Abstract
Background
Multicenter longitudinal objective data for Fontan patients surviving into adulthood are lacking.
Objectives
Describe transplant-free survival and explore relationships between laboratory measures of ventricular performance and functional status over time.
Methods
Exercise testing, echocardiography, B-type natriuretic peptide (BNP), functional health assessment, and medical history abstraction were repeated 9.4 ± 0.4 years after the Fontan Cross-Sectional Study (Fontan 1) compared to previous values. Cox regression analysis explored risk factors for interim death or cardiac transplantation.
Results
From the original cohort of 546 subjects, 466 were recontacted and 373 (80%) were enrolled at 21.2 ± 3.5 years of age. Among subjects with paired testing, percent predicted maximum VO2 decreased (69 ± 14 vs. 61 ± 16, p <0.001, n = 95), ejection fraction decreased (58 ± 11 vs. 55 ± 10, p <0.001, n=259), and BNP increased (Median (IQR) 13 (7,25) vs. 18 (9,36) pg/mol, p <0.001, n = 340). At latest follow-up lower Pediatric Quality of Life Inventory (PedsQL) physical summary score was associated with poorer exercise performance (R2 adjusted = 0.20, p <0.001, n = 274). Cumulative complications since the Fontan included additional cardiac surgery (32%), catheter intervention (62%), arrhythmia treatment (32%), thrombosis (12%), and protein losing enteropathy (8%). Since Fontan 1, 54 subjects (10%) have received a heart transplant (n = 23) or died without transplantation (n = 31). The interval risk of death/transplantation was associated with poorer ventricular performance and functional health status assessed at Fontan 1, but was not associated with ventricular morphology, subject age or type of Fontan connection.
Conclusions
Interim transplant-free survival over 12 years in this Fontan cohort was 90% and was independent of ventricular morphology. Exercise performance decreased and was associated with worse functional health status. Future interventions might focus on preserving exercise capacity. (Clinical Trials Registration #: NCT00132782)
Keywords: Fontan procedure, single ventricle, exercise, adult congenital heart disease, functional health status
Introduction
Functional deterioration and an increasing risk of complications are thought to occur over time in patients with single ventricle physiology who have undergone a Fontan procedure, particularly into adulthood. Previous studies examining vital status over time for Fontan patients and factors associated with death or transplantation have been limited to single center reports that span large time periods and surgical eras (e.g., 25 years) (1), or have included a short length of follow up (e.g., <2 years) (2). The Pediatric Heart Network Fontan Cross-Sectional Study (Fontan 1, 2003–2004) investigated associations between measures of health status, ventricular function, and exercise performance in 546 children and adolescents, then aged 6–18 years (mean age of 11.9± 3.4 years) who had survived a Fontan procedure (3). The cross-sectional design of Fontan 1 limited the ability to assess whether observed differences between older and younger subjects were related to the length of time one lives with Fontan physiology, or to temporal changes in medical, catheter-based or surgical management strategies that had occurred. The Fontan Follow-up Study (Fontan 2) was limited to reassessment of vital status and functional health status and enrolled 85% of the Fontan 1 survivors (n = 428) between 2009–2011. There was 95% interim transplant-free survival and death or transplantation was associated with poorer performance on previously measured functional health status and measures of ventricular performance.(4)
The present study (Fontan 3) is a third assessment of the original Fontan cohort and includes reassessment of vital and health status as well as repeat measures of ventricular function and exercise performance during a time in which many of the original Fontan 1 subjects were transitioning to adulthood. The primary objectives were to: 1) determine trends in laboratory measures of ventricular performance over time (exercise, echocardiography, BNP, underlying rhythm); 2) determine relationships between current laboratory measures of ventricular performance and functional health status using the Pediatric Quality of Life Inventory (PedsQL); 3) determine factors associated with interim death or transplantation since Fontan 1 in this well characterized cohort; and 4) describe access to healthcare over time in this population of subjects who have transitioned into young adulthood.
METHODS
Study design and patient population
The methods for Fontan 1 and Fontan 2 have been previously described (4,5). Eligible patients were identified by a preliminary medical record review of all 546 children who had participated in the Fontan 1 study. Study subjects were included if they agreed to come to 1 of the 7 participating centers for repeat testing. Exclusion criteria included conversion to a biventricular circulation, heart transplantation or death. Each center’s institutional review board approved the protocol. Written informed consent and assent were obtained according to local requirements. Anatomic, clinical and surgical data were collected at enrollment (October 2013 to December 2014) using standardized forms. Structured interviews with the parent/guardian and/or subject were used to assess current clinical state, socioeconomic status, emotional and social functioning, and access to healthcare.
Medical record review and assessment of vital status
For enrolled study subjects, a detailed medical record review was performed using the same standardized forms previously used to abstract data on patient demographics and outcomes for the Fontan 1 and 2 studies (3,4). Vital status was assessed annually in all subjects by direct contact or review of the social security death index for subjects who could not be located.
Measures of functional health status and quality of life
The following instruments were used in this study:
Age-appropriate versions of the Peds-QL with a self-report and a parallel parent proxy report for those subjects ≤18 years of age were used to assess quality of life (6). Peds-QL age groups versions (8–12 years old, 13–18, and 19–25) have only minor differences in language. To preserve sample size and after consultation with the developer of the instrument, we combined these versions for analytic purposes (personal communication, James Varni, MD). Items were linearly transformed to a 0–100 scale, so that higher scores indicate better functioning or quality of life.
The Child Health Questionnaire (CHQ), which includes questionnaires for both the child and the parent, was used for subjects ≤18 years (7).
In subjects aged ≥19 years, functional status was measured with the Short Form Health Survey version 2 (SF-36) (8).
The Peds-QL patient report was used to determine relationships between laboratory measures of ventricular performance and functional health status, since it was the only questionnaire completed across all age groups and it has been shown to correlate highly with both the CHQ and SF-36 (9).
Echocardiographic protocol
Standardized 2-dimensional (2D) and Doppler echocardiograms were centrally interpreted. The measurements were performed by the same observer who analyzed echocardiograms performed at Fontan 1, and all of the measurements were performed using the same method (10). When possible, measurements and derived indices were expressed as Z-scores relative to body surface area (11). Ventricular dominance was assigned as left ventricular, right ventricular, or mixed (e.g., unbalanced atrioventricular canal). End diastolic volume, end-systolic volume, and mass were obtained with the biplane-modified Simpson’s method. For the mixed morphology group, the volume and mass of each ventricle were measured separately, and the combined values were used for data analysis.
