Abstract
Medication nonadherence, a major problem in cardiovascular disease (CVD), contributes yearly to approximately 125,000 preventable deaths, which is partly attributable to only about one-half of CVD patients consistently taking prescribed life-saving medications. Current interest has focused on how labeling and education influence adherence. This paper summarizes the scope of CVD nonadherence, describes key U.S. Food and Drug Administration initiatives, and identifies potential targets for improvement. We describe key adherence factors, methods, and technological applications for simplifying regimens and enhancing adherence, and 4 areas where additional collaborative research and implementation involving the regulatory system and clinical community could substantially reduce nonadherence: 1) identifying monitoring methods; 2) improving the evidence base to better understand adherence; 3) developing patient/health provider team-based engagement strategies; and 4) alleviating health disparities. Alignment of U.S. Food and Drug Administration approaches to dissemination of information about appropriate use with clinical practice could improve adherence, and thereby reduce CVD death and disability.
Keywords: cardiovascular disease, nonadherence, polypill, United States Food and Drug Administration
Patients who do not adhere to prescribed courses of medication are at greater risk for poor outcomes. Although the prevalence of medication nonadherence is difficult to gauge (1), it remains an undermanaged problem (2). Approximately 50% of patients with cardiovascular disease (CVD) had poor adherence to prescribed medications (3). A study of 1,015 patients with stable coronary artery disease showed a 4.4-fold increase in the risk of stroke and a 3.8-fold increase in the risk of death among patients who self-reported as nonadherent (4). Full adherence to guideline-recommended therapies for the treatment of atherosclerotic disease and management post-myocardial infarction (MI), with a threshold >80% in the post-MI population, was associated with a lower rate of major adverse cardiac events, and with cost savings (5).
As part of the Million Hearts Campaign (6), the U.S. Food and Drug Administration (FDA) convened a symposium addressing medication adherence and its effect on the clinical effectiveness of approved drugs, patient outcomes, and health disparities.
In this paper, we summarize key background material from this symposium; provide an overview of the status of current work aimed at improving medication adherence, particularly in the arena of cardiovascular medicine; and discuss possible future directions for this field.
METHODOLOGY
We developed a collaborative initiative with the following objectives: 1) identify key factors that affect medication adherence; 2) describe an innovative collaborative approach to address the medication adherence problem; 3) detail specific features of FDA activities that are applicable to taking innovative steps toward attenuating the problem of medication adherence; 4) describe specific measures to enhance medication adherence; and 5) describe effective interventions for the practicing physician to improve medication adherence. The authors independently searched their respective databases to achieve the collaborative objective. Published studies were included if they were considered to be significant and relevant to the objective set forth in this initiative.
KEY FACTORS AFFECTING ADHERENCE
Patient nonadherence to prescribed medications presents a multifactorial challenge for physicians and other health care providers. In 2011, the American College of Preventive Medicine documented 5 key factors that affect adherence and recommended that research and monitoring efforts be focused on them (7). These include: 1) socioeconomic factors; 2) health care system–related factors; 3) medical condition–related factors; 4) therapy-related factors; and 5) patient-related factors (Table 1). In the following sections, we address each factor in detail.
TABLE 1.
Factors Reported to Affect Adherence
| Social and economic dimension |
| Limited English proficiency |
| Low health literacy |
| Lack of family or social support network |
| Unstable living conditions/homelessness |
| Burdensome schedule |
| Limited access to health care facilities |
| Lack of health care insurance |
| Inability/difficulty accessing pharmacy |
| Medication cost |
| Cultural and lay beliefs about illness and treatment |
|
|
| Health care system dimension |
| Provider-patient relationship |
| Provider communication skills |
| Disparity between health beliefs of patient and provider |
| Lack of positive reinforcement from provider |
| Weak system capacity for patient education and follow-up |
| Lack of knowledge about adherence and interventions for improving it |
| Patient information materials written at too high of a literacy level |
| Changes/restrictions affecting formulary |
| High drug costs/copayments |
| Poor access/missed appointments |
| Long wait times |
| Lack of continuity of care |
|
|
| Condition-related dimension |
| Chronic conditions |
| Lack of symptoms |
| Severity of symptoms |
| Depression |
| Psychotic disorders |
| Mental retardation/developmental disability |
|
|
| Therapy-related dimension |
| Complexity of medication regimen (number of doses, number of concurrent medications) |
| Treatment requires mastery of certain techniques (injection, inhaler) |
| Duration of therapy |
| Frequent changes in medication regimen |
| Lack of immediate therapeutic benefit |
| Medications with associated social stigma |
| Actual/perceived unpleasant side effects |
| Treatment interferes with lifestyle/requires significant behavioral change |
|
|
| Patient-related dimension |
| Physical factors |
| Visual impairment |
| Hearing impairment |
| Cognitive impairment |
| Impaired mobility/dexterity |
| Swallowing problems |
| Psychological/behavioral factors |
| Perceived risk/susceptibility to disease |
| Understanding reason medication is needed |
| Expectations/attitudes toward treatment |
| Perceived benefit of treatment |
| Confidence in ability to follow treatment regimen |
| Motivation |
| Fear of possible adverse effects |
| Fear of dependence |
| Feeling stigmatized by disease |
| Frustration with health care providers |
| Psychosocial stress/anxiety/anger |
| Alcohol/substance abuse |
Adapted with permission from the American College of Preventive Medicine (7).
SOCIOECONOMIC FACTORS
Studies evaluating relationships between social support and medication adherence have shown that weaker social support is associated with poorer adherence. Socioeconomic factors that adversely affect adherence include poverty, illiteracy, unemployment, lack of social support networks, unstable living conditions, greater distance from treatment centers, higher out-of-pocket cost of medications and care, lack of transportation, cultural beliefs reflecting mistrust in the health care system, and family dysfunction (8,9). In some cases, patients attempt to offset the cost of prescription drugs by reducing the dosage and/or frequency of a recommended therapy or by purchasing medications abroad, reinforcing the importance of cost as a factor in adherence (10).
Patient demographic characteristics are also associated with differential rates of medication adherence. African-American race, Hispanic ethnicity, female sex, older age, and greater disability have been associated with lower rates of adherence. Women are less likely than men to adhere to prescribed long-term medications for diabetes mellitus and cardiovascular disease (11). Race and lower socioeconomic status are coexistent factors for non-adherence (12). A retrospective exploratory study of 51,772 hypertensive patients found that adherence rates were lower among African-American patients compared with white patients (13,14). Similarly, a cross-sectional analysis of data from the Medication Event Monitoring System over a 30-day interval showed that African-American race, female sex, and receipt of care from a publicly funded versus a private clinic were all associated with lower rates of adherence (15). In a Veterans Administration study of hypertensive patients, 63% of African-American men were found to have inadequate blood pressure control versus 50% of white men, and African-American men were 81% more likely to be nonadherent (16). Of note, access to medications in the Veterans Administration system is generally equal, regardless of race, ethnicity, or income (17). In a study of adherence in post-MI patients (18), 1-year rates of adherence to beta-blockers were 68%, 66%, 61%, 58%, and 57% for white, Asian, Hispanic, Native American, and African-American patients, respectively; among patients with disabilities, 1-year adherence rates by respective race/ethnicity categories were worse: 59%, 54%, 52%, 47%, and 43%, respectively. Finally, a number of studies have demonstrated higher rates of nonadherence to medication regimens among elderly patients. Barriers to adherence identified in these studies included lower levels of health literacy, reduced cognitive function, polypharmacy, and various logistical barriers to obtaining medications (19).
Levels of education and literacy are also important factors in medication adherence. In a study of 61 patients with heart failure (HF) (20), inability to read prescription labels and lack of understanding were associated with increased cardiovascular-related emergency department visits, as well as emergency visits specifically related to HF. Furthermore, medication education at the time of hospital discharge has shown substantial success in reducing hospital readmission rates (21).
There is significant interaction among these factors, suggesting that efforts to alter nonadherence on an individual or population level must take into account community dynamics that affect the environment in which individuals make decisions about adherence. Efforts that intervene on single factors, or that occur only in a clinical setting removed from the patients’ daily environment, seem unlikely to have the desired level of effect.
