Abstract
The endocrine function of the ovary is dependent upon the ovarian follicle, which on a cellular basis consists of an oocyte surrounded by adjacent somatic cells responsible for generating sex steroid hormones and maintenance of hormonal stasis with the hypothalamic-pituitary axis. As females age, both fertility and the endocrine function of the ovary decline due to waning follicle numbers as well as aging-related cellular dysfunction. Although there is currently no cure for ovarian failure and endocrine disruption, recent advances in ovarian biology centered on ovarian stem cell and progenitor cell populations have brought the prospects of cell- or tissue-based therapeutic strategies closer to fruition. Herein, we review the relative contributions of ovarian stem cells to ovarian function during the reproductive lifespan, and postulate steps toward the development of ovarian stem cell-based approaches to advance fertility treatments, and also importantly to provide a physiological long-term means of endocrine support.
Keywords: Stem cells, Ovary, Female germline stem cell, OSC, Granulosa cell
1. Introduction
In female mammals, fertility and endocrine function rely on a tightly regulated synchronicity within the hypothalamic-pituitary-gonadal (HPG) axis, in which the ovary serves as both the primary source of sex steroid hormones and germ cells (oocytes) required to maintain hormonal stasis and fertility throughout the reproductive lifespan. Predominantly localized to the outer cortex, the ovarian follicles serve as the functional units of the ovaries and consist of an oocyte surrounded by granulosa cells or their precursors, enclosed within an extracellular matrix (ECM)-rich basement membrane, composed of a species- and developmental stage-specific combination of predominantly laminins and collagens (Berkholtz et al., 2006; Heeren et al., 2015; Hummitsch et al., 2013). In rodents it has been demonstrated that the majority of the pregranulosa cells enclosed within primordial follicles in the ovarian cortex are nonproliferative, however mitotically active pregranulosa cells are found within follicles of the medulla, which are likely part of the primordial pool active prior to sexual maturity (Hirshfield and DeSanti, 1995). Following a process of ‘follicle activation’ of quiescent primordial follicles, the granulosa cells transition from squamous to cuboidal and mitotically activate, and a theca cell layer is recruited to surround the basement membrane (Skinner, 2005). In growing follicles, the ovarian granulosa and theca cells work in concert to respond to circulating levels of follicle stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary to generate the sex steroids (i.e. estradiol and progesterone), acting through the FSH receptor (FSHR) and LH receptor (LHR), respectively.
In recent years both germline stem cells, termed ‘female germline stem cells’ (fGSC) or ‘oogonial stem cells’ (OSCs), and somatic ovarian stem cells or progenitors have been reported, generating a renewed enthusiasm for the exploration of strategies to promote ovarian regeneration and/or sustained ovarian function (reviewed in Woods and Tilly, 2015; Grieve et al., 2015; Silvestris et al., 2015). However, despite the identification of ovarian stem cell and progenitor populations along with evidence to support that adult ovaries are amenable to follicle renewal during the reproductive lifespan, ovarian failure remains inevitable due to pathological conditions such as polycystic ovarian syndrome (PCOS) or depletion of follicles as a consequence of surgical ablation, exposure to environmental toxicants or chemotherapeutic agents, or as a result of age. As a major outcome of ovarian failure (in addition to infertility), steroid biosynthesis ceases and the ability of the ovary to feedback to the hypothalamus via inhibin and estradiol is lost. Consequently, circulating levels of FSH (followed by LH) rise sharply in women with menopause, and the pathological conditions associated with ovarian failure ensue. Accordingly, strategies to improve fertility, as well as delay or prevent the endocrine-related symptoms associated with menopause will require a greater understanding of ovarian function and the properties that govern the renewal and regeneration of multiple ovarian cell types. Herein we review germline and somatic stem cell populations in the ovary, a role for pluripotent stem cell-derived somatic cells, and the potential for these cells to maintain ovarian function during the reproductive lifespan, and prospective utility for therapeutics as efforts to extend fertility and prevent or delay menopause come closer to fruition.
2. Ovarian germ cells: primordial germ cells (PGCs) and oogonial stem cells (OSCs) as distinct precursors to oocytes
It has traditionally been accepted that most female mammals, unlike males, or other vertebrate or invertebrate females, are endowed at birth with a non-renewable pool of oocytes, of which a species-specific number will be selected for ovulation throughout the reproductive lifespan until the pool is exhausted (reviewed in Woods and Tilly, 2013c). However, more recent data supports ovarian function and oocyte biology as having a greater degree of plasticity than previously thought. While the oocytes present at birth are the direct progeny of PGCs fetal in origin, an emerging body of work from multiple laboratories worldwide has demonstrated that the ovaries from adult female mammals contain a distinct population of mitotically active germ cells that can generate oocytes during adulthood (Johnson et al., 2004; Zou et al., 2009; Pacchiarotti et al., 2010; Zhang et al., 2011; Zou et al., 2011; White et al., 2012; Imudia et al., 2013; Park et al., 2013; Woods et al., 2013b; Zhou et al., 2014; Xie et al., 2014; Fakih et al., 2015; Grieve et al., 2015; Khosravi-Farsani et al., 2015; Park and Tilly, 2015; Silvestris et al., 2015; Xiong et al., 2015; Ding et al., 2016; Lu et al., 2016; Zhang et al., 2016). Among the defining properties of OSCs are a stable karyotype and germline molecular profile following extended propagation, and, importantly, the ability to spontaneously initiate a differentiation program into oocytes following either culture in vitro or transplantation into ovarian tissue (Zou et al., 2009; Pacchiarotti et al., 2010; White et al., 2012; Ding et al., 2016). In mice, the oocytes formed from transplanted OSCs complete maturation to the metaphase-II stage of development, and can be fertilized yielding viable embryos and offspring (Zou et al., 2009; White et al., 2012; Xiong et al., 2015; Zhang and Wu, 2016). While a number of laboratories have independently successfully isolated OSCs using multiple methodologies, there remains some controversy as to the existence or biological significance of OSCs. These counter-claims to OSCs are largely centered on circumstantial negative findings, (Zhang et al., 2012; Lei and Spradling, 2013), or technical difficulties arising from antibody purification strategies (Zhang et al., 2012; 2015). For example, using a transgenic reporter mouse (Ddx4-Cre:Rosa26rbw/+) in which Ddx4 positive cells were presumed to fluoresce, putative Ddx4-positive cells were identified. However, following subculture, these cells failed to proliferate, inconsistent with what has previously been reported for OSCs (Zhang et al., 2012). Subsequently, the data generated using the Ddx4-Cre mouse reporter line was experimentally re-examined, and it was found that fluorescence was not restricted to the germline as previously claimed, with demonstrated promoter ‘leakiness’ throughout the ovary. Moreover, when ovarian dispersates from this mouse line were combined with antibodies targeting DDX4 and subject to fluorescence activated cell sorting (FACS), a distinct subpopulation of DDX4-tdTm- positive cells having properties consistent with OSCs were isolated and propagated, refuting the earlier claims that DDX4-positive cells from the ovary were not OSCs (Park and Tilly, 2015). The data in these studies and others surrounding the controversy on the existence of OSCs has been extensively reviewed in careful detail by us and others (Woods and Tilly, 2013a, 2015; Grieve et al., 2015; Woods and Tilly, 2015). Although to what extent OSCs contribute under normal physiological circumstances to ovarian function has yet to be demonstrated, the potential utility of OSCs for ovarian therapies is broad (Woods and Tilly, 2013a, 2015; Hummitzsch et al., 2015; Woods and Tilly, 2015). As just one illustration, we have developed a clinically validated fertility treatment, termed autologous germline mitochondrial energy transfer (AUGMENTSM), which utilizes the isolated mitochondria from a woman’s own OSCs injected at the time of intracytoplasmic sperm injection (reviewed in Woods and Tilly, 2015). To date, the findings reported from three clinical sites show a marked improvement in embryo quality and IVF success rates (Casper et al., 2015; Fakih et al., 2015).
Although both PGCs and OSCs give rise to oocytes, differences between the two cell types are clear. During embryonic development PGCs colonize the gonadal ridge and mitotically divide as they populate what will eventually become either an ovary or a testis. In humans, there is a major void in our current understanding of the molecular mechanisms that govern the processes of PGC specification, migration, proliferation, and entry into meiosis due to limitations with sample availability (De Felici, 2013), though histological evidence has shown that PGCs are identifiable during the third week of embryo development in the endoderm of the yolk sac (Witschi, 1948), proliferate extensively between the 3rd and 4th week (Witschi, 1948; Politzer, 1933) and colonize the developing ovary by the end of the 5th or beginning of the 6th week (Makabe et al., 1991). While the earliest stages of embryonic development differ dramatically between humans and mice, much of the contemporary knowledge of mammalian PGC specification, proliferation, migration and colonization has been generated using mice as a model, in which the process is well characterized and can be studied with relative ease. It has been postulated that as in vitro methodology and human modeling using pluripotent stem cell cultures progress that many of the knowledge gaps surrounding human ovarian development will be filled (De Felici et al., 2004). Additionally, as advances in ‘omics’-based approaches move toward lesser input amounts, valuable information can be garnered from samples limited by sources or size, which will dramatically improve our understanding of the molecular events that drive developmental milestones in human ovarian physiology (Truman et al., 2016).