Exercise protocol
Maximal exercise testing was performed with a ramp protocol on an electronically braked cycle ergometer and interpreted locally. A standard 12-lead electrocardiogram was performed prior to the exercise test to assess the underlying heart rhythm. Testing was performed with standard protocols previously used in children, adolescents, and subjects with Fontan physiology (5,12). Six exercise variables were used as outcomes: percent predicted maximum VO2, percent predicted maximum work rate, percent predicted maximum O2 pulse, chronotropic index, percent predicted VO2 at anaerobic threshold, and resting oxygen saturation. Separate analyses of the first four variables were done on those subjects able to achieve maximal effort (peak respiratory exchange ratio ≥1.1). Analysis of chronotropic index was further restricted to non-paced subjects.
Serology
Resting BNP plasma concentration was centrally measured with an immunochemiluminometric assay (LabCorp Clinical Trials, Cranford, New Jersy).
Statistical Methods
The demographic and anatomic characteristics of those enrolled in Fontan 3 were compared to those who were eligible but refused to participate. Subjects with the tests of interest at Fontan 3 (PedsQL patient summary scores, exercise testing, echocardiogram, and BNP) were also compared to those enrolled but without values for that particular test. The groups were compared with respect to their baseline characteristics (demographic profile, medical history, functional status and laboratory testing) from the Fontan 1 study.
The differences between values at Fontan 1 and Fontan 3 were assessed for each outcome for subjects with paired measurements at both time points. Continuous variables were tested using paired Student’s t-tests for means and Wilcoxon’s signed-rank test for medians. Categorical variables were tested using McNemar’s test. Descriptive statistics were provided at each time point.
Regression analyses assessed univariate and multivariable associations of current functional health status (PedsQL physical summary score) with the simultaneously derived variables (exercise, echocardiography and BNP tests, as well as age, gender and type of Fontan procedure). Stepwise regression was used to inform selection of a final model. All stepwise regressions used p=0.15 as a criterion for entry into the model and p=0.05 as a criterion to stay in the model. Bootstrapping was used to estimate the reliability of each independent variable entering into a stepwise regression, equivalent to the percentage of models out of 1000 that contain the variable of interest with a p-value of 0.05 or less. The proportion of variance explained (R2) was calculated and reported after adjusting downward (R2adj) to account for the effect of R2 increasing with number of variables.
The relationship between transplant-free survival and baseline characteristics (assessed at Fontan 1) was examined using Cox proportional hazards regression modeling. The single subject who underwent an interval biventricular conversion was excluded yielding a cohort of 545 Fontan subjects for analysis. Follow-up time was defined as the time from Fontan 1 medical record review until either death/transplantation or the latest available assessment of vital/transplantation status (March 15, 2016). We report hazard ratios (HR) with 95% confidence intervals and p-values associated with the Wald chi-square test. The proportional hazards assumption was evaluated using a supremum test for non-proportionality based on martingale residuals, and interaction terms with time were added if appropriate. Survival and transplantation are illustrated using Kaplan-Meier survival plots. All analyses were performed using SAS statistical software version 9.3 (SAS Institute, Inc., Cary, North Carolinia).
RESULTS
Subject characteristics
At the time of Fontan 3 study enrollment, 466 of the 546 subjects enrolled in the Fontan 1 cohort were found to be eligible; 373 enrolled (80%) and 93 refused participation. Enrolled subjects were slightly younger than those who were eligible but declined participation (21.1 ± 3.5 vs. 22.0 ± 3.4 years, p = 0.01). Gender, race, ethnicity, underlying anatomic diagnosis, and type of Fontan connection performed were not different. No differences were identified in any cardiac characteristics or laboratory measures assessed at Fontan 1 between enrollees and subjects who refused (Data not shown).
Fontan 3 cohort characteristics
The 373 enrolled subjects were studied at a mean of 9.4 ± 0.4 years following the Fontan 1 study, and 17.8 ± 3.4 years after Fontan surgery. Anatomic details of those who enrolled were not different from the original Fontan 1 cohort. The number of patients who underwent each type of test is shown in Figure 1. Of the 373 subjects enrolled, 9 were unable to complete the PedsQL patient report due to physical or mental limitations; therefore 364 subjects formed the cohort to explore associations between functional status and measures of cardiac performance. Patient characteristics are displayed in Online Table 1.
Figure 1. Laboratory testing of subjects enrolled in the Fontan 3 Study.
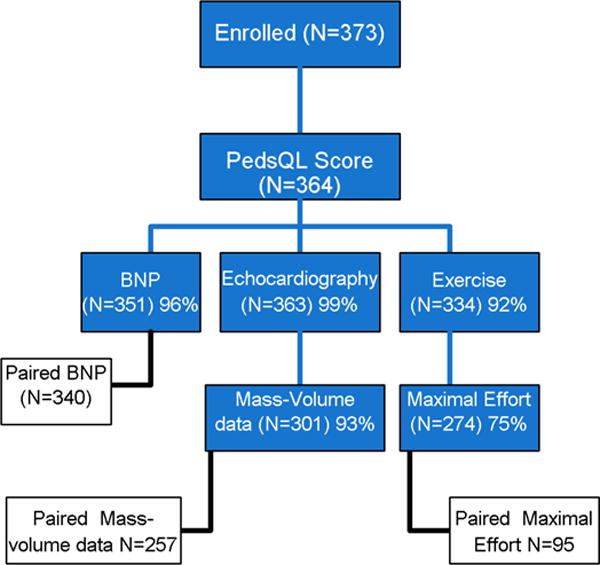
Number of tests performed by enrolled subjects at Fontan 3 is shown in blue. Number of subjects with paired tests performed at both Fontan 1 and Fontan 3 are shown in white.
