Abstract
Injury to the joint provokes a number of local pathophysiological changes, including synthesis of inflammatory cytokines, death of chondrocytes, breakdown of the extra-cellular matrix of cartilage and reduced synthesis of matrix macromolecules. These processes combine to engender the subsequent development of post-traumatic osteoarthritis (PTOA). To prevent this from happening, it is necessary to inhibit these disparate responses to injury; given their heterogeneity, this is challenging. However, dexamethasone has the necessary pleiotropic properties required of a drug for this purpose. Using in vitro models, we have shown that low doses of dexamethasone sustain the synthesis of cartilage proteoglycans while inhibiting their breakdown after injurious compression in the presence or absence of inflammatory cytokines. Under these conditions, dexamethasone is non-toxic and maintains the viability of chondrocytes exposed chronically to such cytokines as interleukin (IL)-1, IL-6 and tumor necrosis factor-α. Moreover, the anti-inflammatory properties of dexamethasone have been appreciated for decades. In view of this information, we have initiated a pilot clinical study to determine whether a single, intra-articular injection of dexamethasone into the wrist shows promise in preventing PTOA after intra-articular fracture of the distal radius.
Introduction
Because post-traumatic osteoarthritis (PTOA) follows an obvious injury to the joint, it offers the opportunity for early therapeutic, even prophylactic, intervention. Recent advances in our understanding of the pathophysiological sequelae triggered by acute injuries to joints have generated a plausible general etiologic schema for PTOA1,2.
The initial injury leads to disruption of the cartilaginous matrix and the rapid death of chondrocytes in the impacted area. Acutely, this may occur via necrosis3 with subsequent apoptotic death of additional cells4. During the next several days, the zone of chondrocyte death expands. Moreover, the surviving chondrocytes further damage their surrounding cartilaginous matrix both by reducing the synthesis of matrix macromolecules and by increasing their degradation5. Osseous disturbances also occur during this period6. These changes occur in response to mechanical insult and are exacerbated by the inflammatory environment of injured joints. Since the work of Cameron et al.7, subsequently confirmed in several additional studies8–12, we know that the expression of several major inflammatory cytokines, including interleukins- (IL-) -1, 6 and -8, tumor necrosis factor-α (TNF-α), interferon-γ and granulocyte-macrophage colony stimulating factor, is elevated soon after injury to the human knee joint. The inflammatory response may be amplified by wear particles13 and various damage-associated molecular pattern molecules (DAMPs) created as a result of injury14. Collectively, these inflammatory mediators contribute to the synovitis, effusion, pain, cartilage degeneration and osseous changes that occur soon after injury. If these imbalances are allowed to persist, cartilage is irreversibly lost and the symptoms of OA ensue, despite a delayed anabolic phase that attempts to restore the cartilaginous matrix.
In this context, it should be noted that although living chondrocytes are able to replenish proteoglycan lost from their extracellular matrix, damage to the type II collagen of cartilage is generally thought to be irreversible15. Moreover, while human articular cartilage is now reported to contain a progenitor cell sub-population16, these cells are unlikely to be capable of replacing the extensive population of dead chondrocytes caused by injury, especially if these progenitors, too, are damaged. Extensive death of chondrocytes and destruction of the collagenous matrix of cartilage may thus determine the point at which degeneration of the cartilage becomes irreversible and PTOA inevitable, rendering futile any subsequent attempt at prophylaxis or restorative therapy17.
Cartilage matrix degradation and suppression of cartilage matrix synthesis, chondrocyte death and inflammation thus feature among the major, early, deleterious events that follow trauma to the joint. Any intervention to prevent the development of PTOA after injury must inhibit as many of these responses as possible, without suppressing endogenous reparative responses or triggering additional adverse events. This article summarizes pre-clinical evidence supporting the use of low-dose, intra-articular dexamethasone in this regard, and describes a consequent clinical trial.