HEALTH CARE SYSTEMS–RELATED FACTORS
The organization of the health care delivery system is believed to play an important role with regard to medication adherence, but empirical data to support this view are scant. Within a given delivery system, physician and caregiver skills are thought to be important in developing supportive patient relationships. Studies have shown that team-based care, in which physician extenders, pharmacists, and nursing team members are engaged in patient education and monitoring of adherence, can be effective in improving adherence and health outcomes (22,23). The role of the pharmacist is critical in the post-hospital setting. Hospital discharge is often associated with multiple changes in medication regimens and discontinuity of care, which is often compounded by inadequate patient education.
Pharmacist intervention focusing on clarifying medication regimens, directions for use, reviewing side effects, and providing patient counseling resulted in a significant reduction in adverse drug events 30 days after hospital discharge (24). In a study of low-income, inner-city patients 50 years of age or older with primary care physician-confirmed HF, a 9-month pharmacist intervention protocol, including verbal instructions, patient monitoring of medication management, and other health care encounters and technical support, yielded an 11% increase in medication adherence (95% confidence interval: 5% to 17%) over standard of care. However, medication adherence declined to that of the standard of care in the 3-month post-intervention follow-up period (25). Other studies have shown that early follow-up visits after hospital discharge, as well as physician continuity, had a significant effect on outcomes (26,27). This was due to various factors, including assessment of adherence to medications.
Substantial evidence shows that nonadherence is associated with increases in hospitalization and use of other medical resources, and these differences in resource use have been demonstrated to translate into substantially higher costs for nonadherent patients and populations (28–30). However, there are currently few studies documenting that specific interventions result in significant cost savings at the societal or health system levels. There is a critical need to understand cost-effectiveness, and the association between medication adherence and health care costs for health system improvements and improved access to care within these systems.
MEDICAL CONDITION–RELATED FACTORS
Concomitant conditions, particularly mental health diseases and cognitive impairment, have been associated with poorer medication adherence in patients with cardiometabolic disease. Depression has been associated with nonadherence after adjustment for potential confounders, such as age, ethnicity, education, social support, and measures of CVD severity (4). In recently hospitalized cardiac patients, improvement in symptoms of depression was consistently and independently associated with superior self-reported adherence to medications and secondary prevention behaviors (i.e., diet, exercise, and stress reduction) across a 6-month span, whereas improvement in anxiety symptoms was not (31).
Furthermore, comorbidities contribute to polypharmacy issues; for example, patients with HF are typically prescribed 6 to 8 daily medications (32). Collaborative disease management programs, in which medications are reviewed with the goal of eliminating unneeded therapies, may be helpful in this regard, but little evidence has been generated to guide recommendations for the most effective interventions and implementation schemes to improve adherence in the setting of complex conditions.
THERAPY-RELATED FACTORS
Therapy-related barriers to adherence appear to be substantial. Side effects, greater complexity of regimen, and longer duration of regimen are all associated with lower rates of adherence, as are frequent changes to the regimen itself (33). In 1 study, nuisance bleeding, which occurred in up to 60% of patients receiving dual antiplatelet therapy, was correlated with non-adherence (34).
In a retrospective cohort study of 255,500 hypertensive patients followed for 2 years, adherence was monitored across the spectrum of antihypertensive medications given as monotherapy or in a fixed-dose combination (35). A total of 56% of the cohort taking monotherapy and 50% of the cohort taking the fixed-dose combination were defined as nonadherent (Table 2). The percentage of nonadherent patients was highest for diuretic therapy, either as monotherapy or in a fixed-dose combination. There was a trend toward an attenuation of nonadherence for fixed-dose combination therapy. This suggested that fixed-dose combination therapy might prove beneficial in promoting medication adherence.
TABLE 2.
Nonadherent Patients Taking Antihypertensive Medications*
| ACE-i | ARB | BB | CCB | Diuretic Agents | Total | |
|---|---|---|---|---|---|---|
| Monotherapy | 31,867 (55.8) | 8,462 (57.6) | 54,559 (55.2) | 18,772 (81.5) | 4,774 (66.3) | 113,136 (56.3) |
| Combination | 12,268 (50.3) | 6,030 (45.9) | 4,808 (49.3) | 43 (33.9) | 4,438 (61.0) | 25,587 (50.4) |
Values are n (%).
Includes 200,787 patients (78.6%) with monotherapy and 54,714 patients (21.4%) with fixed-dose combination therapy. Reprinted with permission from Schulz et al. (35).
ACE-i = angiotensin-converting enzyme inhibitors; ARB = angiotensin II receptor antagonists; BB = beta-blocker; CCB = calcium-channel blocker.
A nonrandomized study of synchronized prescription refills was conducted to evaluate whether filling prescriptions at the same time for common maintenance medications would improve medication adherence (36). This study, although limited (i.e., not a randomized trial, low participation relative to eligible participants, and mail-order pharmacy service only for maintenance prescriptions), suggested the possibility that synchronized refills is a therapy-related streamline mechanism that may improve medication adherence.
Increased number of medications, prescription refill patterns across different time points, not using reminder tools (e.g., pillbox), no insurance, lower level of education, and the need for dialysis were all factors associated with medication discontinuation. These findings suggest a need for common-sense approaches to simplifying regimens and clear logistics as critical factors in better adherence.
PATIENT-RELATED FACTORS
Medication adherence is adversely affected by patient-related factors that are closely linked to the other factors previously discussed. Visual, hearing, cognitive, mobility, and swallowing impairments are obvious barriers, and individual patients with fewer resources or lower literacy levels have more difficulty filling prescriptions and adhering to complex regimens. Additional factors include lack of knowledge or understanding about the disease or need for the prescribed medication, expectations about and perceived benefits of treatment, ability and motivation to follow a medical regimen, frustration, anxiety, and substance or alcohol abuse (37).
COLLABORATIVE APPROACH TO ADDRESS THE MEDICATION ADHERENCE PROBLEM
The multifactorial challenges of medication adherence require a multifactorial solution, and have thus prompted a collaborative effort between the FDA and other agencies or campaigns (i.e., Million Hearts Campaign, National Forum for Heart Disease and Stroke Prevention, National Consumer League [NCL], Duke Adherence Alliance, and the Enhanced Adherence Strategic Initiative [EASi] Consortium) (Central Illustration). This collaborative effort has linked together several independent programs in an effort to formulate an overall strategy to improve medication adherence: knowledge dissemination, patient advocacy, improvement of the evidence base to optimize adherence monitoring and engagement strategies, and alleviation of health disparities that have an adverse effect on medication adherence.
CENTRAL ILLUSTRATION. Multifactorial Approach to Influence Medication Adherence.
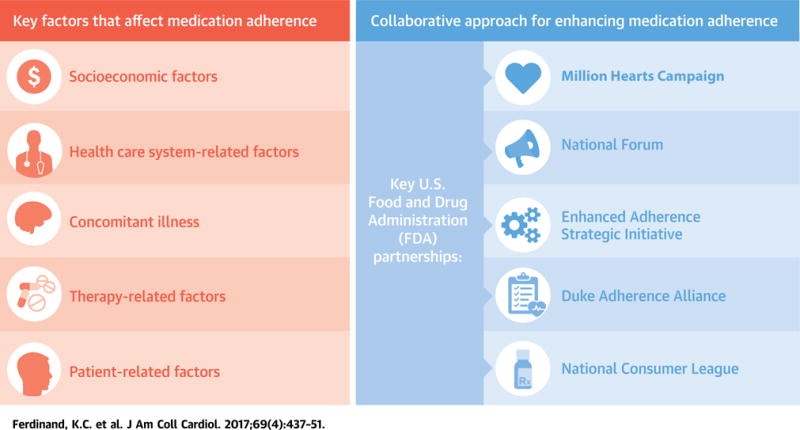
Patient nonadherence to prescribed medications presents a multifactorial challenge for physicians and other health care providers. In 2011, the American College of Preventive Medicine documented 5 key factors that affect adherence (7), and recommended that research and monitoring efforts be focused on them. These factors include: 1) socioeconomic factors; 2) health care system–related factors; 3) medical condition–related factors; 4) therapy-related factors; and 5) patient-related factors. The multifactorial challenges of medication adherence require a multifactorial solution, and have thus prompted a collaborative effort between the Food and Drug Administration and other agencies or campaigns (i.e., Million Hearts Campaign, National Forum for Heart Disease and Stroke Prevention, National Consumer League, Duke Adherence Alliance, and the Enhanced Adherence Strategic Initiative Consortium). This collaborative effort has linked together several independent programs in an effort to formulate an overall strategy to improve medication adherence: knowledge dissemination, patient advocacy, improvement of the evidence base to optimize adherence monitoring and engagement strategies, and alleviation of health disparities that adversely affect medication adherence.