There were no differences in anatomy, demographics, or results of assessments performed at Fontan 1 between those who did not perform exercise testing and the remaining subjects. Subjects with missing mass-volume data derived from echocardiographic measurements included a higher proportion of mixed ventricular dominance (53% vs. 10%) and a lower proportion of RV (8% vs. 36%) and LV (39% vs. 54%) with an overall p <0.001, but did not differ in any other anatomic or demographic variable. There were no differences between characteristics for subjects with and without BNP tests. Of the 364 subjects with PedsQL summary data, 270 (74%) had all laboratory tests performed and 223 had a maximal effort exercise test along with complete echocardiography and BNP testing. Prevalence of interventions and complications since the Fontan procedure increased over the period between Fontan 1 and Fontan 3 (Table 1). The most prevalent type of cardiac surgical procedure performed was related to placement or revision of an electronic device. Among the 58 with electronic devices at Fontan 3, 49 were pacemakers consisting of 17 single chamber atrial and 32 dual chamber pacemakers. An implantable cardioverter defibrillator was present in 9 subjects.
Table 1. Complications and Interventions After Fontan Surgery.
| Fontan 1 N = 546 |
Fontan 2 N = 416 |
Fontan 3 N = 373 |
|
|---|---|---|---|
| Time since Fontan (Years) | 8.7 ± 3.4 | 15.2 ± 3.4 | 17.8 ± 3.4 |
| Cardiac surgery | 23% | 28% | 32% |
| Catheter intervention | 48% | 57% | 62% |
| Electronic device | 13% | 13% | 16% |
| Stroke | 2% | 2% | 4% |
| Seizures | 3% | 5% | 7% |
| Thrombosis | 8% | 9% | 12% |
| Arrhythmia treatment | 20% | 28% | 32% |
| Protein losing enteropathy | 4% | 7% | 8% |
| Cirrhosis | 0.4% | 4% | 8% |
| Plastic bronchitis | 0.1% | 0.5% | 1% |
Paired laboratory performance results over time
Of 336 subjects completing the exercise test in Fontan 3, 275 (82%) achieved maximum effort compared to only 40% (166/411) in Fontan 1. There were 217 subjects who performed exercise testing at both Fontan 1 and Fontan 3; 95 of them reached maximal effort for both tests. The subjects who achieved maximal effort were an average of 2.5 years older than those who had a submaximal effort, p <0.001. After controlling for age, there were no anatomic or demographic differences between the 2 groups. The CHQ-P50 physical summary score at the time of Fontan 1 was lower in the group who did not achieve maximal effort during exercise in the current study, (40 ± 15 vs. 46 ± 20, p = 0.03).
Table 2 presents the comparisons of paired study outcomes at Fontan 1 and Fontan 3. Subjects were included if the outcome was measured at both Fontan 1 and 3 and exercise outcomes were limited to those subjects with maximal effort at both time points (n = 95). Among variables assessed during exercise testing, mean values for the cohort were lower than normal and declined significantly over time for all variables measured except chronotropic index and baseline oxygen saturation. Percent predicted maximum VO2 decreased approximately ½ of a standard deviation from a mean of 69%-61%, P<0.001. We further explored trajectories of the 95 individual subjects with paired maximal effort exercise tests by dividing them into 2 equal-sized cohorts based on age and baseline % predicted maximum VO2. The younger aged group (age at Fontan 1 <13.6 years, n = 48) had a greater decline in % predicted maximum VO2 than the older aged group (mean slope of change over time −1.03 vs. −0.47). The group with better exercise performance at baseline (% predicted maximum VO2 >69%, n = 48) showed a greater decline in % predicted maximum VO2 than those who had poorer exercise performance at baseline (mean slope of change over time −1.31 vs. −0.19). Percent predicted maximum work rate decreased almost one full standard deviation from a mean of 69%–56%, P <0.001. Among echocardiographic outcomes, ejection fraction significantly declined (mean 58% vs. 55%, P <0.001), the result of a greater degree of dilation over time in systole than diastole: end systolic volume z-score (mean change 0.8, P <0.001) and end diastolic volume z-score (mean change of 0.4, P <0.001). Serum BNP was significantly higher at the time of Fontan 3 (median of 18.0 pg/mL vs. 13 pg/mL), p<0.001. Consistent with our earlier report describing BNP in the Fontan 1 cohort (13), mean BNP (adjusted for age and gender) among subjects with atriopulmonary connection was higher than for those with intracardiac lateral tunnel, which in turn was higher than those with an extracardiac conduit.
Table 2. Main Study Outcomes at Fontan 1 and Fontan 3*.
| Characteristic†† | N | Fontan 1 | Fontan 3 | Change | P Value†† |
|---|---|---|---|---|---|
| Age at enrollment, years | 373 | 11.7 ± 3.4 | 21.2 ± 3.5 | 9.5 | ------ |
| Exercise Outcomes | |||||
| Percent predicted VO2 at anaerobic threshold | 196 | 80 ± 25 | 72 ± 25 | −7.9 | <0.001 |
| Percent predicted maximum VO2 † | 95 | 69 ± 14 | 61 ± 16 | −7.1 | <0.001 |
| Percent predicted maximum work rate† | 95 | 69 ± 15 | 56 ± 16 | −13.1 | <0.001 |
| Percent predicted maximum oxygen pulse† | 95 | 91 ± 23 | 80 ± 21 | −11.3 | <0.001 |
| Chronotropic index‡ | 79 | 0.7 ± 0.1 | 0.7 ± 0.2 | −0.1 | 0.64 |
| Oxygen saturation (%) | 267 | 92 ± 5 | 93 ± 5 | 1.1 | 0.23 |
| Echo outcomes | |||||
| Total echo end diastolic volume z score | 259 | −0.7 ± 1.7 | −0.3 ± 2.2 | 0.4 | <0.001 |
| Total echo end systolic volume z score | 259 | 0.2 ± 2.2 | 1.0 ± 3.7 | 0.8 | <0.001 |
| Total echo ventricular mass z score | 257 | 0.9 ± 2.0 | 1.0 ± 2.6 | 0.1 | 0.42 |
| Total echo stroke volume z score | 259 | −1.1 ± 1.7 | −1.0 ± 1.6 | 0.1 | 0.31 |
| Total echo ejection fraction z score | 259 | −1.0 ± 2.0 | −1.5 ± 1.9 | −0.5 | 0.001 |
| Total ejection fraction | 259 | 58 ± 11 | 55 ± 10 | −2.5 | <0.001 |
| Echo mass:volume ratio z score | 257 | 2.6 ± 3.2 | 2.4 ± 3.0 | −0.2 | 0.45 |
| BNP, pg/mL median (IQR) | 340 | 13(7,25) | 18(9,36) | 3.9 | <0.001 |
| Log BNP | 340 | 2.6 ± 0.9 | 2.9 ± 1.1 | 0.3 | <0.001 |
| Sinus rhythm (%) | 355 | 67 | 70 | 3 | 0.30 |
| Paced rhythm (%) | 355 | 7 | 14 | 7 | <0.001 |
Subjects were included if the outcome was measured at both Fontan 1 and 3. Values are mean±SD, %, or median (interquartile range). BNP= B-type natriuretic peptide; VO2=oxygen uptake.