The Injurious Compression of Cartilage, Inflammation and Dexamethasone
A variety of in vitro loading systems have been developed to induce mechanical damage in a manner relevant to in vivo joint injury; these systems have been recently codified and reviewed18. Of these various approaches, we have established a reliable, well-characterized in vitro model with which to investigate the combined effects of injurious mechanical compression in an inflammatory environment on the metabolism of articular cartilage5,19–23. Using this system, it has been confirmed that injurious loading alone leads to degradation of both proteoglycan and collagen by chondrocytes, with suppression of compensatory matrix synthesis. This is accompanied by cell death and leads to loss of the structural and mechanical integrity of cartilage. These effects are dramatically exacerbated in the presence of inflammatory cytokines known to be upregulated in traumatized joints, particularly TNF-α and IL-6 in association with its soluble receptor (sIL-6R)24. This system has enabled an approach to in vitro screening of potential chondro-protective agents21,24–26 as well as intra-articular nano-particle drug delivery methods27.
The multiplicity of mediators generated in response to injury constitutes a major challenge to preventing degenerative responses of cartilage to trauma, and the onset of PTOA in the damaged joint. A partial list of mediators includes the inflammatory cytokines mentioned in the previous section, as well as a variety of proteinases (matrix metalloproteinases (MMPs), a disintegrin and a metalloproteinase with thrombospondin motifs (ADAMTS), serine proteinases), reactive oxygen species, nitric oxide, prostanoids and numerous DAMPs. In the face of such a large and varied cocktail of mediators, an agent that selectively neutralizes a single mediator, or even a class of mediators, is unlikely to provide comprehensive protection from PTOA.
In this context, dexamethasone emerges as an attractive candidate because of its pleiotropic protective properties. In various cell types and animal models it inhibits the induction of MMPs28, prostaglandins29, inflammatory cytokines30, nitric oxide31 and other oxygen-derived radicals32. The literature is inconsistent on whether dexamethasone inhibits or promotes apoptosis in different types of cells33,34 but, as described below, it protects the viability of chondrocytes exposed to IL-1.
Collectively, these properties make dexamethasone a potential prophylactic agent for preventing degenerative responses in cartilage and the onset of PTOA in joints after injury. Moreover, translation is facilitated by its widespread use in routine clinical practice, including intra-articular injection for established OA, with a good understanding of its pharmacology and safety profile.
Dexamethasone is a synthetic member of the glucocorticoid class of steroid drugs, with a potency about 20–30 times that of the naturally occurring hydrocortisone and 4–5 times that of another widely used synthetic analog, prednisolone35. Over seventy years after the first chemical synthesis of cortisone36, corticosteroids remain among the top ten most commonly used drugs. Because they have been widely used to treat a variety of autoimmune diseases much is known about their pharmacology, pharmacokinetics and toxicology37,38. Systemically administered corticosteroids are very effective in controlling inflammatory conditions, but their use is constrained by adverse side effects, the main ones being loss of bone mineral density, avascular necrosis, increased body weight, increased cardiac risk, increased risk of infection and glucose intolerance. Intra-articular administration minimizes these systemic risks39,40.
Although intra-articular steroids are effective in providing symptomatic relief in established OA, there are concerns that repeated use damages the articular cartilage41. For this reason, orthopaedists are reluctant to administer repeated intra-articular steroid injections. However, when used to prevent the development of PTOA, we propose that a limited number of intra-articular injections – possibly as few as one27 – should be effective. This should maintain chondrocyte viability and restore metabolic equilibrium, while suppressing inflammation. From this point onwards, joint homeostasis will have been recovered and, in the absence of further injury, it should not be necessary to inject additional dexamethasone or, indeed, any other agent. Moreover our preliminary data, described below, strongly suggest that even very high doses of dexamethasone have no adverse effects on chondrocyte metabolism during periods of time relevant to a single, intra-articular application. From the surgical perspective, a single, intra-articular injection of dexamethasone would not interfere with any subsequent necessary adjunctive surgery to restore the mechanical integrity and alignment of the joint once the metabolic response to injury has been addressed.
Effects of Dexamethasone on Cartilage exposed to Injurious Compression and Inflammatory Mediators
Lu et al42 were the first to examine the effects of dexamethasone on the metabolism of cartilage after injurious compression in the presence or absence of inflammatory cytokines. They showed that dexamethasone normalized the release of glycosaminoglycans (GAG) from articular cartilage in response to TNF-α (figure 1). This occurred across an exceptionally wide range of dexamethasone concentrations (1–105 nM) and could not be explained by toxicity because the same doses of dexamethasone restored GAG synthesis to control levels (figure 1).
Figure 1. Dose-dependent effects of dexamethasone on GAG loss and GAG synthesis in cartilage explants exposed to TNF-α.