MILLION HEARTS
The Million Hearts Campaign (6) is a national initiative of the Department of Health and Human Services (HHS), whose goal is to prevent 1 million heart attacks and strokes by 2017. This collaborative effort involves multiple government agencies and nongovernmental collaborators. One of its main areas of focus is improving medication adherence through knowledge dissemination, stakeholder activation, creation of incentives, measuring and reporting, improving population health, and research. The initiative seeks to leverage the research capabilities of the National Institutes of Health and the Agency for Healthcare Research and Quality, the payment and quality measures of the Centers for Medicare and Medicaid Services, and the FDA’s expertise in the assessment and regulation of therapeutics.
NATIONAL FORUM
The National Forum for Heart Disease and Stroke Prevention is a consortium of academic and community health care advocacy groups. The FDA’s Office of Health and Constituent Affairs has signed a 5-year memorandum of understanding with the National Forum to promote and increase the use of health knowledge, skills, and practices by the public in their daily lives that are designed to reduce the burden of heart disease and stroke.
NATIONAL CONSUMER LEAGUE
The FDA has partnered with the NCL, a private, nonprofit advocacy group representing consumers in the marketplace and workplace. The NCL’s “Script Your Future” effort (38), launched in 2011, is a national campaign focused on raising awareness of the importance of taking medications as directed. This campaign is the culmination of years of research, collaboration, and contributions from more than 135 committed stakeholders, comprising governmental agencies (including the FDA), health professionals, patient and consumer organizations, industry, and academia.
DUKE ADHERENCE ALLIANCE
The FDA has also joined the Duke Adherence Alliance, a think tank that evaluates the state of medication adherence and makes recommendations to help ensure that Americans get the greatest benefit from advances in pharmacology (39).
ENHANCED ADHERENCE STRATEGIC INITIATIVE
The EASi consortium, which developed alongside the Million Hearts Campaign, has committed its members’ expertise to contribute to this campaign. Collectively, the team plans to fulfill the objectives developed linked to the Million Hearts Campaign and outlined in this paper: 1) identifying methods for monitoring adherence; 2) improving the evidence base to better understand adherence; 3) developing patient/health provider team-based engagement strategies; and 4) alleviating health disparities.
SPECIFIC FDA ACTIVITIES
During a recent meeting involving the FDA, the National Institutes of Health, academicians, and representatives from the medical products industry, an evaluation of medical adherence programs revealed that for long-term treatments, 36 of 83 interventions reported in 70 randomized clinical trials were associated with improvements in adherence, but only 25 interventions led to improvements in at least 1 treatment outcome (40). No single intervention was identified as having a significant effect on adherence. Slight improvements (4% to 11%) were observed in unimodal interventions that included reduction in daily doses, use of motivational strategies, and optimal packaging strategies, with education, monitoring, and feedback from the patients. As noted earlier, multimodal interventions have generally achieved greater success than unimodal ones (41). Interventions that were most effective included combinations of more convenient care, information, reminders, self-monitoring, reinforcement, counseling, family therapy, psychological therapy, crisis intervention, manual telephone follow-up, and supportive care.
In its capacity as both a regulatory and public health agency, the FDA is systematically examining actions and activities that can improve appropriate use of approved medications. However, the FDA is part of a much larger ecosystem whose members must act in concert if adherence is to be substantially improved. In particular, the primary issues involve medical practice, patient commitment, community health services organization and delivery, and involvement in and solutions to access and disparities in health care. Accordingly, the FDA’s efforts are focused on ways in which regulatory strategies, labeling, and patient information can interdigitate with medical practice and health care delivery to improve adherence, as summarized in Table 3.
TABLE 3.
Specific FDA Activities
| Generic medications | (42) |
| Critical Path and Sentinel System | (43–46) |
| Health literacy | (47–54) |
| Medication guides | (55–57) |
| Essential medical information | (58,59) |
| Cardiovascular and Endocrine Liaison Program | (60) |
| FDA Advisory committees-Polypill | (61–64) |
FDA = U.S. Food and Drug Administration.
ROLE OF GENERIC MEDICATIONS
One regulatory strategy being used to address socioeconomic barriers to adherence is to advance access to generic medications, which facilitates greater penetration of medication availability across socioeconomic strata through lower prices. Under the Drug Price Competition and Patent Term Restoration Act (Public Law 98-417), also known as the Hatch-Waxman Act, generic pharmaceutical manufacturers may obtain FDA approval to market a copy of an innovator drug without duplicating the clinical and nonclinical studies required for approval of the innovator. Under the Generic Drug User Fee Act, the FDA has committed to rigorous timelines for review of generic drug applications, and prioritizes applications that deal with therapeutic options lacking in competition (42). The FDA continues to develop strategies to optimize accessibility of medications within this paradigm. Currently, 88% of prescriptions are for generic drugs, including common guideline-recommended cardiovascular therapies, such as statins, aspirin, beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, warfarin, and clopidogrel, suggesting that although such a strategy may be useful, it will not be sufficient in itself to overcome barriers to adherence without being combined with other approaches.
THE CRITICAL PATH AND SENTINEL SYSTEM
In response to societal expectations about drug safety and efficacy, the FDA introduced the Critical Path Initiative, with the intent of modernizing drug development (43). A key facet of this effort is the Sentinel Initiative (44,45), a national electronic safety surveillance system launched in 2008, with the purpose of greatly strengthening the FDA’s ability to conduct post-market safety monitoring of medical products. Prior to Sentinel, the FDA relied on reporting systems such as the FDA Adverse Event Reporting System, Manufacturer and User Facility Device Experience Database, and Vaccine Adverse Event Reporting System, all of which depend on manufacturers, health care providers, and patients to report adverse events related to health care products. Sentinel was designed to complement the existing FDA systems through active monitoring of regulated products. In 2015, Sentinel was used in 17 active protocol assessments. The system’s rapid query capabilities ran close to 2,000 different scenarios. The current Sentinel database includes 200 million individuals and more than 350 million person-years of observation time. The database includes electronic health records provided by hospitals, insurers, and universities (46). As Sentinel evolves, it is anticipated that it will expeditiously produce more knowledge about safety and the risk/benefit balance of medical products. This is anticipated to translate into more appropriate use, and better instructions to prescribing physicians and patients. These features are key parameters in medication adherence, and therefore, it is anticipated that the Sentinel program will contribute to improved adherence. The effect of the Sentinel program on medication adherence will require evaluation through an adequately designed monitoring program.
HEALTH LITERACY
To increase the utility of the label, the FDA revised the label design by adding a “highlights” section to emphasize the drug’s indications and warnings (47). In addition, the FDA advanced a proposal calling for labels to be more transparent by reflecting information the reviewing medical officer used when evaluating the New Drug Application and by displaying any uncertainties in the risk-benefit analysis (43). However, despite the merit of this proposal, incorporating information used by the FDA reviewer may not lead to a better understanding of the drug’s safety and efficacy profile among health care providers and, in turn, patients who are prescribed such drugs. A key policy challenge for advancing high-quality health care is health literacy—the degree to which people have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions (48). Health literacy requires both the patient’s ability to read and understand, as well as the health care provider’s ability to adequately communicate important medical information. A patient-centered approach to designing consumer medication information could improve the comprehensibility of existing warnings in package inserts (49). The use of precise wording on prescription drug label instructions has been shown to improve patient comprehension, but patients with limited literacy were more likely to misinterpret instructions, despite the use of more explicit language (50). In addition, specific implementation strategies designed to provide useful information for patients with limited health literacy, such as a 3-part intervention consisting of automated telephone reminders, picture prescription cards, and pharmacist communication skills training, did not significantly improve refill adherence among inner-city patients. Further exploration of strategies to in-crease health literacy, and thus improve adherence, is clearly warranted (51), and the FDA has an important role to play in fostering improvements in communication that lead to improved adherence.