Limited to those subjects with maximal effort at both time points.
Limited to non-paced subjects with maximal effort at both time points.
Paired T-test for means. Wilcoxon signed rank test for BNP. Mcnemar’s test for rhythm.
Association between PedsQL and lab tests and patient characteristics measured at Fontan 3
To assess associations between PedsQL physical summary score at follow-up and each of the laboratory tests at follow-up, multivariable regression models were fit. The proportion of variation in PedsQL physical functioning summary scores explained by laboratory testing was highest for exercise performance (R2adj = 0.20, model p <0.001, n = 274), and lowest for echocardiographic variables (R2adj = 0.04, p <0.001, n = 301) and BNP (R2 adjusted = 0.06, p <0.001, n = 351). There was a strong association between worse PedsQL scores and lower exercise testing variables (Table 3). Five of 6 univariate relationships were significant, but the multivariable model included only % predicted maximum work rate and gender. For a subset of subjects achieving max effort at both time points (n = 95) the multivariable model included a single variable, % predicted maximum work rate. There was an inverse relationship between PedsQL physical summary score and logBNP. A one-point increase in logBNP was associated with a 2.8 decrease in physical summary score. There were no associations between the PedsQL summary score and echocardiographic variables. (Data not shown) Combining variables from all tests (exercise, echocardiography, BNP) into one model resulted in the same model as for exercise only. (R2adj = 0.20, p<0.001, n=274).
Table 3. Association Between PedsQL Physical Summary Score and Exercise.
| Univariate | Multivariable* | ||||
|---|---|---|---|---|---|
|
| |||||
| Variable | Slope/PE | P-value† | Slope/PE | P-value† | Reliability |
| Gender (Female) | −8.92 | <0.001 | −7.35 | <0.001 | 84% |
| Fontan Type categories | - | 0.077 | |||
| Intracardiac lateral tunnel | Reference | ||||
| Extracardiac conduit | −5.33 | 0.026‡ | |||
| Atriopulmonary connection | −3.66 | 0.279‡ | |||
| Other | 5.81 | 0.352‡ | |||
| Age at Fontan 3 | 0.03 | 0.920 | |||
| %Predicted Max Work Rate | 0.46 | <0.001 | 0.43 | <0.001 | 100% |
| Chronotropic index | 18.44 | 0.001 | |||
| %Predicted Peak VO2 | 0.19 | 0.002 | |||
| Resting oxygen saturation | 0.56 | 0.02 | |||
| %Predicted VO2 at anaerobic | 0.08 | 0.049 | |||
| %Predicted maximum oxygen pulse | 0.05 | 0.311 | |||
PE= parameter estimate-represents the degree of change in the Summary Score associated with one unit change in exercise variable; VO2=oxygen uptake
R-sq=0.21 (R2 adjusted=0.20), p<0.001
F test
T test
Functional health status over time
For subjects who were ≤18 years at Fontan 3, the CHQ-P50 parent-report was administered at 3 time points, Fontan 1, 2, and 3 (n = 106). No change was seen in the physical summary score over 9.3 years between Fontan 1 and Fontan 3, and a statistical (but not clinically meaningful) improvement was seen in the psychosocial summary score as perceived by parents (Table 4). The other instruments (PedsQL and SF-36) were not administered at Fontan 1, so comparisons were made over a mean of 2.8-year period since Fontan 2. No significant changes were seen in any of the summary or component scores of either instrument over this relatively short interval (Table 4).
Table 4. Subjects with Paired Functional Health Status Data at Fontan 1, 2 and 3.
| N | Fontan 1 | Fontan 2 | Fontan 3 | P Value | |
|---|---|---|---|---|---|
| Age (Years) | 11.9 ± 3.4 | 18.4 ± 3.4 | 21.2 ± 3.5 | ||
| CHQ-P50 Physical summary score | 106 | 50 (40,55) | 48 (41,53) | 49 (41,53) | 0.6* |
| CHQ-P50 Psychosocial summary score | 106 | 51 (42,57) | 51 (42,57) | 52 (43,59) | 0.04* |
| PedsQL Physical functioning score | 322 | NA | 78 (63,88) | 75 (63,91) | 0.6† |
| PedsQL Psychosocial health score | 324 | NA | 73 (62,87) | 77 (64,88) | 0.08† |
| SF-36 Aggregated Mental health score | 121 | NA | 75 (65,90) | 80 (65,90) | 0.9† |
| SF-36 Aggregated Physical health score | 120 | NA | 94 (69,100) | 94 (75,100) | 0.4† |
Values are Mean±SD or Median (interquartile range)
Wilcoxon signed-rank test for medians, comparing Fontan 1 to Fontan 3
Wilcoxon signed-rank test for medians, comparing Fontan 2 to Fontan 3
CHQ-P50 = Parent Report Child Health Questionnaire; PedsQL= Pediatric Quality of Life Inventory; SF-36 = Short Form Health Survey Version 2
Associations between interim death or transplantation and patient characteristics assessed at Fontan 1
As of March 15, 2016, 54 subjects (10%) had received a heart transplant (n = 23) or died (n = 31) since Fontan 1 (Central Illustration). We found no differences in the composite risk of death/transplantation based on subject age, gender, race, ethnicity, ventricular morphology, or type of Fontan.
Central Illustration. Survival of Single Ventricle Patients after the Fontan Procedure.