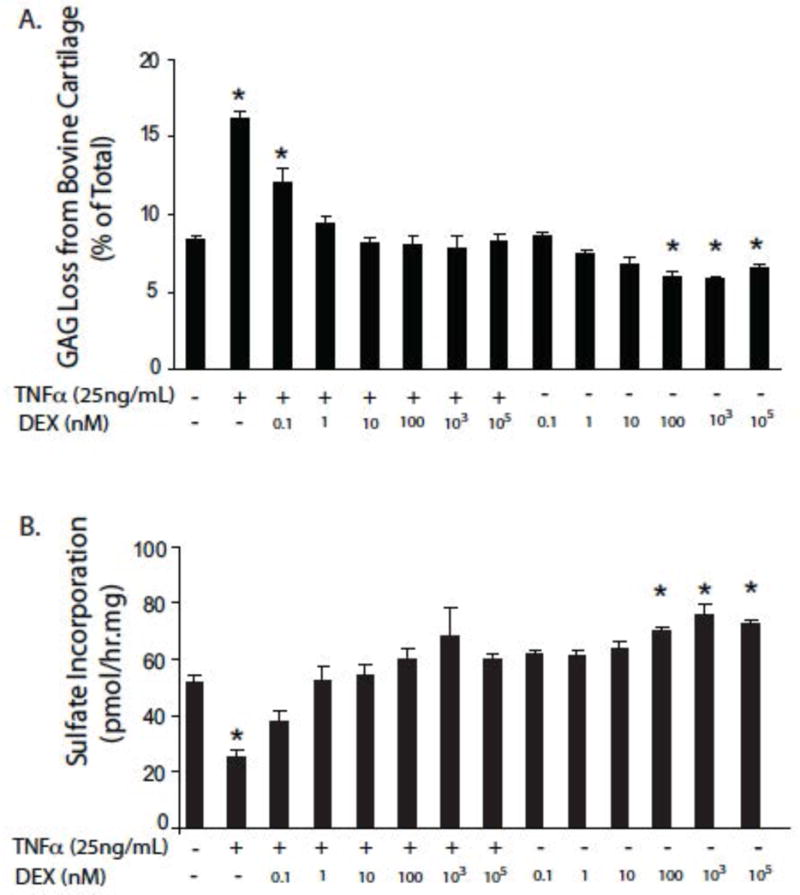
Cartilage samples were maintained in serum-free medium containing low-glucose DMEM, 10 mM HEPES buffer supplemented with 1% ITS: insulin (10 μg)-transferrin (5.5 μg/ml) – selenium (5 ng/ml). Dexamethasone and TNF-α were added at the indicated concentrations, for six days.
Panel A: Effects on GAG loss. Panel B: Effects on GAG synthesis. Values given are means ± SEM, n=5. * = p<0.05 difference from untreated cells.
From reference 42, with permission
Injurious compression of cartilage exposed to TNF-α exacerbated GAG loss, while the addition of IL-6/IL-6sR to this combination provoked even higher GAG release. In every case, GAG synthesis remained at very low levels. Under all conditions, 10 nM dexamethasone normalized GAG synthesis and release rates (figure 2). Of practical clinical relevance, the effects of dexamethasone were still apparent when its administration was delayed until 4 days after injury and the addition of cytokines.
Figure 2. Effect of dexamethasone on GAG loss and GAG synthesis in cartilage explants exposed to combinations of mechanical injury and cytokines.
