Abstract
Rationale
Women represent a vulnerable and growing population with respect to alcohol abuse. Elevated glucocorticoid exposure in adolescence increases addiction risk and stress sensitivity in adulthood. However, little is known about sex differences in ethanol craving-like behavior.
Objective
This study characterized sex differences in ethanol-motivated behavior following ethanol-paired cues and/or acute stimulation of the HPA axis in male and female rats with or without exposure to chronically elevated glucocorticoids in adolescence.
Methods
Adolescent corticosterone-treated (Experiment 1) or naïve (Experiment 2) male and female rats were trained as adults to self-administer ethanol paired with a cue, and tested for the effects of this cue, alone or in combination with yohimbine, on the reinstatement of ethanol seeking.
Results
Females showed elevated ethanol self-administration and seeking compared to males. In Experiment 1, corticosterone exposure in adolescence augmented cue-induced reinstatement of ethanol seeking in females only, and females were more sensitive to yohimbine in promoting reinstatement. Experiment 2 replicated these findings and showed that exposure to both yohimbine and alcohol-related cues enhanced the reinstatement of alcohol seeking, producing additive effects in females. Corticosterone levels were higher in females and in yohimbine-treated rats, and corticosterone and estradiol correlated with responding during reinstatement.
Conclusions
Chronic manipulations in adolescence and acute manipulations in adulthood of the HPA axis increase cue-induced reinstatement of ethanol seeking to a greater degree in females than in males. Elucidating the mechanisms that underlie these effects may lead to the development of sex-specific interventions aimed at mitigating alcohol relapse risk in females.
Keywords: craving, relapse, adolescence, stress, female, ethanol, self-administration
Introduction
Recent epidemiological data suggest that the gender gap in the prevalence of alcohol use disorders is narrowing, with women significantly increasing drinking frequency, but not men (Dawson et al. 2015; Keyes et al. 2011). Moreover, studies indicate that women more rapidly transition to alcohol dependence (“telescoping”) (Diehl et al. 2007; Randall et al. 1999), exhibit greater negative emotions related to their drinking (King et al. 2003), and demonstrate greater stress and cue reactivity, evidenced by increased alcohol craving, when compared to men (Hartwell and Ray 2013; Rubonis et al. 1994). However, despite clear evidence that women represent a vulnerable population with respect to alcohol misuse, females are understudied in clinical and especially preclinical research (Beery and Zucker 2011). Specifically, little is known about sex differences in the sensitivity to stress in promoting alcohol-motivated behaviors, and what is known is inconsistent as very few studies have made explicit comparisons between males and females (Becker et al. 2011).
In addition to sex, age can influence drinking-related outcomes. For example, stress exposure during adolescence, a period of neurodevelopment that is sensitive to glucocorticoids, increases the risk of drug and alcohol dependence later in life (Burke and Miczek 2014; Crews et al. 2007; Eiland and Romeo 2013). In addition, in preclinical models, chronic exposure to psychogenic stress (Toledo-Rodriguez and Sandi 2011) or to corticosterone (CORT) via the drinking water (Torregrossa et al. 2012) during adolescence leads to increased risk-taking/impulsive behaviors, which predict later drug use (de Wit 2009; Jentsch and Taylor 1999). The chronic CORT model produces elevations in plasma corticosterone consistent with mild physiological stressors (Gourley and Taylor 2009) and specifically addresses the role of glucocorticoid signaling in mediating risk for addiction. As some studies have shown female rats to be more sensitive to the effects of stress in adolescence compared to males (Barha et al. 2011; Bourke et al. 2012), and it is known that, in humans, stress exposure can lead to increased alcohol drinking (Becker et al. 2011; Sinha 2001), we hypothesized that females would be more sensitive to the effects of both prior chronic and acute manipulations of the stress axis in motivating alcohol seeking behaviors.
The goal of the present experiments was to characterize sex differences in alcohol-motivated behavior as a function of pharmacological manipulations of the stress system and to alcohol-related cues. In Experiment 1, we sought to determine the effects of chronic CORT exposure during adolescence on operant alcohol self-administration and cue- and yohimbine-induced reinstatement of alcohol seeking in adulthood. It was predicted that CORT-treated rats would exhibit elevated alcohol-motivated behaviors, and that this effect would be enhanced in female rats. In Experiment 2, we built upon previous findings that drug-related cues and stress produced additive effects on reinstatement in male rats trained to self-administer alcohol (Liu and Weiss 2002), and that this effect was enhanced in females trained to self-administer cocaine (Feltenstein et al. 2011), by testing male and female rats for the effects of alcohol-paired cues and yohimbine, alone or in combination, on the reinstatement of alcohol seeking.
Methods
Subjects
Adolescent (aged 27–28 days; Experiment 1) or young adult (aged 67–68 days; Experiment 2) male and female Sprague-Dawley Rats (Harlan, Frederick, MD) were pair-housed and maintained on a regular light:dark cycle in a temperature- and humidity-controlled room. Behavioral testing was conducted during the light cycle in Experiment 1, and during the dark cycle in Experiment 2. Rats were given ad libitum access to water and food except where noted. All procedures were conducted in accordance with the policies set forth by University of Pittsburgh Institutional Animal Care and Use Committee and the National Institutes of Health Guidelines on the Care and Use of Laboratory Animals.
Apparatus
Behavioral testing was conducted in operant conditioning chambers housed in sound-attenuating cubicles (Med Associates, St. Albans, VT). Operant conditioning chambers were equipped with two retractable levers situated on either side of a magazine into which a dipper arm would deliver 0.05ml of the reinforcer; cue lights above each lever; and a tone generator. During testing, the house light was illuminated and an exhaust fan was turned on to mask external noise.
Drugs
Corticosterone (CORT; 4-pregnen-11b,21-diol-3,20-dione 21-hemisuccinate, Steraloids, Newport, RI; 12.5, 25, and 50µg/ml) and ethanol (EtOH; 10%v/v+0.1%w/v saccharin) and saccharin (0.1%w/v saccharin) solutions were dissolved in tap water. Yohimbine HCl (YOH; Sigma-Aldrich, St. Louis, MO; 0.625 and 1.25mg/ml) was dissolved in sterile water.