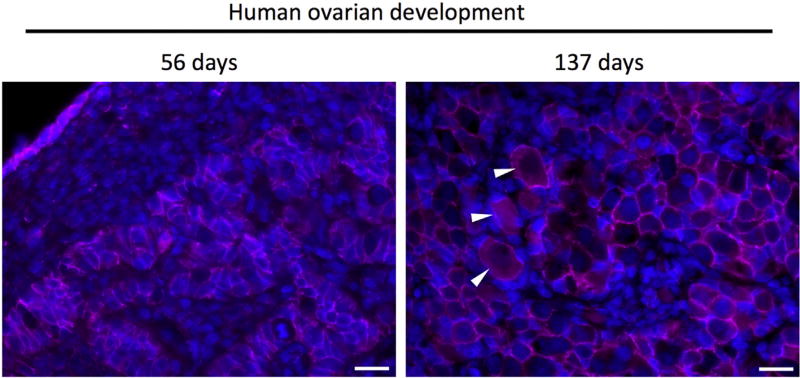
The biological properties of murine PGCs have been extensively reviewed elsewhere (Saitou et al., 2002; De Felici et al., 2004; Wear et al., 2016). In brief, primordial germ cells are identifiable early as 7.25 days post coitum (dpc) as a small cluster of cells positive for alkaline phosphatase; at the end of gastrulation, this small cluster proliferates to approximately 50–80 cells (Chiquoine, 1954; Ginsburg et al., 1990). Mouse PGC migration occurs in several stages, during which PGCs develop in the hindgut, emerge and invade the body wall to move dorsally, and subsequently begin migration toward the genital ridge, and colonize the indifferent gonad at approximately embryonic day e10.5 (Molyneaux et al., 2001; Molyneaux and Wylie, 2004). Following colonization of the gonadal ridge, PGCs rapidly proliferate, reaching approximately 20,000 in number, and become oogonia (Tam and Snow, 1981; Speed, 1982). During colonization, PGCs form nests of closely associated germ cells organized into long ovigerous cords, bordered by a basal lamina which provides a physical separation between the germ cells and the surrounding pre-granulosa and mesenchymal stroma cells (Konishi et al., 1986; Heeren et al., 2015). In mice, formation of the nests begins at e12.5 and continues until meiotic arrest is complete at e16.5 (Hilscher et al., 1974; Menke et al., 2003; Bullejos and Koopman, 2004) and in humans at approximately nine weeks of development (Baker and Franchi, 1967; Motta and Makabe, 1986). Shortly after birth, mouse germ cell nests break down during a process accompanied by significant loss of oogonia as a result of apoptosis (Pepling and Spradling, 2001). However, unlike mice in which the formation of primordial follicles occurs shortly after birth, during human development individual oogonia entering meiosis are cordoned off by pre-granulosa cells to form primordial follicles (beginning at approximately 17–20 weeks of gestation) and maintain this configuration as primordial follicles until follicle activation at puberty (Kurilo, 1981; Konishi et al., 1986; Satoh, 1991; Motta et al., 1997; Pepling and Spradling, 2001) (Fig. 1).
Fig. 1.
Immunofluorescent micrographs of human ovarian tissue during development (56 days, 137 days) and from reproductive-age ovarian tissue reveals break down of the germ cell nests and formation of primordial follicles. At 56 days of development, PGCs/oogonia cluster in cords, segregated from somatic cells. Subsequently, germ cell nests begin to breakdown (shown here at 137 days of development) to create primordial follicles (white arrows in center image) consisting of an oocyte surrounded by several squamous pregranulosa cells. In the adult human ovary, primordial follicles persist as an oocyte surrounded by a single layer of pre-granulosa cells. Scale bar = 20 microns. Immunofluorescence staining for beta-catenin (pink) and counterstained with Hoechst to stain nuclei (blue). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
A number of in vitro experiments utilizing the ability of mouse ovarian dispersates to reform follicles have demonstrated the importance of temporal synchronicity between the developing embryonic ovarian soma and germline. Studies designed to test whether intact fetal ovarian germ and somatic cell cords are requisite for later stages of follicle formation and oocyte development demonstrated that germ cells from e12.5 and e13.5 fail to reorganize into follicles in vitro, and do not further develop following disruption of the gonad, whereas re-agreggated ovarian dispersates prepared from later stages (e.g. e16.5, e17.5) showed robust follicle formation and oocyte survival (Lei et al., 2006; Nicholas et al., 2010). Moreover, combining developmentally mismatched germ cells (e12.5) and somatic cells (e17.5) resulted in developmental incompetency, and inclusion of e12.5 further inhibited the ability of e17.5 ovarian germ- and somatic cells to form follicles (Lei et al., 2006). Taken together, these data strongly support the notion that synchronous, or temporally matched, ovarian germ cells and pre-granulosa somatic cells are critical for normal folliculogenesis during ovarian development.
Additionally, the demonstration that fully functional oocytes can be derived from mouse embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), also relied on ovarian germ cell-somatic cell synchronicity (Hayashi et al., 2012). The in vitro specification of primordial germ cell (PGC)-like cells (PGCLCs) from differentiating mouse ESCs or iPSCs follows a precise time-frame, in which PGCLCs follow a developmental pattern that essentially parallels that of endogenous PGCs (Hubner et al., 2003; Nicholas et al., 2009; Hayashi et al., 2011, 2012, Woods et al., 2013b). Significantly, molecular profiling of PGCLCs formed between 3 and 6 days following directed differentiation in vitro indicates a strong similarity to endogenous PGCs present at e12.5, and PGCLCs at day 6 express the meiotic markers stimulated by retinoic acid 8 (Stra8) and structural maintenance of chromosomes protein 1B (Smc1b) and are capable of reaching the zygotene state of meiotic prophase, analogous to endogenous PGCs at e13.5 – e16.5 (Hayashi et al., 2012). The stem cell-derived PGCLCs were aggregated with dispersed e12.5 ovarian tissue and then transplanted under the ovarian bursa of adult female mice, which presumably provided the necessary environment to support follicle formation, growth and maturation, as previous attempts using similar techniques but transplanted under the kidney capsule were unsuccessful (Nicholas et al., 2009). The stem cell-derived PGCLCs subsequently developed into germinal vesicle stage oocytes that, following retrieval and in vitro maturation (IVM), were fertilized and transferred, with a small percentage (3.9%) surviving gestation and delivered as seemingly healthy pups (Hayashi et al., 2011, 2012), although information on the health or longevity of the offspring during adulthood has not been reported.
Unlike PGCs or pluripotent stem cell-derived PGCLCs, OSCs represent a native population of resident oocyte precursor cells within the post-natal ovary. Having a molecular profile that is distinct from PGCs, but similar to spermatogonial stem cells (SSCs) of the testis (Imudia et al., 2013; Xie et al., 2014), it is unlikely that OSCs are, as some have speculated, resident holdover PGCs that failed to incorporate into follicles during development (Hummitsch et al., 2013; Tingen et al., 2009). Moreover, it should also be noted that, as in fetal development, synchronicity plays an important role in the ability of PGCs to mature into developmentally competent oocytes, as attempts at maturing PGCs in vitro (De Felici, 2000), or transplantation of PGCs into adult ovarian tissue (Zhang et al., 2012), have not resulted in follicle formation, indicating additional significant differences between fetal (PGC) and adult (OSC) oocyte precursors and the specific interactions of each cell type with its respective microenvironment. Comparatively, OSCs have been demonstrated to orchestrate follicle formation both in vitro and in vivo when placed into a reproductive aged-synchronized ovarian microenvironment (e.g. adult ovarian tissue or granulosa cells obtained from adult ovarian tissue), lending additional credence to the hypothesis that OSCs represent a population of germ cells distinct from PGCs.
Importantly, in addition to the demonstrated ability of OSCs to initiate a differentiation program resulting in oogenesis, the capacity to coordinate with granulosa precursor cells to generate follicles following transplantation into adult ovarian tissue establishes that adult mammalian ovaries are amenable to follicle renewal. Initial confirmation of the follicle-forming capability of OSC-derived oocytes was performed in mice, in which OSCs expressing green fluorescent protein (GFP) were directly transplanted into the ovaries of chemosterilized recipients. Subsequent analysis of the transplanted ovarian tissue revealed GFP-expressing oocytes contained within follicles. Additionally, the OSC-derived oocytes matured and produced transgenic GFP-expressing offspring (Zou et al., 2009). In a later study, the ability of OSC-derived oocytes to form follicle-like structures when combined with ovarian granulosa cells in vitro was utilized as an indicator of oocyte identity (Pacchiarotti et al., 2010). Although transplantation and fate-mapping studies are not feasible in humans, a system was devised in which human OSCs expressing GFP were aggregated with dissociated human ovarian cortex and cultured (White et al., 2012), or injected into small pieces of adult human ovarian cortical tissue and then either grafted subcutaneously into immunocompromised mouse hosts (White et al., 2012; Ding et al., 2016) or cultured ex vivo (Woods and Tilly, 2013b) and allowed to further develop. Under each condition, GFP-expressing human OSCs generated GFP-positive immature oocytes. Immunohistological analysis of injected cortical strips revealed GFP-positive oocytes enclosed within defined granulosa cell layers as follicles, separated by the surrounding ovarian environment by a clearly discernable basal lamina (White et al., 2012; Ding et al., 2016). Significantly, these findings demonstrate that human OSCs can directly support new oocyte formation and, crucially, that adult human ovarian tissue from reproductive-aged women remains amenable to de novo follicle formation. However, despite the inherent capacity for renewal in the reproductive-aged ovary, ultimately the follicle pool wanes, and both fertility and the endocrine function of the ovary cease. Since OSCs are present in post-menopausal ovaries (Woods and Tilly, 2015b), this begs the question as to what is the primary driver for ovarian failure, and can intervening strategies be devised to improve fertility and/or prevent or delay menopause?
Although there is clearly a physiological cessation to female fertility, published data argue against an OSC-intrinsic program that results in gradual loss of OSC function coincident with the declining number of follicles. In addition to the finding that OSCs can be readily obtained from post-menopausal ovarian cortex with similar efficiency to that of reproductive aged women, OSCs obtained from aged ovarian cortex proliferate and spontaneously differentiate into oocytes (Woods and Tilly, 2015b). Moreover, the ability of aged mouse OSCs to participate in de novo follicle formation was demonstrated using a heterochronic transplantation model, in which aged mouse ovarian tissue devoid of any discernable follicles obtained from a transgenic mouse line expressing GFP in the germline [OG2, also called TgOG2 or Tg(Pou5f1-EGFP)2Mnn] was grafted onto the ovaries of young wild-type recipients. Interestingly, GFP-expressing oocytes, derived from the aged donors, were found contained within primordial follicles of the young recipients, near the site of engraftment (Niikura et al., 2009). Together, these data provide strong evidence that OSCs from aged ovaries are intrinsically capable of oocyte generation and de novo follicle formation. Whether or not extragonadal influence may play a role in the impaired ability of OSCs to contribute new oocytes in advanced age has also been examined. Upon exposure of young adult mouse ovaries transplanted under kidney capsules to a systemic environment well past the reproductive lifespan (e.g. recipient 24 months of age), a rapid reduction of follicle numbers (approximately 50%) as compared to young adult mouse ovaries implanted in a middle-aged recipient (e.g. recipient 12 months of age) occurred (Niikura et al., 2009). This is suggestive of the possibility that systemic factors generated from an aged environment could impair OSC function, although more work in this area to define a time-frame is needed for further clarity, and it is unknown whether the actions of the systemic environment target specifically OSCs, or rather the ovarian microenvironment.