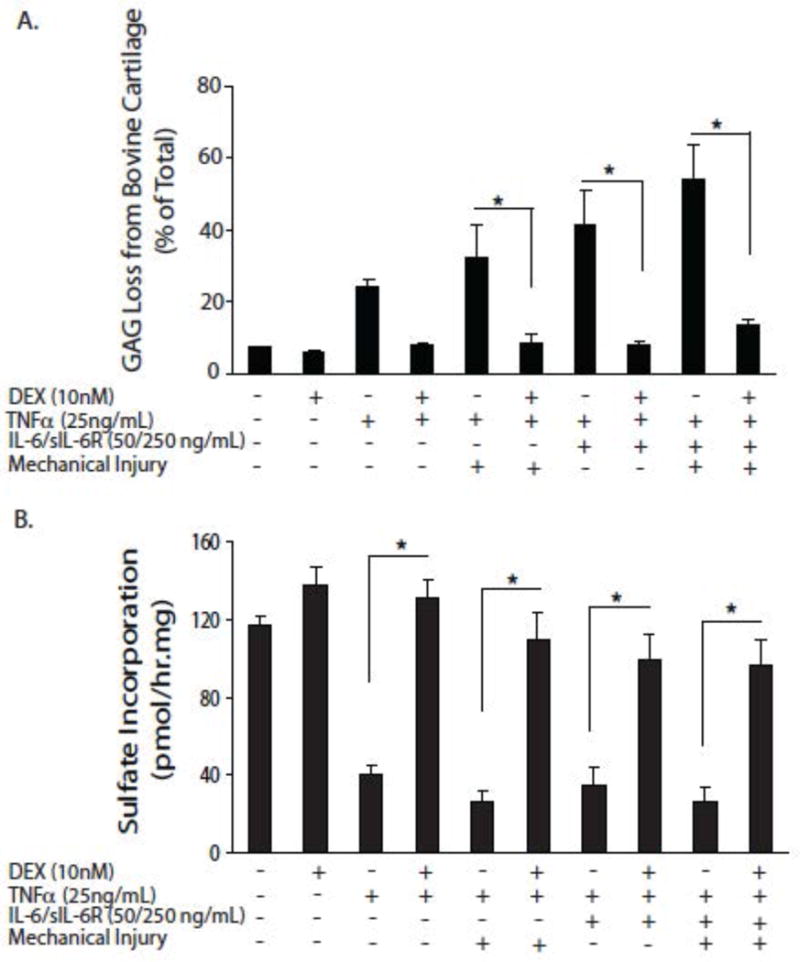
Cartilage samples were maintained in serum-free medium containing low-glucose DMEM, 10 mM HEPES buffer supplemented with 1% ITS: insulin (10 μg)-transferrin (5.5 μg/ml) – selenium (5 ng/ml). Dexamethasone and TNF-α were added at the indicated concentrations, for six days.
Panel A: Effects on GAG loss. Panel B: Effects on GAG synthesis. Values given are means ± SEM, n=5. * = p<0.05.
From reference 42, with permission
As noted, damage to the collagenous component of the cartilaginous matrix places the joint at particular risk of PTOA because such damage is irreversible. The data shown in figure 3 show that, in this in vitro model, loss of collagen from damaged cartilage in response to IL-1 begins at about day 12, after depletion of proteoglycans. Addition of dexamethasone at this time almost completely blocked the subsequent loss of collagen (figure 3).
Figure 3. Effect of dexamethasone on loss of collagen from cartilage explants exposed to IL-1.
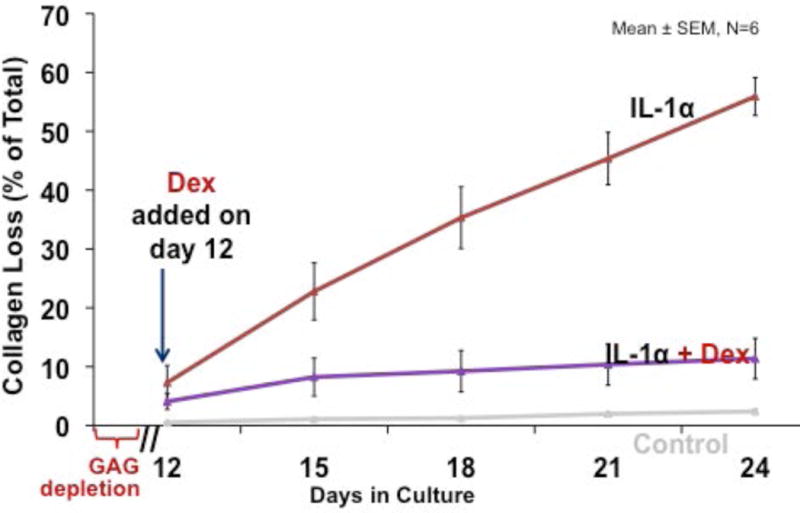
Cartilage samples were maintained in serum-free medium (low-glucose DMEM, 10 mM HEPES) lacking insulin, transferrin, selenium, and in the presence of 1 ng/mL IL-1 throughout culture duration. Dexamethasone was added and maintained at a concentration of 10 nM.
