Abstract
Müller cells are the major supportive and protective glial cells across the retina. Unlike in fish, they have lost the capacity to regenerate the retina in mammals. But, mammalian Müller cells still retain certain retinal stem cell properties with various degree of self-renewal and differentiation potentials, and thereby held a merit in cell-based therapies for treating retinal degeneration diseases. In our laboratory, we use an enzymatic procedure to isolate, purify, and culture mouse Müller cells.
Keywords: Mouse, Müller glial cells, Stem cells, Retinal cell isolation, Primary cell culture, Worthington Papain Kit
Background
Müller glial cell is a major lineage in the retina that functions to maintain retinal homeostasis through synthesis of neurotrophic factors, uptake and recycle of neurotransmitters, spatial buffering of ions, and maintenance of the blood-retinal barrier ( Bringmann et al., 2006 ; De Melo Reis et al., 2008 ). Müller glia serve as retinal progenitor/stem cells in fish and, to a limited extent, in birds (Vihtelic and Hyde, 2000; Fischer and Reh, 2001). But, mammalian Müller cells have lost such a capacity to regenerate the retina, though still retain certain properties of adult stem cells such as proliferation upon a retinal damage. Researches in restoring of the lost capacity of mammalian Müller cells to repair retinal damage and understanding of the underlying mechanism are undertaken in laboratories with primary cells isolated from model animal retinas. Proteolytic enzymes are widely used in Müller cell dissociation and papain is less damaging and more effective than other proteases. Sarthy and Lam developed a method for dissociation and separation of glial cells with papain digestion followed by gentle mechanical dissociation, they found that among the enzymes used for dissociating turtle retina, papain produced the least trauma (Sarthy and Lam, 1978).
Materials and Reagents
Cell culture dishes: 35 x 10 mm (Corning, catalog number: 430166)
15 ml centrifuge tubes (Corning, catalog number: 430790)
Tipone®pipette tips (USA Scientific, catalog number: 1126-7810)
5 ml pipets (Corning, Falcon®, catalog number: 357543)
2 ml cryopreservation vials (Corning, catalog number: 430659)
Alcohol Prep Pads (PDI, catalog number: B33905)
Animals: 2- to 4-week-old mice
-
Worthington Papain Kit (papain dissociation system) (Worthington Biochemical, catalog number: LK003150)
Note: The components of kit include vial 1 (Sterile Earle’s Balanced Salt Solution, EBSS), vial 2 (Papain containing L-cysteine and EDTA), vial 3 (Deoxyribonuclease I, DNase), and vial 4 (Ovomucoid protease inhibitor with bovine serum albumin).
70% ethanol
Phosphate buffered saline (PBS) (Sigma-Aldrich, catalog number: P4417-100TAB)
Penicillin-streptomycin (Pen/Strep) (10,000 μg/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122)
Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, Cellgro®, catalog number: 10-013-CV)
Fetal bovine serum (FBS) (GE Healthcare, HyCloneTM, catalog number: SH30071.03)
Gelatin (Sigma-Aldrich, catalog number: G1890-100G)
0.25% trypsin ethylenediaminetetraacetic acid (EDTA) solution (Mediatech, Cellgro®, catalog number: 25-053-CI)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418)
Cell culture medium (see Recipes)
0.1% gelatin solution (see Recipes)
Cell freezing medium (see Recipes)
Equipment
Pipettes (Eppendorf)
Pipet-aid (Drummond)
Single Edge Blade (Sparco, catalog number: SPR11820)
-
Dissection forceps and scissors
Iris Scissors Sharp Straight (Storz Ophthalmic Instruments, catalog number: E3344)
Castroviejo Suturing Forceps 0.12 mm (Storz Ophthalmic Instruments, catalog number: E1796)
37 °C, 5% CO2 cell culture incubator (Thermo Fisher Scientific, Thermo ScientificTM, model: Model 3250)
37 °C incubator (VWR, model: 1545)
Allegra bench-top centrifuge (Beckman Coulter, model: Allegra® X-15R)
Lab quake rotisserie shaker (Barnstead Thermolyne LabQuake, model: 4152110)
Inverted routine microscope (Nikon Instruments, model: Eclipse TS100)
-
Stereo binocular microscope (Olympus, model: SZ40)
Note: Mice are euthanized by carbon dioxide asphyxiation, a carbon dioxide source, regulated dispenser, and euthanasia chamber. All animal manipulations are conducted in accordance with the policies and guidelines set forth by the Institutional Animal Care and Use Committee (IACUC) were approved by the University of Louisville, Louisville, Kentucky, USA.