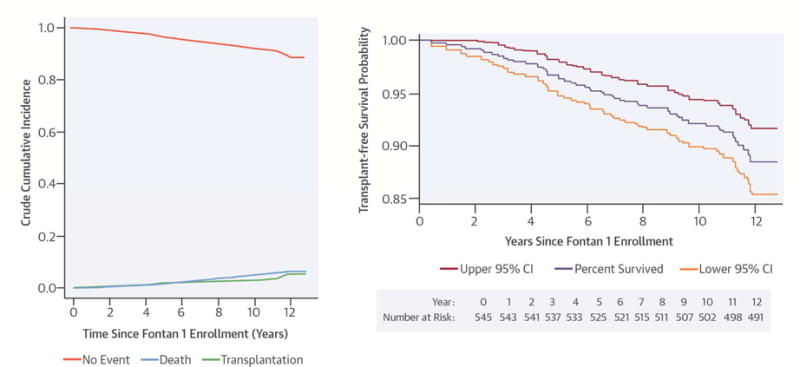
A) Transplant-free survival. Proportion of subjects in each of 3 competing mutually exclusive states: death, transplantation, and alive without transplantation for all 545 subjects. B) Transplant-free survival since Fontan 1 (with 95% confidence intervals) in all subjects with complete follow-up data. For improved resolution, the scale on the y-axis is limited to 0.85–1.0.
The risk of death/transplantation after Fontan 1 was significantly associated in univariate analysis with the following conditions measured at Fontan 1 (Table 5): a poorer CHQ physical summary score, a higher BNP value, not completing an exercise test, decreased resting oxygen saturation, lower percent predicted VO2 at anaerobic threshold, poorer ventricular performance (abnormally low ventricular function and increased (abnormal) ventricular volume). The hazard of death/transplantation for the subjects with the highest tertile BNP (>21 pg/mL) and the lowest tertile CHQ P50 Physical Summary Score (<44) assessed at Fontan 1 was 3.65 times higher than for the rest of the cohort. Analyzed as a whole, family income was not associated with death or transplantation. However, when compared to families with the lowest income, those reporting an income >$100,000 annually had improved outcomes.
Table 5. Cox model for predictors of death/transplant (total N=545, 54 events).
| Variable | Events / Total | Hazard Ratio (95% CI) | P value* |
|---|---|---|---|
| Age at Fontan 1 enrollment | 54 / 545 | 1.01 (0.94–1.09) | 0.79 |
| Age at Fontan 1 enrollment | 54 / 545 | 0.75 | |
| <10 | Reference | ||
| 10–13 | 1.25 (0.65–2.41) | 0.50 | |
| >13 | 1.01 (0.53–1.95) | 0.97 | |
| Male | 54 / 545 | 0.65 (0.38–1.12) | 0.12 |
| Race | 54 / 543 | 0.98 | |
| White | Reference | ||
| Black | 0.85 (0.34–2.15) | 0.74 | |
| Asian | 0.72 (0.10–5.25) | 0.75 | |
| Indian or Alaskan | 1.00 (0.36–2.78) | 0.99 | |
| Hispanic | 52 / 516 | 0.58 (0.14–2.40) | 0.45 |
| Income | 41 / 467 | 0.26 | |
| < $20.000 | Reference | ||
| $20,000–39,999 | 0.47 (0.17–1.31) | 0.15 | |
| $40,000–59,999 | 0.51 (0.18–1.42) | 0.19 | |
| $60,000–79,999 | 0.84 (0.33–2.12) | 0.71 | |
| $80,000–99,999 | 0.60 (0.21–1.69) | 0.33 | |
| > $100,000 | 0.29 (0.10–0.87) | 0.03 | |
| Ventricular type | 54 / 545 | 0.11 | |
| Left | 0.63 (0.30–1.32) | 0.22 | |
| Right | 1.19 (0.59–2.44) | 0.63 | |
| Mixed | Reference | ||
| Fontan type | 54 / 545 | 0.60 | |
| Atriopulmonary Connection | Reference | ||
| Intracardiac Lateral Tunnel | 1.29 (0.50–3.32) | 0.60 | |
| Extracardiac Conduit | 1.75 (0.65–4.75) | 0.27 | |
| Other | 2.00 (0.39–10.31) | 0.41 | |
| Age (years) at latest Fontan surgery as of enrollment in Fontan 1 | 54 / 545 | 1.10 (1.00–1.21) | 0.05 |
| Age (years) >=3 at latest Fontan surgery as of enrollment in Fontan 1 | 54 / 545 | 1.49 (0.87–2.54) | 0.14 |
| Fontan 1 CHQ PF50 Physical summary score | 49 / 510 | 0.97 (0.95–0.98) | <0.001 |
| Fontan 1 CHQ PF50 Physical summary score | 49 / 510 | 0.001 | |
| <44 | 4.78 (1.98–11.59) | <0.001 | |
| 44–52 | 2.59 (1.01–6.63) | 0.047 | |
| >52 | Reference | ||
| Fontan 1 CHQ PF50 Psychosocial summary score | 49 / 510 | 1.00 (0.98–1.03) | 0.91 |
| Fontan 1 exercise test completed | 54 / 545 | 0.51 (0.29–0.88) | 0.015 |
| Resting oxygen saturation (%) | 33 / 404 | 0.86 (0.81–0.91) | <0.001 |
| Percent predicted VO2 at anaerobic threshold (%) | 22 / 316 | 0.98 (0.96–1) | 0.038 |
| With maximum effort | 32 / 400 | 0.82 (0.4–1.68) | 0.59 |
| Percent predicted maximum VO2 (%) | 12 / 165 | 0.99 (0.95–1.02) | 0.46 |
| Percent predicted maximum work rate (%) | 12 / 165 | 0.98 (0.94–1.01) | 0.22 |
| Chronotropic index (0.1 unit increase) | 9 / 140 | 1.85 (1.01–3.38) | 0.045 |
| Fontan 1 echo Mass-volume data | 54 / 545 | 0.54 (0.31–0.93) | 0.028 |
| Total echo end diastolic volume z-score | 34 / 414 | 1.35 (1.18–1.55) | <0.001 |
| Total echo end systolic volume z-score | 34 / 414 | 1.27 (1.18–1.37) | <0.001 |
| Total echo ventricular mass z-score | 33 / 406 | 1.25 (1.11–1.41) | <0.001 |
| Total echo stroke volume z-score | 34 / 414 | 1.11 (0.92–1.32) | 0.27 |
| Total echo ejection fraction z-score | 34 / 414 | 0.76 (0.65–0.88) | <0.001 |
| Total echo ejection fraction | 34 / 414 | 0.95 (0.92–0.97) | <0.001 |
| Total mass-volume ratio | 33 / 406 | 0.70 (0.27–1.82) | 0.47 |
| Total mass-volume ratio z-score | 33 / 406 | 0.95 (0.85–1.07) | 0.41 |
| BNP, pg/mL, adjusted for age and sex | 53 / 509 | 1.01 (1.01–1.01) | <0.001 |
| BNP (pg/mL) | 53 / 509 | 0.039 | |
| <8 | Reference | ||
| 8–21 | 1.25 (0.59–2.67) | 0.56 | |
| >21 | 2.24 (1.13–4.44) | 0.02 | |
| BNP>21 and CHQ P50 physical summary score<44 | 48 / 482 | 3.65 (2.00–6.65) | <0.001 |
BNP= B-type natriuretic peptide; CHQ P50= Parent Report Child Health
Questionnaire; VO2=oxygen uptake
Wald Chi-square test
To determine whether there were differences between subjects who received a heart transplant and those who died without receiving a transplant we compared the Kaplan-Meier survival plots for death and transplantation separately. No differences were noted over time (p = 0.4 log-rank test, p = 0.7 Wilcoxon test). There were no differences in the risk of death when compared to the risk of transplantation based on gender, race, ethnicity, ventricular morphology, or age. There was a trend toward a difference between Fontan surgical types with 5 deaths and no transplantations in the atriopulmonary connection category. (p = 0.059) There were no differences between death and transplantation regarding laboratory testing variables assessed at Fontan 1. The frequencies of deaths since Fontan 1 differed by center, from 3% (3/105 subjects) to 14% (3/22 subjects). The frequencies of transplantations since Fontan 1 varied among centers from 0% (0 of 22 subjects) to 21% (8 of 38 subjects) (Table 6).