From reference 17, with permission
Death of articular chondrocytes further compromises the integrity of articular cartilage and eliminates any possibility of successful endogenous repair. As shown in figure 4, low dose dexamethasone maintained the viability of chondrocytes within cartilage incubated for an extended period of time with IL-1.
Figure 4. Effect of dexamethasone on the viability of chondrocytes within cartilage explants exposed to IL-1.
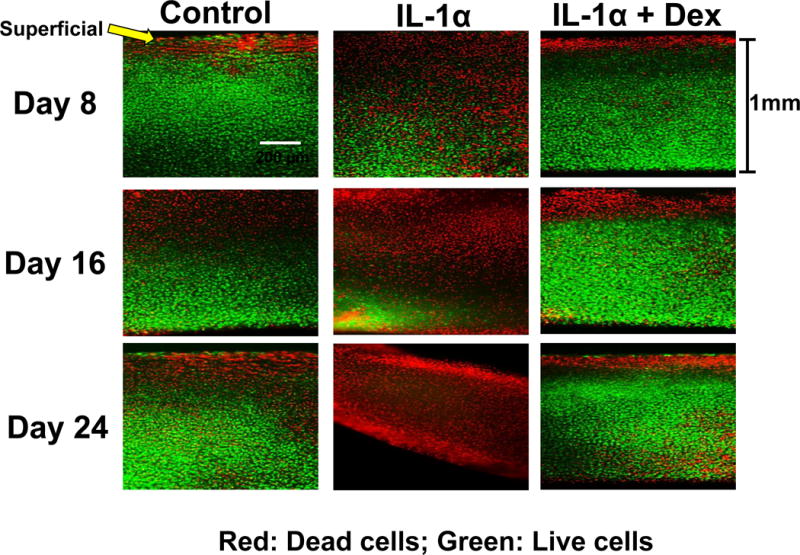
Cartilage samples were maintained in serum-free medium (low-glucose DMEM, 10 mM HEPES) lacking insulin, transferrin, selenium and in the presence of 1 ng/mL IL-1 throughout culture duration. Dexamethasone was added and maintained at a concentration of 10 nM.
From reference 18, with permission
Thus, under these in vitro conditions, low dose dexamethasone reduces the loss of proteoglycan and collagen from the extra-cellular matrix, maintains matrix synthesis and preserves chondrocyte viability. Based on this, it has the properties required of a drug for protecting cartilage in the traumatized joint. Its wide dose-response range and lack of toxicity are particular advantages.
The anti-inflammatory properties of dexamethasone have been appreciated for decades, and it has been widely administered by intra-articular injection to treat inflammation of joints. Its safety in this application is well established. Heard et al43 recently reported data from a rabbit study that addressed the effects of dexamethasone on the inflammatory aspects of PTOA in the absence of blunt trauma. Using a drill-hole model that induced inflammation without mechanical injury to the knee joint, these investigators reported a dramatic effect of a single, intra-articular injection of dexamethasone. Expression of IL-1, IL-6 and IL-8 was reduced and cartilage integrity was maintained.
The First Clinical Trial
Based upon the foregoing information, we have initiated a clinical study to determine whether a single, intra-articular injection of dexamethasone soon after injury can reduce the incidence or severity of PTOA (ClinicalTrials.gov Identifier: NCT02318433). Because we lack the data necessary for a comprehensive power analysis, this is a pilot study designed to provide the quantitative information required for a subsequent interventional trial with adequate statistical strength.
For a feasibility and pilot study of this nature, it was necessary to identify an indication where the incidence of PTOA was high and its development rapid. PTOA following intra-articular fractures of the distal radius seems to satisfy this requirement, where, according to Knirk et al44, there can be radiologic evidence of PTOA in 65% of patients at a mean follow-up of 6.7 years.
Initiated in 2014, the present study is recruiting 40 subjects with an intra-articular fracture of the distal radius. Patients are prospectively randomized into two groups of 20 individuals who receive an intra-articular injection of either 4 mg dexamethasone or an equal volume of saline within 2 weeks of injury. Each subject is being followed for 2 years. The primary outcome measure is incidence of PTOA, determined by joint space narrowing as measured radiologically. Secondary outcomes include functional assessment using several patient-rated evaluations including the Disabilities of the Arm Shoulder and Hand (DASH), the Michigan Hand Questionnaire (MHQ) and the Patient-Rated Wrist Evaluation (PRWE).