To this end, we postulate that the inability of OSCs to solely sustain ovarian function into advanced age may be due, at least in part, to age-related changes in the ovarian microenvironment. It has been established that proper oocyte function depends upon bidirectional communication with surrounding follicular somatic cells (Eppig et al., 2002), most notably, granulosa cells and their precursors. Although mechanisms involved in the formation of follicles in adult ovaries have yet to be determined, Ding et al. recently confirmed our finding that human OSCs participate in de novo follicle formation with granulosa cells when transplanted into human ovarian cortical strips (Ding et al., 2016), and further demonstrated that granulosa cells increase the rate of oogenesis in vitro, as evident by morphological features, an increase in diameter and elevated levels of newborn ovary homeobox protein (NOBOX), lim homeobox gene 8 (LHX8), growth and differentiation factor 9 (GDF9), factor in the germline alpha (FIGLA), zona pellucida protein- (ZP) 1, and ZP3. The increase in oogenesis in the presence of granulosa cells may be due, at least in part, to a physical interaction mediated by gap junction communication between the two cell types, as OSCs projected actin-rich tentacle-like structures expressing connexin-37 (CX37) which made contact with granulosa cells and resulted in transfer of calcein-AM (a gap-junction-permeable fluorescent dye) from granulosa cells to OSCs (Ding et al., 2016). These data provide further evidence that granulosa cells play a critical role in neo-oogenesis, and it stands to reason, as with PGCs, that an appropriately queued somatic microenvironment is essential for OSC function. With age, OSCs persist, yet with follicle loss granulosa cells and their precursors diminish. Although granulosa cell precursors and progenitors have been recently identified in the developing ovary (discussed in detail below), no endogenous granulosa stem cell has yet been discovered in the adult ovary, and the source of the granulosa precursor cells that surround newly formed primordial follicles has not been defined. Furthermore, recent data have demonstrated that oocytes themselves play a critical role in formation of the follicular basal lamina through synthesis of glycoproteins even in the presence of granulosa cells and their precursors (Christensen et al., 2015). Accordingly, a loss of granulosa cells and/or their precursors as occurs with advancing maternal age may be the rate-limiting factor for sustained follicle formation, either from a lack of physical support and interaction for de novo follicle assembly, or as a supply of important secreted regulatory factors, such as estradiol and growth factors.
3. Granulosa cells: origin and plasticity
Granulosa cells, which are direct descendants of the quiescent pre-granulosa cells within primordial follicles, participate in bidirectional communication with oocytes throughout follicular maturation (reviewed extensively in Kidder and Mhawi, 2002), and, as the source of ovarian derived estrogen in women, are critical components of endocrine function. Although the properties of steroid biosynthesis and HPG feedback have been intensely studied and are well characterized (Maggi et al., 2016; Miller and Auchus, 2011; Simpson et al., 2005), only recently has research shed light on the origin of granulosa cells (Hummitzsch et al., 2013; Mork et al., 2012). The source of granulosa cells was once assumed to be of two potential origins: the mesonephros, from which ovarian somatic tissues arise during development, or the ovarian surface epithelium (OSE) (Byskov, 1986; Maheshwari and Fowler, 2008; Motta and Makabe, 1982; Wilhelm et al., 2007) with the most recent evidence derived from multiple animal models favoring an epithelial origin. During ovine ovarian development, electron micrographs depicting morphological changes within the ovary during follicle formation suggest that granulosa cells derive from mesothelial cells originating from the ovarian surface epithelium. The pre-granulosa cells of primordial follicles emerge from within the ovigerous cords, which, until at least 90 days of gestation in the ovine ovary, are open to the ovarian surface epithelium (Juengel et al., 2002; Sawyer et al., 2002). In mice, a lineage tracing approach has demonstrated that granulosa cells are specified in two waves: one prenatally and another postnatally (Mork et al., 2012). These two distinct populations of progenitors commit granulosa cells to two populations of follicles: the first being the medullary follicles which obtain granulosa cells from bipotential forkhead box L2 (FOXL2) expressing cells and are destined to atresia prior to puberty, and the second wave generating the cortical follicles which arise postnatally. Although the origins are distinct, both follicular waves populate with granulosa precursor cells from the surface epithelium (Mork et al., 2012). Together, mounting evidence corroborates that pre-granulosa cells appear to derive from cycling progenitor cells in the surface epithelium of the ovary, at least throughout the initial waves of follicle formation. It is also possible that the originating source or mechanism of derivation of granulosa cells differs between species, as ovarian model organisms often range from rodents to ruminants and primates, with a paucity of information gleaned from mostly histological and molecular characterization studies from human tissue (Anderson et al., 2002; Bayne et al., 2016; Duffin et al., 2009). For example, an alternative mechanism for the origin of granulosa cells has been proposed in the bovine, in which both OSE and granulosa cells derive from a common precursor, termed ‘gonadal ridge epithelial-like’ (GREL) cells (Hummitzsch et al., 2013).
Nonetheless, while there remains some discordance between the specific hypotheses surrounding the origins of granulosa cells during development, it should be noted that there is relatively little information at all regarding the source of granulosa cells or their precursors during the reproductive lifespan (e.g. granulosa precursors that participate in de novo follicle formation with OSCs). However, barring the future discovery of a potential granulosa stem cell present in adult ovaries, it should be noted that granulosa cells themselves have a high degree of plasticity. Thus, it is plausible that granulosa cells or pregranulosa cells contained within existing follicles are the source of new material for freshly formed follicles. While granulosa cells may not indeed be ‘stem cells’ based on characteristics such as asymmetric division or indefinite self-renewal, granulosa cells obtained from human follicular aspirates and cumulus cells, or from porcine antral follicles have been described as having a multi-potent molecular signature, and have been reported to express genes associated with pluripotency such as POU domain, class 5, transcription factor 1 (Pou5f1), Nanog Homeobox (Nanog), SRY (sex determining region y)-box 2 (Sox2), and telomerase reverse transcriptase (TERT), although there is some inconsistency in gene expression patterns reported which may be due to methodological differences in sample collection and/or species (Kossowska-Tomaszczuk et al., 2009; Mattioli et al., 2012; Varras et al., 2012; discussed in Dzafic et al., 2013). Additionally, following prolonged culture under defined conditions similar to those used to maintain other adult stem cell types, human granulosa-luteal cells spontaneously de-differentiate, and progressively lose the steroidogeneic capacity associated with their terminally differentiated phenotype while acquiring the mesenchymal stem cell markers CD29, CD44, CD90, CD105, CD117, and CD166 (Kossowska-Tomaszczuk et al., 2009). Moreover, the plasticity of granulosa cells has also been demonstrated specifically by the ability of granulosa cells to undergo the physiologically rare process of cellular transdifferentiation into neuronal, osteogenic, and chondrogenic lineages (Kossowska-Tomaszczuk et al., 2009; Oki et al., 2012; Mattioli et al., 2012). Transdifferentiation into a sertoli-like phenotype has also been demonstrated. Conditional ablation of the transcriptional regulator Foxl2 in granulosa cells from adult mouse ovaries leads to upregulation of the testis-specific genes Sry and Sry box 9 (Sox9), with these karyotypic XX animals capable of maintaining testosterone levels comparable to their XY littermates (Uhlenhaut et al., 2009). While an interesting phenomenon, the biological purpose underlying granulosa cell plasticity is not yet known, and although existing granulosa cell populations residing within the ovary may be a putative source of granulosa cells for de novo oogenesis, experimental evidence to support this is currently lacking. Other somatic stem cell types, such as those found in the stroma (Gong et al., 2010) may also potentially serve as a source. Finally, while it has been speculated that OSE in adult ovaries is a source of granulosa cells (Bukovsky et al., 2005), we find this hypothesis unlikely as new follicles readily form in human cortical strips in which the OSE has been carefully removed prior to cryopreservation (White et al., 2012).
With age, the endocrine function of the ovary declines as follicle numbers decrease. In addition to an overall reduction in quantity (Gougeon et al., 1994), granulosa cells demonstrate aging-related morphological and functional changes. For instance, analysis of mitochondrial ultrastructure in granulosa cells from older (premenopausal) and younger women revealed a greater degree of vacuolization and cristae malformation in the older granulosa cell cohort, which correlated with a reduction in both superoxide dismutases (SOD1,-2) and catalase activity (Tatone et al., 2006). Increases in mitochondrial DNA (mtDNA) deletion mutations have also been documented in women >38 years of age as compared to women <34 (Seifer et al., 2002), and upregulation of the mitochondrial gene Glutathione S-transferase theta 1 (GSTT1) in granulosa cells with age has been shown (Ito et al., 2008). More recently, global alterations in gene transcription and methylation with age have also been revealed (Yu et al., 2015). Although the mechanisms underlying poor oocyte quality with age have yet to be fully elucidated, evidence suggests that aging granulosa cells may fail to properly support oocyte function. For example, in a comparison between young (7 week old) and aged (34–35) week old mice the rate of oocyte apoptosis was significantly reduced in the aged oocyte cohort when denuded of cumulus cells (Perez and Tilly, 1997). It has also been recently shown in women that granulosa cells from aged IVF patients have characteristic properties consistent with premature luteinization, and that, although preliminary and with the consideration that retrieval protocols and hormone stimulation may impact final interpretation, IVF outcomes in older women (43–47 years of age) can be improved following an ‘early retrieval’ protocol (Wu et al., 2015). Thus, quantity as well as quality of granulosa cells may play a much greater role in maintaining fertility and ovarian function than previously thought.