Table 6. Rate of Death and Transplant since Fontan 1 by Center.
| Center | Death (%) | Transplant (%) | Total (%) |
|---|---|---|---|
| A | 5 (3.6%) | 2 (1.4%) | 7 (5.0%) |
| B | 3 (2.9%) | 7 (6.7%) | 10 (9.5%) |
| C | 3 (3.8%) | 2 (2.5%) | 5 (6.3%) |
| D | 9 (8.7%) | 3 (2.9%) | 12 (11.7%) |
| E | 3 (13.6%) | 0 (0%) | 3 (13.6%) |
| F | 4 (6.7%) | 1 (1.7%) | 5 (8.3%) |
| G | 4 (10.5%) | 8 (21.1%) | 12 (31.6%) |
|
| |||
| Total | 31 (5.7%) | 23 (4.2%) | 54 (9.9%) |
Healthcare utilization
Few differences were observed in the frequencies for relevant healthcare access variables between Fontan 2 and the present data collection. (Online Table 2) The vast majority of subjects (96%) continued to have health insurance with a nearly equal distribution between private and public support. Subjects at the time of Fontan 3 were slightly more likely to have visited an adult cardiologist in the past 2 years (35% vs. 30%) and to have seen an adult congenital heart specialist (33% vs. 24%). Visits to an obstetrician/gynecologist increased among female subjects (41% vs. 32%) while those receiving educational support decreased from 36%–26%.
Discussion
Currently, >1,000 single ventricle patients undergo a Fontan procedure in the U.S annually. This represents 4.1% of all pediatric cardiac surgical procedures performed annually. (Society of Thoracic Surgeons Congenital Heart Disease Database, Spring 2016 Harvest, not shown) A number of reports describe declining survival over time with an increasing and substantial burden of complications (2,14–17). Projections suggest that the number of patients living with a Fontan circulation will double over the next 20 years thereby increasing the demand for care and services (18). Few studies report serial follow-up of individual subjects (19) and most are limited either by the small number of patients followed or by a short period of follow-up. This study comprises the largest to date multicenter study of children and young adults who have undergone a Fontan procedure followed longitudinally over a decade. The novel aspect of the present study was the ability to report the change over time in laboratory measures assessed in an identical manner in individual patients. Virtually all measures of ventricular performance declined over time. The most frequently used metric of aerobic capacity, % predicted maximum VO2 decreased from 69%–61% in those with paired maximal effort tests.
We found an overall 90% transplant-free survival over 12 years since enrollment in the Fontan 1 study. This compares favorably to a previous report, which estimated a 5-year transplant-free survival of 86% (2). Consistent with some single center reports (17), we did not detect a significant difference in the slope of the survival curve over time among these Fontan survivors. Moreover, we did not find a survival benefit in those subjects with dominant left ventricles. When considering functional single ventricles from birth, right ventricular dominance is the most important risk factor for death overall, but this does not appear to be a risk factor beyond the neonatal period (16,17). As we have discovered in other investigations of the PHN Fontan cohort (20), substantial center differences exist. We found a difference in rate of transplantation across centers, ranging from 0%–21%. Significant regional differences in rates of heart transplantation among Fontan patients have recently been reported in Australia and New Zealand (21). Although many factors are likely responsible for this variability, our findings suggest that there remains an unmet need for standardization in criteria used for consideration of heart transplantation in the Fontan population.
We found that 80% of the eligible original Fontan 1 cohort was willing to participate in the present study. Similar to the Fontan 2 study, older patients were less likely to agree to return, which may be due to the known challenges facing subjects as they transition to assuming care for themselves (4,22). For Fontan survivors, significant morbidity is reflected by rates of additional interventions and complications over time. In this cohort, additional catheter interventions occurred in 62%. Many of these may have been planned for Fontan fenestration closure (23). However, catheter interventions continued to increase even 15 years after the Fontan surgery. Complications increased over time, including arrhythmia requiring treatment, which increased from 20%–32% of subjects, and placement of an implanted electronic device, which increased from 13%–16% of subjects. In our previous assessments of the Fontan cross sectional cohort we identified low incidences of some complications associated with the Fontan circulation including rates of stroke, thrombosis, cirrhosis, protein losing enteropathy, and plastic bronchitis. Although not surprising, it is concerning that the rates of each of these complications increased over this decade of follow-up.