Conclusions and Perspective
Laboratory data and clinical experience suggest that a single, intra-articular injection of low dose dexamethasone soon after injury to the joint holds considerable promise as a safe means of preventing the development of PTOA. A small clinical study has been initiated to begin to explore this possibility. Repurposing intra-articular dexamethasone for preventing the development of PTOA after injury will be facilitated by its widespread clinical use in similar applications. This obviates the cost and regulatory burden of developing novel drugs for this purpose. Moreover, dexamethasone is inexpensive, costing only a few dollars per injection. While its clinical development is being pursued, laboratory research is exploring complementary avenues of treatment. One of them seeks to deliver dexamethasone to chondrocytes more efficiently, and another seeks to study the potential of a combination therapy involving dexamethasone and IGF-1.
Although the safety and anti-inflammatory properties of intra-articular dexamethasone have been well established clinically, its ability to influence chondrocyte metabolism in the manner noted here under in vitro conditions may be compromised by the highly hydrophilic nature of cartilaginous matrix and rapid efflux of dexamethasone from the joint45. For these reasons a second-generation product is being developed in which dexamethasone is covalently linked to avidin, a small, highly cationic molecule that rapidly diffuses throughout the whole depth of cartilage and delivers dexamethasone efficiently to chondrocytes27. Additional refinements could include the combination of a protective agent, such as dexamethasone, with an anabolic stimulus such as insulin-like growth factor-126.
Regardless of the drug of choice, it will important to determine the length of time after injury before the pathological changes induced by injury become irreversible and the window of opportunity for preventing PTOA closes.
Clinical significance.
Suppressing the various etiopathophysiological responses to injury in the joint is an attractive strategy for lowering the clinical burden of PTOA. The intra-articular administration of dexamethasone soon after injury offers a simple and inexpensive means of accomplishing this.
Acknowledgments
Supported by NIH-NIAMS grants AR033236 and AR060331 to AJG and a Mayo Clinic Early Career Development Award to SK
Footnotes
Author Contributions
All authors contributed to the writing and editing of this manuscript
References
- 1.Riordan EA, Little C, Hunter D. Pathogenesis of post-traumatic OA with a view to intervention. Best Pract Res Clin Rheumatol. 2014;28:17–30. doi: 10.1016/j.berh.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rundell SA, Baars DC, Phillips DM, Haut RC. The limitation of acute necrosis in retro-patellar cartilage after a severe blunt impact to the in vivo rabbit patello-femoral joint. J Orthop Res. 2005;23:1363–1369. doi: 10.1016/j.orthres.2005.06.001.1100230618. [DOI] [PubMed] [Google Scholar]
- 4.Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381:205–212. doi: 10.1006/abbi.2000.1988. [DOI] [PubMed] [Google Scholar]
- 5.Quinn TM, Grodzinsky AJ, Hunziker EB, Sandy JD. Effects of injurious compression on matrix turnover around individual cells in calf articular cartilage explants. J Orthop Res. 1998;16:490–499. doi: 10.1002/jor.1100160415. [DOI] [PubMed] [Google Scholar]
- 6.Pauly HM, Larson BE, Coatney GA, et al. Assessment of cortical and trabecular bone changes in two models of post-traumatic osteoarthritis. J Orthop Res. 2015;33:1835–1845. doi: 10.1002/jor.22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron ML, Fu FH, Paessler HH, et al. Synovial fluid cytokine concentrations as possible prognostic indicators in the ACL-deficient knee. Knee Surg Sports Traumatol Arthrosc. 1994;2:38–44. doi: 10.1007/BF01552652. [DOI] [PubMed] [Google Scholar]
- 8.Cameron M, Buchgraber A, Passler H, et al. The natural history of the anterior cruciate ligament-deficient knee. Changes in synovial fluid cytokine and keratan sulfate concentrations. Am J Sports Med. 1997;25:751–754. doi: 10.1177/036354659702500605. [DOI] [PubMed] [Google Scholar]