3.1. In vitro derivation of granulosa cells from pluripotent stem cells
As there does not appear to be an endogenous granulosa stem cell, research has turned toward pluripotent stem cells as a potential source. Under the proper directive, pluripotent stem cells such as ESCs and iPSCs can conceivably revolutionize the way we approach and treat endocrine disruption in females by providing an, as of now, untapped source of steroidogenic cells. In an early study, Crawford et al. reported the generation of steroidogenic cells from ESCs and mesenchymal stem cells by inducing expression of nuclear receptor steroidogenic factor 1 (SF-1). The resulting cells generated small amounts of progesterone but were unresponsive to human chorionic gonadotropin (hCG; Crawford et al.,1997). In later studies, mouse ESCs harboring SF-1 under an inducible promoter also differentiated into steroidogenic cells resembling adrenocortical cells (Yazawa et al., 2009, 2010). Additionally, in a number of reports aimed at generating oocyte-like cells from differentiating ESCs, the somatic cells contained within in vitro-derived follicle-like structures functionally and morphologically resemble granulosa cells (Hubner et al., 2003; Novak et al., 2006; Psathaki et al., 2011). Interestingly, in addition to estradiol and progesterone biosynthesis, ultrastructural analysis revealed that these granulosa-like cells physically connect to their enclosed germ cells via intercellular bridges (Psathaki et al., 2011), which highlights the ability of ‘synthetic’ granulosa cells to potentially serve the critical role of oocyte support cells, which are required for normal follicle development. Furthermore, work from our laboratory was the first to isolate and transplant ESC-derived granulosa cells, demonstrating that these cells could participate in follicle formation within a native ovarian environment (Woods et al., 2013a). Employing a dual-fluorescence strategy, in which mouse ESCs were transformed to express both a reporter for germ cell specific Pou5f1-driven green fluorescent reporter and Foxl2-driven Discosoma sp. red (DsRed), ESC-derived germ cells and granulosa cells could be tracked simultaneously. Isolated Foxl2-DsRed positive cells expressed a gene expression profile consistent with early stage granulosa cells, including Foxl2, follistatin (Fst), anti-Mullerian hormone (AMH) and follicle-stimulating hormone recepter (FSHR). Additionally, these cells synthesized progesterone and estradiol in vitro, and incorporated into follicles following transplantation into neonatal mouse ovaries subsequently placed under a recipient kidney capsule (Woods et al., 2013a). Recently, it has been shown that transplanted granulosa-like cells derived from iPSCs can increase estrogen production and maintain ovarian weight in a mouse model for premature ovarian failure (POF; induced by cyclophosphamide) (Liu et al., 2016). However, there is no indication that fertility is restored or sustained, or that hormone levels approach those in an untreated mouse.
A current limitation to the utility of pluripotent cell-derived granulosa cells is the relatively low rate of specification (Woods et al., 2013a). Improvements in directed differentiation are requisite to any future therapeutic application, and hypotheses aimed at developing a targeted approach can be guided by our increasing knowledge of mammalian ovarian development. Such an approach would likely entail inducing the correct signaling pathways following specification of mesoderm. As more information is gleaned from developmental studies in vivo, and as evidence emerges for the molecular mechanisms that govern ovarian lineage specification in humans, it is likely that specific protocols for the directed differentiation of granulosa cells from pluripotent stem cells will be developed. Should methodology to reliably generate granulosa cells materialize, it is critical to ensure the proper steroidogenic stage is reached and not surpassed (i.e. that pregranulosa cells or estrogen producing granulosa cells do not further spontaneously differentiate to luteal cells), potentially through the utilization of co-cultures or formation of follicles with germ cells. Additionally, epigenetic memory in iPSCs can impact cell-fate specification outcomes. It has been demonstrated that mouse and human iPSCs derived from granulosa cells have a greater capacity for steroidogenesis (e.g. estrogen and progesterone) than ESCs or fibroblast-derived iPSCs (Anchan et al., 2015). Although not therapeutically relevant, utilization of this model may provide for more effective and efficient characterization of the specific molecular events that lead to the specification of granulosa cells from pluripotent stem cells, in vitro.
4. Origin of theca cells
Following follicle activation and subsequent granulosa cell proliferation, theca cells are recruited and organized around the growing follicle where they provide structural support, contain the blood supply, and eventually generate the requisite androgens for conversion to estrogen in the granulosa cells in response to LH (reviewed by Young and McNeilly, 2010). Theca cells are thought to be actively recruited to the growing follicle by granulosa cells, and in turn, granulosa cells likely release factors that induce theca cells to obtain LH responsiveness (Orisaka et al., 2009). Once established within the follicle, theca cells participate in bi-directional cellular interactions with granulosa cells by serving as the rate-limiting step in steroidogenesis in response to insulin-like growth factor-1 (IGF1) and kit ligand (KL) synthesized by granulosa cells (Huang et al., 2001; Orisaka et al., 2006). Theca cells also interact with the oocyte, most notably via growth differentiation factor-9 (GDF9), which is released by the oocyte and implicated in theca cell differentiation and androgen production (Elvin et al., 1999; Solovyeva et al., 2000; Spicer et al., 2008). As with granulosa cells, there is much speculation and only limited evidence for the origin of theca cells in the ovary. Initially, it was thought that theca cells derived from the ovarian stroma in response to specifically timed cues from granulosa cells, including IGF1 and KL (Huang et al., 2001). In the only study to date claiming the identification of a source of theca stem cells, it was revealed that cells isolated from neonatal mouse ovary were capable of in vitro propagation in an undifferentiated state and, with step wise induction, expressed markers of theca cell precursors (Honda et al., 2007). Defined culture conditions, including supplementation with LH, IGF1, and stem cell factor (SCF) resulted in a morphological transition to theca-like cells, and resulted in an 8-fold increase in androstenedione. Further treatment of these intermediate cells with granulosa cell-conditioned medium or co-culture with granulosa cells resulted in an additional elevation in androstenedione production, as well as increase in gene expression of the mature theca cell marker, patched 2 (Ptch2). Following transplantation into nontransgenic C57BL/6 recipient mouse ovaries, GFP expressing putative thecal stem cells (derived from the reporter strain C57BL/6-Tg(CAG-EGFP)C14-Y01-FM131Osb) actively proliferated and were found associated with or incorporated into the inner and outer thecal layers of follicles of undefined size (Honda et al., 2007). Alternatively, recent lineage-tracing analysis in mice has shed light on putative sources of theca progenitor cells, identifying two independent sources of theca cells (Liu et al., 2015). Relying on GLI-Kruppel family member GLI1 (Gli1; an effector of hedgehog signaling) as a marker of the theca cell lineage, Gli1-CreERT2; Rosa-LSL-tdTomato mice were injected with tamoxifen during pregnancy, and tdTomato-positive cells were identified in the ovaries of embryos immediately prior to parturition, indicating that the Gli1-positive cells identified in the mesonephros become at least a small portion of the theca cells in the ovary. Additionally, early somatic cell progenitors in the ovary express Wilms’ tumour 1 (Wt1), and employing a similar lineage tracing strategy, Wt1-CreERT2; Rosa-LSL-tdTomato reporter mice were utilized to trace Wt1-positive lineages from the gonadal primordium into the ovarian interstitium. Wt1-positive cells surrounded primary follicles postnatally and acquired Gli1 expression, indicative of theca cells. The vast majority of theca cells identified in this study were ovarian in origin, as indicated by the onset of Gli1 expression subsequent to appearance in the ovary, while Gli1 positive progenitors from the mesonephros supply only a limited quantity of theca cells, restricted to a small number of follicles (Liu et al., 2015). Although not yet evaluated, should Wt1 and Gli1 be conserved as markers of theca progenitors across species, including humans, these may eventually be used for guiding directed differentiation strategies to develop theca cell models from pluripotent stem cells.
5. Ovarian replacement as a putative therapeutic for loss of endocrine function in ovaries
Ovarian aging occurs relatively early in the chronological lifespan of women and has been the subject of scientific inquiry for decades. To this end, researchers have employed the use of ‘heterochronic’ transplantations to evaluate ovarian aging in a systemic context. Early work demonstrated that placement of a ‘young’ mouse ovary into an ovariectomized ‘old’ mouse restored estrus, although it failed to restore fertility completely (Krohn, 1962). Later work expanded this experimental design and refined this observation with modern techniques in a study in which 11 month old ovariectomized mice received an ovary transplant from 2-month-old sexually mature females (Cargill et al., 2003). Remarkably, the recipient females demonstrated an increase in lifespan by 60% compared to ovariectomized controls (Cargill et al., 2003). It has also been demonstrated that the age of the ovary at ‘donation’ directly impacts the effect on longevity, as prepubertal donor ovaries do not increase lifespan, while mice ovariectomized at 11 months of age receiving a donor ovary from a 60-day-old mouse exhibited the greatest increase in lifespan of all mice studied (Mason et al., 2009). The positive impact of prolonged ovarian function on health has also been documented. In a study utilizing a strain of mice deficient for the proapoptotic Bax gene, Bax knockout (KO) mice exhibited prolonged fertility in addition to improvements in other aging-related issues, including minimized bone and muscle loss, less fat deposition, decreased frequency of alopecia, cataracts, and deafness, as well as decreased anxiety and attention deficit (Perez et al., 2007). While the KO mice did not exhibit an increase in lifespan, overall condition of the mice indicates that sustained ovarian function is correlated with improvements to overall health. Collectively, these studies lend strong support the notion that ovarian function and female health are tightly linked, and provide strong impetus to pursue strategies to prevent or delay ovarian failure.
While extreme, ovarian transplantation has implications for restoration of endocrine function and, as a result, healthier aging without the myriad of health concerns associated with menopause. Although whole ovary transplantation is not generally feasible in human females, the concept of rejuvenating the ovarian environment to provide hormonal support is applicable, and the aforementioned studies in mice support the potential health benefits of sustained ovarian function. There are clinical case reports citing restoration of fertility in humans via ovarian tissue transplantation in which patients elected to have tissue removed and cryopreserved for grafting at a later date, or transplanted directly into a heterotrophic site (Oktay, 2001; Oktay et al., 2004). Multiple clinics have now reported restoration of ovarian function following ovarian tissue grafting, including a rise in serum estradiol concentrations and reduction in circulating FSH levels, occurring on average between 3.5 and 6.5 months post transplantation (Donnez et al., 2004, 2010, 2011; Macklon et al., 2014; Suzuki et al., 2015; Rodriguez-Wallberg et al., 2015; Oktay et al., 2016). Notably, in many instances the ovarian tissue grafts continue to function for several years following engraftment (Anderson et al., 2012). In a highly publicized example, a successful human ovarian transplant occurred between monozygotic twins, one of whom experienced POF and was unable to conceive. She received a portion of her twins’ ovarian cortex, and later reestablished menstruation and conceived a child naturally (Silber et al., 2005). Currently, ovarian cryopreservation and tissue vitrification are at the forefront of ovarian repair strategies after an expected premature ovarian failure (Silber, 2012; Suzuki et al., 2015; Oktay et al., 2016) but are not employed to extend ovarian endocrine function in women.
