Abstract
Blockade of dopamine (DA) reuptake via the dopamine transporter (DAT) is a primary mechanism identified as underlying the therapeutic actions of (±)-modafinil (modafinil) and its R-enantiomer, armodafinil. Herein, we explored the neurochemical and behavioral actions of modafinil to better characterize its psychostimulant profile. Swiss-Webster mice were implanted with microdialysis probes in the nucleus accumbens shell (NAS) or core (NAC) to evaluate changes in DA levels related to acute reinforcing actions of drugs of abuse. Additionally, subjective effects were studied in mice trained to discriminate 10 mg/kg cocaine (i.p.) from saline. Modafinil (17–300 mg/kg, i.p.) significantly increased NAS and NAC DA levels that at the highest doses reached ~300% at 1 hour, and lasted >6 hours in duration. These elevated DA levels did not show statistically significant regional differences between the NAS and NAC. Modafinil produced cocaine-like subjective effects at 56–100 mg/kg when administered at 5 and 60 minutes before the start of the session, and enhanced cocaine effects when the two were administered in combination. Despite sharing subjective effects with cocaine, modafinil’s psychostimulant profile was unique compared to that of cocaine and like compounds. Modafinil had lower potency and efficacy than cocaine in stimulating NAS DA. Additionally, the cocaine-like subjective effects of modafinil were obtained at lower doses and earlier onset times than expected based on its dopaminergic effects. These studies suggest that although inhibition of DA reuptake may be a primary mechanism underlying modafinil’s therapeutic actions, non DA-dependent actions may be playing a role in its psychostimulant profile.
Keywords: cocaine abuse and addiction, dopamine microdialysis, nucleus accumbens shell, ADHD, Modafinil, Methylphenidate
Introduction
Several recent studies suggest (±)-modafinil (modafinil) (Provigil®; (2-[(diphenylmethyl)]sulfinyl] acetamide) as a potential treatment for dependence on stimulants, such as cocaine and methamphetamine, in certain populations of subjects (Dackis et al., 2003; Dackis et al., 2005; Hart et al., 2008; Kalechstein et al., 2010; Kampman et al., 2015). Modafinil was approved by the US Food and Drug Administration as a wakefulness-promoting agent, and is listed as a Schedule IV compound in the Controlled Substances Act (Food and Drug Administration, 2007). However, preclinical and clinical studies suggest that modafinil lacks the abuse potential of amphetamine and methylphenidate (Martinez-Raga et al., 2008) and case reports of modafinil abuse are rare (Ozturk & Deveci, 2014; Krishnan & Chary, 2015).
Preclinical assessments of the abuse potential of modafinil are noteworthy for inconsistent results. For example, Gold and Balster (1996) reported intravenous self-administration of modafinil when substituted for cocaine in rhesus monkeys. However, a subsequent report (Deroche-Gamonet et al., 2002) indicated that modafinil did not produce reinforcing effects in rats. In a study with human subjects (Stoops et al., 2005) break points on progressive-ratio schedules increased with increasing dose, but only if the subjects were required to complete simple arithmetic problems after taking the drug. Further, studies of modafinil place conditioning have yielded similarly inconsistent results with some showing positive (Wuo-Silva et al., 2011; Shuman et al., 2012) and some negative (Deroche-Gamonet et al., 2002; Quisenberry et al., 2013b) outcomes. Finally, studies of drug discrimination have found partial (Gold & Balster, 1996; Dopheide et al., 2007; Quisenberry et al., 2013a) or full substitution (Paterson et al., 2010; Loland et al., 2012) for cocaine or d-amphetamine. In subjects trained to discriminate modafinil from saline injections cocaine methylphenidate and GBR 12909 fully substituted whereas d-amphetamine did not (Quisenberry & Baker, 2015).
Consistent with abuse liability is evidence that modafinil increases in vivo levels of DA by blocking the DAT, as do standard psychostimulants such as methylphenidate or cocaine (Volkow et al., 2009; Zolkowska et al., 2009; Loland et al., 2012). However, standard psychostimulants have higher affinity for the DAT when it is in a conformation open to the extracellular compared to the intracellular space (Ferrer & Javitch, 1998; Loland et al., 2008). In contrast, the affinity of modafinil for the DAT in an outward conformation is similar to its affinity for an inward facing DAT (Loland et al., 2012), which is reminiscent of binding profiles for other compounds that have been considered atypical DAT inhibitors (Loland et al., 2008; Newman & Katz, 2009; Tanda et al., 2009b; Schmitt & Reith, 2011; Schmitt et al., 2013). Thus the lack of pronounced selectivity of binding to DAT conformations may contribute to the unique in vivo pharmacological profile of modafinil. For example, although modafinil and its R- and S-enantiomers stimulate extracellular levels of DA in the NAS, in mice (Loland et al., 2012), a brain region related to the reinforcing effects of psychostimulants (Pontieri et al., 1995; Tanda et al., 1997), the stimulation showed relatively long time courses and significantly lower maxima compared to that for cocaine (Loland et al., 2012).
When evaluating the abuse potential of modafinil in human subjects, it is important to consider that it is practically insoluble in water and highly unstable when heated at high temperatures. Insolubility and the vehicle used in preclinical studies may contribute to the inconsistent outcomes obtained and make the compound impractical for intravenous injection, a primary route of administration for abuse of cocaine or methamphetamine. Nonetheless, the use of modafinil in non-medical settings has increased in recent years. Indeed, orally taken modafinil use as a “smart drug,” in student populations, but also by professionals to improve cognitive performance, has been the subject of several commentaries (Greely et al., 2008; Cakic, 2009; Partridge et al., 2011). Thus, a better understanding of the behavioral actions and related neurochemistry of modafinil is necessary to monitor its potential for abuse alone and in combination with illicit psychostimulants. To that end, the present study compared the effects of modafinil with those of cocaine in order to assess its relative potential to increase brain DA levels, as assessed by in vivo microdialysis, and to assess subjective effects, assessed in mice trained to discriminate cocaine. Additionally a novel vehicle was used that increased bioavailability of modafinil as evidenced by a leftward shift in the dose-effect curve for increasing extracellular DA levels.
Materials and Methods
Subjects
Male, Swiss-Webster mice (Taconic), weighing 30–40 g and experimentally naïve at the start of the study, were group housed (four mice per cage) in temperature- and humidity controlled rooms on a 12-h light/dark cycle (lights on from 0700–1900h). Mice had free access to food and water at all times except during microdialysis test sessions performed between 09:00 and 18:00 hours. Mice used for drug discrimination procedures had free access to food and water until training started, when they were individually housed with free access to water and with food access designed to maintain them at ~85% of their unrestricted-feeding weights for the duration of the study. All testing was performed between 9:00 AM and 1:00 PM 5 days/week. Subjects were fed 15 g of Purina chow 30 min after sessions. Care of the subjects was in accordance with the guidelines of the National Institutes of Health and the National Institute on Drug Abuse Intramural Research Program, Animal Care and use Program, which is fully accredited by AAALAC International.