Poor exercise capacity has been associated with a greater risk of morbidity (2) and mortality (24) in patients with a Fontan circulation. We previously reported a weak association between functional health status (CHQ-P50 Physical Functioning Summary Score) and exercise at Fontan 1 (25). In the present study we found an even stronger association between functional health status (PedsQL) and exercise performance. Lower exercise capacity at initial testing was the best predictor of the decrease seen in exercise at subsequent testing. We did not find associations between exercise performance over time with either ventricular morphology or age at which the Fontan was performed (26,27). A few studies have documented acute improvements in exercise function in children with heart disease (28), including Fontan patients (29,30) after participation in a formal exercise training program. A recent pilot study has shown improvement in quality of life with a home based physical activity program in this population (31). Our study did not collect detailed descriptions of activity level or utilize activity trackers. Future studies should investigate whether exercise programs can lead to sustained improvements in exercise capacity that are linked with decreases in morbidity and mortality.
The results of this study must be viewed in light of certain limitations. The original Fontan 1 cohort was by necessity a subset of subjects with single ventricle physiology who survived multi-stage surgical palliation culminating in the modified Fontan procedure and who were well enough to complete most of study testing. The timeframe between Fontan 1 and 3 may have been a period of relative clinical stability and too short to observe meaningful changes in functional health status, medical conditions, or laboratory measures of ventricular performance beyond those reported here. We have previously reported some inherent limitations of the echocardiographic evaluation of Fontan subjects, especitally as they age (32). However, even though 2D echocardiographic measurements systematically underestimate ventricular volume measurements acquired by magnetic resonance imaging, the reproducibility of measurements are comparable by both modalities (10). Our assessment was also restricted to the 80% of eligible Fontan 1 survivors who agreed to participate in this follow-up study. In this longitudinal analysis, we report mean differences in this cohort over time that may not be applicable to each individual subject. Characterizing individual subjects with better or worse functional outcomes remains poorly defined (19).
Conclusions
In this follow-up study of a large, well characterized cohort of Fontan survivors there was a significant decline in exercise performance, which was associated with poorer functional health status at follow-up. In addition, there was an increase in the proportion of subjects undergoing interventions and suffering complications over nearly a decade. Transplant-free survival from the time of initial enrollment was 90% at 12 years and was independent of underlying ventricular morphology or age. Further study might focus on interventions aimed at preserving or enhancing exercise capacity.
Supplementary Material
PERSPECTIVES.
Competency in Patient Care
Among survivors of the Fontan procedure, complications and the need for additional interventions increase over time, but transplant-free survival is independent of ventricular morphology or age.
Translational Outlook
Further research is needed to develop more effective strategies to improve transplant-free survival in patients with single ventricle.
Acknowledgments
National Heart, Lung, and Blood Institute: Gail Pearson, Mario Stylianou, Jonathan Kaltman, Victoria Pemberton, Kristin Burns, Ellen Rosenberg*
Data Coordinating Center: New England Research Institutes, Lynn Sleeper*, Steven Colan, Dianne Gallagher*, Victor Zak, Peter Shrader, Nicholas D’agincourt
Protocol Chair: Lynn Mahony, University of Texas Southwestern Medical Center
Clinical Site Investigators: Children’s Hospital Boston, Jane Newburger (Principal Investigator), Roger Breitbart, Carolyn Dunbar-Masterson, Jill Handisides, Lisa-Jean Buckley, Bethany Trainor; Children’s Hospital of New York, Wyman W. Lai* (Principal Investigator), Rosalind Korsin; Children’s Hospital of Philadelphia, Robert Shaddy, J William Gaynor, Stephen M Paridon, (Principal Investigators), David Goldberg, Kaitlyn Daniels, Tonia Morrison, Nicole Mirarchi*; Duke University, Jennifer S. Li (Principal Investigator), Piers Barker, Mingfen Xu; Medical University of South Carolina, Andrew M. Atz (Principal Investigator), Patricia Infinger, Ann Harvey Frampton; Primary Children’s Hospital, Salt Lake City, Utah, LuAnn Minich (Principal Investigator), Richard Williams, Linda Lambert; Hospital for Sick Children, Toronto, Brian McCrindle (Principal Investigator), Elizabeth Radojewski*, Svetlana Khaikin, Patricia Walter; Cincinnati Children’s Medical Center, Bradley Marino*, Karen Uzark*
Protocol Review Committee: Michael Artman, Chair; Frank Evans, Jogu Gobburu, Timothy Feltes, G. Paul Matherne
Data and Safety Monitoring Board: John Kugler, Chair; David J. Driscoll, Frank Evans, Mark Galantowicz, Sally A. Hunsberger, Holly Taylor, Thomas J. Knight
Funding: Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288) This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health/National Heart, Lung, and Blood Institute.
Abbreviations
- BNP
brain natriuretic peptide
- CHQ
Childhood Health Questionnaire
- CHQ-PF50
Parent Report Child Health Questionnaire
- Peds-QL
The Pediatric Quality of Life Inventory
- SF-36
Short Form Health Survey Version 2
- VO2
oxygen uptake
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No relationship with industry exists.