In 2010, the HHS initiated a national action plan to improve health literacy (52) on the basis of 2 core principles: 1) all people have the right to health information that helps them make informed decisions; and 2) health services should be delivered in ways that are easy to understand and that improve health, longevity, and quality of life. As part of this national plan, the FDA is working to develop language for product labeling that patients can easily understand. The Labeling Development Team, located in the Office of New Drugs in the Center for Drug Evaluation and Research at the FDA, has been tasked with ensuring that prescribing information: 1) is a useful communication tool for health care providers; 2) is on the basis of regulations and guidance; and 3) conveys the essential scientific information needed to prescribe and use drugs and biological products safely and effectively (53). A 2014 FDA guidance described Section 17 in prescription drug product labeling (Patient Counseling Information), which summarizes information that a health care provider should convey to a patient or caregiver during a counseling discussion (54). This HHS health literacy initiative is a work in progress, and its effects on adherence have yet to be assessed.
MEDICATION GUIDES
As part of a strategy to improve adherence by improving medication knowledge, the FDA has required medication guides designed to be issued to consumers along with certain prescribed medications (55). Medication guides are handouts that address issues specific to particular drugs and drug classes, and are written in nontechnical and understandable language, on the basis of approved professional labeling for the drug. The FDA requires medication guides to be issued when the Agency determines that 1 or more of the following circumstances exists: 1) the drug product is one for which patient labeling could help prevent serious adverse effects; 2) the drug product is one that has serious risk(s) (relative to benefits), of which patients should be made aware because information concerning the risk(s) could affect patients’ decisions to use, or continue to use, the product; or 3) the drug product is important to health and has specific directions for its use that require patient adherence (such as keep away from light, store in refrigerator, do not chew, take only with food, and so on). A key feature of the medication guide is the heading, “What is the most important information I should know about (name of drug)?” followed by a statement describing the health concern that necessitated the medication guide. The communication features associated with medication guides are postulated to favorably affect medication adherence, specifically in a manner that enhances the benefit-risk ratio of such therapy.
An evaluation of the medication guides program (56) revealed that none of the 40 guides available at that time (written from 2001 to 2006) met federal recommendations for readability and use of plain language. All exceeded the recommended seventh- to eighth-grade level for reading comprehensibility; only 10% provided a summary of content; and 76% failed to limit the scope of information; furthermore, the guides ranged in length from 667 to 4,900 words. Only 23% of patients reported having looked at the guides or accompanying patient information material. Patients with low literacy were less likely to look at the medication guides, and they are likely ineffective in these patients.
Between March 2008 and January 2011, the FDA approved more than 150 medication guides for products approved under a New Drug Application and Biologics License Application as part of a risk evaluation and mitigation strategy; currently, 14 cardio-vascular drugs are accompanied by such guides. A sample guide for prasugrel shows that the language is directed toward a general U.S. population (57), but it has not been ascertained whether adherence, adverse events, or efficacy have been affected by the medication guide. Efforts should be focused on determining whether there has been improvement in this decade on adherence attributable to the use of medication guides.
ESSENTIAL MEDICAL INFORMATION
The FDA is developing a framework to provide patients with high-quality, up-to-date essential prescription drug product information (58) by convening public workshops on the basis of a cooperative agreement between the Agency and the Brookings Institution’s Engelberg Center for Health Care Reform. Currently available forms of prescription drug information intended for patients include the Patient Package Insert, the Medication Guide, and Consumer Medication Information. From these workshops, a consensus emerged in favor of a new framework for providing an easy-to-read, FDA-driven Patient Medication Information document.
A study on the best way to display information that accompanies prescription medication was conducted by interviewing patients with select immune disorders (n = 30), other chronic diseases (n = 30), and the general public (n = 30) (59). Respondent preferences varied according to age, education, and health status. The FDA is committed to continued efforts to create a focused, single standardized document that: 1) comes in an easily understood format; 2) is consumer-tested; 3) is provided when a patient receives a prescription medication; 4) is intended to be used at home, but does not replace or affect professional labeling, instructions for use, or patient counseling; and 5) is freely available and easily accessible to health care providers and patients.
Adherence is increasingly likely to improve to the extent that this publicly vetted information from the FDA is: 1) consistent with the messages from clinicians and educators; and 2) can be adapted to be appropriate for local circumstances and the needs of individual patients.
FDA OFFICE OF HEALTH AND CONSTITUENT AFFAIRS
The FDA’s Office of Health and Constituent Affairs (OHCA) serves as the liaison between the FDA and stakeholder organizations. The OHCA provides coordination for activities related to patient advocacy, health professional organizations, consumer groups, state organizations, and industry groups on high-priority topics, such as serious and life-threatening diseases, imminent public health needs, and other special health issues, such as medication adherence. By working with these organizations and patient advocacy groups, the OHCA may better understand patient-specific concerns and work directly with these groups on initiatives for implementation.
CARDIOVASCULAR AND ENDOCRINE LIAISON PROGRAM
The Cardiovascular and Endocrine Liaison Program (CELP) has been under development since November 2011 (60). The CELP was designed to serve as a liaison among the FDA, cardiovascular and endocrine health professionals, and patient communities. CELP encourages and supports the community’s active participation in developing FDA policies that promote healthy dietary and nutritional practices and advance the safety and effectiveness of medical products that treat diabetes, hypertension, heart disease, and obesity.
Forums of participation include FDA open public meetings, stakeholder calls, listening sessions, and workshops and conferences, as well as providing written or electronic comments to the FDA’s published documents via open public dockets (Get Illness/Condition Information). FDA-mediated patient engagement across the health care continuum directly addresses a key issue (i.e., patient engagement and education) that was shown to improve medication adherence (22,23). Feedback from the patient’s perspective may help FDA policies to evolve regarding drug development by addressing patient concerns and thus increasing the likelihood of medication adherence.
FDA ADVISORY COMMITTEES
Advisory Committees are used to provide advice on individual product approvals and also to consider major policy issues, including health information and useful approaches to product development to meet needs, such as the need for greater adherence. A recent FDA Advisory Committee (61) addressed the cardiovascular polypill, a single-dose regimen that typically combines a low-dose statin, a beta-blocker, an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, and low-dose aspirin, in an effort to improve adherence by reducing patient pill burdens. The basic question at issue was whether people are better off receiving some reasonable dose of drugs for secondary prevention of CVD rather than none, even if they are not getting what is believed to be optimal dosing. The advisory committee agreed that there was a significant group of patients who are not seen frequently for medical care, and for whom taking the polypill might offer a real advantage. There was concern, however, that discontinuing the polypill due to a side effect associated with just 1 component (e.g., statin intolerance) would thereby result in discontinuing all other components. The committee’s consensus was that many in the general population might be suitable candidates for the polypill, which could improve adherence. Clinical studies of poly-pills, including the UMPIRE (Use of a Multidrug Pill In Reducing Cardiovascular Events) (62) and FOCUS (Fixed-Dose Combination Drug for Secondary Cardiovascular Prevention) (63) trials, have shown improved medication adherence, but the investigators suggested that further research with appropriate trial designs were required to validate this approach. This sentiment was echoed in the conclusion of the advisory committee.
The HOPE-3 (Heart Outcomes Prevention Evaluation-3) trial demonstrated a significantly lower rate of cardiovascular events in intermediate-risk subjects without cardiovascular disease for the combination of rosuvastatin and candesartan/hydrochlorothiazide compared with dual placebo (64). In 12,705 racially/ethnically diverse subjects who were taking combination therapy, adherence was 83.6% at 2 years and 74.6% at the end of the trial (median follow-up 5.6 years), which was similar to dual placebo, despite a higher incidence of muscle weakness and dizziness. Although the study was not designed to evaluate medical adherence, the data suggested that combination therapy would not attenuate adherence, despite additive side effects.
SPECIFIC MEASURES TO ENHANCE MEDICATION ADHERENCE
Four broad areas where additional focus could significantly contribute to reducing the scope of the medication adherence problem are summarized in Table 4 and are described in the following sections.