Compounds
(±)-Modafinil was synthesized in the Department of Medicinal Chemistry, University of Kansas and in the Medicinal Chemistry Section, NIDA-IRP according to published procedures (Prisinzano et al., 2004; Cao et al., 2011), and dissolved in a vehicle containing DMSO 10%, Tween-80 15%, and sterile saline 75% (V/V/V). (-)-Cocaine hydrochloride (Sigma-Aldrich, St.Louis, MO or NIDA Drug Supply Program) or methylphenidate hydrochloride (racemic) (NIDA Drug Supply Program), were dissolved in sterile saline. In some experiments, detailed below, methylphenidate was prepared for injection using the vehicle used for injection of modafinil. Pretreatment times and doses of drugs used in the present study are described below and were chosen based on preliminary data obtained in this laboratory.
Microdialysis studies
Mice were anesthetized with a mixture of ketamine and xylazine (60 and 12 mg/kg, respectively), and then placed in a stereotaxic frame (Kopf Instruments, CA, USA); the skull was surgically exposed and a hole was drilled to expose the dura. Concentric dialysis probes were implanted randomly in the right or the left NAS or NAC (Uncorrected coordinates from Paxinos and Franklin (2001) expressed in mm from bregma were: Anterior = +1.5, Lateral = ±0.6, Vertical = −5.2 for the NAS; Anterior = +1.3, Lateral = ±1.3, Vertical = −4.9 for the NAC) as described previously (Mereu et al., 2015). See Figure 1 for placements. After surgery, subjects were given a subcutaneous injection of saline to replenish body fluids and were allowed to recover overnight in square Plexiglas cages (Med Associates). Cages were equipped with overhead quartz-lined fluid swivels (Instech Laboratories Inc., Plymouth Meeting, PA) for connections to the dialysis probes. All subsequent studies were conducted in these cages (Mereu et al., 2015).
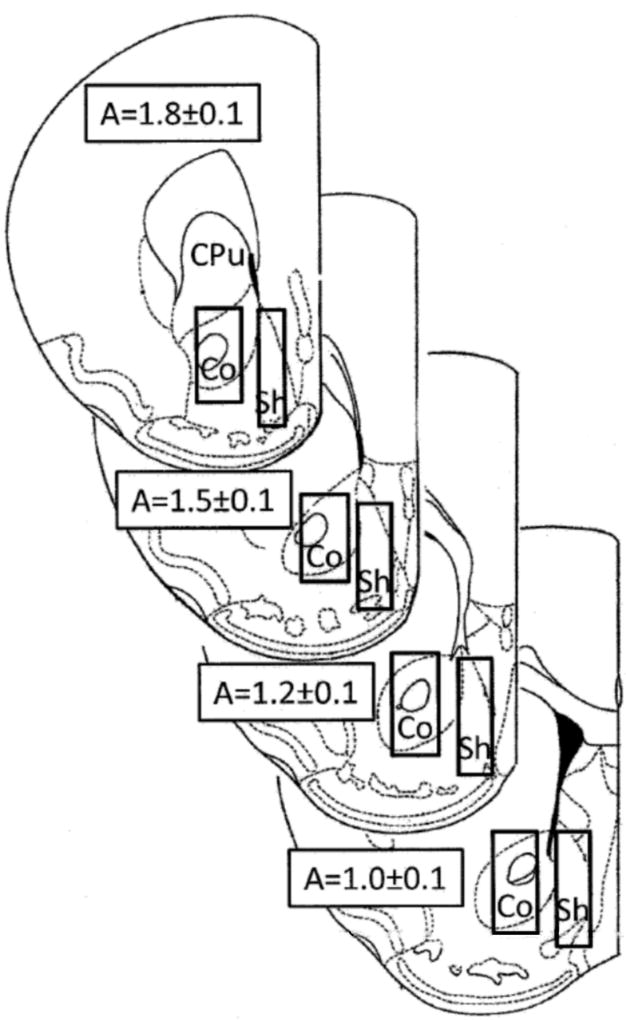
Figure 1.
Forebrain sections, redrawn from Paxinos and Watson (1987), show the limits of the positions of the dialyzing portions of the microdialysis probes (superimposed rectangles). The anterior coordinate (measured from bregma) is indicated on each section. CPU, caudate putamen; Co, nucleus accumbens core; Sh, nucleus accumbens shell.
Concentric dialysis probes were prepared using AN69 dialyzing membranes (Hospal Dasco, Bologna, Italy) as described previously (Tanda et al., 2009a; Mereu et al., 2015). The exposed dialyzing surface of the membrane, i.e. that not covered by glue, was limited to the lowest 1.0 mm portion of the probes that were less than 18 mm in total length. Experiments were performed in freely moving animals in the same cages in which they recovered from surgery. Microdialysis test sessions started at 9.00 a.m., approximately 42–47 hours after the surgical procedures. Probes were connected to fluid with swivels (375/D/22QM; Instech Laboratories, Plymouth Meeting, PA, USA) and perfused with Ringer’s solution (147.0 mM NaCl, 2.2 mM CaCl2, and 4.0 mM KCl) delivered by a 1.0 ml syringe, operated by a BAS Bee Syringe Pump Controller (BAS West Lafayette, IN, USA), through the dialysis probes at a constant flow rate of 1 µl/min. Collection of dialysate samples (10 µl) started after about 30 min, and samples collected every 10 min were immediately analyzed for DA content.
After establishment of a DA baseline, 2–4 samples differing no more than 15% (approximately after 1 hour), different groups of naïve mice were injected with one dose of modafinil (17, 30, 100, 300, 560 mg/kg i.p.), or vehicle (10 ml/kg i.p. containing, DMSO 10%, Tween-80 15%, and sterile saline 75% by volume). Doses of modafinil were based on effects described previously (Ferraro et al., 1997; Cao et al., 2011; Loland et al., 2012). Sample collection continued every 10 min typically during the first 3 hours after treatment, and every 20 min thereafter (but only 10 of the 20 µl collected were analyzed).