no longer at the institution listed
References
- 1.Khairy P, Fernandes SM, Mayer JE, Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 2.Diller GP, Giardini A, Dimopoulos K, et al. Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J. 2010;31:3073–83. doi: 10.1093/eurheartj/ehq356. [DOI] [PubMed] [Google Scholar]
- 3.Anderson PA, Sleeper LA, Mahony L, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atz AM, Zak V, Mahony L, et al. Survival data and predictors of functional outcome an average of 15 years after the Fontan procedure: the pediatric heart network Fontan cohort. Congenit Heart Dis. 2015;10:E30–42. doi: 10.1111/chd.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sleeper LA, Anderson P, Hsu DT, et al. Design of a large cross-sectional study to facilitate future clinical trials in children with the Fontan palliation. Am Heart J. 2006;152:427–433. doi: 10.1016/j.ahj.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Landgraf JM, Abetz L, Ware JE. The Child Health Questionnaire (CHQ): A User’s Manual: The Health Insitute. New England Medical Center. 1996 [Google Scholar]
- 8.Turner-Bowker DM, Bartley PJ, Ware JE. SF-36 Health Survey & SF Bibliography: Third Edition 1988–2000. 2002. [Google Scholar]
- 9.Uzark K, Zak V, Shrader P, et al. Assessment of Quality of Life in Young Patients with Single Ventricle after the Fontan Operation. J Pediatrics. 2016;170:166–172 e1. doi: 10.1016/j.jpeds.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margossian R, Schwartz ML, Prakash A, et al. Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study) Am J Cardiol. 2009;104:419–28. doi: 10.1016/j.amjcard.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–457. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 12.Paridon SM, Mitchell PD, Colan SD, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52:99–107. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 13.Atz AM, Zak V, Breitbart RE, et al. Factors associated with serum brain natriuretic peptide levels after the Fontan procedure. Congenit Heart Dis. 2011;6:313–321. doi: 10.1111/j.1747-0803.2011.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J. 2008;29:1681–7. doi: 10.1093/eurheartj/ehn215. [DOI] [PubMed] [Google Scholar]
- 15.Ohuchi H, Yasuda K, Miyazaki A, et al. Comparison of prognostic variables in children and adults with Fontan circulation. Int J Cardiol. 2014;173:277–83. doi: 10.1016/j.ijcard.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 16.d’Udekem Y, Xu MY, Galati JC, et al. Predictors of survival after single-ventricle palliation: the impact of right ventricular dominance. J Am Coll Cardiol. 2012;59:1178–85. doi: 10.1016/j.jacc.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 17.Pundi KN, Johnson JN, Dearani JA, et al. 40-Year Follow-Up After the Fontan Operation: Long-Term Outcomes of 1,052 Patients. J Am Coll Cardiol. 2015;66:1700–10. doi: 10.1016/j.jacc.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 18.Schilling C, Dalziel K, Nunn R, et al. The Fontan epidemic: Population projections from the Australia and New Zealand Fontan Registry. Int J Cardiol. 2016;219:14–9. doi: 10.1016/j.ijcard.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 19.Ohuchi H, Ono S, Tanabe Y, et al. Long-term serial aerobic exercise capacity and hemodynamic properties in clinically and hemodynamically good, “excellent”, Fontan survivors. Circ J. 2012;76:195–203. doi: 10.1253/circj.cj-11-0540. [DOI] [PubMed] [Google Scholar]
- 20.Anderson PA, Breitbart RE, McCrindle BW, et al. The Fontan patient: inconsistencies in medication therapy across seven pediatric heart network centers. Pediatric Cardiol. 2010;31:1219–28. doi: 10.1007/s00246-010-9807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi WY, Yong MS, McGiffin DC, et al. Heart transplantation in Fontan patients across Australia and New Zealand. Heart. 2016;102:1120–1126. doi: 10.1136/heartjnl-2015-308848. [DOI] [PubMed] [Google Scholar]
- 22.Goossens E, Stephani I, Hilderson D, et al. Transfer of adolescents with congenital heart disease from pediatric cardiology to adult health care: an analysis of transfer destinations. J Am Coll Cardiol. 2011;57:2368–74. doi: 10.1016/j.jacc.2010.11.068. [DOI] [PubMed] [Google Scholar]
- 23.Atz AM, Travison TG, McCrindle BW, et al. Late status of Fontan patients with persistent surgical fenestration. J Am Coll Cardiol. 2011;57:2437–2443. doi: 10.1016/j.jacc.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes SM, Alexander ME, Graham DA, et al. Exercise testing identifies patients at increased risk for morbidity and mortality following Fontan surgery. Congenit Heart Dis. 2011;6:294–303. doi: 10.1111/j.1747-0803.2011.00500.x. [DOI] [PubMed] [Google Scholar]
- 25.McCrindle BW, Zak V, Breitbart RE, et al. The relationship of patient medical and laboratory characteristics to changes in functional health status in children and adolescents after the Fontan procedure. Pediatr Cardiol. 2014;35:632–40. doi: 10.1007/s00246-013-0831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiraishi S, Yagihara T, Kagisaki K, et al. Impact of age at Fontan completion on postoperative hemodynamics and long-term aerobic exercise capacity in patients with dominant left ventricle. Ann Thorac Surg. 2009;87:555–60. doi: 10.1016/j.athoracsur.2008.11.015. discussion 560–1. [DOI] [PubMed] [Google Scholar]
- 27.Ovroutski S, Ewert P, Miera O, et al. Long-term cardiopulmonary exercise capacity after modified Fontan operation. Eur J Cardio-thorac Surg. 2010;37:204–9. doi: 10.1016/j.ejcts.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 28.Rhodes J, Curran TJ, Camil L, et al. Impact of cardiac rehabilitation on the exercise function of children with serious congenital heart disease. Pediatrics. 2005;116:1339–45. doi: 10.1542/peds.2004-2697. [DOI] [PubMed] [Google Scholar]
- 29.Opocher F, Varnier M, Sanders SP, et al. Effects of aerobic exercise training in children after the Fontan operation. Am J Cardiol. 2005;95:150–2. doi: 10.1016/j.amjcard.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 30.Minamisawa S, Nakazawa M, Momma K, Imai Y, Satomi G. Effect of aerobic training on exercise performance in patients after the Fontan operation. Am J Cardiol. 2001;88:695–8. doi: 10.1016/s0002-9149(01)01822-7. [DOI] [PubMed] [Google Scholar]
- 31.Jacobsen RM, Ginde S, Mussatto K, Neubauer J, Earing M, Danduran M. Can a Home-based Cardiac Physical Activity Program Improve the Physical Function Quality of Life in Children with Fontan Circulation? Congenit Heart Dis. 2016;11:175–82. doi: 10.1111/chd.12330. [DOI] [PubMed] [Google Scholar]
- 32.Williams RV, Margossian R, Lu M, et al. Factors impacting echocardiographic imaging after the Fontan procedure: a report from the pediatric heart network fontan cross-sectional study. Echocardiography. 2013;30:1098–106. doi: 10.1111/echo.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


