Abstract
Objective:
The prognosis of bladder cancer patients with positive lymph node (LN) disease is affected by both the extent of lymphadenectomy and LN density retrieved during radical cystectomy. This study aimed at assessing the differences in LN metastasis between patients who presented primarily with muscle-invasive transitional cell carcinoma of the bladder “de novo disease” versus “progressive disease.” The latter is defined as patients who progressed to muscle-invasive bladder cancer (MIBC) following prior conservative management of a non-muscle-invasive disease.
Methods:
Data were prospectively collected from consecutive 41 radical cystectomies that were divided into two groups: Group I included de novo MIBC cases and Group II included progressive MIBC cases.
Results:
The median age was 60 years (44-75). Thirty-four patients exhibited de novo disease versus 7 patients who presented as progressive MIBC with a median duration of 9 months between the resection of the first non-invasive tumor and the diagnosis of progressive MIBC (range: 6-56 months). The median number of retrieved LNs in both groups was 15 LNs (range: 4-36). Ten patients (24.39%) had positive pathological LN disease; distributed as 9 patients in Group I and 1 patient in Group II. The median LN density of LN-positive patients was 15.73% (6.46 % in Group I, 28.57% in Group II). Five patients had LN density >20%.
Conclusion:
Although non-muscle-invasive urothelial bladder tumor may progress to muscle-invasive disease, it still carries less aggressive course than de novo MIBC based on differences in LN metastasis and density.
Keywords: Bladder cancer, lymph node density, non-muscle-invasive bladder tumor, progressive muscle-invasive bladder tumor
Introduction
Urinary bladder cancer (BCa) is the most common urinary tract malignancy, with transitional cell carcinoma (TCC) being the most common pathologic subtype. Approximately, 15% of patients who present with muscle-invasive bladder cancer (MIBC) have a history of non-muscle-invasive cancer.1 However, most of the diagnosed cases of MIBC (80% to 90%) present de novo (i.e., without history of non-invasive disease).1
Radical cystectomy (RC) with bilateral pelvic lymph node dissection (PLND) remains the gold standard treatment of MIBC,2 which comprises 25% of BCa patients.3 Approximately, 25% of patients, who undergo RC for clinical N0M0 disease, present with LN-positive disease according to pathologic staging.4 The goal of PLND during the radical surgery is to remove the regional LN, particularly the typically affected sites.4,5
Herr6 and Stein et al.7 first described the concept of lymph node (LN) density for BCa, which is the number of LNs containing metastatic deposits divided by the total number of nodes removed and examined. It takes into account two important prognostic factors; the LN tumor burden and the meticulousness/extent of the LN dissection.8
Recently, it has been stated that extranodal extension in LN-positive patients, who underwent RC for urothelial MIBC, is an independent predictor of both cancer recurrence and cancer-specific mortality.9 This finding, together with final histopathological results and the extent of LN removal, may help with clinical decision of enrollment of such patients in trials of adjuvant therapy together with tailored follow-up post RC.9
The aim of our series was to study the possible differences of LN metastasis in muscle-invasive TCC of urinary bladder between de novo and progressive MIBC disease.
Methods
The study was designed as a prospective consecutive study in which clinical and histopathological data were gathered from 41 patients who presented to our tertiary center with muscle-invasive TCC of the urinary bladder between May 2013 and November 2014. All patients underwent RC with bilateral standard PLND after full informed consent. This study was reviewed and approved by the research ethics committee of the Alexandria University. Each patient was subjected to detailed history taking (BCa risk factors, previous transurethral resection [transurethral resection of bladder tumor (TURBT)], and intravesical therapy), clinical examination, laboratory workup, performance status assessment, radiological evaluation (multiphasic computed tomography ± magnetic resonance imaging), and bimanual examination under anesthesia. Patients who received neoadjuvant chemotherapy were excluded from the study.
Patients were divided into two groups:
Group I de novo MIBC: Included 34 patients who presented primarily with muscle invasive TCC of the bladder.
Group II progressive MIBC: Included 7 patients who progressed to MIBC following prior history of conservative treatment for non-muscle-invasive disease (NMIBC). All patients underwent RC after stage pT2 disease had been documented pathologically from the specimen collected at the last TURBT.