TABLE 4.
Specific Measures to Enhance Medication Adherence
METHODS FOR MEASURING AND MONITORING ADHERENCE
A fundamental issue in developing effective approaches to improve adherence is the difficulty of measuring it. As discussed previously, the series of behaviors required to fill a prescription and take the right dose of the right medicine at the right time are complex; furthermore, self-reporting is subject to numerous, well-documented biases and inaccuracies. Studies reporting on approaches that used substantial focus and concentration of supportive resources have shown improved estimates, but the generalizability of such methods to practice where additional resources are not available is questionable. New methods, including novel technologies combined with patient-reported outcome measures, are increasingly being used to monitor and measure adherence. These new methods offer the prospect of much more accurate measurement, with fidelity at the level of the individual for health care, and for population assessment in research designed to develop new interventions. Validated methods for analyzing, monitoring, and ranking identified predictors of nonadherence are critical to these efforts.
A plethora of new biomonitoring and Internet reporting systems have emerged to target adherence (e.g., Telehomecare). Tools, such as special watches with alarms, pillbox timers, “smart pill” containers, and automated pill dispensers, are readily available (65). Electronic monitoring has been favored over pill counts by many authorities as producing the most accurate data on adherence (66). Although traditional pillboxes are useful in improving adherence, advances in digital pillboxes can improve medication adherence by engaging patients in innovative ways. In 1 study, a wireless electronic pill bottle, which reminded hypertensive patients to take their medication by emitting light and sound, increased medication adherence by 27% (67). The FDA’s approach to regulating wireless technology in medical devices is evolving in an effort to balance the need for innovation with the responsibility for assuring the public that devices are safe and effective (68).
IMPROVING THE EVIDENCE BASE FOR EFFECTIVE THERAPIES, RISK FACTORS FOR POOR ADHERENCE, AND INTERVENTIONS TO IMPROVE ADHERENCE
Simple and clear directions for medications are enhanced by consensus in the professional community about the body of evidence supporting therapeutics. The development of the evidence-based medicine paradigm, defined as the systematic collation, synthesis, and application of high-quality empirical evidence, has been adversely affected by an unmanageably high volume of evidence; statistically significant benefits that have marginal effect in clinical practice; rules and practice prompts that produce management-driven, rather than patient-centered care; and poor mapping of evidence-based guidelines to complex multimorbidity (69).
The developing strategies to improve adherence have likely been influenced by the challenges of the developing evidence-based medicine paradigm. Advances in information technology will facilitate new approaches to monitoring and improving medication adherence, through combinations of structured and unstructured data. As these interventions advance, attention to rigorous science, transparency, and completeness of reporting will be crucial, because much of the adherence intervention research involves behavioral interventions that are not subject to federal requirements for reporting in the ClinicalTrials.gov registry.
A poor-quality evidence base about interventions can lead to unsettled science and to differences of opinion among clinicians about the appropriate use or duration of therapy; this can often affect public opinion, which can pose challenges to adherence. A recent Danish study (70) showed that when negative news appeared about statins appeared in the media, patients discontinued their statins and had worse outcomes.
The Agency for Healthcare Research and Quality recently articulated its research priorities for improving the database by tracking: 1) the days covered and prescriptions filled; 2) medication possession versus medication prescription; 3) adherence by class related to multiple chronic conditions; and 4) patient-reported self-adherence evaluation systems, and associating prescription data with pharmacy/payer data to track adherence (71). The cohesiveness of the database and the quality of evidence that populates it are central to an effective understanding of adherence and how to improve it.
PATIENT/HEALTH CARE PROVIDER TEAM-BASED ENGAGEMENT STRATEGIES
When we consider challenges posed in this arena, the evidence points to a complex problem involving individual, social, and health care–related causes. For this reason, it is likely that the entire health care team, including the patient and family, must be engaged in efforts to improve adherence.
Cardiac rehabilitation represents an engagement strategy with the potential to improve medication adherence. This health care resource usually follows hospitalization for acute coronary syndrome. Post-hospitalization referral to cardiac rehabilitation is a pivotal moment in the patient’s management, and it is likely to have a significant effect on medication adherence via patient engagement. A survey of cardiac rehabilitation programs listed in the American Association of Cardiovascular and Pulmonary Rehabilitation database showed that only 28% of eligible patients used cardiac rehabilitation services. If all 812 of these programs functioned at maximum capacity, they could service a maximum of 47% of qualifying U.S. patients (72). A study of cardiac rehabilitation program adherence among women evaluated 3 rehabilitation models: supervised mixed-sex, supervised women-only, or home-based cardiac rehabilitation. Adherence was defined as the recorded number of on-site or telephone sessions (in the case of home-based programs). The overall adherence was 64%. The results did not clearly favor any model (73). This study suggested that offering women alternative models for cardiac rehabilitation services did not promote adherence. In a study of 822 consecutive patients referred to cardiac rehabilitation at the Montefiore Medical Center in the Bronx, New York, nonwhite patients initiated cardiac rehabilitation less often than white patients (54% vs. 65%; p = 0.003). Copayment did not influence initiation. White patients were 61% more likely to complete the prescribed 36 sessions. Cardiac rehabilitation initiation was associated with cardiologist involvement. Initiation was associated with a survival benefit, and there were no differences between white and nonwhite patients in survival benefit for those patients initiating rehabilitation (74). Even in a community of a predominantly minority population, racial disparity exists among cardiac rehabilitation participants. This study suggested that greater involvement by the cardiologist was pivotal for rehabilitation initiation and adherence, with consequent survival benefit.
Medicaid, Indian Health Service programs, and some community health center programs provide devices for disease-monitoring purposes, e-mail messaging, clinical reminders, and medication education. In addition to educating patients about the importance of adherence, these tools can help patients share concerns about side effects, cost barriers, or other factors that may contribute to nonadherence. Call centers for personal communications from a care team member are used when patients fail to respond to e-mail or text messages.
Patient web portals have also been used, and they hold promise to increase patient health understanding and shared decision making. Nevertheless, although most users (60%) consider portals to be very useful (75), overall use remains relatively low, with only 28% of patients being offered this tool as of 2013. Optimally, patient portals could be used to host e-visits and telemedicine, with interoperability across multiple providers (e.g., record sharing) and health coaching (76). In addition, mobile technology and associated health applications (apps) have gained popularity, with a growing market in recent years. The most common method for engaging primary users appears to be self-monitoring (77). However, a recent study that validated blood pressure measurements from a highly popular mHealth app (>148,000 units sold) found that the app’s measurements were highly inaccurate. In particular, the app demonstrated poor sensitivity for hypertensive measurements, with the result that approximately four-fifths (77.5%) of individuals with blood pressure measurements in the hypertensive range would receive a false assurance that their blood pressure was within the nonhypertensive range (78). These types of interventions need to be carefully evaluated so as to optimize engagement strategies.
ALLEVIATION OF HEALTH DISPARITY
As previously noted, poor adherence is embedded in and exacerbates health care disparities. Some ongoing projects to address this include an initiative started in September 2015 by the Morehouse School of Medicine and Emory University. This initiative explores the risk of heart disease and stroke among minority patients by establishing the Morehouse School of Medicine/Emory Cardiovascular Center for Health Equity, with funding from the American Heart Association’s (AHA’s) new Strategically Focused Research Network (79).
Another initiative is the African American Health Disparity Project, which is designed to improve the health status of African Americans and to eliminate institutional racism in the health care system of San Francisco, California. Some of the project’s accomplishments include no-cost treatment for African Americans diagnosed with breast cancer, establishment of an African American Health Initiative, community grants to address health disparities in disproportionately affected neighborhoods, maintaining posting of pledge statements, providing educational seminars, and adding a cultural diversity competency component to the employee evaluation tool. Some key obstacles included the availability of funding, the immensity and complexity of the factors affecting health disparities, and mistrust in the health system among the African-American community, as well as lack of communication. Key lessons included the need for partnership with the external community, the importance of developing community trust by building relationships, and the critical need to leverage greater resources for a multidisciplinary effort, to educate staff, and to develop cultural sensitivity (80).