Dialysate samples (10 µl) were injected without purification into a high-performance liquid chromatography apparatus equipped with a MD 150 × 3.2 mm column, particle size 3.0 µm (ESA, Chelmsford, MA) and a coulometric detector (5200a Coulochem II, or Coulochem III, ESA) to quantify DA. Potentials for the oxidation and reduction electrodes of the analytical cell (5014B; ESA) were set at +125 mV and −125 mV, respectively. The mobile phase, containing 100 mM NaH2PO4, 0.1 mM Na2EDTA, 0.5 mM n-octyl sulfate, and 18% (v/v) methanol (pH adjusted to 5.5 with Na2HPO4), was pumped by an ESA 582 solvent delivery module at 0.50 ml/min. Assay sensitivity for DA was 2 fmoles per sample.
At the end of the experiment, mice were euthanized by pentobarbital overdose, brains were removed and left to fix in 4% formaldehyde in saline solution (Tanda et al., 2009a). Brains were sliced, using a vibratome (Vibratome Plus, The Vibratome Company, St. Louis, MO), in serial coronal slices oriented according to the atlas cited above, in order to identify the location of the probes. Data were only used from subjects for which probe tracks were within the correct NAS or NAC boundaries.
Microdialysis data are shown as increases above basal DA values, calculated as the mean of 2–4 consecutive samples immediately preceding the first drug or vehicle injection. All results are presented as group means (± SEM). Statistical analysis was carried out with Statistica Software (Tulsa, Oklahoma) using a three-way ANOVA for repeated measures over time, with drug dose, brain area and time as factors. Results from treatments showing overall changes were subjected to post-hoc Tukey`s test. Changes were considered to be significant when p<0.05.
Drug Discrimination
Experimental sessions were conducted daily (Monday-Friday) with mice were placed in 29.2 × 24.2 × 21 cm operant-conditioning chambers (modified ENV-001; MED Associates, St. Albans, VT) containing two response levers (requiring a downward force of 0.4 N) with pairs of green and yellow light-emitting diodes above each. Food pellets (20 mg, BioServ, Frenchtown, NJ) were dispensed to a tray located between the response levers. Overall illumination was provided by a light mounted near the chamber ceiling. The chambers were enclosed in ventilated enclosures that provided sound attenuation, and were provided with white noise to further mask extraneous noise.
Subjects were initially trained with food reinforcement to press both levers. They were then trained to press one lever after cocaine (10 mg/kg, i.p.) and the other after saline (i.p.) injection. All responses produced audible clicks of a relay mounted behind the front wall of the chamber. The ratio of responses to food pellets (fixed ratio or FR) was gradually increased until, under the final conditions, the completion of 10 consecutive responses on the cocaine- or saline-appropriate lever produced food. Incorrect responses reset the FR response requirement. The right- versus left-assignment of cocaine and saline levers was counterbalanced among subjects. Subjects were injected and placed in chambers, and sessions started after a 5-min time-out period during which lights were off and responses had no consequences, other than the audible click. After the time-out the house light was turned on until the completion of the 10-response requirement and the presentation of food. Sessions ended after 20 food presentations or 15 min, whichever occurred first, with cocaine or saline sessions scheduled in a double alternation sequence (e.g. …CSSCSS…).
Testing with different doses of cocaine, modafinil, or their combination was initiated after subjects met the training criteria of at least 85% cocaine- or saline-appropriate responding on four consecutive sessions (two sessions of each) over the entire session, and the first FR of the session. Test sessions were conducted at most every third daily session only if subjects met the training criterion for one saline and one cocaine session conducted on days immediately before the test session. Tests were conducted with the pre-session injections of different doses of cocaine, modafinil or their combination 5 min before the test. The test sessions were identical to training sessions with the exception that 10-consecutive responses on either lever were reinforced. The percentage of responses emitted on the cocaine-appropriate lever was assessed as an indicator of the similarity of the subjective effects of the test drug/dose compared to the training dose. The rate of responding in time was also assessed as an indicator of a potential generalized behavioral disruptive effect of the drug. Test doses of modafinil were based on previous reports (Cao et al., 2011; Loland et al., 2012). Tests with different doses of methylphenidate were performed as a control for modafinil effects.
Results
In-vivo brain microdialysis
Basal values of DA in NAS and NAC are reported in the figure captions for each experimental group. No significant differences were found between shell and core DA values in mice treated with different doses of modafinil (ANOVA: F7,46 = 1.42, p= 0.218).
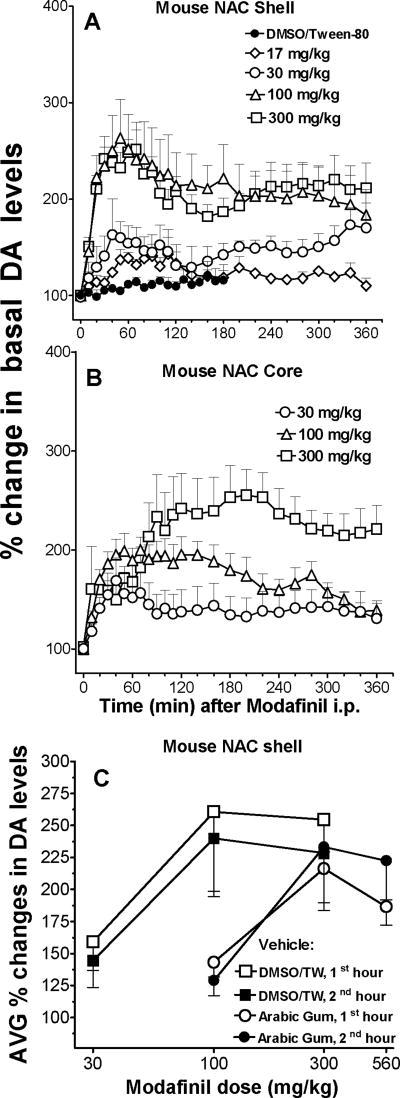
As shown in figure 2A and B, the acute administration of modafinil significantly increased extracellular levels of DA in the NAS and NAC. Moreover, the vehicle had no significant effects on DA levels in the NAC shell (Figure 2A, filled symbols). The lowest dose tested (17 mg/kg) did not significantly modify DA levels, while the 30 mg/kg dose produced a modest increase (approximately 65 to 70 % above basal levels after 40 min). Higher doses induced larger and longer lasting (> 4 hours) increases in DA levels in both NAS and NAC, with maxima reaching about 270 % of basal values in both brain areas. Maximal increases were obtained more rapidly in the NAS (~60 min) compared to the NAC (~180 min). The increases in DA levels produced by modafinil were significantly related to dose in both NAS and NAC, but there were no statistically significant differences between the two brain areas at any dose tested. A 3-way ANOVA showed significant main effect of dose (F2,30=5.13, p=0.012) and time (F24,720=9.74, p<0.001), with a non-significant main effect of brain area (F1,30=1.533, p=0.225). There also were significant interactions of dose by time (F48,720=1.78, p=0.0011), and time by brain area (F24,720=1.62, p= 0.031), and non-significant interactions of brain area by dose, or time by dose by brain area.