High volume surgeons (3 surgeons) performed RC and bilateral standard PLND. The cystectomy specimen and dissected LNs were sent in separate labeled bottles to a single experienced uropathologist. Pathologic evaluation fulfilled the following points: BCa stage, grade, pathologic subtype, total number of retrieved LNs, and number of positive LNs, if any. LN density was calculated using the following formula: Number of positive LNs times 100 divided by the total number of removed LNs.
Results
Our series included 41 patients with mean age of 58.90 ± 7.10 years (44-75). Table 1 describes the demographic criteria and performance status of both studied groups. Thirty-four patients (83%) exhibited de novo MIBC Group I. Group II included 7 patients (17%) who started with NMIBC disease and progressed to MIBC. Both groups were matched for age, sex, and Karnofsky performance status. In Group II, the median and mean duration between the resection of the first non-invasive tumor and the diagnosis of stage ≥T2 disease was 9 and 17.85 ± 16.64 months, respectively (range 6-56 months). The initial tumor grade and stage in the progressive group were low-grade papillary pT1 in one patient, high-grade non-papillary pT1 in 5 patients, and extensive dysplasia with carcinoma in situ in another patient. All patients with progressive MIBC received at least one course (6 doses) of intravesical Bacillus Calmette-Guerin therapy. However, none of them received maintenance doses.
Table 1.
Demographic criteria and performance status (%) of both study groups
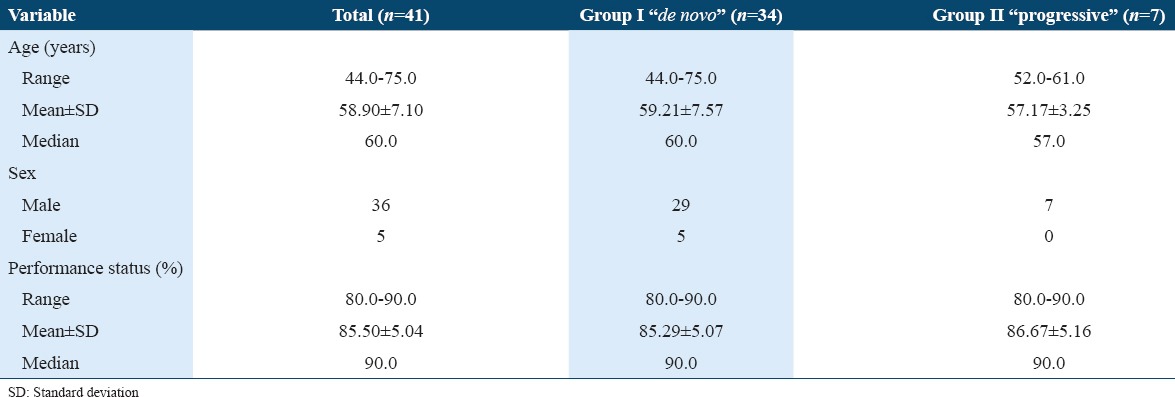
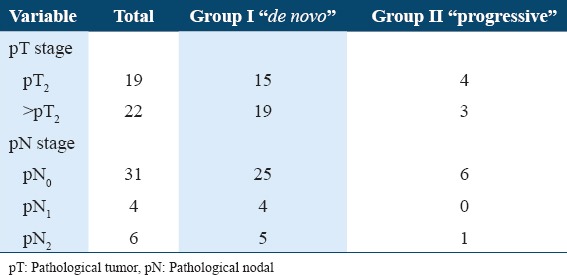
Fifteen patients (15/34) were staged as pT2 in Group I versus 4 patients (4/7) in Group II. Nineteen patients (19/34) in Group I were staged as >pT2 versus 3 patients (3/7) in Group II. The mean number of overall retrieved LNs was 16.70 ± 8.72 (4-36). Group I showed 9 patients with positive LNs representing 21.95% (9/41) of the whole cohort. Four had single regional LN metastasis (pN1) while the other five were staged as (pN2). Group II showed only one patient who had nodal metastasis (Table 2). All positive LNs showed subcapsular sinusoidal involvement. Negative LNs showed reactive follicular hyperplasia and sinus histiocytosis.
Table 2.