6. Conclusion
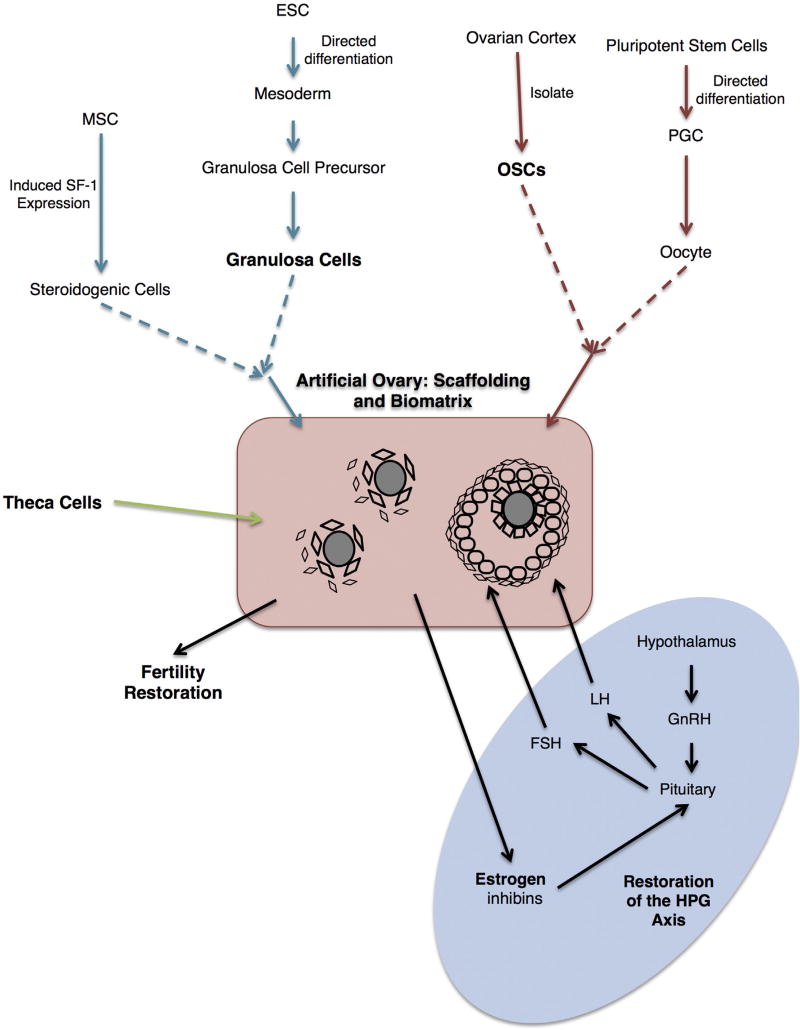
Although the use of elective ovarian cryopreservation and transplantation for the purpose of sustaining fertility has been subject to ethical debate, utilization as a therapeutic intervention for menopause to improve quality of life and healthy aging remains an intriguing possibility. However, clinical data for reversal of age-associated menopause from ovarian transplants have not been published and the availability of viable patient autologous tissue would be a likely limiting factor, as tissue would have to be removed at a young age and stored for a decade or more until transplantation. Accordingly, stem cell-based strategies that would eliminate the need for donor tissue or tissue banking are an attractive possible alternative. Achieving this goal would likely include combining stem cell-derived ovarian cells with one or more of the many strategies currently under development for the maturation of follicles from endogenous sources (Shea et al., 2014; Telfer and McLaughlin, 2011). For example, using a 3-dimensional (3D) in vitro maturation (IVM) culture system, it has been demonstrated that combining the follicular subtypes (e.g. granulosa and oocytes, with or without theca cells) creates an ‘artificial’ ovarian environment which supports human oocyte maturation (Krotz et al., 2010). Similar approaches have been reported using cells and tissues from mice, rats, non-human primates and humans (Xu et al., 2006; Laronda et al., 2014; Hornick et al., 2012; Xu et al., 2009; Luyckx et al 2013, 2014; Díaz-García and Herraiz, 2014, Liu et al., 2013, 2014; Kniazeva et al., 2015), albeit with the goal of follicle maturation rather than endocrine function. The potential utility in restoration of endocrine function has only recently been explored. Utilizing a previously described alginate encapsulated 3D follicle culture system, (Vanacker et al., 2012), data demonstrated that encapsulated multilayered co-cultures of theca and granulosa cells from rat ovaries could be sustained en vivo for up to a month. During this timeframe, the encapsulated co-cultures functioned in a similar capacity to that of native follicles demonstrated by the synthesis of estradiol and progesterone following gonadotropin stimulation (Liu et al., 2013, 2014). Eventually, combining bioengineering approaches, such as alginate hydrogels and collagen support matrices with OSCs and ‘synthetic’ stem cell-derived granulosa and theca cells could culminate into a fully functional ‘prosthetic ovary’ having dramatic implications not only for fertility preservation, but also for the restoration of estrogen synthesis and maintenance or reparation of the HPG-axis (Fig. 2).
Fig. 2.
Schematic summary of ovarian cell types and putative cell sources required for the production of an “artificial” ovary with the dual purpose of the generation of fertilization competent oocytes, as well as the capability of functional communication with the hypothalamic-pituitary axis. Pluripotent stem cell derived ovarian somatic cells (granulosa and/or theca) can be combined with OSCs or PGCLCs within a biomatrix to create the “prosthetic” ovary. Abbreviations: MSC - mesenchymal stem cells, SF-1 – steroidogenic factor 1, ESC – embryonic stem cell, OSC – oogonial stem cell, PGC – primordial germ cell, GnRH – gonadotropin releasing hormone, LH – luteinizing hormone, FSH - follicle stimulating hormone, HPG – hypothalamic-pituitary-gonadal.
Although currently ovarian failure is inevitable, advances in reproductive sciences are moving toward the development of strategies that will eventually thwart menopause and endocrine disruption. As we translate the information gained from basic scientific studies on ovarian organogenesis to the development of in vitro strategies to derive stem-cell based ovarian somatic cells, bioengineer new matrices to support sustained ovarian function, and discover new ways to drive oogenesis, we progress closer to this goal. Data from mice and also evidence in women have highlighted the efficacy of ovarian transplants to rescue fertility and hormone production; a critical next step in this process will be the elimination of the requirement for healthy donor tissue as a source of somatic components.
Acknowledgments
A Method to Extend Research in Time (MERIT) Award from the National Institute on Aging (NIH R37-AG012279), and a grant from the Glenn Foundation for Medical Research, supported work conducted by the authors discussed herein.
Footnotes
Competing financial interests
Dori C. Woods declares interest in intellectual property described in U.S. Patent 8,642,329 and U.S. Patent 8,647,869, and is a recipient of a corporate-sponsored research award from OvaScience, Inc. (Waltham, MA). Jonathan L. Tilly discloses interest in intellectual property described in U.S. Patent 7,195,775, U.S. Patent 7,850,984, U. S. Patent 7,955,846, U.S. Patent 8,642,329, U.S. Patent 8,647,869, and U.S. Patent 8,652,840, and is a scientific cofounder and a current member of the Scientific Advisory Board of OvaScience, Inc.
References
- Anchan R, Germani-Naini B, Lindsey J, Ho JWK, Kiezun A, Lipskin S, Ng N, LiCausi JA, Kim CS, Brezina P, Tuschi T, Maas R, Kearns WG, Willaims Z. Efficient differentiation of steroidogenic and germ-like cells from epigenetically-related iPSCs derived from ovarian granulosa cells. Plos One. 2015;10(3) doi: 10.1371/journal.pone.0119275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Robinson LL, Brooks J, Spears N. Neurotropins and their receptors are expressed in the human fetal ovary. J. Clin. Endocrinol. Metab. 2002;87(2):890–897. doi: 10.1210/jcem.87.2.8221. [DOI] [PubMed] [Google Scholar]
- Andersen CY, Silber SJ, Bergholdt SH, Jorgensen JS, Ernst E. Long-term duration of function of ovarian tissue transplants: case reports. Reprod. Biomed. Online. 2012 Aug;25(2):128–132. doi: 10.1016/j.rbmo.2012.03.014. http://dx.doi.org/10.1016/j.rbmo.2012.03.014. Epub 2012 Apr 5. [DOI] [PubMed] [Google Scholar]
- Baker TG, Franchi LL. The fine structure of oogonia and oocytes in human ovaries. J. Cell Sci. 1967;2(2):213–224. doi: 10.1242/jcs.2.2.213. [DOI] [PubMed] [Google Scholar]
- Bayne RA, Donnachie DJ, Kinnell HL, Childs AJ, Anderson RA. BMP signalling in human fetal ovary somatic cells is modulated in a gene-specific fashion by GREM1 and GREM2. Mol. Hum. Reprod. 2016 doi: 10.1093/molehr/gaw044. http://dx.doi.org/10.1093/molehr/gaw044. [DOI] [PMC free article] [PubMed]
- Berkholtz CB, Lai BE, Woodruff TK, Shea LD. Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem Cell Biol. 2006;126(5):583–592. doi: 10.1007/s00418-006-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukovsky A, Svetlikova M, Caudle MR. Oogenesis in cultures from adult human ovaries. Reprod. Biol. Endocrinol. 2005;3:17. doi: 10.1186/1477-7827-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Germ cells enter meiosis in a rostro-caudal wave during development of the mouse ovary. Mol. Reprod. Dev. 2004;68(4):422–428. doi: 10.1002/mrd.20105. [DOI] [PubMed] [Google Scholar]
- Byskov AG. Differentiation of mammalian embryonic gonad. Physiol. Rev. 1986;66(1):71–117. doi: 10.1152/physrev.1986.66.1.71. [DOI] [PubMed] [Google Scholar]
- Cargill SL, Carey JR, Muller HG, Anderson G. Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging Cell. 2003;2(3):185–190. doi: 10.1046/j.1474-9728.2003.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper RF, Dela Cruz DB, Mitri F, Hershko A, Bentov Y, Chang P, Esfandiari N. Preliminary results with autologous egg precursor cell mitochondrial injection during Intracytoplasmic Sperm Injection (ICSI) in women with previous poor embryo development; Poster Presented at: Society for Reproductive Investigation, 62nd Annual Scientific Meeting; 2015 March 25–28; San Francisco, CA. 2015. https://www.sri-online.org/UserFiles/file/SRI15_Abstracts_LB_Final.pdf. [Google Scholar]
- Chiquoine AD. The identification, origin, and migration of the primordial germ cells in the mouse embryo. Anat. Rec. 1954;118(2):135–146. doi: 10.1002/ar.1091180202. [DOI] [PubMed] [Google Scholar]
- Christensen AP, Patel SH, Grasa P, Christian HC, Williams SA. Oocyte glycoproteins regulate the form and function of the follicle basal lamina and theca cells. Dev. Biol. 2015;401(2):287–298. doi: 10.1016/j.ydbio.2014.12.024. [DOI] [PubMed] [Google Scholar]
- Crawford PA, Sadovsky Y, Milbrandt J. Nuclear receptor steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Mol. Cell Biol. 1997;17(7):3997–4006. doi: 10.1128/mcb.17.7.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felici M. Regulation of primordial germ cell development in the mouse. Int. J. Dev. Biol. 2000;44(6):575–580. [PubMed] [Google Scholar]
- De Felici M. Origin, migration, and proliferation of human primordial germ cells. In: Coticchio C, Albertini FD, De Santis L, editors. Oogenesis. Springer London; London: 2013. pp. 19–37. [Google Scholar]
- De Felici M, Scaldaferri ML, Lobascio M, Iona S, Nazzicone V, Klinger FG, Farini D. Experimental approaches to the study of primordial germ cell lineage and proliferation. Hum. Reprod. Update. 2004;10(3):197–206. doi: 10.1093/humupd/dmh020. [DOI] [PubMed] [Google Scholar]
- Díaz-García C, Herraiz S. The artificial ovary: any new step is a step forward. Fertil. Steril. 2014;101(4):940. doi: 10.1016/j.fertnstert.2014.01.057. [DOI] [PubMed] [Google Scholar]