Procedure
-
Preparation of papain dissociation system
Prepare Worthington Papain Kit according to the manufacturer’s instructions.
Add 32 ml of EBSS (vial 1) to the albumin ovomucoid inhibitor mixture (vial 4). Completely dissolve, then store in a 4 °C refrigerator and use it within 1 week.
Add 5 ml of EBSS (vial 1) to a papain vial (vial 2), place vial 2 in a 37 °C incubator (or water bath) for 10 min or until the papain is completely dissolved.
Add 500 µl of EBSS (vial 1) to a DNase vial (vial 3), mix gently, and then add 250 µl of the mixture to the papain vial 2 saved at step A3. The remaining 250 µl will be used in Procedure C, step C5.
Transfer the above mixed vial 2 to a 15 ml conical centrifuge tube.
-
Mouse eye dissection
Disinfect dissection forceps and scissors with 70% ethanol and burn them to dry.
Prepare three dishes containing 2 ml of PBS and 1% penicillin-streptomycin, and mark them with numbers as shown in Figure 1.
At least 2 mice are euthanized by CO2 asphyxiation and then placed onto a sterile surgical pad. Disinfect surface of the eyelid with 70% alcohol preparation pads.
Mouse eyeballs with the optical bundles are extracted by inserting the forceps along the socket until the optical nerve bundle, and placed in the first dish for 10 min (Figure 2A).
Under a stereo binocular microscope, carefully remove the muscle, fascia and conjunctiva tissues around the eyeballs in the first dish, and then transfer them to the second dish (Figure 2B, Video 1).
Eyeball dissection: remove the anterior portion of the eye, including the cornea, the iris and the lens, reserve the posterior eye cup in the second dish (Figure 2C, Video 1).
From the remaining posterior eyecup, the retinas are dissected free of the retinal pigment epithelium (RPE) in the third dish (Figure 2D, Video 1).
-
Isolation and culture of Müller cell
Place the retina in a 15 ml conical centrifuge tube containing papain and DNase solution saved at step A5. Cut the retina into pieces as small as possible with a single edge blade (Figure 3A).
Incubate the tube containing the tissue in a 37 °C incubator with gently shaking by a constant shaker for 60 min until the solution becomes clear (Figure 3B).
Triturate the mixture with a 5 ml pipette for 2-3 times. Allow any pieces of undigested tissue residues to settle to the bottom of the tube.
Carefully transfer the cloudy cell suspension to a 15-ml conical centrifuge tube, and centrifuge at 300 × g for 5 min at room temperature. During this time, prepare cell culture medium to resuspend the pelleted cells.
Mix medium in a sterile tube with 2.7 ml of EBSS (vial 1), 300 µl of reconstituted albumin-ovomucoid inhibitor solution (vial 4) saved at step A2, 150 µl of DNase solution (vial 3) saved at step A4.
Discard the supernatant and then immediately resuspend the cell pellet by adding the medium mix at step C5.
Add 5.0 ml of the albumin-inhibitor solution (vial 4) to the centrifuge tube, carefully lay the cell suspension on top, then centrifuge at 70 × g for 6 min at room temperature. The interface between the two layers of the gradients should be clearly visible. Dissociated cell pellet is at the bottom of the tube while the membrane fragments remain at the interface.