Procedures
Acquisition and Extinction of EtOH Self-Administration
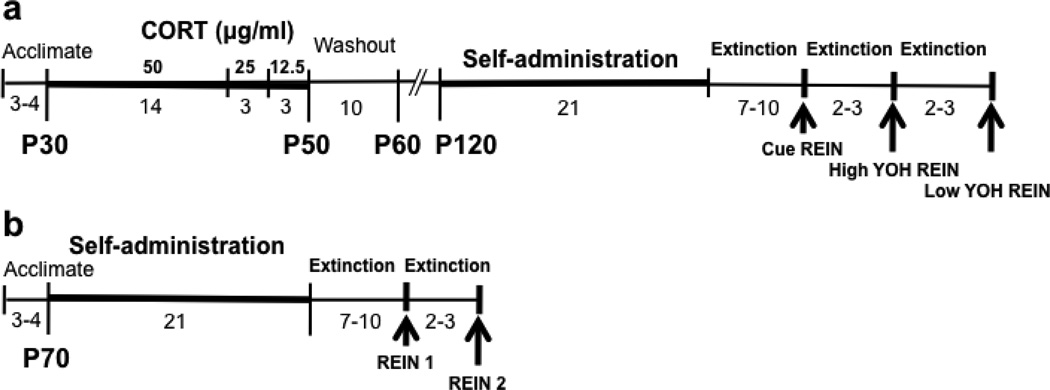
A timeline of the experimental procedures is described in Fig. 1. Starting on postnatal day 70 (or later), rats were restricted to ~85% of their free feeding weight and habituated to the EtOH solution in a novel cage. Rats then received a 30-min magazine training session, during which EtOH was presented every 30-s. EtOH self-administration training was conducted on a fixed ratio 1 schedule of reinforcement. Responses on the active lever produced 10-s presentation of EtOH, a 75dB tone, and a cue light, followed by a 10-s timeout. Inactive lever responses had no programmed consequences. Troughs were weighed before and after each session to measure fluid intake. Sessions were one hour in length, and rats received a total of 21 self-administration sessions. Food restriction was gradually reduced such that rats were free feeding by the final week of training. Following successful acquisition (average of ≥20 reinforcers earned over the last 3 days of training), instrumental lever extinction training began, during which responses had no programmed consequences. Rats were given a minimum of 7 extinction sessions to meet criterion (≤25 active lever presses over two consecutive days) before reinstatement testing (described below). The majority of rats met this criterion within 10 sessions, and no systematic differences in the time to reach criterion were evident across groups.
Fig. 1.
Timeline of experimental procedures for Experiment 1 (A) and Experiment 2 (B). The number of days of testing and the age of the rat are indicated below each timeline, and the concentration of CORT (µg/ml) is indicated above timeline A. Thick bars represent CORT and ethanol drinking periods. Arrows represent reinstatement (REIN) days. Low YOH=0.625 mg/kg YOH; High YOH=1.25 mg/kg YOH
Experiment 1: Sex differences in the effects of chronic corticosterone exposure during adolescence on EtOH self-administration and reinstatement of EtOH seeking
Chronic CORT exposure in adolescence
From postnatal day 30–50, rats received ad libitum access to food and either CORT or water as their sole fluid. Intake was measured daily, and rats were weighed every other day. CORT-treated rats received 50µg/ml CORT for 14 days, followed by 25 and 12.5µg/ml for 3 days each. Rats were then given a 10-day washout period to allow for the normalization of HPA axis function (Gourley and Taylor 2009).
Reinstatement
Reinstatement tests were conducted in the absence of reinforcement. Rats were first tested for cue-induced reinstatement, wherein responding on the active lever produced the light+tone cue. Next, rats were tested for reinstatement following injection of either 0.625 or 1.25mg/kg YOH (−10 min) in separate tests. Between tests, rats underwent at least two extinction sessions to ensure that the extinction criterion was met. Only rats that met both the self-administration and extinction criteria were included in the reinstatement analyses.
Saccharin self-administration
To determine if the effects of sex and/or adolescent corticosterone exposure were selective for EtOH, a separate group of male and female rats underwent the same CORT exposure and self-administration protocol described above, with the exception that they responded for a solution of 0.1% saccharin.
Experiment 2: Sex differences in cue-, yohimbine-, and cue+yohimbine-induced reinstatement of EtOH seeking
To determine if sex differences observed in Experiment 1 were associated with handling during adolescence, a separate group of young adult rats were trained to self-administer EtOH and were extinguished as described above. Vaginal lavage samples were taken from female rats immediately following the final self-administration session, and estrous cycle phase was confirmed by examining vaginal cytology. Rats were exposed to two of the following conditions: vehicle injection/no cue; vehicle injection/cue; 1.25mg/kg YOH/no cue; 1.25mg/kg YOH/cue; meeting extinction criterion between reinstatement tests.
Hormone Assays
Rats in Experiment 2 were euthanized via rapid decapitation immediately following the second reinstatement test. Trunk blood samples were collected in heparinized 1.5ml centrifuge tubes. Plasma was analyzed for corticosterone and estradiol using commercially available EIA kits (Enzo Life Sciences, Farmingdale, NY) according to the manufacturer’s instructions.
Statistics
Statistical analyses were conducted using IBM SPSS Statistics v21 (IBM Corporation, Armonk, NY). Missing values (e.g., fluid spillage) were interpolated from the day preceding and following that data point. Fluid intake, reinforcers earned, active and inactive lever presses, and body weight were subjected to mixed factorial ANOVAs (p < 0.05), followed by Bonferroni post-hoc comparisons where appropriate. In Experiment 1, sex and adolescent treatment (CORT vs. H2O) were between-subjects factors for measures taken during CORT exposure, and day (across self-administration sessions or extinction vs. reinstatement) was the within-subjects factor during EtOH and saccharin self-administration, extinction, and reinstatement. In Experiment 2, ANOVAs with sex as the between-subjects factor and reinstatement order (first vs. second reinstatement) as the within-subjects factor failed to find order effects; as such, reinstatement test days were collapsed and subsequent ANOVAs with sex, cue (cue vs. no cue), injection (vehicle vs. YOH) as the between-subjects factors and day (extinction vs. reinstatement) were conducted. Active lever responding on the final reinstatement day was correlated with plasma corticosterone and estradiol levels collected immediately after testing.
Results
Experiment 1: Sex differences in the effects of chronic corticosterone exposure during adolescence on EtOH self-administration and reinstatement of EtOH seeking
Chronic CORT exposure in adolescence
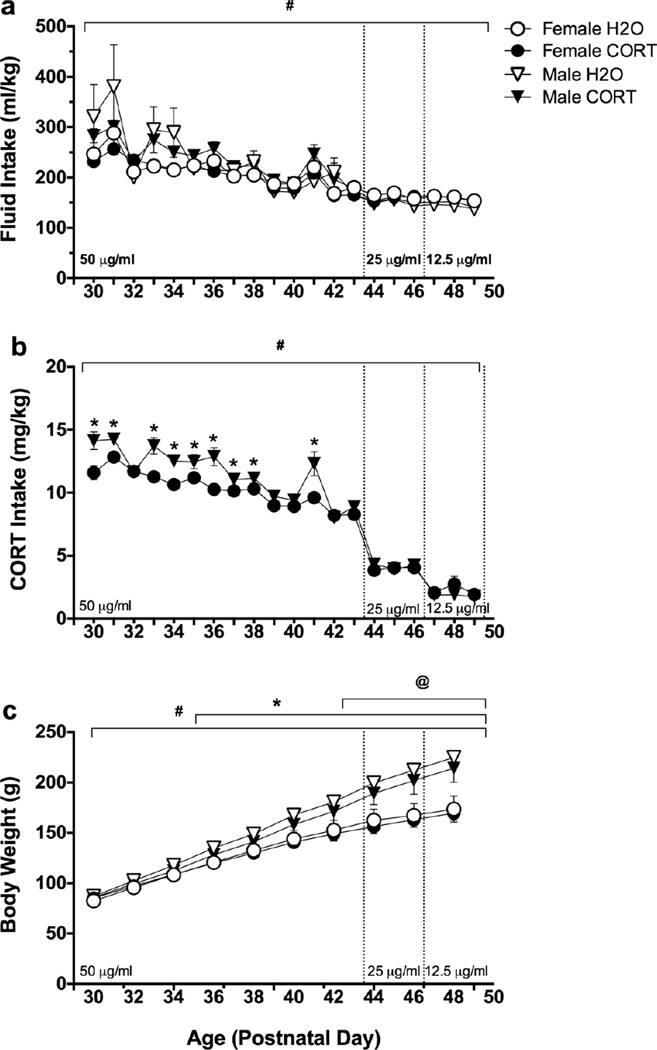
Fluid intake (ml/kg) increased across the treatment period [F(19,1178)=36.18, p<0.001], but no differences as a function of sex or fluid type were found (Fig. 2a). CORT intake (mg/kg) decreased as the concentration decreased, and males showed small, but significantly greater CORT intake compared to females at the highest dose, corresponding with postnatal days ~30–40 (day×sex interaction [F(19,570)=5.98, p<0.001], Fig. 2b). Main effects of day [F(19,1178)=3994.61, p <0.001], sex [F(1,62)=105.21, p <0.001], and adolescent treatment [F(1,62)=5.18, p=0.026], as well as day×sex [F(19,1178)=184.57, p < 0.001] and day×treatment [F(19,1178)=7.59, p < 0.001] interactions (Fig. 2c) were found for body weight, illustrating the expected weight gain over the course of treatment, and that males weighed more than females. CORT-treated rats showed slightly, but significantly lower body weights compared to H2O-treated controls.