The BARBER-1 (Effectiveness of a Barbershop-Based Program to Improve High Blood Pressure Control and Awareness in Black Men) study (81) demonstrated a neighborhood-based intervention strategy involving black-owned barbershops with >95% black male clientele. The cluster-randomized study assigned barbershops to receive either AHA pamphlets only, with no other intervention, or to receive interventions including blood pressure checks and health messaging through the barber and peers (but no AHA pamphlets). The results demonstrated a significant increase in hypertension control in the intervention arm (p = 0.035). These results suggest that neighborhood programs designed to improve blood pressure outcome in African-American men may have clinical utility. These results may be applicable to other ethnic groups. Further research into this neighborhood-based strategy is warranted.
A review of interventions, and state and national policy initiatives, aimed at reducing racial and ethnic disparities in blood pressure control showed mixed results (82). The 39 interventions that were examined mostly focused on patient education, communication, behavioral intervention, multilevel settings, cultural competency training, community intervention, and/or social support. Of these, 27 resulted in some improvement of blood pressure control; 7 did not. Among 6 studies that focused on disparities, 3 had interventions that led to reduced disparity, 2 precipitated increased levels of disparity, and 1 showed no effect on disparity. Although several effective interventions have been identified to improve blood pressure in racial and ethnic minorities, data on whether these interventions will reduce disparity are limited. The Million Hearts Campaign is addressing this issue, as is a joint series of projects undertaken by the National Heart, Lung, and Blood Institute and the Patient-Centered Outcomes Research Institute (83).
Another critically important issue is to ensure that research cohorts are diverse in terms of race/ethnicity, sex, and age, as well as socioeconomic status. Better information about adherence in the context of clinical trials from representative populations should enable the development of strategies tailored to the needs of disadvantaged populations. The collaborative initiative of the EASi consortium will address health disparities and its effect on medication adherence as a priority item. The magnitude of this problem requires more than intervention by the FDA; a concerted effort that engages the entire community is needed to ensure success.
EFFECTIVE INTERVENTIONS FOR THE PRACTICING CLINICIAN TO IMPROVE ADHERENCE
The state-of-the-art paradigm for enhancing medication adherence is complex and involves multiple hierarchies. Collaborative efforts at the national level and FDA activities steered toward enhancing medication adherence are tools for the practicing clinician. Practical guidelines for the clinician to improve medication adherence are shown in Table 5. Given our findings, patient engagement via communication with the patient in a literacy-sensitive manner is a significant parameter. Phone calls to the patient and providing educational services have been shown to improve adherence. FDA label highlights have facilitated implementation of this guideline. Greater use of generics to reduce cost is warranted. Use of the Sentinel program will enhance knowledge about drug safety and consequent risk/benefit, thereby facilitating the physician knowledge base in optimizing communication with patients. The clinician should communicate with the FDA on key issues affecting adherence through the CELP.
TABLE 5.
Intervention by the Practicing Clinician to Improve Adherence
| Intervention | Reference |
|---|---|
| Improve patient health literacy: label highlights and conveyance of literacy-sensitive information to patients. Engage the patient for greater health care provider-patient interaction: follow-up communication with the patient (e.g., cardiac rehabilitation), clinical reminders, phone calls, communication with the pharmacist, medical education, and use of patient web portals. | (43,47–54,76,77) |
| Use of generics. | (42) |
| Use of the Sentinel System for reporting adverse events. | (43–46) |
| Communicate with the FDA on key issues from your practice via the Cardiovascular and Endocrine Liaison Program. | (60) |
| Develop an office-based strategy to monitor adherence. | (65) |
| Be judicious about poor-quality evidence. | (70) |
| Establish greater cognizance of inherent health care disparities: build community trust. | (80,81) |
FDA = U.S. Food and Drug Administration.
The office practice should design a monitoring strategy using current technologies. The physician should be judicious about poor-quality evidence that can lead to unsettled science, resulting in inappropriate medication discontinuation and worsening of outcomes. There should be heightened sensitivity to health care disparity, and measures should be taken to attenuate this inherent issue.
SUMMARY AND CONCLUSIONS
Improving medication adherence could be one of the most effective and efficient ways of improving health outcomes, especially for cardiometabolic disease, the leading cause of mortality in the United States. Because less than one-half of patients with chronic cardiovascular conditions take their prescriptions consistently, it is critical that we develop an effective and generalizable adherence strategy for patients. Approaches for enhancing adherence are multifaceted, requiring the participation of governmental agencies, academia, and organizations devoted to optimizing health care. The FDA and collaborators have taken substantial steps to enhance medication adherence, and are working closely with stakeholders on a variety of activities. Nevertheless, because of the many social and economic causes of nonadherence, much work remains before we can claim to have met the challenge of medication adherence. Alignment between therapeutic evaluation and labeling, and the efforts of clinicians and health systems to improve adherence, could ultimately have a major effect on health outcomes.
Acknowledgments
The authors are grateful to Jonathan McCall, MS, of the Duke Clinical Research Institute for his invaluable assistance in preparing this paper. Mr. McCall received no compensation for his work on this paper other than usual salary. The authors would also like to thank Dr. Norman Stock-bridge, Director of the Division of Cardiovascular and Renal Products at the FDA, for facilitating the activities of the EASi Board. Finally, the authors would like to thank Julie Harvill, National Forum, for her tireless coordination of collaboration meetings and planning.
Dr. Ferdinand has received a grant from Boehringer Ingelheim; and serves as consultant for Amgen, Sanofi, Boehringer Ingelheim, and Eli Lilly. Dr. Cryer has served as a consultant for Amgen. Dr. Califf currently holds the post of Commissioner of Food and Drugs, U.S. Food and Drug Administration (FDA); before his appointment to the FDA, he received research grant funding from the Patient-Centered Outcomes Research Institute, National Institutes of Health, FDA, Amylin, and Eli Lilly and Company; received research grants and consulting payments from Bristol-Myers Squibb, Janssen Research and Development, Merck, and Novartis; received consulting payments from Amgen, Bayer Healthcare, BMEB Services, Genentech, GlaxoSmithKline, Heart.org–Daiichi Sankyo, Kowa, Les Laboratoires Servier, Medscape/Heart.org, Regado, and Roche; and held equity in N30 Pharma and Portola.
ABBREVIATIONS AND ACRONYMS
- CVD
cardiovascular disease
- EASi
Enhanced Adherence Strategic Initiative
- FDA
U.S. Food and Drug Administration
- HF
heart failure
- OHCA
Office of Health and Constituent Affairs
Footnotes
Listen to this manuscript’s audio summary by JACC Editor-in-Chief Dr. Valentin Fuster.
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Kolandaivelu K, Leiden BB, O’Gara PT, et al. Non-adherence to cardiovascular medications. Eur Heart J. 2014;35:3267–76. doi: 10.1093/eurheartj/ehu364. [DOI] [PubMed] [Google Scholar]
- 2.Baroletti S, Dell’Orfano H. Medication adherence in cardiovascular disease. Circulation. 2010;121:1455–8. doi: 10.1161/CIRCULATIONAHA.109.904003. [DOI] [PubMed] [Google Scholar]
- 3.Kronish I, Ye S. Adherence to cardiovascular medication: lessons learned and future direction. Prog Cardiovasc Dis. 2013;55:590–600. doi: 10.1016/j.pcad.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gehi AK, Ali S, Na B, et al. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2007;167:1798–803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansilal S, Castellano JM, Garrido E. Assessing the impact of medication adherence on long-term cardiovascular outcomes. J Am Coll Cardiol. 2016;68:789–801. doi: 10.1016/j.jacc.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Million Hearts. Available at: http://millionhearts.hhs.gov. Accessed November 23, 2016.
- 7.American College of Preventive Medicine. Medication adherence—improving health outcomes. A resource from the American College of Preventive Medicine. 2011 Available at: http://www.acpm.org/?Adherence. Accessed November 22, 2016.