- Ding X, Liu G, Xu B, Wu C, Hui N, Ni X, Wang J, Du M, Teng X, Wu J. Human GV oocytes generated by mitotically active germ cells obtained from follicular aspirates. Sci. Rep. 2016;6:28218. doi: 10.1038/srep28218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, Van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- Donnez J, Squifflet J, Pirard C, Jadoul P, Dolmans MM. Restoration of ovarian function after allografting of ovarian cortex between genetically non-identical sisters, 2010 Oct Hum. Reprod. 2010;25(10):2489–2495. doi: 10.1093/humrep/deq186. [DOI] [PubMed] [Google Scholar]
- Donnez J, Squifflet J, Pirard C, Demylle D, Delbaere A, Armenio L, Englert Y, Cheron AC, Jadoul P, Dolmans MM. Live birth after allografting of ovarian cortex between genetically non-identical sisters, 2011 Jun Hum. Reprod. 2011;26(6):1384–1388. doi: 10.1093/humrep/der089. [DOI] [PubMed] [Google Scholar]
- Duffin K, Bayne RA, Childs AJ, Collins C, Anderson RA. The forkhead transcription factor FOXL2 is expressed in somatic cells of the human ovary prior to follicle formation. Mol. Hum. Reprod. 2009;12:771–777. doi: 10.1093/molehr/gap065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzafic E, Stimpfel M, Virant-Klun I. Plasticity of granulosa cells: on the crossroad of stemness and transdifferentiation potential. J. Assist. Reprod. Genet. 2013;30(10):1255–1261. doi: 10.1007/s10815-013-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol. Endocrinol. 1999;13(6):1018–1034. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc. Natl. Acad. Sci. U. S. A. 2002;99(5):2890–2894. doi: 10.1073/pnas.052658699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakih MH, El Shmoury M, Szeptycki J, dela Cruz DB, Lux C, Verjee S, Burgess CM, Cohn GM, Casper RF. The AUGMENTSM treatment: physician reported outcomes of the initial global patient experience. JFIV Reprod. Med. Genet. 2015;3:3. [Google Scholar]
- Ginsburg M, Snow MH, McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990;110(2):521–528. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- Gong SP, Lee ST, Lee EJ, Kim DY, Lee G, Chi SG, Ryu BK, Lee CH, Yum KE, Lee HJ, Han JY, Tilly JL, Lim JM. Embryonic stem cell-like cells established by culture of adult ovarian cells in mice. Fertil. Steril. 2010;93(8):2594–2601. doi: 10.1016/j.fertnstert.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougeon A, Ecochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol. Reprod. 1994;50(3):653–663. doi: 10.1095/biolreprod50.3.653. [DOI] [PubMed] [Google Scholar]
- Grieve KM, McLaughlin M, Dunlop CE, Telfer EE, Anderson RA. The controversial existence and functional potential of oogonial stem cells. Maturitas. 2015;82(3):278–281. doi: 10.1016/j.maturitas.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338(6109):971–975. doi: 10.1126/science.1226889. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146(4):519–532. doi: 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- Heeren AM, van Iperen L, Klootwijk DB, de Melo Bernardo A, Roost MS, Gomes Fernandes MM, Louwe LA, Hilders CG, Helmerhorst FM, van der Westerlaken LA, Chuva de Lopes SM. Development of the follicular basement membrane during human gametogenesis and early folliculogenesis. BMC Dev. Biol. 2015;15:4. doi: 10.1186/s12861-015-0054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilscher B, Hilscher W, Bulthoff-Ohnolz B, Kramer U, Birke A, Pelzer H, Gauss G. Kinetics of gametogenesis. I. Comparative histological and autoradiographic studies of oocytes and transitional prospermatogonia during oogenesis and prespermatogenesis. Cell Tissue Res. 1974;154(4):443–470. doi: 10.1007/BF00219667. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN, DeSanti A. Patterns of ovarian cell proliferation in rats during the embryonic period and the first three weeks postpartum. Bio Reprod. 1995;53:1208–1221. doi: 10.1095/biolreprod53.5.1208. [DOI] [PubMed] [Google Scholar]
- Honda A, Hirose M, Hara K, Matoba S, Inoue K, Miki H, Hiura H, Kanatsu-Shinohara M, Kanai Y, Kono T, Shinohara T, Ogura A. Isolation, characterization, and in vitro and in vivo differentiation of putative thecal stem cells. Procc Natl. Acad. Sci. U. S. A. 2007;104(30):12389–12394. doi: 10.1073/pnas.0703787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum. Reprod. 2012;27(6):1801–1810. doi: 10.1093/humrep/der468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF, 3rd, Boiani M, Scholer HR. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300(5623):1251–1256. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- Hummitsch K, Irving-Rodgers HF, Hatzirodos N, Bonner W, Sabatier L, Reinhardt DP, Sado Y, Ninomiya Y, Wilhelm D, Rodgers RJ. A new model of development of the mammalian ovary and follicles. PLOS One. 2013;8:2. doi: 10.1371/journal.pone.0055578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CT, Weitsman SR, Dykes BN, Magoffin DA. Stem cell factor and insulin-like growth factor-I stimulate luteinizing hormone-independent differentiation of rat ovarian theca cells. Biol. Reprod. 2001;64(2):451–456. doi: 10.1095/biolreprod64.2.451. [DOI] [PubMed] [Google Scholar]
- Imudia AN, Wang N, Tanaka Y, White YA, Woods DC, Tilly JL. Comparative gene expression profiling of adult mouse ovary-derived oogonial stem cells supports a distinct cellular identity. Fertil. Steril. 2013;100(5):1451–1458. doi: 10.1016/j.fertnstert.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Muraki M, Takahashi Y, Imai M, Tsukui T, Yamakawa N, Nakagawa K, Ohgi S, Horikawa T, Iwasaki W, Iida A, Nishi Y, Yanase T, Nawata H, Miyado K, Kono T, Hosoi Y, Saito H. Glutathione S-transferase theta 1 expressed in granulosa cells as a biomarker for oocyte quality in age-related infertility. Fertil. Steril. 2008;90(4):1026–1035. doi: 10.1016/j.fertnstert.2007.07.1389. [DOI] [PubMed] [Google Scholar]
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Sawyer HR, Smith PR, Quirke LD, Heath DA, Lun S, Wakefield SJ, McNatty KP. Origins of follicular cells and ontogeny of steroidogenesis in ovine fetal ovaries. Mol. Cell Endocrinol. 2002;191(1):1–10. doi: 10.1016/s0303-7207(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Khosravi-Farsani S, Amidi F, Habibi Roudkenar M, Sobhani A. Isolation and enrichment of mouse female germ line stem cells. Cell J. 2015;16(4):406–415. doi: 10.22074/cellj.2015.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidder GM, Mhawi AA. Gap junctions and ovarian folliculogenesis. Reproduction. 2002;123(5):613–620. doi: 10.1530/rep.0.1230613. [DOI] [PubMed] [Google Scholar]
- Kniazeva E, Hardy AN, Boukaidi SA, Woodruff TK, Jeruss JS, Shea LD. Primordial follicle transplantation within designer biomaterial grafts produce live births in a mouse infertility model. Sci. Rep. 2015;5:17709. doi: 10.1038/srep17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi I, Fujii S, Okamura H, Parmley T, Mori T. Development of interstitial cells and ovigerous cords in the human fetal ovary: an ultrastructural study. J. Anat. 1986;148:121–135. [PMC free article] [PubMed] [Google Scholar]
- Kossowska-Tomaszczuk K, De Geyter C, De Geyter M, Martin I, Holzgreve W, Scherberich A, Zhang H. The multipotency of luteinizing granulosa cells collected from mature ovarian follicles. Stem Cells. 2009;27(1):210–219. doi: 10.1634/stemcells.2008-0233. [DOI] [PubMed] [Google Scholar]
- Krohn PL. Review lectures on senescence II. Heterochronic transplantation in the study of ageing. R. Soc. 1962:157. doi: 10.1098/rspb.1962.0066. [DOI] [PubMed] [Google Scholar]
- Krotz SP, Robins JC, Ferruccio TM, Moore R, Steinhoff MM, Morgan JR, Carson S. In vitro maturation of oocytes via the pre-fabricated self-assembled artificial human ovary. J. Assist. Reprod. Genet. 2010;27(12):743–750. doi: 10.1007/s10815-010-9468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilo LF. Oogenesis in antenatal development in man. Hum. Genet. 1981;57(1) doi: 10.1007/BF00271175. [DOI] [PubMed] [Google Scholar]
- Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA, Shea LD, Woodruff TK. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. Assist. Reprod. Genet. 2014;8:1013–1028. doi: 10.1007/s10815-014-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Spradling AC. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. PNAS. 2013;110(21):8585–8590. doi: 10.1073/pnas.1306189110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Zhang H, Jin S, Wang F, Fu M, Wang H, Xia G. Stage-specific germ-somatic cell interaction directs the primordial folliculogenesis in mouse fetal ovaries. J. Cell Physiol. 2006;208(3):640–647. doi: 10.1002/jcp.20702. [DOI] [PubMed] [Google Scholar]
- Liu C, Xia X, Miao W, Luan X, Sun L, Jin Y, Liu L. An ovarian cell microcapsule system simulating follicle structure for providing endogenous female hormones. Int. J. Pharm. 2013;455(1–2):312–319. doi: 10.1016/j.ijpharm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Liu C, Luan X, He Y, Xia X, Sun L, Miao W, Jin Y, Liu L. Endogenous release of female hormones from co-microencapsulated rat granulosa and theca cells. Biomed. Microdevices. 2014;16(2):209–216. doi: 10.1007/s10544-013-9824-2. [DOI] [PubMed] [Google Scholar]
- Liu C, Peng J, Matzuk MM, Yao HH. “Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells”. Nat. Commun. 2015;6:6934. doi: 10.1038/ncomms7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Li Q, Wang S, Chen C, Zheng J. Transplantation of ovarian granulosalike cells derived from human induced pluripotent stem cells for the treatment of murine premature ovarian failure. Mol. Med. Rep. 2016;13(6):5053–5058. doi: 10.3892/mmr.2016.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Wu M, Zhang J, Xiong J, Cheng J, Shen W, Luo A, Fang L, Wang S. Improvement in isolation and identification of mouse oogonial stem cells. Stem Cells Int. 2016 doi: 10.1155/2016/2749461. Article ID 2749461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyckx V, Dolmans MM, Vanacker J, Legat C, Fortuño Moya C, Donnez J, Amorim CA. A new step toward the artificial ovary: survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil. Steril. 2014;101(4):1149–1156. doi: 10.1016/j.fertnstert.2013.12.025. [DOI] [PubMed] [Google Scholar]
