestinal tract of mice exposed to dietary AHR ligands [54]. While, the microbiota is now established as a determinant of overall physiology and associated with many disease states, the contribution of AHR ligand generation by the microbiota is only now being investigated.
Figure 3. Tryptophan metabolism by the gastrointestinal microbiota represents a source of AHR ligands and ligand precursors.
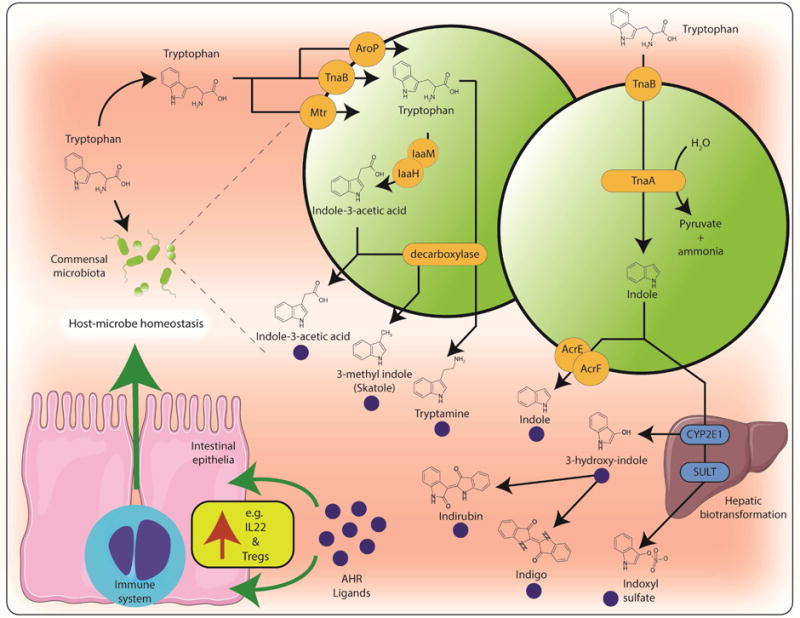
The uptake of luminal tryptophan by the resident gastrointestinal microbiota though amino acid transporters e.g. Mtr, TnaB and AroP provides the substrate for enzymatic conversion by bacterial monooxygenases (IaaM), acetimide hydrolases (IaaH), decarboxylases to generate AHR ligands. In addition, microbial tryptophanse (TnaA) activity can generate the AHR ligand indole. Efflux of bacterial indole by transporters e.g. AcrE and AcrF provides a substrate for host metabolism e.g. CYP2E1 and sulfotransferases (SULT) resulting in the generation of additional AHR ligands and ligand precursors. The repertoire of tryptophan-derived microbial AHR ligands has the capacity to influence host-microbe homeostasis through the AHR-dependent modulation of IL22 expression and immunosuppressive Treg differentiation.
Inter-species variability and intrinsic AHR ligands
Despite the evolutionary conservation of AHR expression from invertebrates to mammals, the gene encoding AHR has undergone functionally significant divergence across and within species [55–57]. Evidence highlights a marked variability regarding the potential for a given ligand to induce AHR activity across different species e.g. indirubin, indoxyl sulfate, indole, kynurenine, kynurenic acid and xanthurenic acid all exhibit greater potential to activate the human AHR when compared to mouse AHR [37,43,53,58,59]. Factors independent of the AHR likely contribute to the variation in AHR ligand-mediated physiology across species. However, evolutionary divergence of functionally critical residues within the AHR protein, particularly the ligand binding domain, likely represent the dominant determinant. In vivo studies performed in rodents supporting a role for intrinsic AHR ligands in physiology, particularly immune regulation, may not present an accurate representation of AHR ligand function in humans. Indeed, data demonstrating that many endogenous and pseudo-endogenous AHR ligands have greater affinity for the human AHR than that of mouse would argue that human sensitivity and physiological responses to such ligands are underestimated. The enhanced sensitivity of the human AHR for such endogenous and pseudo-endogenous ligands relative to rodent AHR is in stark contrast to the situation with exogenous xenobiotic toxicants such as TCDD, which exhibit greater than ten-fold increased affinity for rodent AHR species [60]. Thus, in contrast to the potential underestimation of endogenous ligand-mediated AHR activity, the toxic effects of TCDD and other xenobiotic pollutants to humans are likely overestimated.