- 8.Lewis LM, Ogedegbe C, Ogedegbe G. Enhancing adherence of antihypertensive regimens in hypertensive African-Americans: current and future prospects. Expert Rev Cardiovasc Ther. 2012;10:1375–80. doi: 10.1586/erc.12.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braverman J, Dedier J. Predictors of medication adherence for African American patients diagnosed with hypertension. Ethn Dis. 2009;19:396–400. [PubMed] [Google Scholar]
- 10.Cohen RA, Villarroel MA. Strategies Used by Adults to Reduce Their Prescription Drug Costs: United States, 2013. Hyattsville, MD: National Center for Health Statistics; 2015. (NCHS Data Brief No 184). [PubMed] [Google Scholar]
- 11.Manteuffel M, Williams S, Chen W, et al. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J Womens Health (Larchmt) 2014;23:112–9. doi: 10.1089/jwh.2012.3972. [DOI] [PubMed] [Google Scholar]
- 12.Rolnick SJ, Pawloski PA, Hedblom BD, et al. Patient characteristics associated with medication adherence. Clin Med Res. 2013;11:54–65. doi: 10.3121/cmr.2013.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes HM, Luo R, Hanlon JT, et al. Ethnic disparities in adherence to antihypertensive medications of Medicare Part D beneficiaries. J Am Geriatr Soc. 2012;60:1298–303. doi: 10.1111/j.1532-5415.2012.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishisaka DY, Jukes T, Romanelli RJ, et al. Disparities in adherence to and persistence with antihypertensive regimens: an exploratory analysis from a community-based provider network. J Am Soc Hypertens. 2012;6:201–9. doi: 10.1016/j.jash.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Grigoryan L, Pavlik VN, Hyman DJ. Predictors of antihypertensive medication adherence in two urban health-care systems. Am J Hypertens. 2012;25:735–8. doi: 10.1038/ajh.2012.30. [DOI] [PubMed] [Google Scholar]
- 16.Bosworth HB, Dudley T, Olsen MK, et al. Racial differences in blood pressure control: potential explanatory factors. Am J Med. 2006;119:70.e9–e15. doi: 10.1016/j.amjmed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Lewis LM, Schoenthaler AM, Ogedegbe G. Patient factors, but not provider and health care system factors, predict medication adherence in hypertensive black men. J Clin Hypertens (Greenwich) 2012;14:250–5. doi: 10.1111/j.1751-7176.2012.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Baik SH. Race/ethnicity, disability, and medication adherence among Medicare beneficiaries with heart failure. J Gen Intern Med. 2013;29:602–7. doi: 10.1007/s11606-013-2692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gellad WF, Grenard JL, Marcum ZA. A systematic review of barriers to medication adherence in the elderly: looking beyond cost and regimen complexity. Am J Geriatr Pharmacother. 2011;9:11–23. doi: 10.1016/j.amjopharm.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hope CJ, Wu J, Tu W, et al. Association of medication adherence, knowledge, and skills with emergency department visits by adults 50 years or older with congestive heart failure. Am J Health Syst Pharm. 2004;61:2043–9. doi: 10.1093/ajhp/61.19.2043. [DOI] [PubMed] [Google Scholar]
- 21.Bradley EH, Curry L, Horwitz LI, et al. Contemporary evidence about hospital strategies for reducing 30-day readmissions: a national study. J Am Coll Cardiol. 2012;60:607–14. doi: 10.1016/j.jacc.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granger BB, Ekman I, Hernandez AF, et al. Results of the Chronic Heart Failure Intervention to Improve MEdication Adherence study: a randomized intervention in high-risk patients. Am Heart J. 2015;169:539–48. doi: 10.1016/j.ahj.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66:2129–39. doi: 10.1016/j.jacc.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565–71. doi: 10.1001/archinte.166.5.565. [DOI] [PubMed] [Google Scholar]
- 25.Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure. Ann Intern Med. 2007;146:714–25. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–22. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 27.Sidhu RS, Youngson E, McAlister FA. Physician continuity improves outcomes for heart failure patients treated and released from the emergency department. J Am Coll Cardiol HF. 2014;2:368–76. doi: 10.1016/j.jchf.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Granger BB, Swedberg K, Ekman I, et al. for the CHARM Investigators Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–11. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez AF, Hammill BG, Peterson ED, et al. Relationships between emerging measures of heart failure processes of care and clinical outcomes. Am Heart J. 2010;159:406–13. doi: 10.1016/j.ahj.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Retrum JH, Boggs J, Hersh A, et al. Patient-identified factors related to heart failure read-missions. Circ Cardiovasc Qual Outcomes. 2013;6:171–7. doi: 10.1161/CIRCOUTCOMES.112.967356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer LK, Caro MA, Beach SR, et al. Effects of depression and anxiety improvement on adherence to medication and health behaviors in recently hospitalized cardiac patients. Am J Cardiol. 2012;109:1266–71. doi: 10.1016/j.amjcard.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Wong CY, Chaudhry SI, Desai MM, et al. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med. 2011;124:136–43. doi: 10.1016/j.amjmed.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fried T, Tinetti ME, Towle V, et al. Effects of benefits and harms on older persons’ willingness to take medications for primary cardiovascular prevention. Arch Intern Med. 2011;171:923–8. doi: 10.1001/archinternmed.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serebruany V, Rao SV, Silva MA, et al. Correlation of inhibition of platelet aggregation after clopidogrel with post discharge bleeding events; assessment by different bleeding classifications. Eur Heart J. 2010;31:227–35. doi: 10.1093/eurheartj/ehp434. [DOI] [PubMed] [Google Scholar]
- 35.Schulz M, Krueger K, et al. Medication adherence and persistence according to different antihypertensive drug classes: a retrospective cohort study of 255,500 patients. Int J Cardiol. 2016;220:668–76. doi: 10.1016/j.ijcard.2016.06.263. [DOI] [PubMed] [Google Scholar]
- 36.Doshi J, Lim R, Li P, et al. A synchronized prescription refill program improved medication adherence. Health Aff (Millwood) 2016;35:1504–12. doi: 10.1377/hlthaff.2015.1456. [DOI] [PubMed] [Google Scholar]
- 37.Brown MT, Bussell JK. Medication adherence: WHO cares. Mayo Clin Proc. 2011;86:304–14. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Consumers League. Script Your Future. 2016 Available at: http://www.scriptyourfuture.org. Accessed November 23, 2016.
- 39.Mozaffarian D, Benjamin EJ, Go AS, et al. for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2015 update: a report of the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 40.Bosworth HB, Granger BB, Mendys P, et al. Medical adherence: a call for action. Am Heart J. 2011;162:412–24. doi: 10.1016/j.ahj.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–35. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 42.U.S. Food and Drug Administration. Generic Drug User Fee Act program performance goals and procedures. Available at: http://www.fda.gov/downloads/ForIndustry/UserFees/GenericDrugUserFees/UCM282505.pdf. Accessed November 22, 2016.
- 43.Woodcock J, Woosley R. The FDA Critical Path Initiative and its influence on new drug development. Ann Rev Med. 2008;59:1–12. doi: 10.1146/annurev.med.59.090506.155819. [DOI] [PubMed] [Google Scholar]
- 44.Robb M, Racoosin JA, Sherman RE, et al. The US Food and Drug Administration’s Sentinel Initiative: expanding the horizons of medical product safety. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):9–11. doi: 10.1002/pds.2311. [DOI] [PubMed] [Google Scholar]
- 45.U.S. Food and Drug Administration. FDA’s Sentinel Initiative–News and Events. 2016 Available at: http://www.fda.gov/Safety/FDAsSentinelInitiative/ucm149341.htm. Accessed November 23, 2016.
- 46.Mezher M. Woodcock: Drug Safety Surveillance System Ready for Full Operation. RAPS. 2016 Feb 3; Available at: http://raps.org/Regulatory-Focus/News/2016/02/03/24248/Woodcock-Drug-Safety-Surveillance-System-Ready-for-Full-Operation/. Accessed November 23, 2016.