Fig. 2.
Outcome measures in female (circles) and male (triangles) rats during adolescent CORT (closed symbols) or H2O treatment (open symbols); n=15 female H2O, n=16 female CORT, n=14 male H2O, n=14 male CORT. (A) Fluid Intake (ml/kg), #p<0.05=main effect of day; (B) CORT Intake (mg/kg), #p<0.05=main effect of day, *p<0.05 vs. female; (C) Body Weight (g), #p<0.05=main effect of day, *p<0.05=male vs. female, @p<0.05=H2O vs. CORT.
Acquisition and extinction of EtOH self-administration
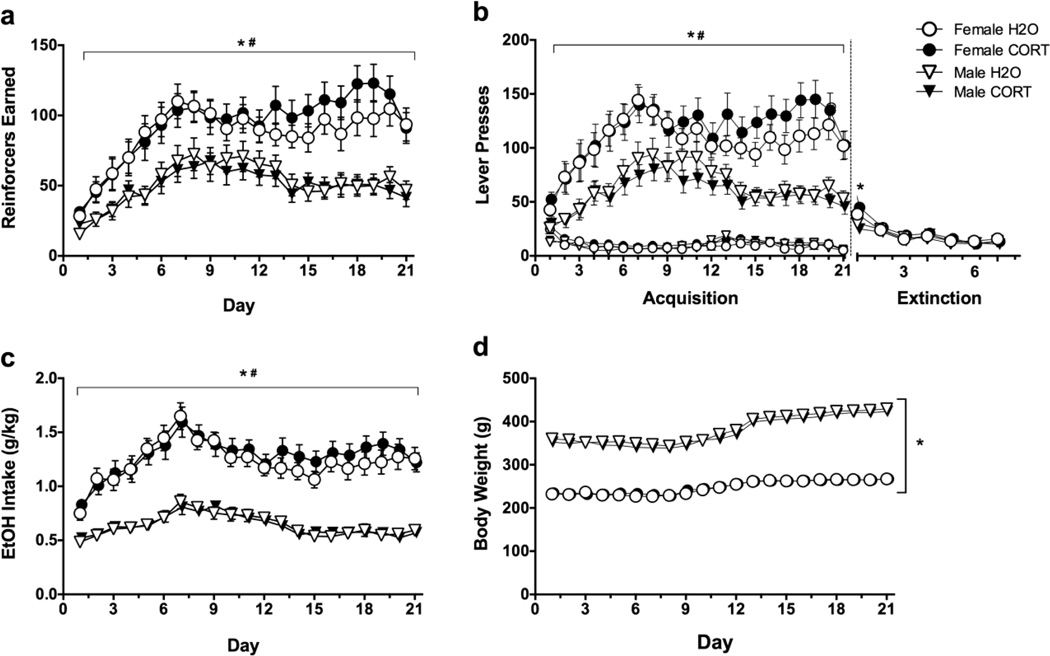
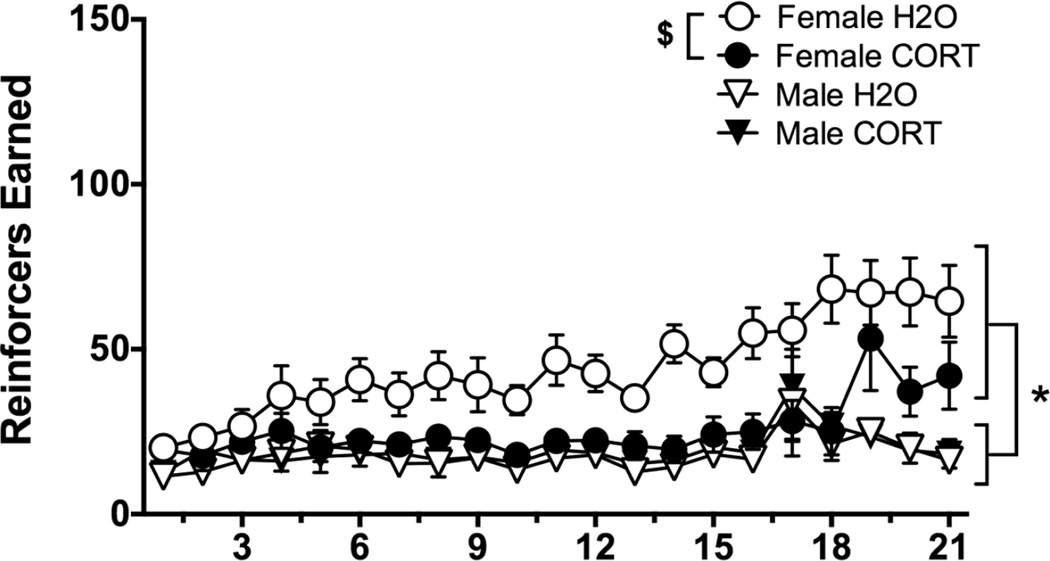
Analysis of sex and adolescent treatment effects on ethanol self-administration revealed that females earned more reinforcers than males (main effects of day [F(20,1100)=38.22, p<0.001], sex [F(1,55)=22.8, p<0.001], and a day×sex interaction [F(20,1100)=6.18, p<0.001]; Fig. 3a) Similar results were found for active lever presses (main effects of day [F(20,1100)=22.71, p<0.001], sex [F(1,55)=24.51, p<0.001], and a day×sex interaction [F(20,1100)=3.34, p<0.001]; Fig. 3b, large symbols), and inactive lever presses (main effect of day [F(20,1100)=11.92, p<0.001] and a day×sex interaction [F(20,1100)=3.45, p<0.001]; Fig. 3b, small symbols), though these differences were driven by high inactive lever presses in females on the first day of self-administration. Ethanol intake (g/kg) followed a similar pattern, with main effects of day [F(20,1100)=31.87, p<0.001], sex [F(1,55)=79.91, p<0.001], and a day×sex interaction [F(20,1100)=7.33, p<0.001] indicating higher intake in females compared to males (Fig. 3c). During extinction, rats showed the expected decrease in responding across days (main effect of day [F(6,330)=63.19, p<0.001]; Fig. 3b, right panel). A day×sex interaction [F(6,330)=6.39, p<0.001] indicated that females responded more on the first day of extinction [t(57)=5.916, p<0.001], but this was likely driven by the higher response rates in females during self-administration, not an extinction deficit.