AHR and immune signaling
Systemically, activation of the AHR has been shown to either enhance or repress inflammatory signaling and inflammation in a context and cell type specific manner [61–63]. However, in the gastrointestinal tract most studies support an anti-inflammatory role for ligand mediated AHR activation. While the level of AHR activation and the type of AHR ligands present also will likely play a role in the response observed. It is important to point out that many of the studies that propose that the AHR is anti-inflammatory utilized Ahr−/− mice and thus were independent of ligand activation status or the ability of the AHR to exhibit activity in the absence of ligands [64]. Part of the maintenance of a normal host-microbiota axis in the gut involves proper immune surveillance by the host. For example, cytokines and chemokines are pleotropic factors that are key mediators of innate immunity and barrier function. Indeed, the AHR has been shown capable of modulating expression of Il1B, Il6, Ccl20, Ccl1 [65–68]. In addition, prostaglandin G/H synthase 2 (Ptgs2) is also directly regulated by the AHR in combination with other transcription factors [69]. Considering the importance of prostaglandin E2 produced by PTGS2 to maintaining gut homeostasis, the role of the AHR in this pathway needs to be further explored [70]. Thus, the production of AHR ligands either by microbiota or by immune cells could act as a sensor mechanism leading to heightened immune surveillance.
Upon enhanced expression of inflammatory cytokines in the intestinal tract, retinoic acid, along with activation of the AHR, can mediate a dramatic increase in Th17 cell development from CD4+ T cells. Th17 cells express both IL17 and IL22 while AHR agonist treatment leads to an increase in IL22 expressing cells [71,72]. IL22 is an important cytokine in wound healing through stimulating survival of intestinal stem cells and enhancing anti-bacterial peptide production from epithelial cells [73,74]. The precise mechanism of AHR-mediated IL22 expression is not clear, however AHR agonist can stimulate IL22 expression in cultured CD+ T cells in a Notch-dependent manner [75]. Another proposed mechanisms of IL22 induction is through AHR-induced IL23 expression in infected macrophages, which in turn stimulates T cells to produce IL22 [65]. Importantly, the endogenous AHR ligand cinnabarinic acid has been shown to induce IL22 production in CD4+ T cells placed in conditions that lead to Th17 T cell polarization [38].
Intestinal challenge models and AHR ligands
Dextran sodium sulfate (DSS) exposure is often used as a mouse model to study factors that influence inflammatory bowel disease and there is some evidence that this model is relevant to humans [76]. Potent AHR ligands (e.g. TCDD, FICZ, β-naphthoflavone) have been shown to attenuate the severity of symptoms from DSS exposure [77–81]. In these studies a number of possible mechanisms for AHR mediated repression of DSS-mediated toxicity were suggested, including an increase in IL22, PGE2, and regulatory T (Treg) cell levels. Dietary administration of I3C or DIM lessens DSS-mediated colonic inflammation and disease severity, suggesting that production of AHR ligands such as ICZ are protective through AHR activation. This would suggest that foods rich in AHR ligands or ligand precursors would protect against chemically-induced gastrointestinal injury. Dietary cruciferous vegetables are capable of inducing AHR activity and have been shown to attenuate DSS induced toxicity, however, whether these effects are mediated by AHR activation has not been established [82]. The probiotic bacteria Propionibacterium freudenreichii produces the AHR ligand 1,4-dihydroxy-2-naphthoic acid, which has been shown to inhibit DSS-induced colitis [83]. This illustrates the possibility that intestinal bacteria may be capable of producing AHR ligands to improve intestinal health.
Ahr−/− mice are more susceptible to Listeria monocytogenes and Citrobacter rodentium infection compared to Ahr+/+ mice [84,85]. These studies, along with the general chemopreventive properties of AHR ligand treatment on gastrointestinal health would suggest that AHR ligands in the diet may protect against opportunistic bacterial infections. Indeed, a recent study demonstrated that dietary I3C can decrease the level of Clostridium difficile induced lethality in mice, in part through activation of the AHR [86]. It will be important to test other AHR ligands in this infection model. Macrophages are a critical cell type in the intestinal tract directly involved in clearance of bacteria. AHR expression is critical to maintain macrophage survival in the presence of pathogenic bacteria and for robust production of reactive oxygen species (ROS) [87]. In addition, treatment with induces ROS in mitochondria [88]. Studies in Mycobacterium tuberculosis infected macrophages have revealed that activation of the AHR by the endogenous ligand kynurenine reduced bacterial viability [65]. Treatment with kynurenine also induced IL23A in macrophages, which in turn can lead to IL22 production by intraepithelial lymphocytes. These observations would support the concept that the presence of dietary- or bacterially-generated AHR ligands may also aid in the response to pathogenic bacteria.
The 2,4,6-trinitrobenzene sulfonic acid (TNBS)- induced colitis model is widely used to mimic Crohn’s disease and CD4+ T cells play a critical role in the development of disease in this model. Treatment of mice orally with TCDD in the TNBS murine model resulted in decreased inflammation and an increase in Treg cells, which may in part explain the mechanism mediating inhibition of inflammation [89]. FICZ has also been shown to significantly reduce inflammation in a TNBS model, while treatment with an AHR antagonist increases colitis severity [79]. A possible mechanism of these observations is the ability of AHR ligand to increase IL22 expression. A likely mechanism of Treg production is the ability of AHR ligand to induce IDO1 and IDO2 expression in dendritic cells [41].