- 47.Schwartz L, Woloshin S. Lost in transmission—FDA drug information that never reaches clinicians. N Engl J Med. 2009;361:1717–20. doi: 10.1056/NEJMp0907708. [DOI] [PubMed] [Google Scholar]
- 48.Parker RM, Ratzan SC, Lurie N. Health literacy: a policy challenge for advancing high-quality health care. Health Aff (Millwood) 2003;22:147–53. doi: 10.1377/hlthaff.22.4.147. [DOI] [PubMed] [Google Scholar]
- 49.Webb J, Davis TC, Bernadella P, et al. Patient-centered approach for improving prescription drug warning labels. Patient Educ Couns. 2008;72:443–9. doi: 10.1016/j.pec.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 50.Davis TC, Federman AD, Bass PF, III, et al. Improving patient understanding of prescription drug label instructions. J Gen Intern Med. 2009;24:57–62. doi: 10.1007/s11606-008-0833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gazmararian J, Jacobson KL, Pan Y, et al. Effect of pharmacy-based health literacy intervention and patient characteristics on medication refill adherence in an urban health system. Ann Pharmacother. 2010;44:80–7. doi: 10.1345/aph.1M328. [DOI] [PubMed] [Google Scholar]
- 52.U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National Action Plan to Improve Health Literacy. Washington, DC: US Department of Health and Human Services; 2010. [Google Scholar]
- 53.U.S. Food and Drug Administration. Labeling Development Team. 2015 Available at: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm443026.htm. Accessed November 22, 2016.
- 54.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) Patient counseling information section of labeling for human prescription drugs and biological products–content and format. Guidance for industry. 2014 Dec; Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM368602.pdf Accessed November 22, 2016.
- 55.Electronic Code of Federal Regulations. Title 21. Chapter I, Subchapter C, Part 208. Medication guides for prescription drug products. Available at: http://www.ecfr.gov/cgi-bin/text-idx?SID=a4598f2153bbff076bd49ee19fbbe5eb&mc=true&node=pt21.4.208&rgn=div5. Accessed December 4, 2016.
- 56.Wolf MS, Davis TC, Osborn CY, et al. Literacy, self-efficacy, and HIV medication adherence. Patient Educ Couns. 2007;65:253–60. doi: 10.1016/j.pec.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 57.US Food and Drug Administration. Medication guide—Effient (prasugrel) tablets. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM238428.pdf. Accessed March 4, 2016.
- 58.Pearsall BM, Araojo R, Hinton D. Essential medication information for patients: Ensuring access. Ther Innov Regul Sci. 2014;48:162–4. doi: 10.1177/2168479013507437. [DOI] [PubMed] [Google Scholar]
- 59.Kish-Doto J, Scales M, Eguino-Medina P, et al. Preferences for patient medication information: what do patients want? J Health Commun. 2014;19(Suppl 2):77–88. doi: 10.1080/10810730.2014.946114. [DOI] [PubMed] [Google Scholar]
- 60.U.S. Food and Drug Administration. Cardiovascular and Endocrine Liaison Program (CELP) 2015 Available at: http://www.fda.gov/forhealthprofessionals/liaisonactivities/ucm402222.htm. Accessed November 22, 2016.
- 61.Food and Drug Administration, Center for Drug Evaluation and Research. Summary minutes of the cardiovascular and renal drugs advisory committee meeting; September 10, 2014; Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM456600.pdf. Accessed November 22, 2016. [Google Scholar]
- 62.Thom S, Poulter N, Field J, et al. for the UMPIRE Collaborative Group Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE Randomized Clinical Trial. JAMA. 2013;310:918–29. doi: 10.1001/jama.2013.277064. [DOI] [PubMed] [Google Scholar]
- 63.Castellano JM, Sanz G, Peñalvo JL, et al. A polypill strategy to improve adherence: results from the FOCUS project. J Am Coll Cardiol. 2014;64:2071–82. doi: 10.1016/j.jacc.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 64.Yusuf S, Lonn E, Pais P, et al. for the HOPE-3 Investigators Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med. 2016;374:2032–43. doi: 10.1056/NEJMoa1600177. [DOI] [PubMed] [Google Scholar]
- 65.Figge HL. Electronic tools to measure and enhance medication adherence. US Pharm. 2010;36(Suppl):6–10. 4 Compliance & Adherence. [Google Scholar]
- 66.Urquhart J. The electronic medication event monitor. Lessons for pharmacotherapy. Clin Pharmacokinet. 1997;32:345–56. doi: 10.2165/00003088-199732050-00001. [DOI] [PubMed] [Google Scholar]
- 67.Dolan B. Wireless Medication Adherence Study Conducted at the Partners Center for Connected Health Shows Promising Initial Findings [press release] Boston, MA: Center for Connected Health; 2010. [Google Scholar]
- 68.U.S. Food and Drug Administration. FDASIA Health IT Report. Proposed strategy and recommendations for a risk-based framework. 2014 Apr; Available at: http://www.fda.gov/downloads/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cdrh/cdrhreports/ucm391521.pdf. Accessed November 22, 2016.
- 69.Greenhalgh T, Howick J, Maskrey N, for the Evidence Based Medicine Renaissance Group Evidence based medicine: a movement in crisis? BMJ. 2014;348:g3725. doi: 10.1136/bmj.g3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nielsen SF, Nordestgaard BG. Negative statin-related news stories decrease statin persistence and increase myocardial infarction and cardiovascular mortality: a nationwide prospective cohort study. Eur Heart J. 2016;37:908–16. doi: 10.1093/eurheartj/ehv641. [DOI] [PubMed] [Google Scholar]
- 71.Agency for Healthcare Research and Quality. Medication adherence. doi: 10.1080/15360280802537332. Available at: http://healthit.ahrq.gov/ahrq-funded-projects/emerging-lessons/medication-adherence. Accessed November 22, 2016. [DOI] [PubMed]
- 72.Pack QR, Squires RW, Lopez-Jimenez F, et al. The current and potential capacity for cardiac rehabilitation utilization in the United States. J Cardiopulm Rehabil Prev. 2014;34:318–26. doi: 10.1097/HCR.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 73.Grace S, Midence L, Brister S, et al. Cardiac rehabilitation program adherence and functional capacity among women: a randomized controlled trial. Mayo Clinic Proc. 2016;91:140–8. doi: 10.1016/j.mayocp.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 74.Prince DZ, Sobolev M, Gao J, et al. Racial disparities in cardiac rehabilitation initiation and the effect on survival. PM R. 2014;6:486–92. doi: 10.1016/j.pmrj.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 75.Patel V, Barker W, Siminerio E. Individuals’ Access and Use of Their Online Medical Record Nationwide. Washington, DC: Office of the National Coordinator for Health Information Technology; 2014. (ONC Data Brief No 20). [Google Scholar]
- 76.Jacobs J. Best practices for patient portals. 2014 HIMSS.org. Available at: http://www.himss.org/News/NewsDetail.aspx?ItemNumber=43127. Accessed November 22, 2016.
- 77.Sama PR, Eapen ZJ, Weinfurt KP, et al. An evaluation of mobile health application tools. JMIR MHealth Uhealth. 2014;2:e19. doi: 10.2196/mhealth.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Plante TB, Urrea B, MacFarlane ZT, et al. Validation of the Instant Blood Pressure smartphone app. JAMA Intern Med. 2016;176:700–2. doi: 10.1001/jamainternmed.2016.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morehouse School of Medicine and Emory Explore Risk for Heart Disease and Stroke Among Minorities [press release] Atlanta, GA: Morehouse School of Medicine; Sep 1, 2015. [Google Scholar]
- 80.African American Health Disparity Project–San Francisco. Four-year project report. Available at: http://www.sfhip.org/javascript/htmleditor/uploads/AAHD_Four_Year_Report.pdf. Accessed November 22, 2016.
- 81.Victor RG, Ravenell JE, Freeman A, et al. Effectiveness of a barber-based intervention for improving hypertension control in black men: the BARBER-1 study: a cluster randomized trial. Arch Intern Med. 2011;171:342–50. doi: 10.1001/archinternmed.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mueller M, Purnell TS, Mensah GA, et al. Reducing racial and ethnic disparities in hyper-tension prevention and control: what will it take to translate research into practice and policy? Am J Hypertens. 2015;28:699–716. doi: 10.1093/ajh/hpu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.National Heart, Lung, and Blood Institute. Testing multi-level interventions to improve blood pressure control in minority racial/ethnic, low socioeconomic status, and/or rural populations FAQs. 2014 Available at: http://www.nhlbi.nih.gov/research/funding/opportunities/faq-foa-HL-15-021. Accessed November 22, 2016.