Discard the supernatant and immediately resuspend the pelleted cells in the culture medium (DMEM) with 10% fetal bovine serum (FBS) and 1% Pen/strep antibiotics (see Recipes).
Prepare 100 ml of 0.1% gelatin in PBS (see Recipes), add 1 ml or enough to cover the bottom of a 35-mm dish to coat the surface, leave the coating dish with the lid on in a 37 °C cell culture incubator for over 30 min, aspirate the coating solution and the coated dish is ready for use.
The dissociated cells are collected and cultured in the above gelatin-coated dish at 5.5% CO2. The primary cells before passage (P0) are actually a mixture of Müller cells, photoreceptors, and other retinal neurons.
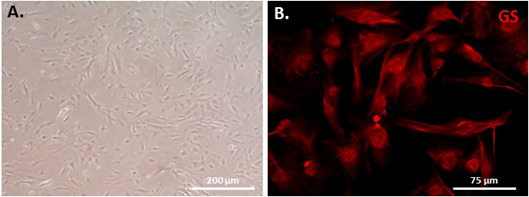
After plating, adherent cells are maintained for 7 days at 37 °C. The medium is replaced every 5 days until cells are confluent and passaged at 1:1 ratio to a fresh dish coated with 0.1% gelatin (Figure 4A). Adherent Müller glial cells become relatively pure after 2 to 3 passages identified by cell morphology and immunostaining with glutamine synthetase (GS, Müller glial cell marker) (Figure 4B).
-
Cell cryopreservation in liquid nitrogen
Remove the culture medium from confluent monolayer-culture dish.
Rinse the dish twice with PBS at room temperature.
Add 0.75 ml or 2 ml of 0.25% trypsin solution to a 6-cm or 10-cm dish, respectively, or enough amount to cover the monolayer.
Incubate in a 37 °C incubator for 1-2 min until cells come off the dish after gently striking the dish to a bench edge.
Add the culture medium that contains 10% FBS, 2-3 times of the amount of the added trypsin, and gently mix for a minute, and transfer to a 15 ml tube.
Centrifuge at 500 × g for 10 min to collect cell pellet.
Discard the supernatant, and re-suspend the cell pellet in 1-3 ml of cell freezing medium (see Recipes) (usually 1 ml per 2 x 105 cells).
Aliquot into 2 ml cryopreservation vial.
Place the vial in a -20 °C freezer for 1 day, then transfer to -80 °C for 1 day, and finally store in a liquid nitrogen tank for long-term storage.
Figure 1. Prepare three dishes, and mark them with numbers.
Figure 2. Eyeball dissection.
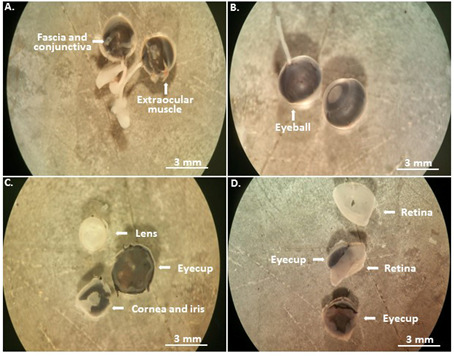
The enucleated mouse eyeballs are first immersed in PBS with the antibiotics for disinfection (A); and then transferred to the second dish (B) to remove the most anterior eye tissues (C), and to isolate the retina in the third dish (D).
Video 1. Mouse eyeball dissection.
Figure 3. The medium turns to be cloudy before incubation (A) and most pieces of the retina disappear after incubation (B).
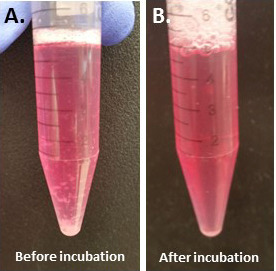
Figure 4. Mouse Müller monolayer cells cultured for 10 days after one passage (A) and the cultured cells are stained with Müller cell-specific marker GS (B).