Fig. 3.
Outcome measures during acquisition and extinction of ethanol self-administration in female (circles) and male (triangles) H2O (open symbols) or CORT (closed symbols)-treated rats. (A) Reinforcers earned; (B) Active (large symbols) vs. Inactive (small symbols) Lever Presses; (C) Ethanol Intake (g/kg); (D) Body Weight (g); #p<0.05=main effect of day, *p<0.05=male vs. female, for all panels.
Cue-induced reinstatement
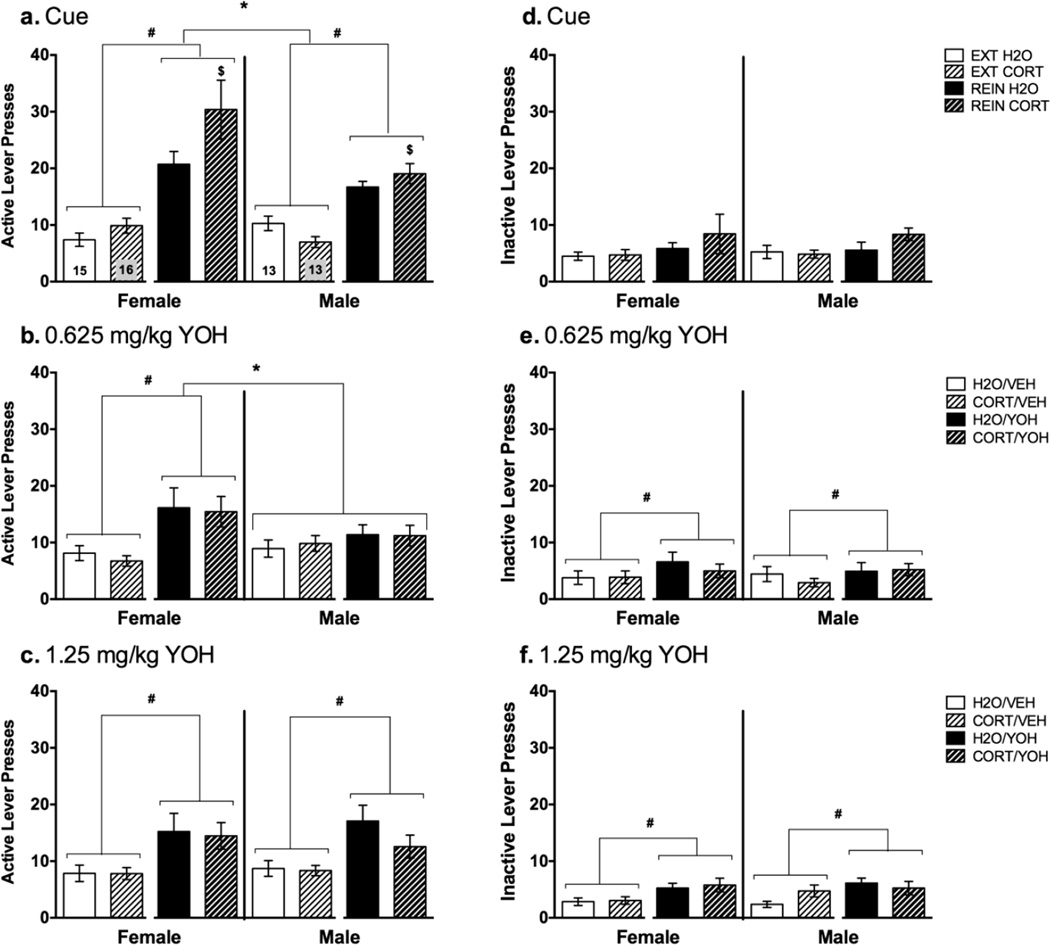
Analysis of cue-induced reinstatement test day responding revealed main effects of day [F(1,55)=78.68, p<0.001], sex [F(1,55)=4.01, p=0.05], and day×sex [F(1,55)=6.76, p=0.012] and day×adolescent treatment [F(1,55)=4.71, p=0.034] interactions (Fig. 4a), indicating that responding was higher during reinstatement compared to extinction, and that adolescent CORT-treated (vs. H2O-treated) and female rats (vs. males), exhibited greater reinstatement. During reinstatement, there was a trend for increased responding in CORT-treated rats (vs. H2O; p=0.06), and significantly higher responding in female rats (vs. males; [t(68)=2.45, p=0.011]. Further, a trend (p=0.09) for a sex×adolescent treatment interaction suggested that CORT-treated females, but not males, responded more than their H2O-treated counterparts. No significant differences in inactive lever responding were evident during cue-induced reinstatement (Fig. 4d).
Fig. 4.
Responding during reinstatement testing in female (left panels) and male (right panels) H2O (solid bars) and CORT (hashed bars)-treated rats; n=15 female H2O, n=16 female CORT, n=13 male H2O; n=13 male CORT. (A) Active and (D) inactive lever presses during extinction (EXT; white bars) and reinstatement (REIN; black bars) of cue-induced reinstatement; *p<0.05=male vs. female, #p<0.05 EXT vs. REIN, $p<0.05 vs. H2O (REIN day only; p=0.06 H2O vs. CORT overall). (B) Active and (E) inactive lever presses following vehicle (VEH; white bars) or 0.625mg/kg yohimbine (YOH; black bars) injection; *p<0.05=male vs. female, #p<0.05 VEH vs. YOH. (C) Active and (F) inactive lever presses following vehicle (VEH; white bars) or 1.25mg/kg yohimbine (YOH; black bars) injection; *p<0.05=male vs. female, #p<0.05 VEH vs. YOH.
Yohimbine (0.625mg/kg)-induced reinstatement
In response to a low dose of YOH we observed a main effect of injection [F(1,53)=16.53, p<0.001] and a sex×injection interaction [F(1,53)=5.42, p=0.024]. Post hoc analysis revealed that only females significantly increased active lever responding to yohimbine [t(30)=3.99, p<0.001] (Fig. 4b). A main effect of injection on inactive lever presses [F(1,53)=8.02, p=0.007] suggests that YOH can increase general responding in both sexes. Nevertheless, the magnitude of increase in active lever responding following YOH is larger (63%) than for the inactive lever (45%) (Fig. 4e).
Yohimbine (1.25mg/kg)-induced reinstatement
Reinstatement to a higher dose of YOH was observed in both sexes as we found a main effect of drug injection [F(1,53)=27.35, p<0.001] but no interaction with sex (Fig. 4c). A main effect of injection was also found for inactive lever presses [F(1,53)=819.72, p<0.001]. Removal of rats exhibiting inactive lever responding exceeding 2 standard deviations above the mean did not eliminate this effect; however, the magnitude of the increase in active lever responding following YOH was larger (85%) than for inactive lever responding (69%; Fig. 4f).