Conclusions
Studies in rodents clearly support the concept that ligand activation of the AHR is protective from toxic insults that mimic chronic and acute gastrointestinal diseases in rodent models. From a mechanistic standpoint these results appear to be due at least in part to an increase in intraepithelial lymphocyte residence time, elevated IL22 expression and expansion of Treg cell levels within the intestinal tract. While treatment with relatively high levels of AHR levels is chemoprotective, it is not clear whether this protection is conferred from dietary consumption of AHR ligands found in foods consumed in a healthy diet. The role of AHR antagonist, selective AHR modulators, and agonist activities in the gut will need to be further explored. Can the microbiota produce enough AHR ligands to be protective in the intestinal tract, what are the precursors that can lead to AHR ligand production? These questions will need to be addressed in order to assess how knowledge of AHR ligands can lead to selecting foods or therapeutic agents to maintain intestinal and overall health. One important point to consider is that dietary ligands may exhibit the greatest activity in the small intestine prior to absorption, while microbiota generated ligands would be predominantly in the cecum and large intestine. Another issue that has not been adequately addressed is the role of the AHR in intestinal cells of epithelial origin. To further complicate studies on endogenous and microbiota generated ligands is the difference in ligand specificity between the human versus mouse AHR, suggesting that the human AHR may be more responsive to many of these ligands[90]. This observation would indicate that an appropriate humanized AHR mouse model is needed to address these issues. Yet another issue that needs to be considered further is whether high AHR ligand exposure can shift T cell populations to an immune tolerant status, which would be advantageous to attenuate autoimmune diseases. However, this attenuating effect in turn might increase susceptibility to cancer (e.g. colon cancer), and thus this issue should be considered in future studies [91].
Acknowledgments
The authors thank Marcia H. Perdew for excellent editorial assistance. This work was partially supported by National Institutes of Health grants RO1ES004869 and RO1ES019964. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Agriculture and Food Research Initiative Competitive Grant no. 2014-06624 from the USDA National Institute of Food and Agriculture also supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared
Pseudo-endogenous AHR ligands are defined as any metabolite that is produced by the microbiota and are capable of binding to the AHR.
References
- 1.Jeuken A, Keser BJ, Khan E, Brouwer A, Koeman J, Denison MS. Activation of the ah receptor by extracts of dietary herbal supplements, vegetables, and fruits. J Agric Food Chem. 2003;51(18):5478–5487. doi: 10.1021/jf030252u. [DOI] [PubMed] [Google Scholar]
- 2.Wattenberg LW, Loub WD. Inhibition of polycyclic aromatic hydrocarbon-induced neoplasia by naturally occurring indoles. Cancer Res. 1978;38(5):1410–1413. [PubMed] [Google Scholar]
- 3.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44(1):44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 4.Amakura Y, Tsutsumi T, Sasaki K, Nakamura M, Yoshida T, Maitani T. Influence of food polyphenols on aryl hydrocarbon receptor-signaling pathway estimated by in vitro bioassay. Phytochemistry. 2008;69(18):3117–3130. doi: 10.1016/j.phytochem.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 5.De Waard WJ, Aarts JM, Peijnenburg AC, De Kok TM, Van Schooten FJ, Hoogenboom LA. Ah receptor agonist activity in frequently consumed food items. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008;25(6):779–787. doi: 10.1080/02652030701798880. [DOI] [PubMed] [Google Scholar]
- 6.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56(1):5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 7.Johansson G, Gillner M, Hogberg B, Gustafsson JA. The tcdd receptor in rat intestinal mucosa and its possible dietary ligands. Nutr Cancer. 1982;3(3):134–144. doi: 10.1080/01635588109513715. [DOI] [PubMed] [Google Scholar]