- Luyckx V, Dolmans MM, Vanacker J, Scalercio SR, Donnez J, Amorim CA. First step in developing a 3D biodegradable fibrin scaffold for an artificial ovary. J. Ovarian Res. 2013;6(1):83. doi: 10.1186/1757-2215-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklon KT, Jensen AK, Loft A, Ernst E, Andersen CY. Treatment history and outcome of 24 deliveries worldwide after autotransplantation of cryopreserved ovarian tissue, including two new Danish deliveries years after autotransplantation, 2014 Nov J. Assist. Reprod. Genet. 2014;31(11):1557–1564. doi: 10.1007/s10815-014-0331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi R, Cariboni AM, Marelli MM, Moretti RM, Andre V, Marzagalli M, Limonta P. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system. Hum. Reprod. Update. 2016;22(3) doi: 10.1093/humupd/dmv059. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Fowler PA. Primordial follicular assembly in humans - revisited. Zygote. 2008;16(4):285–296. doi: 10.1017/S0967199408004802. [DOI] [PubMed] [Google Scholar]
- Mason JB, Cargill SL, Anderson GB, Carey JR. Transplantation of young ovaries to old mice increased life span in transplant recipients. Journals Gerontology Ser. A Biol. Sci. Med. Sci. 2009;64A(12):1207–1211. doi: 10.1093/gerona/glp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makabe S, Naguro T, Nottola SA, Pereda J, Motta PM. Migration of germ cells, development of the ovary, and folliculogenesis. Ultrastruct. Ovary. Volume 9 of the series Electron Microscopy in Biology and Medicine. 1989:1–27. [Google Scholar]
- Mattioli M, Gloria A, Turriani M, Berardinelli P, Russo V, Nardinocchi D, Curini V, Baratta M, Martignani E, Barboni B. Osteo-regenerative potential of ovarian granulosa cells: an in vitro and in vivo study. Thereogeneology. 2012;77(7):1425–1437. doi: 10.1016/j.theriogenology.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev. Biol. 2003;262(2):303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011;32(1):81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux K, Wylie C. Primordial germ cell migration. Int. J. Dev. Biol. 2004;48(5–6):537–544. doi: 10.1387/ijdb.041833km. [DOI] [PubMed] [Google Scholar]
- Molyneaux KA, Stallock J, Schaible K, Wylie C. Time-lapse analysis of living mouse germ cell migration. Dev. Biol. 2001;240(2):488–498. doi: 10.1006/dbio.2001.0436. [DOI] [PubMed] [Google Scholar]
- Mork L, Maatouk DM, McMahon JA, Guo J, Zhang P, McMahon AP, Capel B. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol. Reprod. 2012;86(2):37. doi: 10.1095/biolreprod.111.095208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta PM, Makabe S. Development of the ovarian surface and associated germ cells in the human fetus. Cell Tissue Res. 1982;226(3):493–510. doi: 10.1007/BF00214779. [DOI] [PubMed] [Google Scholar]
- Motta PM, Makabe S. Elimination of germ cells during differentiation of the human ovary: an electron microscopic study. Eur. J. Obstet. Gynecol. Reprod. Biol. 1986;22(5–6):271–286. doi: 10.1016/0028-2243(86)90115-2. [DOI] [PubMed] [Google Scholar]
- Motta PM, Makabe S, Nottola SA. The ultrastructure of human reproduction. I. The natural history of the female germ cell: origin, migration and differentiation inside the developing ovary. Hum. Reprod. Update. 1997;3(3):281–295. doi: 10.1093/humupd/3.3.281. [DOI] [PubMed] [Google Scholar]
- Nicholas CR, Haston KM, Grewall AK, Longacre TA, Reijo Pera RA. Transplantation directs oocyte maturation from embryonic stem cells and provides a therapeutic strategy for female infertility. Hum. Mol. Genet. 2009;18(22):4376–4389. doi: 10.1093/hmg/ddp393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas CR, Haston KM, Pera RA. Intact fetal ovarian cord formation promotes mouse oocyte survival and development. BMC Dev. Biol. 2010;10:2. doi: 10.1186/1471-213X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura Y, Niikura T, Tilly JL. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging. 2009;1(12):971–978. doi: 10.18632/aging.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak I, Lightfoot DA, Wang H, Eriksson A, Mahdy E, Höög C. Mouse embryonic stem cells form follicle-like ovarian structures but do not progress through meiosis. Stem Cells. 2006;24(8):1931–1936. doi: 10.1634/stemcells.2005-0520. [DOI] [PubMed] [Google Scholar]
- Oki Y, Ono H, Motohashi T, Sugiura N, Nobusue H, Kano K. Dedifferentiated follicular granulosa cells derived from pig ovary can transdifferentiate into osteoblasts. Biochem. J. 2012;447(2):239–248. doi: 10.1042/BJ20120172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay K. Ovarian tissue cryopreservation and transplantation: preliminary findings and implications for cancer patients. Hum. Reprod. Update. 2001;7(6):526–534. doi: 10.1093/humupd/7.6.526. [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, Opsahl M, Rosenwaks Z. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363(9412):837–840. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozenbanked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am. J. Obstet. Gynecol. 2016;214(1):94.e1–94e9. doi: 10.1016/j.ajog.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orisaka M, Tajima K, Tsang BK, Kotsuji F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J. Ovarian Res. 2009;2(1):1–7. doi: 10.1186/1757-2215-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orisaka M, Tajima K, Mizutani T, Miyamoto K, Tsang BK, Fukuda S, Yoshida Y, Kotsuji F. Granulosa cells promote differentiation of cortical stromal cells into theca cells in the bovine ovary. Biol. Reprod. 2006;75(5):734–740. doi: 10.1095/biolreprod.105.050344. [DOI] [PubMed] [Google Scholar]
- Pacchiarotti J, Maki C, Ramos T, Marh J, Howerton K, Wong J, Pham J, Anorve S, Chow YC, Izadyar F. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79(3):159–170. doi: 10.1016/j.diff.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Park ES, Tilly JL. Use of DEAD-box polypeptide-4 (Ddx4) gene promoter-driven fluorescent reporter mice to identify mitotically active germ cells in post-natal mouse ovaries. Mol. Hum. Reprod. 2015;21(1):58–65. doi: 10.1093/molehr/gau071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ES, Woods DC, Tilly JL. Bone morphogenetic protein 4 promotes mammalian oogonial stem cell differentiation via Smad1/5/8 signaling. Fertil. Steril. 2013;100(5):1468–1475. doi: 10.1016/j.fertnstert.2013.07.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 2001;234(2):339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- Perez GI, Tilly JL. Cumulus cells are required for the increased apoptotic potential in oocytes of aged mice. Hum. Reprod. 1997;12(12):2781–2783. doi: 10.1093/humrep/12.12.2781. [DOI] [PubMed] [Google Scholar]
- Perez GI, Jurisicova A, Wise L, Lipina T, Kanisek M, Bechard A, Takai Y, Hunt P, Roder J, Grynpas M, Tilly JL. Absence of the proapoptotic Bax protein extends fertility and alleviates age-related health complications in female mice. Proc. Natl. Acad. Sci. U. S. A. 2007;104(12):5229–5234. doi: 10.1073/pnas.0608557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politzer G. Die Keimbahn des Menshen. Z Anat. EntwGesch. 1933;1933(100):331–336. [Google Scholar]
- Psathaki OE, Hübner K, Sabour D, Sebastiano V, Wu G, Sugawa F, Wieacker P, Pennekamp P, Schöler HR. Ultrastructural characterization of mouse embryonic stem cell-derived oocytes and granulosa cells. Stem Cells Dev. 2011;20(12):2205–2215. doi: 10.1089/scd.2010.0575. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Wallberg KA, Karlström PO, Rezapour M, Castellanos E, Hreinsson J, Rasmussen C, Sheikhi M, Ouvrier B, Bozóky B, Olofsson JI, Lundqvist M, Hovatta O. Full-term newborn after repeated ovarian tissue transplants in a patient treated for Ewing sarcoma by sterilizing pelvic irradiation and chemotherapy. Acta Obstet. Gynecol. Scand. 2015;94(3):324–328. doi: 10.1111/aogs.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Barton SC, Surani MA. A molecular programme for the specification of germ cell fate in mice. Nature. 2002;418(6895):293–300. doi: 10.1038/nature00927. [DOI] [PubMed] [Google Scholar]
- Satoh M. Histogenesis and organogenesis of the gonad in human embryos. J. Anat. 1991;177:85–107. [PMC free article] [PubMed] [Google Scholar]
- Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield S, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Bio Reprod. 2002;66(4):1134–1150. doi: 10.1095/biolreprod66.4.1134. [DOI] [PubMed] [Google Scholar]
- Seifer DB, DeJesus V, Hubbard K. Mitochondrial deletions in luteinized granulosa cells as a function of age in women undergoing in vitro fertilization. Fertil. Steril. 2002;78(5):1046–1048. doi: 10.1016/s0015-0282(02)04214-0. [DOI] [PubMed] [Google Scholar]
- Shea L, Woodruff TK, Shikanov A. Bioengineering the ovarian follicle microenvironment. Annu. Rev. Biomed. Eng. 2014 Jul;11(16):29–52. doi: 10.1146/annurev-bioeng-071813-105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber SJ, Lenahan KM, Levine DJ, Pineda JA, Gorman KS, Friez MJ, Crawford EC, Gosden RG. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N. Engl. J. Med. 2005;353(1):58–63. doi: 10.1056/NEJMoa043157. [DOI] [PubMed] [Google Scholar]
- Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol. Hum. Reprod. 2012;18(2):59–67. doi: 10.1093/molehr/gar082. [DOI] [PubMed] [Google Scholar]