Saccharin self-administration
To control for any potential effects of the addition of saccharin to the EtOH solution, we also analyzed self-administration of a saccharin only solution. As with EtOH, females earned more reinforcers than males, and there was an increase in the number of reinforcers earned across training (main effects of day [F(20,500)=7.08, p<0.001] and sex [F(1,25)=18.2, p<0.001]; Fig. 5). However, a sex×adolescent treatment interaction was also uncovered [F(20,500)=7.08, p=0.013], and further analysis indicated that adolescent CORT treated females exhibited significantly decreased saccharin self-administration than control females [F(1,15)=10.33, p=0.006]. Similar results were found for active lever presses and saccharin intake (ml/kg; data not shown). Importantly, the magnitude of the sex differences in H2O-treated rats responding for saccharin was comparable to those responding for EtOH, but saccharin-trained rats earned half the amount of reinforcers of EtOH-trained rats.
Fig. 5.
Reinforcers earned during acquisition of saccharin self-administration in female (circles) and male (triangles; *p<0.05=male vs. female) rats treated with H2O (open symbols) or CORT (closed circles; $p<0.05=H2O vs. CORT, females only); n=9 female H2O, n=8 female CORT; n=6 male H2O and CORT.
Experiment 2: Sex differences in cue-, yohimbine-, and cue+yohimbine-induced reinstatement of EtOH seeking
Acquisition and extinction of EtOH self-administration
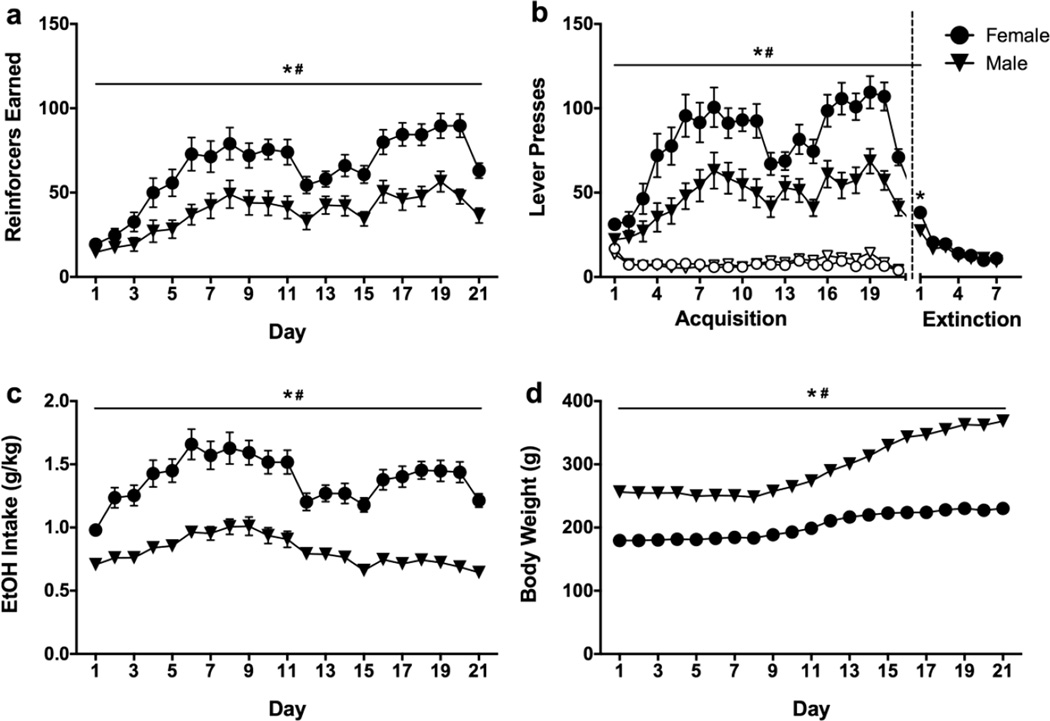
Analysis of sex differences in EtOH self-administration without a history of adolescent treatment revealed similar sex differences as observed in Experiment 1. As expected, active lever presses, reinforcers earned, EtOH intake (g/kg), and body weight all increased across training, while inactive lever presses decreased across training (all main effects p<0.001; Fig. 6a–d). In addition, significant main effects of sex were found for reinforcers earned [F(1,43)=14.993, p<0.001], active lever presses [F(1,43)=15.537, p<0.001], and EtOH intake [F(1,43)=55.531, p<0.001], but not inactive lever presses, indicating that increased EtOH self-administration in females was specific to EtOH seeking and drinking and not simply increased general locomotor activity. Significant sex×day interactions were also observed for all measures, suggesting that females had accelerated acquisition of self-administration (all ps<0.01). Thus, the sex differences in self-administration observed in Experiment 1 were replicated in Experiment 2. During extinction, main effects of day [F(6,258)=49.429, p<0.001] and sex [F(1,43)=4.226, p=0.046] and a day×sex interaction [F(6,258)=2.757, p=0.013] were found (Fig. 6b, right panel). The sex effects were driven by increased responding by females only on day 1 of extinction [t(43)=2.827, p=0.007].
Fig. 6.
Outcome measures in female (circles) and male (triangles) rats (n=24 for each sex) during acquisition and extinction of ethanol self-administration. Main effects of sex (*p<0.05=male vs. female) and day (#p<0.05) were found for (A) Reinforcers Earned, (B) Active (closed symbols) and Inactive (open symbols) Lever Presses, (C) Ethanol Intake (g/kg), and (D) Body Weight (g).