- 8.Ito S, Chen C, Satoh J, Yim S, Gonzalez FJ. Dietary phytochemicals regulate whole-body cyp1a1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J Clin Invest. 2007;117(7):1940–1950. doi: 10.1172/JCI31647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Julliard W, Fechner JH, Mezrich JD. The aryl hydrocarbon receptor meets immunology: Friend or foe? A little of both. Front Immunol. 2014;5(458) doi: 10.3389/fimmu.2014.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67(2):259–279. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- 11**.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–385. doi: 10.1016/j.immuni.2013.08.003. Identified a bacterially generated tryptophan metabolite that is an AHR ligand that mediates IL22 production and protect against yeast infection. This study established the importance of bacterial metabolism for the production of AHR ligands that aid in maintaining intestinal homeostasis. [DOI] [PubMed] [Google Scholar]
- 12.Gillner M, Bergman J, Cambillau C, Alexandersson M, Fernstrom B, Gustafsson JA. Interactions of indolo[3,2-b]carbazoles and related polycyclic aromatic hydrocarbons with specific binding sites for 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat liver. Mol Pharmacol. 1993;44(2):336–345. [PubMed] [Google Scholar]
- 13**.Bjeldanes LF, Kim JY, Grose KR, Bartholomew JC, Bradfield CA. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: Comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci U S A. 1991;88(21):9543–9547. doi: 10.1073/pnas.88.21.9543. The first identification and characterization of the major indole-3-carbinol acid mediated condensation products capable of activating the AHR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradfield CA, Bjeldanes LF. Modification of carcinogen metabolism by indolylic autolysis products of brassica oleraceae. Adv Exp Med Biol. 1991;289:153–163. doi: 10.1007/978-1-4899-2626-5_13. [DOI] [PubMed] [Google Scholar]
- 15*.Perdew GH, Babbs CF. Production of ah receptor ligands in rat fecal suspensions containing tryptophan or indole-3-carbinol. Nutr Cancer. 1991;16(3–4):209–218. doi: 10.1080/01635589109514159. Demonstrated for the first time that fecal bacteria can metabolize tryptophan to AHR ligands. [DOI] [PubMed] [Google Scholar]
- 16.Jellinck PH, Forkert PG, Riddick DS, Okey AB, Michnovicz JJ, Bradlow HL. Ah receptor binding properties of indole carbinols and induction of hepatic estradiol hydroxylation. Biochem Pharmacol. 1993;45(5):1129–1136. doi: 10.1016/0006-2952(93)90258-x. [DOI] [PubMed] [Google Scholar]
- 17.De Kruif CA, Marsman JW, Venekamp JC, Falke HE, Noordhoek J, Blaauboer BJ, Wortelboer HM. Structure elucidation of acid reaction products of indole-3-carbinol: Detection in vivo and enzyme induction in vitro. Chem Biol Interact. 1991;80(3):303–315. doi: 10.1016/0009-2797(91)90090-t. [DOI] [PubMed] [Google Scholar]
- 18.Bradfield CA, Bjeldanes LF. Structure-activity relationships of dietary indoles: A proposed mechanism of action as modifiers of xenobiotic metabolism. J Toxicol Environ Health. 1987;21(3):311–323. doi: 10.1080/15287398709531021. [DOI] [PubMed] [Google Scholar]
- 19.Anderton MJ, Manson MM, Verschoyle RD, Gescher A, Lamb JH, Farmer PB, Steward WP, Williams ML. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin Cancer Res. 2004;10(15):5233–5241. doi: 10.1158/1078-0432.CCR-04-0163. [DOI] [PubMed] [Google Scholar]
- 20.Reed GA, Arneson DW, Putnam WC, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A. Single-dose and multiple-dose administration of indole-3-carbinol to women: Pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2477–2481. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- 21.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 22.Ashida H, Fukuda I, Yamashita T, Kanazawa K. Flavones and flavonols at dietary levels inhibit a transformation of aryl hydrocarbon receptor induced by dioxin. FEBS Lett. 2000;476(3):213–217. doi: 10.1016/s0014-5793(00)01730-0. [DOI] [PubMed] [Google Scholar]
- 23.Gradelet S, Astorg P, Leclerc J, Chevalier J, Vernevaut MF, Siess MH. Effects of canthaxanthin, astaxanthin, lycopene and lutein on liver xenobiotic-metabolizing enzymes in the rat. Xenobiotica. 1996;26(1):49–63. doi: 10.3109/00498259609046688. [DOI] [PubMed] [Google Scholar]
- 24.Ciolino HP, Daschner PJ, Yeh GC. Resveratrol inhibits transcription of cyp1a1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998;58(24):5707–5712. [PubMed] [Google Scholar]
- 25*.Zhang S, Qin C, Safe SH. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: Effects of structure and cell context. Environmental health perspectives. 2003;111(16):1877–1882. doi: 10.1289/ehp.6322. Examined the ability of a number of dietary flavonoids to exhibit AHR agonist or antagonist activity that is context specific. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goerz G, Merk H, Bolsen K, Tsambaos D, Berger H. Influence of chronic uv-light exposure on hepatic and cutaneous monooxygenases. Experientia. 1983;39(4):385–386. doi: 10.1007/BF01963137. [DOI] [PubMed] [Google Scholar]