Data analysis
Using the above procedure, we successfully obtained large number of Müller cells isolated from 4 C57BL/6 mice (total 8 eye cups) by continuously passaging the primary cells at 1:1 ratio until passage 6 (P6) when most cells manifested a stress-induce premature senescence (SIPS) phenotype–large and flat with an obvious heterochromatin foci nucleus and positive for β-galactosidase activity. These senescent Müller cells were not proliferative, but could survive in culture for a long period of time if keeping medium timely refreshed, but eventually died in 3-4 months. Cells should be preserved in liquid nitrogen tank for a long-term storage if not used for month, and could be recovered by directly thawing the frozen cell vials in a 37 °C water bath and thereafter seeding cells in culture with little loss of viability. The percentage of the Müller cell purity differs from passage to passage because other retinal neural cells will not survive under such a culture condition. The estimation of the purity after 2 times of passage is more than 95%.
Notes
All tissue and cell manipulations must be under sterile condition either in a tissue dissecting or cell culture hood with surface disinfected by 70% ethanol.
We strongly recommend to collect and dissect two eyeballs together from the same mouse, and to isolate and culture Müller cells separately from other mice to minimize possible cross contamination.
The primary cells are including Müller cells, photoreceptors and other neurons. Photoreceptor and other neuron cells cannot be adapted for adherent culture and will go apoptosis after passage 2 (P2).
The dish for culture is specifically coated with 0.1% gelatin to allow Müller cells to better adhere.
Recipes
-
Cell culture medium
Dulbecco’s modified Eagle’s medium (DMEM)
10% fetal bovine serum (FBS)
1% penicillin-streptomycin (Pen/Strep)
-
0.1% gelatin solution
100 ml of dH2O plus 0.1 g of gelatin, autoclaved
-
Cell freezing medium
Dulbecco’s modified Eagle’s medium (DMEM)
20% fetal bovine serum (FBS)
1% penicillin-streptomycin (Pen/Strep)
10% dimethyl sulfoxide (DMSO)
Acknowledgments
This protocol was initially adopted in the laboratory for pig ( Xu et al., 2017 ), and now modified for mouse. The work was supported by National Institute of General Medical Sciences (P20GM103453 to Y. L.), University of Louisville School of Medicine (E0819 to Y. L.), and Research to Prevent Blindness (to the Department of Ophthalmology and Visual Sciences at Louisville).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Bringmann A., Pannicke T., Grosche J., Francke M., Wiedemann P., Skatchkov S. N., Osborne N. N. and Reichenbach A.(2006). Müller cells in the healthy and diseased retina. Prog Retin Eye Res 25: 397-424. [DOI] [PubMed] [Google Scholar]
- 2.De Melo Reis R. A., Ventura A. L., Schitine C. S., de Mello M. C. and de Mello F. G.(2008). Müller glial as an active compartment modulating nervous activity in the vertebrate retina: neurotransmitters and trophic factors. Neurochem Res 33: 1466-1474. [DOI] [PubMed] [Google Scholar]
- 3.Fischer A. J. and Reh T. A.(2001). Müller glial glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci 4: 247-252. [DOI] [PubMed] [Google Scholar]
- 4.Sarthy P. V. and Lam D. M.(1978). Biochemical studies of isolated glial(Müller) cells from the turtle retina. J Cell Biol 78(3): 675-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vihtelic T. S. and Hyde D. R.(2000). Light-induced rod and cone cell death and regeneration in the adult albino zebrafish(Danio rerio) retina. J Neurobiol 44(3): 289-307. [DOI] [PubMed] [Google Scholar]
- 6.Xu N., Chen Y., Dean K. C., Lu X., Liu X., Wang W., Dean D. C., Kaplan H. J., Gao L., Dong F. and Liu Y.(2017). Sphere-induced rejuvenation of swine and human muller glia is primarily caused by telomere elongation. Stem Cells 25(6): 1579-1591. [DOI] [PubMed] [Google Scholar]