Reinstatement testing
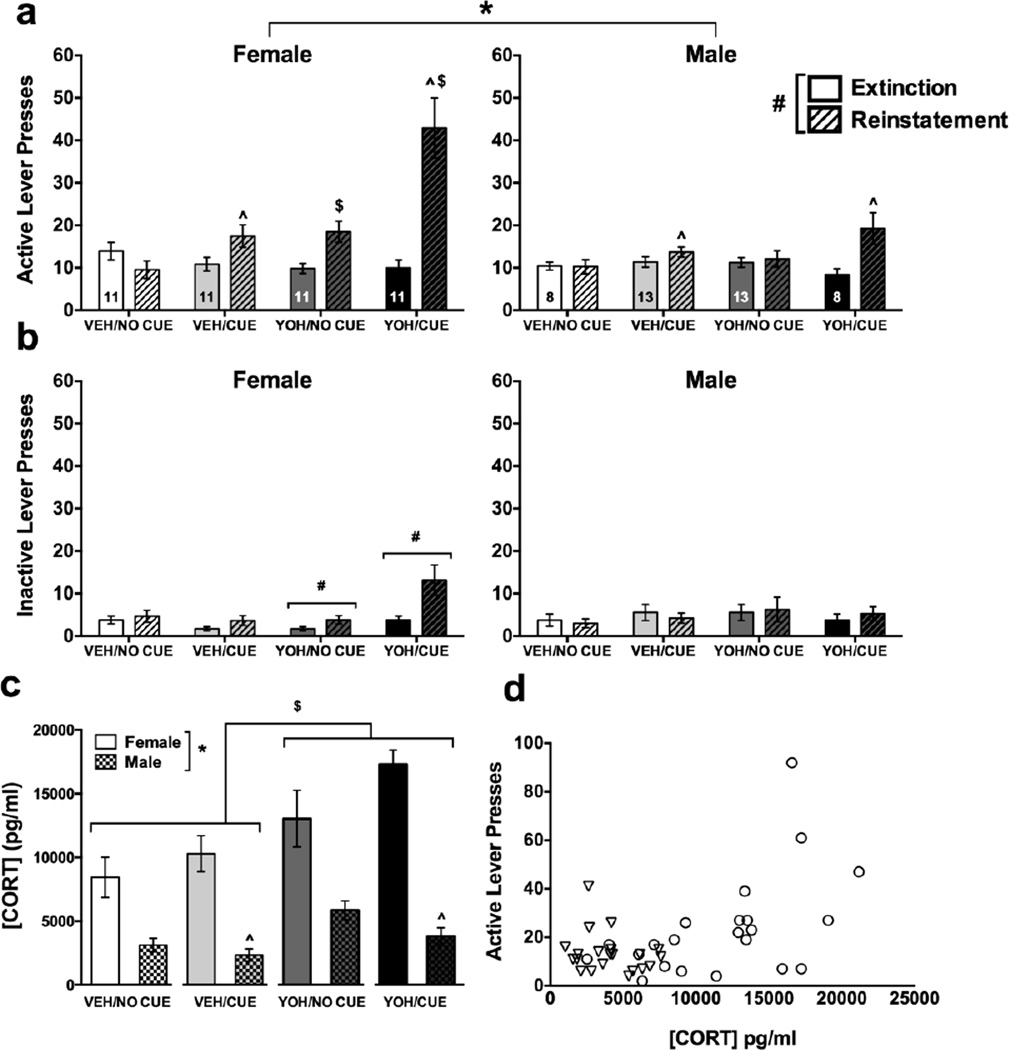
Analysis of active lever presses during cue, YOH, and cue+YOH reinstatement tests revealed main effects of sex [F(1,78)=9.18, p=0.003] in response to all stimuli (Fig. 7a), indicating increased reinstatement responding in females compared to males. Day×sex [F(1,78)=12.24, p=0.001], day×cue [F(1,78)=31.26, p<0.001], day×injection [F(1,78)=32.58, p<0.001], day×sex×cue [F(1,78)=7.12, p=0.009] and day×sex×injection [F(1,78)=12.16, p=0.001] interactions were also found. Follow-up tests found a main effect of cue [F(1,38)=6.13, p=0.018] in males, and effects of injection approached significance [p=0.093]. In contrast, females demonstrated significant main effects of cue [F(1,40)=15.6, p<0.001] and injection [F(1,40)=17.59, p<0.001], as well as a cue×injection interaction [F(1,40)=64.078, p=0.05], revealing increased responding when females were exposed to a combination of cue- and YOH-induced reinstatement relative to either stimulus alone. The increased reinstatement response to the combination treatment was also greater in females relative to males. Analysis of inactive lever responses revealed day×sex [F(1,78)=5.61, p=0.02] and day×injection [F(1,78)=4.67, p=0.034] interactions (Fig. 7b), and subsequent analyses showed that females [F(1,43)=9.84, p=0.003] and rats injected with YOH [F(1,43)=6.04, p=0.018] increased inactive lever presses, suggesting that females are more sensitive to the effects of YOH in eliciting nonspecific behaviors.
Fig. 7.
Responding and corticosterone levels during reinstatement; n=11 females in each condition; n=8 male NO CUE/VEH (vehicle), CUE/YOH (yohimbine); n=13 male NO CUE/YOH, CUE/VEH. (A) Active and (B) Inactive Lever Presses in female (left panels) and male (right panels) rats on the last day of extinction (solid bars) vs. the reinstatement day (hashed bars); *p<0.05=male vs. female, #p<0.05=extinction vs. reinstatement, ^p<0.05 vs. no cue, $p<0.05 vs. VEH. (C) Plasma CORT concentrations (pg/ml) immediately following the final reinstatement test in female (solid bars) and male (checked bars) rats, *p<0.05=male vs. female, ^p<0.05 vs. no cue, $p<0.05=YOH vs. VEH. (D) An overall significant (p<0.05) positive correlation was found between active lever presses and CORT (pg/ml), an effect evident in female (circles) but not male (triangles) rats.
Corticosterone Assay
Plasma CORT levels were measured following the second reinstatement test, and analyses revealed main effects of sex [F(1,35)=72.52, p<0.001], injection [F(1,35)=14.37, p=0.001], and a sex×cue [F(1,35)=7.77, p=0.009] interaction (Fig. 7c). CORT levels were twice as high in females as males, and YOH significantly increased CORT concentrations in both sexes. However, male rats showed significantly elevated CORT levels in the no cue conditions relative to the cue conditions [t(19)=2.772, p=0.012], whereas females tended to show elevated CORT levels in the cue conditions (p=0.1). Further, a significant positive correlation was found between active lever presses and CORT concentrations overall [r(41)=0.499, p<0.001]; however, further analysis indicated that this effect was driven by the females [r(22)=0.52, p=0.013, males only p=0.3; Fig. 7d]. No such correlation was found for inactive lever presses [r(41)=0.21, p=0.19, data not shown]. Therefore, increased circulating CORT appears to be related to the degree of active EtOH seeking responses, particularly in females.
Estradiol Assay
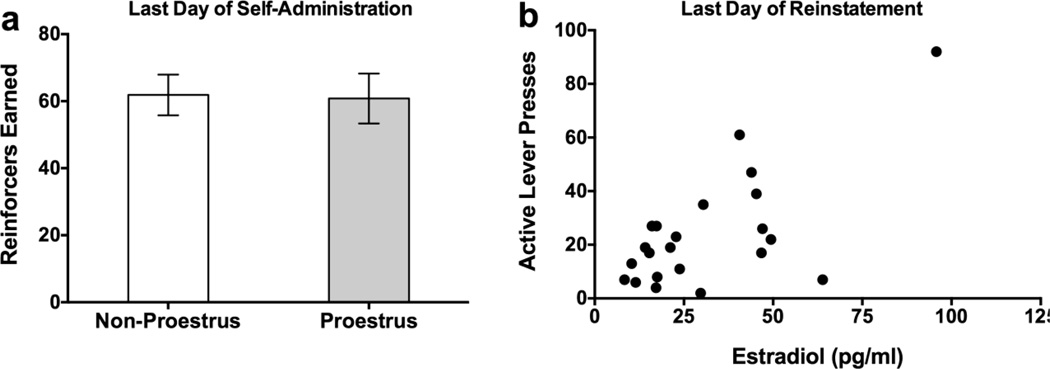
No significant main effects of injection or cue on estradiol levels immediately following the second reinstatement test were found (Fig. 8a). However, collapsed across treatment, a significant positive correlation was found for active lever presses during reinstatement and estradiol levels [r(23)=0.66, p=0.001, Fig. 8b], suggesting a potential role for circulating estradiol in regulating the degree of reinstatement to EtOH seeking.
Fig. 8.