- 27.Helferich WG, Denison MS. Ultraviolet photoproducts of tryptophan can act as dioxin agonists. Mol Pharmacol. 1991;40(5):674–678. [PubMed] [Google Scholar]
- 28.Kiyohara C, Hirohata T. Environmental factors and aryl hydrocarbon hydroxylase activity (cyp1a1 phenotype) in human lymphocytes. J Epidemiol. 1997;7(4):244–250. doi: 10.2188/jea.7.244. [DOI] [PubMed] [Google Scholar]
- 29.Oberg M, Bergander L, Hakansson H, Rannug U, Rannug A. Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol Sci. 2005;85(2):935–943. doi: 10.1093/toxsci/kfi154. [DOI] [PubMed] [Google Scholar]
- 30**.Rannug A, Rannug U, Rosenkranz HS, Winqvist L, Westerholm R, Agurell E, Grafstrom AK. Certain photooxidized derivatives of tryptophan bind with very high affinity to the ah receptor and are likely to be endogenous signal substances. J Biol Chem. 1987;262(32):15422–15427. Established that UV light can produce products from tryptophan that have high affinity for the AHR. [PubMed] [Google Scholar]
- 31.Rannug U, Rannug A, Sjoberg U, Li H, Westerholm R, Bergman J. Structure elucidation of two tryptophan-derived, high affinity ah receptor ligands. Chem Biol. 1995;2(12):841–845. doi: 10.1016/1074-5521(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 32.Wei YD, Rannug U, Rannug A. Uv-induced cyp1a1 gene expression in human cells is mediated by tryptophan. Chem Biol Interact. 1999;118(2):127–140. doi: 10.1016/s0009-2797(98)00118-5. [DOI] [PubMed] [Google Scholar]
- 33.Smirnova A, Wincent E, Vikstrom Bergander L, Alsberg T, Bergman J, Rannug A, Rannug U. Evidence for new light-independent pathways for generation of the endogenous aryl hydrocarbon receptor agonist ficz. Chem Res Toxicol. 2016;29(1):75–86. doi: 10.1021/acs.chemrestox.5b00416. [DOI] [PubMed] [Google Scholar]
- 34.Leklem JE. Quantitative aspects of tryptophan metabolism in humans and other species: A review. The American journal of clinical nutrition. 1971;24(6):659–672. doi: 10.1093/ajcn/24.6.659. [DOI] [PubMed] [Google Scholar]
- 35.Ball HJ, Jusof FF, Bakmiwewa SM, Hunt NH, Yuasa HJ. Tryptophan-catabolizing enzymes – party of three. Front Immunol. 2014;5:485. doi: 10.3389/fimmu.2014.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491. Provided evidence that the tryptophan-2, 3-dioxygenase product, kynurenine, and AHR expression are associated with malignant progression of brain tumors. [DOI] [PubMed] [Google Scholar]
- 37.Dinatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. Kynurenic acid is a potent endogenous ah receptor ligand that synergistically induces interleukin 6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115(1):89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowe MM, Mold JE, Kanwar B, Huang Y, Louie A, Pollastri MP, Wang C, Patel G, Franks DG, Schlezinger J, Sherr DH, et al. Identification of cinnabarinic acid as a novel endogenous aryl hydrocarbon receptor ligand that drives il-22 production. PLoS One. 2014;9(2):e87877. doi: 10.1371/journal.pone.0087877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory t cells. J Immunol. 2010;185(6):3190–3198. doi: 10.4049/jimmunol.0903670. Determined that the IDO metabolite kynurenine is an AHR ligand that can induce AHR-dependent generation of Treg cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litzenburger UM, Opitz CA, Sahm F, Rauschenbach KJ, Trump S, Winter M, Ott M, Ochs K, Lutz C, Liu X, Anastasov N, et al. Constitutive ido expression in human cancer is sustained by an autocrine signaling loop involving il-6, stat3 and the ahr. Oncotarget. 2014;5(4):1038–1051. doi: 10.18632/oncotarget.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375(3):331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Q, Harden JL, Anderson CD, Egilmez NK. Tolerogenic phenotype of ifn-gamma-induced ido+ dendritic cells is maintained via an autocrine ido-kynurenine/ahr-ido loop. J Immunol. 2016;197(3):962–970. doi: 10.4049/jimmunol.1502615. [DOI] [PubMed] [Google Scholar]
- 43.Hubbard TD, Murray IA, Bisson WH, Lahoti TS, Gowda K, Amin SG, Patterson AD, Perdew GH. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci Rep. 2015;5:12689. doi: 10.1038/srep12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R, Safe S. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol. 2014;85(5):777–788. doi: 10.1124/mol.113.091165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heath-Pagliuso S, Rogers WJ, Tullis K, Seidel SD, Cenijn PH, Brouwer A, Denison MS. Activation of the ah receptor by tryptophan and tryptophan metabolites. Biochemistry. 1998;37(33):11508–11515. doi: 10.1021/bi980087p. [DOI] [PubMed] [Google Scholar]