- 9.Irie K, Uchiyama E, Iwaso H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee. 2003;10:93–96. doi: 10.1016/s0968-0160(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi H, Shirakura K, Kimura M, et al. Changes in biochemical parameters after anterior cruciate ligament injury. Int Orthop. 2006;30:43–47. doi: 10.1007/s00264-005-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuellar VG, Cuellar JM, Golish SR, et al. Cytokine profiling in acute anterior cruciate ligament injury. Arthroscopy. 2010;26:1296–1301. doi: 10.1016/j.arthro.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Sward P, Frobell R, Englund M, et al. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis)–a cross-sectional analysis. Osteoarthritis Cartilage. 2012;20:1302–1308. doi: 10.1016/j.joca.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Evans CH, Mazzocchi RA, Nelson DD, Rubash HE. Experimental arthritis induced by intraarticular injection of allogenic cartilaginous particles into rabbit knees. Arthritis Rheum. 1984;27:200–207. doi: 10.1002/art.1780270212. [DOI] [PubMed] [Google Scholar]
- 14.Venereau E, Ceriotti C, Bianchi ME. DAMPs from Cell Death to New Life. Front Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 16.Williams R, Khan IM, Richardson K, et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010;5:e13246. doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Grodzinsky AJ. The response of cartilage to injury. In: Olson SA, Guilak F, editors. Post-Traumatic Arthritis, Pathogenesis, Diagnosis and Management. New York: Springer; 2015. [Google Scholar]
- 18.Chen CT, Torzilli PA. In vitro cartilage explant injury models. In: Olson SA, Guilak F, editors. Post-Traumatic Arthritis, Pathogenesis, Diagnosis and Management. New York: Springer; 2015. [Google Scholar]
- 19.Patwari P, Fay J, Cook MN, et al. In vitro models for investigation of the effects of acute mechanical injury on cartilage. Clin Orthop Relat Res. 2001:S61–71. doi: 10.1097/00003086-200110001-00007. [DOI] [PubMed] [Google Scholar]
- 20.Patwari P, Lin SN, Kurz B, et al. Potent inhibition of cartilage biosynthesis by coincubation with joint capsule through an IL-1-independent pathway. Scand J Med Sci Sports. 2009;19:528–535. doi: 10.1111/j.1600-0838.2009.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens AL, Wishnok JS, White FM, et al. Mechanical injury and cytokines cause loss of cartilage integrity and upregulate proteins associated with catabolism, immunity, inflammation, and repair. Mol Cell Proteomics. 2009;8:1475–1489. doi: 10.1074/mcp.M800181-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank EH, Jin M, Loening AM, et al. A versatile shear and compression apparatus for mechanical stimulation of tissue culture explants. J Biomech. 2000;33:1523–1527. doi: 10.1016/s0021-9290(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 23.Sah RL, Doong JY, Grodzinsky AJ, et al. Effects of compression on the loss of newly synthesized proteoglycans and proteins from cartilage explants. Arch Biochem Biophys. 1991;286:20–29. doi: 10.1016/0003-9861(91)90004-3. [DOI] [PubMed] [Google Scholar]
- 24.Sui Y, Lee JH, DiMicco MA, et al. Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor alpha in immature bovine and adult human articular cartilage. Arthritis Rheum. 2009;60:2985–2996. doi: 10.1002/art.24857. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler CA, Jafarzadeh SR, Rocke DM, Grodzinsky AJ. IGF-1 does not moderate the time-dependent transcriptional patterns of key homeostatic genes induced by sustained compression of bovine cartilage. Osteoarthritis Cartilage. 2009;17:944–952. doi: 10.1016/j.joca.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Wang Y, Chubinskaya S, et al. Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: relevance to post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23:266–274. doi: 10.1016/j.joca.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajpayee AG, Quadir MA, Hammond PT, Grodzinsky AJ. Charge based intra-cartilage delivery of single dose dexamethasone using Avidin nano-carriers suppresses cytokine-induced catabolism long term. Osteoarthritis Cartilage. 2016;24:71–81. doi: 10.1016/j.joca.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson DW, Dodge GR. Dose-dependent effects of corticosteroids on the expression of matrix-related genes in normal and cytokine-treated articular chondrocytes. Inflamm Res. 2003;52:39–49. doi: 10.1007/s000110300012. [DOI] [PubMed] [Google Scholar]