Estrogen-related outcomes. (A) Number of reinforcers earned on the last day of EtOH self-administration as a function of low (Non-Proestrus) or high (Proestrus) estradiol phases of the estrous cycle. (B) An overall significant (p<0.05) positive correlation was found between active lever presses and estradiol (pg/ml) during the final reinstatement test.
Discussion
The results of the present study reveal pronounced sex differences in EtOH-motivated behaviors at baseline that are enhanced following pharmacological manipulations of the stress system and exposure to alcohol-related cues. Across both experiments, females showed elevated EtOH self-administration and reinstatement of EtOH seeking compared to males. In Experiment 1, chronic CORT exposure in adolescence tended to increase EtOH drinking and augmented cue-induced reinstatement of EtOH seeking to a greater degree in females than in males. Females exhibited increased sensitivity to YOH-induced reinstatement evidenced by significant elevations in responding following a dose that had no effect in males. Experiment 2 replicated these findings in rats not exposed to experimental manipulations in adolescence, and further showed that exposure to both YOH and alcohol-related cues elicited an additive effect on reinstatement of EtOH seeking in females only. Although enhanced reinforcement of other drugs of abuse in females has been consistently shown (Anker and Carroll 2011; Becker et al. 2007), this is the first report, to our knowledge, confirming that female rats show increased reinstatement of EtOH seeking compared to males. Importantly, these findings provide further support that females represent a population that is vulnerable to stress-related changes in EtOH-motivated behavior and may require different treatment strategies.
Chronically elevated glucocorticoid levels in adolescence can have enduring effects on behavior in adulthood, and heightened EtOH seeking in female rats exposed to CORT in adolescence in the current study is consistent with this finding. However, other studies have shown that adult male (Skelly et al. 2015), but not female rats (Butler et al. 2014) display increased EtOH intake following adolescent social isolation stress. Inconsistencies regarding the direction of CORT responses to adolescent stress as a function of sex (Cruz et al., 2012; McCormick et al., 2008; Weintraub et al., 2010) make it difficult to interpret whether these sex-specific responses in the present study are due to underlying changes in HPA axis reactivity. Further, in our control study, adolescent CORT treatment reduced saccharin self-administration in female, but not male rats, which appears to dissociate the effects of persistent glucocorticoid elevations on responding for drug vs. nondrug reinforcers. Additional studies are needed to fully characterize the degree to which adolescent exposure to excess glucocorticoids alters sensitivity to reward in female vs. male rats.
While YOH has been shown to reinstate EtOH seeking in male rats (Bertholomey et al. 2013; Cippitelli et al. 2010; Le et al. 2005; Simms et al. 2011), and other drugs of abuse in female rats and women (Anker and Carroll 2010; Cox et al. 2013; Feltenstein et al. 2012; Feltenstein et al. 2011; Moran-Santa Maria et al. 2014), our results reveal that female rats show YOH-induced reinstatement of EtOH seeking at lower doses and to a greater degree than males. Moreover, reinstatement is correlated with CORT levels, consistent with previous studies (Marinelli et al. 2007; Simms et al. 2012; Simms et al. 2011). However, responding during reinstatement did not correlate with prior EtOH self-administration, consistent with findings from the clinical literature indicating that alcohol use frequency is not related with craving following exposure to stress-related or alcohol cues (Chaplin et al., 2008). Responding during reinstatement did, however, correlate with both CORT and estradiol levels in females, suggesting that overlapping stress and gonadal hormones may be involved in our observed sex differences. Enhanced craving-like behavior in female rats exposed to both a stressor and alcohol-related cues in the present study is consistent with some studies in humans (Amlung and MacKillop 2014; Coffey et al. 2002) but not others (Nesic and Duka 2006; Thomas et al. 2011). Though both males and females showed sensitivity to the combination of YOH and alcohol-related cues, this effect was far more pronounced in females, suggesting that females may require specialized interventions aimed at mitigating the interactive effects of these factors.
Overlapping gonadal and stress hormone systems likely underlie the enhanced vulnerability to the effects of elevated glucocorticoids on EtOH-motivated behaviors in females. While some studies have shown that estradiol is critical for EtOH (Ford et al. 2004) and cocaine self-administration (Lynch et al. 2001) and reinstatement (Feltenstein et al. 2011; Larson and Carroll 2007), others have failed to demonstrate consistent effects of circulating ovarian hormones (Ford et al. 2002; Roberts et al. 1998; Vetter-O'Hagen and Spear 2011). Though estrous cycle was not continually monitored in the present study, the variability in EtOH self-administration across days in females was similar to males, and thus does not appear to be heavily influenced by estrous cycle phase. Further, while assessment of vaginal cytology on the final day of EtOH self-administration in Experiment 2 did not reveal effects of estrous cycle phase (proestrus versus all other phases) on EtOH consumed, responding during reinstatement was significantly correlated with plasma estradiol levels. However, this finding should be interpreted with caution due to low sample sizes and high variability. Ongoing studies are aimed at determining the specific role of estradiol in EtOH-motivated behaviors, as well as identifying neural circuits that are differentially regulated by alcohol-paired cues or stress in male versus female rats.
There are a few limitations to the present experiments. First, while food restriction is used to promote self-administration of other drugs of abuse, this approach can be problematic for initiating EtOH self-administration due to the potential for caloric compensation. However, rats were eased off of the food restriction to free-feeding by the final week of self-administration, and remained in a non-deprived state for the majority of testing, thus potentially mitigating compensatory effects. Similarly, the addition of saccharin to the EtOH solution may contribute to sex differences in EtOH-motivated behavior. However, the magnitude of the sex difference for saccharin self-administration in H2O-treated controls was similar to that for EtOH self-administration, and rats exhibited twice the level self-administration of EtOH compared to saccharin, indicating that the reinforcing effects of EtOH are likely driving behavior, though studies using unsweetened EtOH are warranted. The lack of analysis of blood EtOH concentrations in the present study preclude confirming the degree to which these rats were under the pharmacological effects of EtOH. While intake in males is relatively low, the amount of ethanol consumed consistently supports self-administration behavior, and intake in both sexes is consistent with other reinstatement studies in Sprague-Dawley rats (Bito-Onon et al. 2011). The order of reinstatement testing in Experiment 1 might have contributed to enhanced cue- and YOH-induced reinstatement in adolescent-treated males relative to those not treated until adulthood. However, based on relatively consistent observations in our lab that this dose of YOH evokes reinstatement in males, variability in the YOH response may be interacting with unknown environmental factors to mitigate this response in the rats in Experiment 2. Variability in the response to YOH could also contribute to the increased responding on the inactive lever during reinstatement, though this behavior is not uncommon (Bertholomey et al., 2013; Marinelli et al., 2007; Simms et al., 2012), and could reflect the engagement of broader response strategies aimed at gaining access to the reinforcer.
In conclusion, our findings provide further support for increased EtOH self-administration in female compared to male rats, extend the findings of the chronic CORT model to show that females are more sensitive to elevated glucocorticoid levels in adolescence on EtOH-motivated behavior in adulthood, and demonstrate that reinstatement of EtOH seeking is enhanced in female rats. These results lay the foundation for future studies aimed at determining the mechanisms underlying female sensitivity to environmental factors in promoting alcohol craving/relapse-like behavior, which likely implicate overlapping gonadal and stress hormone systems. Identification of these circuits could potentially lead to the development of genetic, pharmacologic, and behavioral interventions aimed at mitigating the risk of stress and cue exposure in stimulating relapse to alcohol seeking in females.