Figure 2.
Time courses for the effects of modafinil (17, 30, 100, or 300 mg/kg i.p.) administered at time 0 on extracellular levels of DA in dialysates from the NAS (A), or NAC (B). Each point represents means (with vertical bars representing S.E.M.) of the amount of DA in 10-min dialysate samples, expressed as percentage of basal values, uncorrected for probe recovery. Basal values (fmoles/sample ± SEM) and number of mice per group were as follows: 46.4±12.5, n=4; 44.0±9.2, n=5; 53.0±5.7, n=8; 60.8±5.2, n=9, for NAS placements in modafinil 17, 32, 100, and 300 mg/kg treated animals respectively; and 55.2±14.6, n=6; 78.2±15.8, n=5; 64.2±13.6, for NAC placements in modafinil 32, 100, and 300 mg/kg treated animals respectively. Percentage values for DA are represented on the figure at the point corresponding to the end of the sampling period.
The DMSO/Tween-80 vehicle substantially improved the activity of modafinil as compared to the Arabic gum vehicle often used in studies in the literature, likely by increasing its CNS bioavailability (Olsen et al., 1973; Mantilla-Plata & Harbison, 1975). As shown in Figure 2C, and Supplemental Figure 1, modafinil was about 3-fold more potent in its effects on DA levels in the NAC shell when dissolved in the DMSO/Tween-80 vehicle compared to when suspended in Arabic Gum.
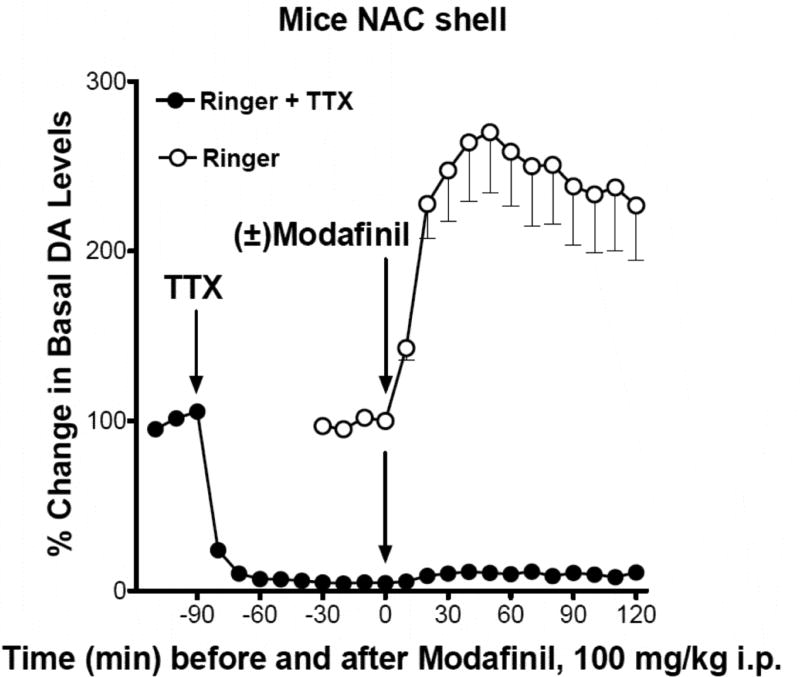
The effects of modafinil on stimulation of extracellular levels of DA were also assessed with a 1 µM concentration of TTX in the perfusion Ringer’s solution. Drugs that increase DA levels by blocking uptake are dependent on, and those that release DA independent of neuronal firing. As TTX blocks firing of neurons in the area immediately surrounding the microdialysis probe (Carboni et al., 1989; Solinas et al., 2006), it was used to determine if the increases in DA produced by modafinil were through a blockade of uptake or release of DA (Di Chiara et al., 1996). As shown in Figure 3, within 30 min of TTX perfusion DA levels decreased from 100 to about 5 percent or less in the NAC shell. When modafinil was administered 90 min after TTX perfusion, it had no effect on DA levels (Fig. 3).
Figure 3.
Time course of effects of modafinil (100 mg/kg i.p.) on stimulation of NAS DA levels with and without the addition of TTX 1µM in the perfusion Ringer solution. Basal values (fmoles/sample ± SEM) and number of mice in the TTX treated group were: 46.9±11.6, n=5. See Fig. 2 for more information about DA data-points.
Cocaine discrimination
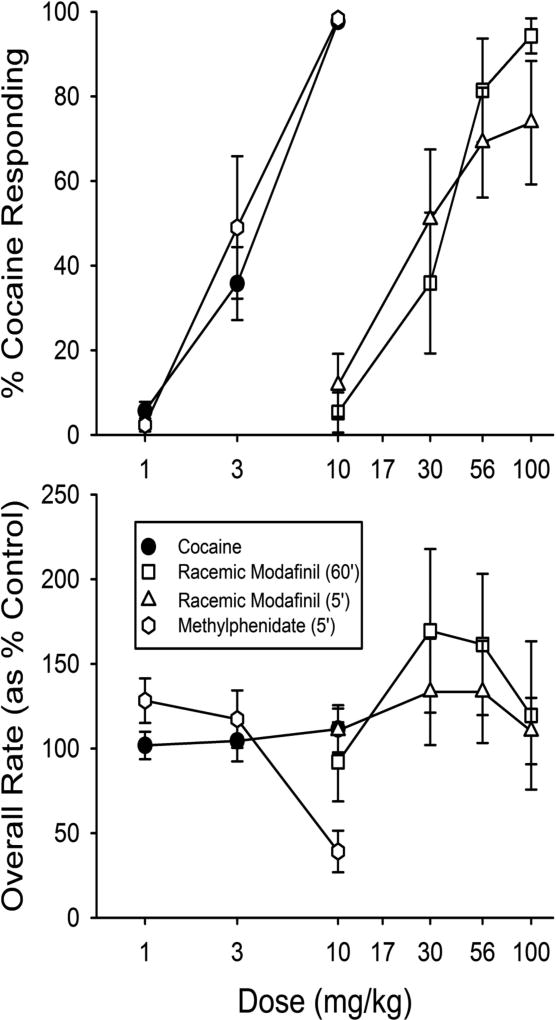
Cocaine injected (i.p.) 5 min before the session dose-dependently increased the percentage of drug-appropriate responding in mice trained to discriminate cocaine (10 mg/kg, i.p.) from saline injections (Fig. 4, upper panel, filled symbols). Methylphenidate (Fig. 4, open circles) fully substituted for cocaine with a potency equal to that of cocaine. Modafinil substitution for cocaine (Fig. 4, triangles) approached 80% at doses of 56 and 100 mg/kg when administered 5 min before the session. When administered 60 min before the session (Fig. 4, squares), complete substitution was more reliably obtained without a change in potency. Modafinil was about 10-fold less potent than cocaine. Only the highest dose of methylphenidate produced substantive decreases in response rates (Fig. 4, lower panel) suggesting a lack of complete cross tolerance between methylphenidate and the alternatingly administered cocaine.