- 29.Shimpo H, Sakai T, Kondo S, et al. Regulation of prostaglandin E(2) synthesis in cells derived from chondrocytes of patients with osteoarthritis. J Orthop Sci. 2009;14:611–617. doi: 10.1007/s00776-009-1370-7. [DOI] [PubMed] [Google Scholar]
- 30.Uddin MN, Siddiq A, Oettinger CW, D’Souza MJ. Potentiation of pro-inflammatory cytokine suppression and survival by microencapsulated dexamethasone in the treatment of experimental sepsis. J Drug Target. 2011;19:752–760. doi: 10.3109/1061186X.2011.561856. [DOI] [PubMed] [Google Scholar]
- 31.Pudrith C, Martin D, Kim YH, et al. Glucocorticoids reduce nitric oxide concentration in middle ear effusion from lipopolysaccharide induced otitis media. Int J Pediatr Otorhinolaryngol. 2010;74:384–386. doi: 10.1016/j.ijporl.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Huo Y, Rangarajan P, Ling EA, Dheen ST. Dexamethasone inhibits the Nox-dependent ROS production via suppression of MKP-1-dependent MAPK pathways in activated microglia. BMC Neurosci. 2011;12:49. doi: 10.1186/1471-2202-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao B, Xie GJ, Li RF, et al. Dexamethasone protects normal human liver cells from apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand by upregulating the expression of P-glycoproteins. Mol Med Rep. 2015;12:8093–8100. doi: 10.3892/mmr.2015.4458. [DOI] [PubMed] [Google Scholar]
- 34.Xing K, Gu B, Zhang P, Wu X. Dexamethasone enhances programmed cell death 1 (PD-1) expression during T cell activation: an insight into the optimum application of glucocorticoids in anti-cancer therapy. BMC Immunol. 2015;16:39. doi: 10.1186/s12865-015-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollander JL. Clinical use of dexamethasone: role in treatment of patients with arthritis. J Am Med Assoc. 1960;172:306–310. doi: 10.1001/jama.1960.03020040004002. [DOI] [PubMed] [Google Scholar]
- 36.Sarett LH. Partial synthesis of pregnene-4-triol-17(beta), 20(beta), 21-dione-3,11 and pregnene-4-diol-17(beta), 21-trione-3,11,20 monoacetate. J Biol Chem. 1946;162:601–631. [PubMed] [Google Scholar]
- 37.Toth GG, Kloosterman C, Uges DR, Jonkman MF. Pharmacokinetics of high-dose oral and intravenous dexamethasone. Ther Drug Monit. 1999;21:532–535. doi: 10.1097/00007691-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Czock D, Keller F, Rasche FM, Haussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005;44:61–98. doi: 10.2165/00003088-200544010-00003. [DOI] [PubMed] [Google Scholar]
- 39.Makrygiannakis D, Revu S, Engstrom M, et al. Local administration of glucocorticoids decreases synovial citrullination in rheumatoid arthritis. Arthritis Res Ther. 2012;14:R20. doi: 10.1186/ar3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez-Palazzi F, Caviglia HA, Salazar JR, et al. Intraarticular dexamethasone in advanced chronic synovitis in hemophilia. Clin Orthop Relat Res. 1997:25–29. [PubMed] [Google Scholar]
- 41.Wernecke C, Braun HJ, Dragoo JL. The Effect of Intra-articular Corticosteroids on Articular Cartilage: A Systematic Review. Orthop J Sports Med. 2015;3 doi: 10.1177/2325967115581163. 2325967115581163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu YC, Evans CH, Grodzinsky AJ. Effects of short-term glucocorticoid treatment on changes in cartilage matrix degradation and chondrocyte gene expression induced by mechanical injury and inflammatory cytokines. Arthritis Res Ther. 2011;13:R142. doi: 10.1186/ar3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heard BJ, Barton KI, Chung M, et al. Single intra-articular dexamethasone injection immediately post-surgery in a rabbit model mitigates early inflammatory responses and post-traumatic osteoarthritis-like alterations. J Orthop Res. 2015;33:1826–1834. doi: 10.1002/jor.22972. [DOI] [PubMed] [Google Scholar]
- 44.Knirk JL, Jupiter JB. Intra-articular fractures of the distal end of the radius in young adults. J Bone Joint Surg Am. 1986;68:647–659. [PubMed] [Google Scholar]
- 45.Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol. 2014;10:11–22. doi: 10.1038/nrrheum.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]