- Silvestris E, D’Oronzo S, Cafforio P, D’Amato G, Loverro G. Perspective in infertility: the ovarian stem cells. J. Ovarian Res. 2015;8:55. doi: 10.1186/s13048-015-0184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson ER, Misso M, Hewitt KN, Hill RA, Boon WC, Jones ME, Kovacic A, Zhou J, Clyne CD. Estrogen-the good, the bad, and the unexpected. Endocr. Rev. 2005;26(3):322–330. doi: 10.1210/er.2004-0020. [DOI] [PubMed] [Google Scholar]
- Skinner MK. Regulation of primordial follicle assembly and development. Hum. Reprod. Update. 2005;11(5):461–471. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- Solovyeva EV, Hayashi M, Margi K, Barkats C, Klein C, Amsterdam A, Hsueh AJ, Tsafriri A. Growth differentiation factor-9 stimulates rat theca-interstitial cell androgen biosynthesis. Biol. Reprod. 2000;63(4):1214–1218. doi: 10.1095/biolreprod63.4.1214. [DOI] [PubMed] [Google Scholar]
- Speed RM. Meiosis in the foetal mouse ovary. I. An analysis at the light microscope level using surface-spreading. Chromosoma. 1982;85(3):427–437. doi: 10.1007/BF00330366. [DOI] [PubMed] [Google Scholar]
- Spicer LJ, Aad PY, Allen DT, Mazerbourg S, Payne AH, Hsueh AJ. Growth differentiation factor 9 (GDF9) stimulates proliferation and inhibits steroidogenesis by bovine theca cells: influence of follicle size on responses to GDF9. Biol. Reprod. 2008;78(2):243–253. doi: 10.1095/biolreprod.107.063446. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, Morimoto Y, Kawamura K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency, 2015 Mar Hum. Reprod. 2015;30(3):608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- Tam PP, Snow M. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J. Embryol. Exp. Morphol. 1981;64:133–147. [PubMed] [Google Scholar]
- Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, Marci R, Pandolfi A, Ragnelli AM, Amicarelli F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol. Hum. Reprod. 2006;11:655–660. doi: 10.1093/molehr/gal080. [DOI] [PubMed] [Google Scholar]
- Telfer E, McLaughlin M. In vitro development of ovarian follicles. Semin. Reprod. Med. 2011 Jan;29(1):15–23. doi: 10.1055/s-0030-1268700. http://dx.doi.org/10.1055/s-0030-1268700. [DOI] [PubMed] [Google Scholar]
- Tingen C, Kim A, Woodruff TK. The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol. Hum. Reprod. 2009;15(12):795–803. doi: 10.1093/molehr/gap073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman A, Izdebski M, Takai Y, Seki H, Ishihara O, Tilly J, Woods D. Dynamics of WNT signaling during human ovarian development. Reprod. Sci. 2016;23:51A–344A. http://dx.doi.org/10.1177/1933719116641257. [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schütz G, Cooney AJ, Lovell-Badge R, Treier M. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139(6):1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Vanacker J, Luyckx V, Dolmans MM, Des Rieux A, Jaeger J, Van Langendonckt A, Donnez J, Amorim CA. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials. 2012 Sep;33(26):6079–6085. doi: 10.1016/j.biomaterials.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Varras M, Griva T, Kalles V, Akrivis C, Paparisteidis N. Markers of stem cells in human ovarian granulosa cells: is there a clinical significance in ART? J. Ovarian Res. 2012;5(1):36. doi: 10.1186/1757-2215-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wear HM, McPike MJ, Watanabe KH. From primordial germ cells to primordial follicles: a review and visual representation of early ovarian development in mice. J. Ovarian Res. 2016;9(1):36. doi: 10.1186/s13048-016-0246-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat. Med. 2012;18(3):413–421. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol. Rev. 2007;87(1):1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- Witschi E. Migration of the Germ Cells of Human Embryos Freom the Yolk Sac to the Primitive Gonadal Folds (Washington) 1948 [Google Scholar]
- Woods DC, Tilly JL. Germline stem cells in adult mammalian ovaries. In: Sanders S, editor. Ten Critical Topics in Reproductive Medicine, vol 2013. Science/AAAS. Washington, DC: 2013a. pp. 10–12. [Google Scholar]
- Woods DC, Tilly JL. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat. Protoc. 2013b;8(5):966–988. doi: 10.1038/nprot.2013.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DC, Tilly JL. An evolutionary perspective on adult female germline stem cell function from flies to humans. Semin. Reprod. Med. 2013c;31(01):024–032. doi: 10.1055/s-0032-1331794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DC, Tilly JL. Autologous germline mitochondrial energy transfer (AUGMENT) in human assisted reproduction. Semin. Reprod. Med. 2015a;33(6):410–421. doi: 10.1055/s-0035-1567826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DC, Tilly JL. Reply to Adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat. Med. 2015b;21:1118–1121. doi: 10.1038/nm.3775. [DOI] [PubMed] [Google Scholar]
- Woods DC, White YAR, Niikura Y, Kiatpongsan S, Lee JH, Tilly JL. Embryonic stem cell-derived granulosa cells participate in ovarian follicle formation in vitro and in vivo. Reprod. Sci. 2013a;20(5):524–535. doi: 10.1177/1933719113483017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DC, White YA, Tilly JL. Purification of oogonial stem cells from adult mouse and human ovaries: an assessment of the literature and a view toward the future. Reprod. Sci. 2013b;20(1):7–15. doi: 10.1177/1933719112462632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YG, Barad DH, Kushnir VA, Lazzaroni E, Wang Q, Albertini DF, Gleicher N. Aging-related premature luteinization of granulosa cells is avoided by early oocyte retrieval. J. Endocrinol. 2015;226(3):167–180. doi: 10.1530/JOE-15-0246. [DOI] [PubMed] [Google Scholar]
- Xie W, Wang H, Wu J. “Similar morphological and molecular signatures shared by female and male germline stem cells”. Sci. Rep. 2014;4:5580. doi: 10.1038/srep05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Lu Z, Wu M, Zhang J, Cheng J, Luo A, Shen W, Fang L, Zhou S, Wang S. Intraovarian transplantation of female germline stem cells rescue ovarian function in chemotherapy-injured ovaries. PLoS ONE. 2015;10(10):e0139824. doi: 10.1371/journal.pone.0139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol. Bioeng. 2009;103(2):378–386. doi: 10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol. Reprod. 2006;75(6):916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- Yazawa T, Inanoka Y, Mizutani T, Kuribayashi M, Umezawa A, Miyamoto K. Liver receptor homolog-1 regulates the transcription of steroidogenic enzymes and induces the differentiation of mesenchymal stem cells into steroidogenic cells. Endocrinology. 2009;150(8):3885–3893. doi: 10.1210/en.2008-1310. [DOI] [PubMed] [Google Scholar]
- Yazawa T, Kawabe S, Inaoka Y, Okada R, Mizutani T, Imamichi Y, Ju Y, Yamazaki Y, Usami Y, Kuribayashi M, Umezawa A, Miyamoto K. Differentiation of mesenchymal stem cells and embryonic stem cells into steroidogenic cells using steroidogenic factor-1 and liver receptor homolog-1. Mol. Cell Endocrinol. 2010;336(1–2):127–132. doi: 10.1016/j.mce.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140(4):489–504. doi: 10.1530/REP-10-0094. [DOI] [PubMed] [Google Scholar]
- Yu B, Russanova VR, Gravina S, Hartley S, Mullikin JC, Ignezweski A, Graham J, Segars JH, DeCherney AH, Howard BH. DNA methylome and transcriptome sequencing in human ovarian granulosa cells links age-related changes in gene expression to gene body methylation and 3’-end GC density. Oncotarget. 2015;6(6):3627–3643. doi: 10.18632/oncotarget.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zheng W, Shen Y, Adhikari D, Ueno H, Liu K. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc. Natl. Acad. Sci. U. S. A. 2012;109(31):12580–12585. doi: 10.1073/pnas.1206600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Panula S, Petropoulos S, Edsgärd D, Busayavalasa K, Liu L, Li X, Risal S, Shen Y, Shao J, Liu M, Li S, Zhang D, Zhang X, Raphaela Gerner R, Sheikhi M, Damdimopoulou P, Sandberg R, Douagi I, Gustafsson J, Liu L, Lanner F, Hovatta O, Liu K. Adult Human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat. Med. 2015;21:1116–1118. doi: 10.1038/nm.3775. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang Z, Yang Y, Wang S, Shi L, Xie W, Sun K, Zou K, Wang L, Xiong J, Xiang J, Wu J. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J. Mol. Cell Biol. 2011;3(2):132–141. doi: 10.1093/jmcb/mjq043. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wu J. Production of offspring from a germline stem cell line derived from prepubertal ovaries of germline reporter mice. Mol. Hum. Reprod. 2016;22(7):457–464. doi: 10.1093/molehr/gaw030. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Wu J, Wang J, Shen T, Li H, Lu J, Gu Y, Kang Y, Wong CH, Ngan CY, Shao Z, Wu J, Zhao X. Integrative epigenomic analysis reveals unique epigenetic signatures involved in unipotency of mouse female germline stem cells. Genome Biol. 2016;17(1):162. doi: 10.1186/s13059-016-1023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Wang L, Kang JX, Xie W, Li X, Wu C, Xu B, Wu J. Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol. Hum. Reprod. 2014;20(3):271–281. doi: 10.1093/molehr/gat081. [DOI] [PubMed] [Google Scholar]
- Zou K, Hou L, Sun K, Xie W, Wu J. Improved efficiency of female germline stem cell purification using fragilis-based magnetic bead sorting. Stem Cells Dev. 2011;20(12):2197–2204. doi: 10.1089/scd.2011.0091. [DOI] [PubMed] [Google Scholar]
- Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, Hou R, Wu J. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat. Cell Biol. 2009;11(5):631–636. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]