Acknowledgments
This research was supported by the NIH grant K01DA071345, the American Heart Association grant AHA13BGIA16850030, the Pennsylvania Department of Health (all to MMT), and the DSF Charitable Foundation 132RA03 (training fellowship to MLB). The authors would like to thank Bryan McElroy, Hayley Busch, and Jenna Hebert for their skilled technical assistance.
Footnotes
The authors report no conflicts of interest.
References
- Amlung M, MacKillop J. Understanding the effects of stress and alcohol cues on motivation for alcohol via behavioral economics. Alcohol Clin Exp Res. 2014;38:1780–1789. doi: 10.1111/acer.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010;107:264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Barha CK, Brummelte S, Lieblich SE, Galea LA. Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus. 2011;21:1216–1227. doi: 10.1002/hipo.20829. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Monteggia LM, Perrot-Sinal TS, Romeo RD, Taylor JR, Yehuda R, Bale TL. Stress and disease: is being female a predisposing factor? J Neurosci. 2007;27:11851–11855. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey ML, Verplaetse TL, Czachowski CL. Alterations in ethanol seeking and self-administration following yohimbine in selectively bred alcohol-preferring (P) and high alcohol drinking (HAD-2) rats. Behav Brain Res. 2013;238:252–258. doi: 10.1016/j.bbr.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addict Biol. 2011;16:440–449. doi: 10.1111/j.1369-1600.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Horm Behav. 2012;62:210–218. doi: 10.1016/j.yhbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology (Berl) 2014;231:1557–1580. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Carter E, Weiner JL. Adolescent social isolation does not lead to persistent increases in anxiety- like behavior or ethanol intake in female long-evans rats. Alcohol Clin Exp Res. 2014;38:2199–2207. doi: 10.1111/acer.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Hong K, Bergquist K, Sinha R. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin Exp Res. 2008;32:1242–1250. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, Thorsell A, Heilig M. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacology (Berl) 2010;208:417–426. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Saladin ME, Drobes DJ, Brady KT, Dansky BS, Kilpatrick DG. Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug Alcohol Depend. 2002;65:115–127. doi: 10.1016/s0376-8716(01)00157-0. [DOI] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM. Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology. 2013;38:2343–2353. doi: 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, DeLucia R, Planeta CS. Effects of chronic stress on nicotine-induced locomotor activity and corticosterone release in adult and adolescent rats. Addict Biol. 2008;13:63–69. doi: 10.1111/j.1369-1600.2007.00080.x. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Saha TD, Grant BF. Changes in alcohol consumption: United States, 2001–2002 to 2012–2013. Drug Alcohol Depend. 2015;148:56–61. doi: 10.1016/j.drugalcdep.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl A, Croissant B, Batra A, Mundle G, Nakovics H, Mann K. Alcoholism in women: is it different in onset and outcome compared to men? Eur Arch Psychiatry Clin Neurosci. 2007;257:344–351. doi: 10.1007/s00406-007-0737-z. [DOI] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121:240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 2011;216:53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Microanalysis of ethanol self-administration: estrous cycle phase-related changes in consumption patterns. Alcohol Clin Exp Res. 2002;26:635–643. [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Determination of an estradiol dose-response relationship in the modulation of ethanol intake. Alcohol Clin Exp Res. 2004;28:20–28. doi: 10.1097/01.ALC.0000108647.62718.5A. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Taylor JR. Recapitulation and reversal of a persistent depression-like syndrome in rodents. Curr Protoc Neurosci Chapter. 2009;Chapter 9(Unit 9):32. doi: 10.1002/0471142301.ns0932s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell EE, Ray LA. Sex moderates stress reactivity in heavy drinkers. Addict Behav. 2013;38:2643–2646. doi: 10.1016/j.addbeh.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Li G, Hasin DS. Birth cohort effects and gender differences in alcohol epidemiology: a review and synthesis. Alcohol Clin Exp Res. 2011;35:2101–2112. doi: 10.1111/j.1530-0277.2011.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Bernardy NC, Hauner K. Stressful events, personality, and mood disturbance: gender differences in alcoholics and problem drinkers. Addict Behav. 2003;28:171–187. doi: 10.1016/s0306-4603(01)00264-7. [DOI] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. Estrogen receptor beta, but not alpha, mediates estrogen's effect on cocaine-induced reinstatement of extinguished cocaine-seeking behavior in ovariectomized female rats. Neuropsychopharmacology. 2007;32:1334–1345. doi: 10.1038/sj.npp.1301249. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, McRae-Clark A, Baker NL, Ramakrishnan V, Brady KT. Yohimbine administration and cue-reactivity in cocaine-dependent individuals. Psychopharmacology (Berl) 2014;231:4157–4165. doi: 10.1007/s00213-014-3555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesic J, Duka T. Gender specific effects of a mild stressor on alcohol cue reactivity in heavy social drinkers. Pharmacol Biochem Behav. 2006;83:239–248. doi: 10.1016/j.pbb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol. 1999;60:252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res. 1998;22:1564–1569. [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD. Alcohol cue reactivity and mood induction in male and female alcoholics. J Stud Alcohol. 1994;55:487–494. doi: 10.15288/jsa.1994.55.487. [DOI] [PubMed] [Google Scholar]
- Simms JA, Haass-Koffler CL, Bito-Onon J, Li R, Bartlett SE. Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. Neuropsychopharmacology. 2012;37:906–918. doi: 10.1038/npp.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Richards JK, Mill D, Kanholm I, Holgate JY, Bartlett SE. Induction of multiple reinstatements of ethanol- and sucrose-seeking behavior in Long-Evans rats by the alpha-2 adrenoreceptor antagonist yohimbine. Psychopharmacology (Berl) 2011;218:101–110. doi: 10.1007/s00213-011-2451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Chappell AE, Carter E, Weiner JL. Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: Possible role of disrupted noradrenergic signaling. Neuropharmacology. 2015;97:149–159. doi: 10.1016/j.neuropharm.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Randall PK, Brady K, See RE, Drobes DJ. An acute psychosocial stressor does not potentiate alcohol cue reactivity in non-treatment-seeking alcoholics. Alcohol Clin Exp Res. 2011;35:464–473. doi: 10.1111/j.1530-0277.2010.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C. Stress during Adolescence Increases Novelty Seeking and Risk-Taking Behavior in Male and Female Rats. Front Behav Neurosci. 2011;5:17. doi: 10.3389/fnbeh.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Xie M, Taylor JR. Chronic corticosterone exposure during adolescence reduces impulsive action but increases impulsive choice and sensitivity to yohimbine in male Sprague-Dawley rats. Neuropsychopharmacology. 2012;37:1656–1670. doi: 10.1038/npp.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O'Hagen CS, Spear LP. The effects of gonadectomy on sex- and age-typical responses to novelty and ethanol-induced social inhibition in adult male and female Sprague-Dawley rats. Behav Brain Res. 2011;227:224–232. doi: 10.1016/j.bbr.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]