Figure 4.
Dose-dependent effects of cocaine, modafinil, and methylphenidate in mice trained to discriminate cocaine from saline. Each point represents the mean of six subjects with SEM represented by the error bars. Abscissae: Dose of cocaine, modafinil, or methylphenidate in mg/kg. Top panel ordinates: Percentage of responses on the cocaine-appropriate lever. Bottom panel ordinates: Rates of responding as a percentage of vehicle control rates. Note that modafinil (10–100 mg/kg i.p.) almost fully (~75 % cocaine lever responding) substituted for cocaine when injected 5 minutes before the session, and completely (>90 % cocaine lever responding) substituted for cocaine when injected 60 minutes before the sessions (top panel). The effects of methylphenidate (1–10 mg/kg i.p.) administration 5 minutes before the session are also shown. Overall rate of responding was not significantly modified by modafinil or cocaine at any dose tested though the highest dose of methylphenidate decreased response rates (bottom panel).
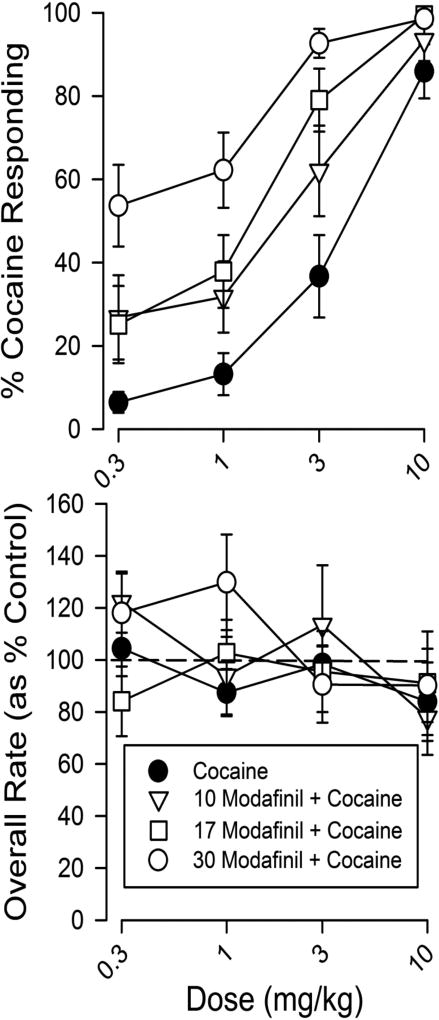
Modafinil treatment produced significant leftward shifts in the dose-effect curve for the discriminative-stimulus effects of cocaine (Fig. 5, upper panel). The changes in cocaine effects produced by modafinil were dose related, with higher doses shifting the cocaine curve progressively further to the left. The potency of cocaine in combination with 30 mg/kg of modafinil was more than 10-fold greater than that for cocaine alone (Fig. 5, upper panel). The ED50 value of 3.61 mg/kg for cocaine when administered alone decreased progressively to 1.46, 1.10, and 0.257 mg/kg, with increasing doses of modafinil. The latter two values were significantly different from those obtained with cocaine alone (95% confidence intervals did not overlap). Additionally, modafinil pretreatments decreased the slope of the cocaine dose-effect curve due to increases in the percentage of cocaine-appropriate responses at the lowest doses from the additional modafinil effects. None of the dose combinations produced substantive decreases in response rates (Fig. 5, lower panel).
Figure 5.
Dose-dependent effects of cocaine in mice trained to discriminate cocaine from saline when cocaine was administered alone or in combination with several doses of modafinil. Each point represents the mean of six subjects with SEM represented by the error bars. Abscissae: Dose of cocaine in mg/kg. Top panel ordinates: Percentage of responses on the cocaine-appropriate lever. Bottom panel ordinates: Rates of responding as a percentage of vehicle control rates. Note that modafinil significantly shifted the discriminative-stimulus effects of cocaine to the left (top panel). Modafinil did not modify the overall rate of responding even at doses in which it enhanced the subjective effects of cocaine (bottom panel).
Discussion
Three main findings on the psychostimulant actions of modafinil are described in the present report. First, under the present experimental conditions, modafinil dose-dependently affected DA extracellular levels in the NAS and NAC, but its effects were not statistically different between the two accumbens sub-regions. In behavioral studies, intraperitoneal injections of modafinil almost fully substituted for the subjective effects of cocaine when injected 5 minutes before the session, and fully generalized with the cocaine cue when injected 60 minutes before the session. Finally, modafinil pretreatment potentiated the effects of cocaine in drug-discrimination studies, with significant leftward shifts of the dose-response function.
Thus, our results confirm and extend our knowledge about the unique psychostimulant profile of modafinil. As shown in microdialysis studies, modafinil effects on stimulation of extracellular DA levels overlap with those of typical psychostimulants that are known to increase DA levels in striatal areas (Tanda et al., 2009a; Mereu et al., 2015). These effects of modafinil on DA levels were blocked by addition of TTX to the perfusion ringer solution. In these conditions there is no coupling between firing of DA neurons and exocytotic release of DA (Carboni et al., 1989; Di Chiara et al., 1996; Solinas et al., 2006). Only DA releasers like amphetamines would be able to stimulate DA levels under these conditions (Westerink et al., 1987). Lack of stimulation suggests that the effects of modafinil on DA levels are due to blockade of the DA reuptake after a firing-dependent, exocytotic vesicular and physiological release of DA. Also, behavioral effects of modafinil, like the ability to produce cocaine-like subjective effects, or to potentiate cocaine discriminative-stimulus effects, are typical of several abused psychostimulants (Witkin et al., 1991; Li et al., 2006). However, important instances in which modafinil effects do not overlap with those of known psychostimulants (Simon et al., 1995; Zolkowska et al., 2009) have also been described. For example, though modafinil significantly increased DA levels in the NAS, in contrast with abused psychostimulants and other drugs abused by humans, the effect was not significantly different from that obtained in the NAC (Pontieri et al., 1995; Pontieri et al., 1996).