- 46.Cheng Y, Jin UH, Allred CD, Jayaraman A, Chapkin RS, Safe S. Aryl hydrocarbon receptor activity of tryptophan metabolites in young adult mouse colonocytes. Drug Metab Dispos. 2015;43(10):1536–1543. doi: 10.1124/dmd.115.063677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vikstrom Bergander L, Cai W, Klocke B, Seifert M, Pongratz I. Tryptamine serves as a proligand of the ahr transcriptional pathway whose activation is dependent of monoamine oxidases. Mol Endocrinol. 2012;26(9):1542–1551. doi: 10.1210/me.2011-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rasmussen MK, Balaguer P, Ekstrand B, Daujat-Chavanieu M, Gerbal-Chaloin S. Skatole (3-methylindole) is a partial aryl hydrocarbon receptor agonist and induces cyp1a1/2 and cyp1b1 expression in primary human hepatocytes. PLoS One. 2016;11(5):e0154629. doi: 10.1371/journal.pone.0154629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schrenk D, Riebniger D, Till M, Vetter S, Fiedler HP. Tryptanthrins and other tryptophan-derived agonists of the dioxin receptor. Adv Exp Med Biol. 1999;467:403–408. doi: 10.1007/978-1-4615-4709-9_51. [DOI] [PubMed] [Google Scholar]
- 50.Vlachos C, Schulte BM, Magiatis P, Adema GJ, Gaitanis G. Malassezia-derived indoles activate the aryl hydrocarbon receptor and inhibit toll-like receptor-induced maturation in monocyte-derived dendritic cells. Br J Dermatol. 2012;167(3):496–505. doi: 10.1111/j.1365-2133.2012.11014.x. [DOI] [PubMed] [Google Scholar]
- 51.Banoglu E, Jha GG, King RS. Hepatic microsomal metabolism of indole to indoxyl, a precursor of indoxyl sulfate. Eur J Drug Metab Pharmacokinet. 2001;26(4):235–240. doi: 10.1007/BF03226377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adachi J, Mori Y, Matsui S, Takigami H, Fujino J, Kitagawa H, Miller CA, 3rd, Kato T, Saeki K, Matsuda T. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J Biol Chem. 2001;276(34):31475–31478. doi: 10.1074/jbc.C100238200. [DOI] [PubMed] [Google Scholar]
- 53*.Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S, Perdew GH. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry. 2010;49(2):393–400. doi: 10.1021/bi901786x. The uremic toxin 3-indoxyl sulfate was determined to be a relatively high affinity ligand for the human AHR and exhibited lower affinity for the mouse AHR. Structure-activity studies revealed that the presence of the sulfate group greatly increased AHR activation potential; this in contrast to the view that high affinity AHR ligands are hydrophobic planar neutral compounds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54**.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629–640. doi: 10.1016/j.cell.2011.09.025. Intraepithelial lymphocytes (IELs) are important immune cells in barrier tissues; the AHR helps maintain the appropriate number of cells within the intestinal tract. Dietary activation of the AHR in IELs is critical during development to establish intestinal homeostasis, especially in reference to the overall microbial load and composition. [DOI] [PubMed] [Google Scholar]
- 55.Wirgin I, Roy NK, Loftus M, Chambers RC, Franks DG, Hahn ME. Mechanistic basis of resistance to pcbs in atlantic tomcod from the hudson river. Science. 2011;331(6022):1322–1325. doi: 10.1126/science.1197296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hahn ME. Aryl hydrocarbon receptors: Diversity and evolution. Chem Biol Interact. 2002;141(1–2):131–160. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 57*.Hubbard TD, Murray IA, Bisson WH, Sullivan AP, Sebastian A, Perry GH, Jablonski NG, Perdew GH. Divergent ah receptor ligand selectivity during hominin evolution. Mol Biol Evol. 2016;33(10):2648–2658. doi: 10.1093/molbev/msw143. Evidence is provided that the AHR evolved in primates to bind indole derivatives such as indoxy-3-sulfate. The human AHR is unique among primates in expressing an AHR variant that has a dramatic lower affinity for polycyclic aromatic hydrocarbons, while the binding of indole derivatives was conserved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flaveny CA, Murray IA, Chiaro CR, Perdew GH. Ligand selectivity and gene regulation by the human aryl hydrocarbon receptor in transgenic mice. Mol Pharmacol. 2009;75(6):1412–1420. doi: 10.1124/mol.109.054825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flaveny CA, Perdew GH. Transgenic humanized ahr mouse reveals differences between human and mouse ahr ligand selectivity. Molecular and cellular pharmacology. 2009;1(3):119–123. doi: 10.4255/mcpharmacol.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramadoss P, Perdew GH. Use of 2-azido-3-[125i]iodo-7,8-dibromodibenzo-p-dioxin as a probe to determine the relative ligand affinity of human versus mouse aryl hydrocarbon receptor in cultured cells. Mol Pharmacol. 2004;66(1):129–136. doi: 10.1124/mol.66.1.129. [DOI] [PubMed] [Google Scholar]