Comparison between the two studied groups according to pT and pN stages
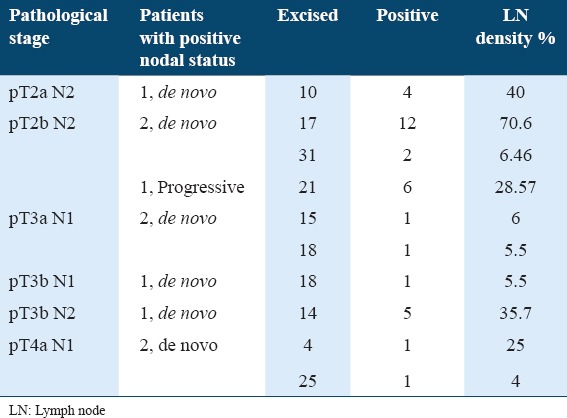
The mean and median LN density of pN+ patients was 22.79% ± 21.78 and 15.73%, respectively (range: 4-70.6%). Using LN density with a cutoff value of 20%, which revealed an independent influence on cancer specific survival, 5 out of 10 pN+ patients displayed LN density >20%. Table 3 shows the comparison between the two studied groups according to total number of dissected LNs, tumor-positive LNs, and LN density. Table 4 illustrates pathological characteristics of patients with LN disease at cystectomy.
Table 3.
Comparison between the two studied groups according to total number of dissected LNs, tumor-positive LNs, and LN density
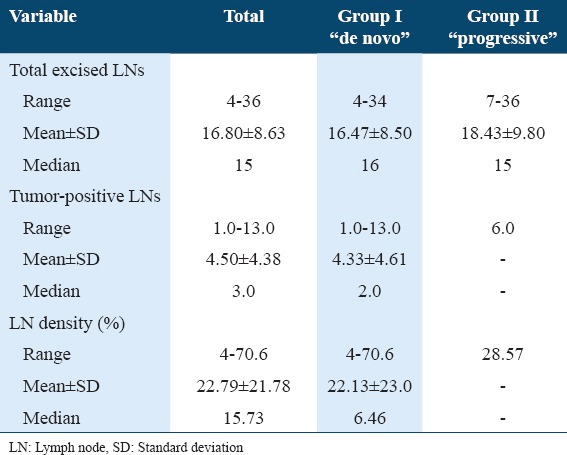
Table 4.
Pathological characteristics of patients with LN disease at cystectomy
Discussion
To the best of our knowledge, the current prospective study is the first to investigate the difference of LN density between de novo and progressive muscle-invasive TCC of the urinary bladder. We were only concerned to study LN malignant infiltration in both groups to clarify the actual need of neoadjuvant chemotherapy for either disease entity; de novo or progressive urothelial MIBC before RC.
Neoadjuvant chemotherapy is recommended in patients with clinically operable, muscle-invasive N0 M0, urothelial BCa before definitive surgery.10 There are many advantages of neoadjuvant chemotherapy. It is delivered at the earliest time-point when the low burden of micrometastatic disease is expected. Tolerability of chemotherapy is expected to be better before cystectomy rather than after. Micrometastatic disease might respond to neoadjuvant therapy and reveal favorable pathologic status including negative LN status and negative surgical margins.11-13 However, there are also potential disadvantages of neoadjuvant chemotherapy including that approximately 50% of clinically N0 M0 patients are without micrometastatic disease, thus receiving unnecessary treatment. Furthermore, the delay in RC may compromise outcomes in non-chemotherapy-responsive patients.11-13
A population-based study of the effect of neoadjuvant chemotherapy on perioperative outcomes in BCa patients, treated with RC, revealed neoadjuvant chemotherapy, when compared to RC alone, is not associated with higher perioperative morbidity or mortality.14 Our prospective study revealed significant difference in LN metastasis of de novo compared to progressive invasive tumors (26.5% and 14.3%, respectively). Based on this result, neoadjuvant chemotherapy may be considered as overtreatment for progressive invasive patients; however, a larger cohort study is recommended to justify such statement.