The nucleus accumbens is known to play an important role in brain mechanisms mediating drug-reinforcement including the transition from abuse to addiction (Di Chiara et al., 1999; Koob, 1999). In rodents, it has been repeatedly shown that acute administration of drugs abused by humans, elicits an increase in DA levels in the NAS, an effect that appears selective or preferential as compared to NAC DA stimulation (Pontieri et al., 1995; Pontieri et al., 1996; Tanda et al., 1997; Cadoni et al., 2000; Mereu et al., 2015) suggesting that the reinforcing effects of these drugs are more related to a dopaminergic response in the NAS than in the NAC after acute administration (Di Chiara et al., 1999). The inconsistency in producing significant differences in DA stimulation in NAS compared to NAC may play a role in the low abuse liability of modafinil. Indeed, modafinil does not have reinforcing effects in tests of its self-administration (Deroche-Gamonet et al., 2002) even though it binds to the DAT at clinically used doses (Volkow et al. 2009). Also, because of its particular chemical structure, modafinil is only administered orally in human subjects. Indeed, it is not soluble in water (cannot be injected), and it is thermosensitive (cannot be smoked). Thus its reinforcing actions (if any) in humans might be even lower than those found in experimental animals, for which special vehicles are used for systemic i.p. and i.v. administration.
As previously shown for modafinil and its R- and S-enantiomers (Loland et al., 2012), the presence of a “ceiling effect” for stimulation of DA levels in the accumbens shell may be related to the disconnect between DA reuptake blockade and their lack of reinforcing effects. We have now confirmed this plateau to the increases in DA levels in the NAC as well as the NAS. We also suggest that this ceiling effect on DA stimulation might be one of the factors playing a role in the lack of preferential DA response in the NAS. Indeed, the dose response effect of modafinil shows a smaller and less robust increase in DA levels as compared to cocaine. The rapid increase in NAS DA levels obtained at the highest doses tested might suggest a preferential increase in this area compared to the NAC, which has not been confirmed by the statistical analysis. However, the limit of NAS and NAC DA stimulation under the described ceiling conditions would reduce the amplitude of the increase in DA, thus blunting the potential differences between these brain areas.
It is noteworthy that in our hands, DA levels could be significantly increased by lower doses of modafinil than previously found (Ferraro et al., 1997). Ferraro et al., (1997) reported that modafinil stimulates DA levels to values lower than those produced by GBR12909, nomifensine and amphetamine. However, in their study only a single dose of modafinil was used to treat anesthetized rats and they did not evaluate the relationship between DA release and behavioral effects. The lower effects previously found might be explained by differences in modafinil bioavailability from the vehicle used in the experiments. In fact, because of the low solubility of modafinil, we tested different vehicles and found that modafinil administered in the vehicle used in several previous studies (Tanganelli et al., 1994; Ferraro et al., 1996; Ferraro et al., 1997; Ferraro et al., 1999; de Saint Hilaire et al., 2001) (Arabic gum suspension in saline) produced similar but less potent effects than modafinil dissolved in our present vehicle (DMSO/Tween-80/Saline), suggesting a lower bioavailability with the former.
In agreement with previous studies (Dopheide et al., 2007; Loland et al., 2012) in mice trained to discriminate the subjective effects of cocaine, 10 mg/kg i.p., from those of saline, modafinil produced near full generalization (75%) to the cocaine discriminative stimulus when injected 5 minutes before the session. The mesolimbic DA system has been implicated in mediating cocaine’s subjective effects (Callahan et al., 1997). We have also found that the amount of stimulation of DA levels in the NAS elicited by cocaine could be strictly related to its subjective effects determined in discrimination procedures (Kohut et al., 2014). In that study doses of cocaine-like drugs that increased DA to levels equal to/greater than ~200% to 225% of basal values produced consistent cocaine-like subjective effects (Kohut et al., 2014). It should be noted that in the present experiments, modafinil approached or produced full generalization with the cocaine cue at a dose of 56 mg/kg i.p. administered 5 or 60 minute before the start of the session. Based on the data available from the present microdialysis experiments in mice, 30 and 100 mg/kg, we can infer that the dose of 56 mg/kg of modafinil would not produce an increase in DA during the first 20 minutes after injection that compares with the average increase in DA levels produced by a 10 mg/kg dose of cocaine. If translated into human dosage, 56 mg/kg of modafinil administered in mice would correspond to about 4.6 mg/kg (FDA, 2005). This dosage is actually not far from the daily prescribed dosage of 200 or 400 mg, equivalent to 3.33 and 6.66 mg/kg for a 60 kg person, respectively. However, one limitation of our study is that only the acute effects of modafinil have been reported. Thus it is hard to provide a complete meaningful equivalent of doses in our present study compared to human doses that are chronically administered. Thus modafinil demonstrates higher behavioral efficacy than expected at low doses (generalization with discriminative cues), and lower neurochemical efficacy at higher doses (ceiling in DA stimulation). Moreover, pretreatments with varying doses of modafinil, which did not elicit significant increases in DA, enhanced the dose-response effects of cocaine in mice trained to discriminate cocaine from saline. These results suggest that together with blockade of the DAT, possibly changes in glutamate and orexin brain levels, for example, may be at play and involved in the behavioral actions of modafinil (see review by Mereu et al., 2013).
Recent clinical trials have shown promising results for modafinil as a treatment for stimulant use disorders (Dackis et al., 2005; Hart et al., 2008; Anderson et al., 2009; Heinzerling et al., 2010; Kampman et al., 2015). Such clinical results support further elucidation of modafinil’s mechanism(s) of action, especially as these may inspire the development of novel medications for the treatment of psychostimulant abuse and other neuropsychiatric disorders.
Supplementary Material
Acknowledgments
Animal experimentation described in this manuscript was approved by the local ACUC of the NIDA/IRP, Baltimore, MD. The animals in the present study were maintained in an AAALAC International accredited facility in accordance with NIH Policy Manual 3040-2, Animal Care and Use in the Intramural Program (released 1 November 1999). Care of the subjects was in accordance with the guidelines of the National Institutes of Health and the National Institute on Drug Abuse Intramural Research Program Animal Care and Use Program, which is fully accredited by AAALAC International. This work was funded by the NIDA Intramural Research Program.
The present manuscript has been funded by the Medication Development Program, NIDA-IRP, NIH/DHHS.
List of nonstandard abbreviations
- ANOVA
analysis of variance
- CSF
cerebrospinal fluid
- DA
dopamine
- DAT
dopamine transporter
- EXT
extinction
- FR
fixed ratio
- i.p.
intraperitoneally
- i.v.
intravenously
- LEDs
light-emitting diodes
- NS
non-significant
- NAS
nucleus accumbens shell
- NAC
nucleus accumbens core
- S.E.M.
standard error of the mean
- TO
timeout
- modafinil
(±)modafinil
Footnotes
Disclosure of funding and conflict of interests.