- 61.Sekine H, Mimura J, Oshima M, Okawa H, Kanno J, Igarashi K, Gonzalez FJ, Ikuta T, Kawajiri K, Fujii-Kuriyama Y. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol Cell Biol. 2009;29(24):6391–6400. doi: 10.1128/MCB.00337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee YH, Lin CH, Hsu PC, Sun YY, Huang YJ, Zhuo JH, Wang CY, Gan YL, Hung CC, Kuan CY, Shie FS. Aryl hydrocarbon receptor mediates both proinflammatory and anti-inflammatory effects in lipopolysaccharide-activated microglia. Glia. 2015;63(7):1138–1154. doi: 10.1002/glia.22805. [DOI] [PubMed] [Google Scholar]
- 63.Kim HO, Kim JH, Chung BY, Choi MG, Park CW. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23(4):278–281. doi: 10.1111/exd.12350. [DOI] [PubMed] [Google Scholar]
- 64.Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor in combination with stat1 regulates lps-induced inflammatory responses. J Exp Med. 2009;206(9):2027–2035. doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Memari B, Bouttier M, Dimitrov V, Ouellette M, Behr MA, Fritz JH, White JH. Engagement of the aryl hydrocarbon receptor in mycobacterium tuberculosis-infected macrophages has pleiotropic effects on innate immune signaling. J Immunol. 2015;195(9):4479–4491. doi: 10.4049/jimmunol.1501141. [DOI] [PubMed] [Google Scholar]
- 66*.DiNatale BC, Schroeder JC, Francey LJ, Kusnadi A, Perdew GH. Mechanistic insights into the events that lead to synergistic induction of interleukin 6 transcription upon activation of the aryl hydrocarbon receptor and inflammatory signaling. J Biol Chem. 2010;285(32):24388–24397. doi: 10.1074/jbc.M110.118570. Demonstrated that the AHR can work in concert with inflammatory transcription factors on the Il6 promoter to synergistically induce Il-6 expression. Indicating that AHR can directly participate in inflammatory signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lahoti TS, Boyer JA, Kusnadi A, Muku GE, Murray IA, Perdew GH. Aryl hydrocarbon receptor activation synergistically induces lipopolysaccharide-mediated expression of proinflammatory chemokine (c-c motif) ligand 20. Toxicol Sci. 2015;148(1):229–240. doi: 10.1093/toxsci/kfv178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.N’Diaye M, Le Ferrec E, Lagadic-Gossmann D, Corre S, Gilot D, Lecureur V, Monteiro P, Rauch C, Galibert MD, Fardel O. Aryl hydrocarbon receptor- and calcium-dependent induction of the chemokine ccl1 by the environmental contaminant benzo[a]pyrene. J Biol Chem. 2006;281(29):19906–19915. doi: 10.1074/jbc.M601192200. [DOI] [PubMed] [Google Scholar]
- 69.Degner SC, Kemp MQ, Hockings JK, Romagnolo DF. Cyclooxygenase-2 promoter activation by the aromatic hydrocarbon receptor in breast cancer mcf-7 cells: Repressive effects of conjugated linoleic acid. Nutr Cancer. 2007;59(2):248–257. doi: 10.1080/01635580701485585. [DOI] [PubMed] [Google Scholar]
- 70.Duffin R, O’Connor RA, Crittenden S, Forster T, Yu C, Zheng X, Smyth D, Robb CT, Rossi F, Skouras C, Tang S, et al. Prostaglandin e(2) constrains systemic inflammation through an innate lymphoid cell-il-22 axis. Science. 2016;351(6279):1333–1338. doi: 10.1126/science.aad9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper t cell population that has abundant production of interleukin 22 and is distinct from t(h)-17, t(h)1 and t(h)2 cells. Nat Immunol. 2009;10(8):864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 72.Brembilla NC, Ramirez JM, Chicheportiche R, Sorg O, Saurat JH, Chizzolini C. In vivo dioxin favors interleukin-22 production by human cd4+ t cells in an aryl hydrocarbon receptor (ahr)-dependent manner. PLoS One. 2011;6(4):e18741. doi: 10.1371/journal.pone.0018741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rubino SJ, Geddes K, Girardin SE. Innate il-17 and il-22 responses to enteric bacterial pathogens. Trends Immunol. 2012;33(3):112–118. doi: 10.1016/j.it.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Aparicio-Domingo P, Romera-Hernandez M, Karrich JJ, Cornelissen F, Papazian N, Lindenbergh-Kortleve DJ, Butler JA, Boon L, Coles MC, Samsom JN, Cupedo T. Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage. J Exp Med. 2015;212(11):1783–1791. doi: 10.1084/jem.20150318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alam MS, Maekawa Y, Kitamura A, Tanigaki K, Yoshimoto T, Kishihara K, Yasutomo K. Notch signaling drives il-22 secretion in cd4+ t cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2010;107(13):5943–5948. doi: 10.1073/pnas.0911755107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melgar S, Karlsson L, Rehnstrom E, Karlsson A, Utkovic H, Jansson L, Michaelsson E. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int Immunopharmacol. 2008;8(6):836–844. doi: 10.1016/j.intimp.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 77.Takamura T, Harama D, Matsuoka S, Shimokawa N, Nakamura Y, Okumura K, Ogawa H, Kitamura M, Nakao A. Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunol Cell Biol. 2010;88(6):685–689. doi: 10.1038/icb.2010.35. [DOI] [PubMed] [Google Scholar]