Many clinical studies evaluated the prognosis of patients with muscle-invasive TCC after RC. However, only few series studied the prognostic difference between de novo and progressive MIBC tumors. Still, patients with de novo and progressive MIBC are treated equally in normal daily practice where RC and bilateral PLND remain the gold standard. In 2001, Vaidya et al.15 found a 2-year survival rate of 49% for those with de novo invasive tumors and 79% for those with progressive lesions. However, in 2003, these data were challenged by Schrier et al.1 who found a statistically significant decreased survival for patients with progressive tumors (the 3- and 5-year survival rates are 67% and 55%, respectively, for patients with a primary invasive tumor and 37% and 28%, respectively, for patients with a progressive invasive tumor).
In 2004, May et al.16 found the overall survival rate after 3 and 5 years was 59% and 50% for progressive tumors and 52% and 46% for de novo tumors. They did not observe any statistically significant difference between these parameters concluding that progressive tumors do not have a better prognosis than initially muscle-invasive tumors. In 2007, Türkölmez et al. could not reveal a significant difference between the two groups. Two-year cancer-specific survival rate for the de novo and progressive tumors was 75% and 72%, respectively. During follow-up, they mentioned that the decrease in the survival rate was greater in the progressive group although no statistical significance was observed (5-year cancer-specific survival rate of 54% and 43%, respectively).17
In 2012, Kotb et al. conducted a retrospective study on 1150 patients and concluded that patients with progressive MIBC had better clinical and pathological outcomes than patients presenting with de novo MIBC.18 All aforementioned studies were retrospective. The current study is a prospective one, and it revealed that LN metastasis was readily more identifiable in the de novo MIBC. However, it is noteworthy that the group of progressive MIBC included a single pN+ patient who initially presented with high-grade non-papillary pT1 BCa.
The LN positive status was studied also by El-Abbady et al.19 They compared 16 patients with progressive MIBC with 20 patients who were diagnosed with de novo MIBC, all undergoing cystectomy. On meticulous histopathological examination, they found that patients who underwent previous transurethral resections had significantly more local spread of malignant cells into the bladder muscle as compared to patients with de novo invasive tumors. Since they could demonstrate that intravesical pressure reaches pressures as high as 80 cm water, they suggested some malignant cells penetrate through the denuded urothelium during resection as a result of high intravesical pressures.
Various studies pointed to the prognostic value of LN density in MIBC. LN density is stated to be superior to TNM nodal status in predicting disease-specific survival following RC for BCa.20 Because the number of nodes excised and pathologically examined may directly influence the TNM nodal stage and the absolute number of positive LNs, LN density is thought to be a superior prognostic factor that is less influenced by confounding from the number of reported LNs. We used a cutoff value of 20% for LN density to stratify pN+ patients into two groups, either >20% or <20%. Five patients had LN density >20%, and the only pN+ progressive case showed density of 28.57%.
Conclusion
Although non-muscle-invasive urothelial bladder tumor may progress to a muscle-invasive disease, it still may carry less aggressive course rather than de novo MIBC based on the difference in risk of LN metastasis and density.
References
- 1.Schrier BP, Hollander MP, van Rhijn BW, Kiemeney LA, Witjes JA. Prognosis of muscle-invasive bladder cancer:Difference between primary and progressive tumours and implications for therapy. Eur Urol. 2004;45:292–6. doi: 10.1016/j.eururo.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Yafi FA, Steinberg JR, Kassouf W. Contemporary management of muscle-invasive bladder cancer. Int J Clin Oncol. 2008;13:504–9. doi: 10.1007/s10147-008-0788-9. [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J. European Association of Urology (EAU). EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008;54:303–14. doi: 10.1016/j.eururo.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 4.Leissner J, Ghoneim MA, Abol-Enein H, Thüroff JW, Franzaring L, Fisch M, et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer:Results of a prospective multicenter study. J Urol. 2004;171:139–44. doi: 10.1097/01.ju.0000102302.26806.fb. [DOI] [PubMed] [Google Scholar]