All Authors declare that there are not competing financial interests of any kind in relation to the work described in the present manuscript.
References
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, 3rd, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Di Chiara G. Psychostimulant sensitization: differential changes in accumbal shell and core dopamine. Eur J Pharmacol. 2000;388:69–76. doi: 10.1016/s0014-2999(99)00824-9. [DOI] [PubMed] [Google Scholar]
- Cakic V. Smart drugs for cognitive enhancement: ethical and pragmatic considerations in the era of cosmetic neurology. J Med Ethics. 2009;35:611–615. doi: 10.1136/jme.2009.030882. [DOI] [PubMed] [Google Scholar]
- Callahan PM, De La Garza R, 2nd, Cunningham KA. Mediation of the discriminative stimulus properties of cocaine by mesocorticolimbic dopamine systems. Pharmacol Biochem Behav. 1997;57:601–607. doi: 10.1016/s0091-3057(96)00434-0. [DOI] [PubMed] [Google Scholar]
- Cao J, Prisinzano TE, Okunola OM, Kopajtic T, Shook M, Katz JL, Newman AH. Structure-Activity Relationships at the Monoamine Transporters for a Novel Series of Modafinil (2-[(diphenylmethyl)sulfinyl]acetamide) Analogues. ACS Med Chem Lett. 2011;2:48–52. doi: 10.1021/ml1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Cadoni C, Tanda GL, Di Chiara G. Calcium-dependent, tetrodotoxin-sensitive stimulation of cortical serotonin release after a tryptophan load. J Neurochem. 1989;53:976–978. doi: 10.1111/j.1471-4159.1989.tb11802.x. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, Rowan A, Poole S, White L, O'Brien CP. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- de Saint Hilaire Z, Orosco M, Rouch C, Blanc G, Nicolaidis S. Variations in extracellular monoamines in the prefrontal cortex and medial hypothalamus after modafinil administration: a microdialysis study in rats. Neuroreport. 2001;12:3533–3537. doi: 10.1097/00001756-200111160-00032. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Darnaudery M, Bruins-Slot L, Piat F, Le Moal M, Piazza PV. Study of the addictive potential of modafinil in naive and cocaine-experienced rats. Psychopharmacology (Berl) 2002;161:387–395. doi: 10.1007/s00213-002-1080-8. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Carboni E. Estimation of in-vivo neurotransmitter release by brain microdialysis: the issue of validity. Behav Pharmacol. 1996;7:640–657. [PubMed] [Google Scholar]
- Dopheide MM, Morgan RE, Rodvelt KR, Schachtman TR, Miller DK. Modafinil evokes striatal [(3)H]dopamine release and alters the subjective properties of stimulants. Eur J Pharmacol. 2007;568:112–123. doi: 10.1016/j.ejphar.2007.03.044. [DOI] [PubMed] [Google Scholar]
- FDA. U.S. Department of Health and Human Services. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Office of Training and Communications, Division of Drug Information; HFD-240 Rockville, MD, USA: 2005. Food and Drug Administration. Center for Drug Evaluation and Research (CDER) http://www.fda.gov/cder/guidance/index.htm. [Google Scholar]
- Ferraro L, Antonelli T, O'Connor WT, Tanganelli S, Rambert FA, Fuxe K. Modafinil: an antinarcoleptic drug with a different neurochemical profile to d-amphetamine and dopamine uptake blockers. Biol Psychiatry. 1997;42:1181–1183. doi: 10.1016/s0006-3223(97)00353-3. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, Tanganelli S, O'Connor WT, Perez de la Mora M, Mendez-Franco J, Rambert FA, Fuxe K. The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABAA receptor blockade. Neuropsychopharmacology. 1999;20:346–356. doi: 10.1016/S0893-133X(98)00085-2. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tanganelli S, O'Connor WT, Antonelli T, Rambert F, Fuxe K. The vigilance promoting drug modafinil decreases GABA release in the medial preoptic area and in the posterior hypothalamus of the awake rat: possible involvement of the serotonergic 5-HT3 receptor. Neurosci Lett. 1996;220:5–8. doi: 10.1016/s0304-3940(96)13212-2. [DOI] [PubMed] [Google Scholar]
- Ferrer JV, Javitch JA. Cocaine alters the accessibility of endogenous cysteines in putative extracellular and intracellular loops of the human dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:9238–9243. doi: 10.1073/pnas.95.16.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration, U. FDA Approved Labeling, PROVIGIL® (modafinil) Tablets 2007 [Google Scholar]
- Gold LH, Balster RL. Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology (Berl) 1996;126:286–292. doi: 10.1007/BF02247379. [DOI] [PubMed] [Google Scholar]
- Greely H, Sahakian B, Harris J, Kessler RC, Gazzaniga M, Campbell P, Farah MJ. Towards responsible use of cognitive-enhancing drugs by the healthy. Nature. 2008;456:702–705. doi: 10.1038/456702a. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson AN, Kim S, Cederblom L, Moe A, Ling W, Shoptaw S. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109:20–29. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalechstein AD, De La Garza R, 2nd, Newton TF. Modafinil administration improves working memory in methamphetamine-dependent individuals who demonstrate baseline impairment. Am J Addict. 2010;19:340–344. doi: 10.1111/j.1521-0391.2010.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM, Lynch KG, Pettinati HM, Spratt K, Wierzbicki MR, Dackis C, O'Brien CP. A double blind, placebo controlled trial of modafinil for the treatment of cocaine dependence without co-morbid alcohol dependence. Drug Alcohol Depend. 2015;155:105–110. doi: 10.1016/j.drugalcdep.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut SJ, Hiranita T, Hong SK, Ebbs AL, Tronci V, Green J, Garces-Ramirez L, Chun LE, Mereu M, Newman AH, Katz JL, Tanda G. Preference for distinct functional conformations of the dopamine transporter alters the relationship between subjective effects of cocaine and stimulation of mesolimbic dopamine. Biol Psychiatry. 2014;76:802–809. doi: 10.1016/j.biopsych.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Ann N Y Acad Sci. 1999;877:445–460. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Chary KV. A rare case modafinil dependence. J Pharmacol Pharmacother. 2015;6:49–50. doi: 10.4103/0976-500X.149149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SM, Campbell BL, Katz JL. Interactions of cocaine with dopamine uptake inhibitors or dopamine releasers in rats discriminating cocaine. J Pharmacol Exp Ther. 2006;317:1088–1096. doi: 10.1124/jpet.105.100594. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol. 2008;73:813–823. doi: 10.1124/mol.107.039800. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Mereu M, Okunola OM, Cao J, Prisinzano TE, Mazier S, Kopajtic T, Shi L, Katz JL, Tanda G, Newman AH. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72:405–413. doi: 10.1016/j.biopsych.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla-Plata B, Harbison RD. Distribution studies of (14C)delta-9-tetrahydrocannabinol in mice: effect of vehicle, route of administration, and duration of treatment. Toxicol Appl Pharmacol. 1975;34:292–300. doi: 10.1016/0041-008x(75)90034-4. [DOI] [PubMed] [Google Scholar]