- 78.Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Activation of aryl hydrocarbon receptor (ahr) leads to reciprocal epigenetic regulation of foxp3 and il-17 expression and amelioration of experimental colitis. PLoS One. 2011;6(8):e23522. doi: 10.1371/journal.pone.0023522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79*.Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, MacDonald TT, Pallone F, Monteleone G. Aryl hydrocarbon receptor-induced signals up-regulate il-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141(1):237–248. 248 e231. doi: 10.1053/j.gastro.2011.04.007. Activation of the AHR with the endogenous ligand FICZ leads to enhanced IL-22 expression and inhibition of TNBS-mediated colitis in mice. [DOI] [PubMed] [Google Scholar]
- 80.Ji T, Xu C, Sun L, Yu M, Peng K, Qiu Y, Xiao W, Yang H. Aryl hydrocarbon receptor activation down-regulates il-7 and reduces inflammation in a mouse model of dss-induced colitis. Dig Dis Sci. 2015;60(7):1958–1966. doi: 10.1007/s10620-015-3632-x. [DOI] [PubMed] [Google Scholar]
- 81.Furumatsu K, Nishiumi S, Kawano Y, Ooi M, Yoshie T, Shiomi Y, Kutsumi H, Ashida H, Fujii-Kuriyama Y, Azuma T, Yoshida M. A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig Dis Sci. 2011;56(9):2532–2544. doi: 10.1007/s10620-011-1643-9. [DOI] [PubMed] [Google Scholar]
- 82.Lippmann D, Lehmann C, Florian S, Barknowitz G, Haack M, Mewis I, Wiesner M, Schreiner M, Glatt H, Brigelius-Flohe R, Kipp AP. Glucosinolates from pak choi and broccoli induce enzymes and inhibit inflammation and colon cancer differently. Food Funct. 2014;5(6):1073–1081. doi: 10.1039/c3fo60676g. [DOI] [PubMed] [Google Scholar]
- 83.Fukumoto S, Toshimitsu T, Matsuoka S, Maruyama A, Oh-Oka K, Takamura T, Nakamura Y, Ishimaru K, Fujii-Kuriyama Y, Ikegami S, Itou H, et al. Identification of a probiotic bacteria-derived activator of the aryl hydrocarbon receptor that inhibits colitis. Immunol Cell Biol. 2014;92(5):460–465. doi: 10.1038/icb.2014.2. [DOI] [PubMed] [Google Scholar]
- 84.Shi LZ, Faith NG, Nakayama Y, Suresh M, Steinberg H, Czuprynski CJ. The aryl hydrocarbon receptor is required for optimal resistance to listeria monocytogenes infection in mice. J Immunol. 2007;179(10):6952–6962. doi: 10.4049/jimmunol.179.10.6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85**.Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334(6062):1561–1565. doi: 10.1126/science.1214914. Demonstrated in mice dietary that AHR activation controls post-natal expansion of RORγt(+) innate lymphoid cells. [DOI] [PubMed] [Google Scholar]
- 86.Julliard W, De Wolfe TJ, Fechner JH, Safdar N, Agni R, Mezrich JD. Amelioration of clostridium difficile infection in mice by dietary supplementation with indole-3-carbinol. Ann Surg. 2016 doi: 10.1097/SLA.0000000000001830. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kimura A, Abe H, Tsuruta S, Chiba S, Fujii-Kuriyama Y, Sekiya T, Morita R, Yoshimura A. Aryl hydrocarbon receptor protects against bacterial infection by promoting macrophage survival and reactive oxygen species production. International immunology. 2014;26(4):209–220. doi: 10.1093/intimm/dxt067. [DOI] [PubMed] [Google Scholar]
- 88.Senft AP, Dalton TP, Nebert DW, Genter MB, Puga A, Hutchinson RJ, Kerzee JK, Uno S, Shertzer HG. Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free radical biology & medicine. 2002;33(9):1268–1278. doi: 10.1016/s0891-5849(02)01014-6. [DOI] [PubMed] [Google Scholar]
- 89.Benson JM, Shepherd DM. Aryl hydrocarbon receptor activation by tcdd reduces inflammation associated with crohn’s disease. Toxicol Sci. 2011;120(1):68–78. doi: 10.1093/toxsci/kfq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hubbard TD, Murray IA, Perdew GH. Indole and tryptophan metabolism: Endogenous and dietary routes to ah receptor activation. Drug Metab Dispos. 2015;43(10):1522–1535. doi: 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barth SD, Schulze JJ, Kuhn T, Raschke E, Husing A, Johnson T, Kaaks R, Olek S. Treg-mediated immune tolerance and the risk of solid cancers: Findings from epic-heidelberg. Journal of the National Cancer Institute. 2015;107(11):djv224. doi: 10.1093/jnci/djv224. [DOI] [PubMed] [Google Scholar]