- 5.Fang AC, Ahmad AE, Whitson JM, Ferrell LD, Carroll PR, Konety BR. Effect of a minimum lymph node policy in radical cystectomy and pelvic lymphadenectomy on lymph node yields, lymph node positivity rates, lymph node density, and survivorship in patients with bladder cancer. Cancer. 2010;116:1901–8. doi: 10.1002/cncr.25011. [DOI] [PubMed] [Google Scholar]
- 6.Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol. 2003;169:943–5. doi: 10.1097/01.ju.0000032474.22093.06. [DOI] [PubMed] [Google Scholar]
- 7.Stein JP, Cai J, Groshen S, Skinner DG. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy:Concept of lymph node density. J Urol. 2003;170:35–41. doi: 10.1097/01.ju.0000072422.69286.0e. [DOI] [PubMed] [Google Scholar]
- 8.Quek ML, Flanigan RC. The role of lymph node density in bladder cancer prognostication. World J Urol. 2009;27:27–32. doi: 10.1007/s00345-008-0347-z. [DOI] [PubMed] [Google Scholar]
- 9.Fajkovic H, Cha EK, Jeldres C, Robinson BD, Rink M, Xylinas E, et al. Extranodal extension is a powerful prognostic factor in bladder cancer patients with lymph node metastasis. Eur Urol. 2013;64:837–45. doi: 10.1016/j.eururo.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Stenzl A, Cowan NC, De Santis M, Kuczyk MA, Merseburger AS, Ribal MJ, et al. Treatment of muscle-invasive and metastatic bladder cancer:Update of the EAU guidelines. Eur Urol. 2011;59:1009–18. doi: 10.1016/j.eururo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Sternberg CN, Pansadoro V, Calabrò F, Schnetzer S, Giannarelli D, Emiliozzi P, et al. Can patient selection for bladder preservation be based on response to chemotherapy? Cancer. 2003;97:1644–52. doi: 10.1002/cncr.11232. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Ortiz RF, Huang WC, Mick R, Van Arsdalen KN, Wein AJ, Malkowicz SB. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol. 2003;169:110–5. doi: 10.1016/S0022-5347(05)64047-5. [DOI] [PubMed] [Google Scholar]
- 13.Stein JP. Contemporary concepts of radical cystectomy and the treatment of bladder cancer. J Urol. 2003;169:116–7. doi: 10.1016/S0022-5347(05)64048-7. [DOI] [PubMed] [Google Scholar]
- 14.Gandaglia G, Popa I, Abdollah F, Schiffmann J, Shariat SF, Briganti A, et al. The effect of neoadjuvant chemotherapy on perioperative outcomes in patients who have bladder cancer treated with radical cystectomy:A population-based study. Eur Urol. 2014;66:561–8. doi: 10.1016/j.eururo.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Vaidya A, Soloway MS, Hawke C, Tiguert R, Civantos F. De novo muscle invasive bladder cancer:Is there a change in trend? J Urol. 2001;165:47–50. doi: 10.1097/00005392-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 16.May M, Helke C, Nitzke T, Vogler H, Hoschke B. Survival rates after radical cystectomy according to tumor stage of bladder carcinoma at first presentation. Urol Int. 2004;72:103–11. doi: 10.1159/000075962. [DOI] [PubMed] [Google Scholar]
- 17.Türkölmez K, Tokgöz H, Resorlu B, Köse K, Bedük Y. Muscle-invasive bladder cancer:Predictive factors and prognostic difference between primary and progressive tumors. Urology. 2007;70:477–81. doi: 10.1016/j.urology.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Kotb AF, Kovac E, Kassouf W, Chin J, Fradet Y, Izawa J, et al. Radical cystectomy for clinically muscle invasive bladder cancer:Does prior non-invasive disease affect clinical outcomes? orld J Urol. 2012;30:761–7. doi: 10.1007/s00345-012-0832-2. [DOI] [PubMed] [Google Scholar]
- 19.El-Abbady AA, Shoukry MS, Hanno AG, Younis LK, Abdel-Rahman M. Repeated transurethral resection of recurrent superficial bladder tumors - Does it affect the spread and stage of the tumor? Scand J Urol Nephrol. 2002;36:60–4. doi: 10.1080/003655902317259382. [DOI] [PubMed] [Google Scholar]
- 20.Kassouf W, Agarwal PK, Herr HW, Munsell MF, Spiess PE, Brown GA, et al. Lymph node density is superior to TNM nodal status in predicting disease-specific survival after radical cystectomy for bladder cancer:Analysis of pooled data from MDACC and MSKCC. J Clin Oncol. 2008;26:121–6. doi: 10.1200/JCO.2007.12.9247. [DOI] [PubMed] [Google Scholar]