- Martinez-Raga J, Knecht C, Cepeda S. Modafinil: a useful medication for cocaine addiction? Review of the evidence from neuropharmacological, experimental and clinical studies. Curr Drug Abuse Rev. 2008;1:213–221. doi: 10.2174/1874473710801020213. [DOI] [PubMed] [Google Scholar]
- Mereu M, Bonci A, Newman AH, Tanda G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology (Berl) 2013;229:415–434. doi: 10.1007/s00213-013-3232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu M, Tronci V, Chun LE, Thomas AM, Green JL, Katz JL, Tanda G. Cocaine-induced endocannabinoid release modulates behavioral and neurochemical sensitization in mice. Addict Biol. 2015;20:91–103. doi: 10.1111/adb.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Katz JL. Atypical dopamine uptake inhibitors that provide clues about cocaine's mechanism at the dopamine transporter. Topics in medicinal chemistry. 2009;4:95–129. [Google Scholar]
- Olsen JL, Makhani M, Davis KH, Wall ME. Preparation of 9-tetrahydrocannabinol for intravenous injection. J Pharm Pharmacol. 1973;25:344. doi: 10.1111/j.2042-7158.1973.tb10023.x. [DOI] [PubMed] [Google Scholar]
- Ozturk A, Deveci E. Drug Abuse of Modafinil by a Cannabis User. Bulletin of Clinical Psychopharmacology. 2014;24:405–407. [Google Scholar]
- Partridge BJ, Bell SK, Lucke JC, Yeates S, Hall WD. Smart drugs "as common as coffee": media hype about neuroenhancement. PLoS One. 2011;6:e28416. doi: 10.1371/journal.pone.0028416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Fedolak A, Olivier B, Hanania T, Ghavami A, Caldarone B. Psychostimulant-like discriminative stimulus and locomotor sensitization properties of the wake-promoting agent modafinil in rodents. Pharmacol Biochem Behav. 2010;95:449–456. doi: 10.1016/j.pbb.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press; New York, New York: 2001. [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the "shell" as compared with the "core" of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Prisinzano TE, Tidgewell K, Podobinski J, Luo M, Swenson D. Synthesis and determination of the absolute configuration of the enantiomers of modafinil. Tetrahedron-Asymmetr. 2004;15:1053–1058. [Google Scholar]
- Quisenberry AJ, Baker LE. Dopaminergic mediation of the discriminative stimulus functions of modafinil in rats. Psychopharmacology (Berl) 2015;232:4411–4419. doi: 10.1007/s00213-015-4065-0. [DOI] [PubMed] [Google Scholar]
- Quisenberry AJ, Prisinzano T, Baker LE. Combined effects of modafinil and d-amphetamine in male Sprague-Dawley rats trained to discriminate d-amphetamine. Pharmacol Biochem Behav. 2013a;110:208–215. doi: 10.1016/j.pbb.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Quisenberry AJ, Prisinzano TE, Baker LE. Modafinil alone and in combination with low dose amphetamine does not establish conditioned place preference in male Sprague-Dawley rats. Exp Clin Psychopharmacol. 2013b;21:252–258. doi: 10.1037/a0031832. [DOI] [PubMed] [Google Scholar]
- Schmitt KC, Reith ME. The atypical stimulant and nootropic modafinil interacts with the dopamine transporter in a different manner than classical cocaine-like inhibitors. PLoS One. 2011;6:e25790. doi: 10.1371/journal.pone.0025790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Rothman RB, Reith ME. Nonclassical pharmacology of the dopamine transporter: atypical inhibitors, allosteric modulators, and partial substrates. J Pharmacol Exp Ther. 2013;346:2–10. doi: 10.1124/jpet.111.191056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman T, Cai DJ, Sage JR, Anagnostaras SG. Interactions between modafinil and cocaine during the induction of conditioned place preference and locomotor sensitization in mice: implications for addiction. Behav Brain Res. 2012;235:105–112. doi: 10.1016/j.bbr.2012.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P, Hemet C, Ramassamy C, Costentin J. Non-amphetaminic mechanism of stimulant locomotor effect of modafinil in mice. Eur Neuropsychopharmacol. 1995;5:509–514. doi: 10.1016/0924-977x(95)00041-m. [DOI] [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of modafinil: influence of dose and behavioral demands following drug administration. Psychopharmacology (Berl) 2005;182:186–193. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- Tanda G, Newman AH, Ebbs AL, Tronci V, Green JL, Tallarida RJ, Katz JL. Combinations of cocaine with other dopamine uptake inhibitors: assessment of additivity. J Pharmacol Exp Ther. 2009a;330:802–809. doi: 10.1124/jpet.109.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Newman AH, Katz JL. Discovery of drugs to treat cocaine dependence: behavioral and neurochemical effects of atypical dopamine transport inhibitors. Adv Pharmacol. 2009b;57:253–289. doi: 10.1016/S1054-3589(08)57007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Tanganelli S, Ferraro L, Bianchi C, Fuxe K. 6-hydroxy-dopamine treatment counteracts the reduction of cortical GABA release produced by the vigilance promoting drug modafinil in the awake freely moving guinea-pig. Neurosci Lett. 1994;171:201–204. doi: 10.1016/0304-3940(94)90639-4. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. Jama. 2009;301:1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink BH, Tuntler J, Damsma G, Rollema H, de Vries JB. The use of tetrodotoxin for the characterization of drug-enhanced dopamine release in conscious rats studied by brain dialysis. Naunyn Schmiedebergs Arch Pharmacol. 1987;336:502–507. doi: 10.1007/BF00169306. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Nichols DE, Terry P, Katz JL. Behavioral effects of selective dopaminergic compounds in rats discriminating cocaine injections. J Pharmacol Exp Ther. 1991;257:706–713. [PubMed] [Google Scholar]
- Wuo-Silva R, Fukushiro DF, Borcoi AR, Fernandes HA, Procopio-Souza R, Hollais AW, Santos R, Ribeiro LT, Correa JM, Talhati F, Saito LP, Aramini TC, Kameda SR, Bittencourt LR, Tufik S, Frussa-Filho R. Addictive potential of modafinil and cross-sensitization with cocaine: a pre-clinical study. Addict Biol. 2011;16:565–579. doi: 10.1111/j.1369-1600.2011.00341.x. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.