Abstract
Objective
Identify the progression of specific signs of multi-organ dysfunction among infants with fatal sepsis.
Study design
Cohort study of 679 infants who died within 3 days of the start of a late-onset sepsis (LOS) episode in neonatal intensive care units from 1997–2012. We extracted clinical and laboratory data on the day of death (day 0) and the preceding 5 days (days −5 to −1).
Results
Median (25th percentile-75th percentile) gestational age was 25 (24–28) weeks. Compared with day −1, day 0 was characterized by an increased requirement for mechanical ventilation and higher mean fraction of inspired oxygen. Measures of cardiorespiratory support and the proportion of infants with neutropenia began to rise on day −2.
Conclusions
Hospitalized infants with fatal LOS manifest respiratory, cardiovascular, renal, immune, and hematologic dysfunction. Knowledge of these factors and their timing may be important for the development and testing of novel therapeutics to reduce sepsis mortality.
Keywords: sepsis, infant, neonate, multi-organ dysfunction, death
Late-onset sepsis (LOS), or sepsis occurring after day of life 3, results in significant morbidity and mortality among infants hospitalized in neonatal intensive care units (NICUs).1–4 The frequency of LOS varies inversely with gestational age at birth, with up to 36% of infants <28 weeks’ gestation at birth affected.5 Treatment of LOS remains limited to supportive care and antimicrobial therapy. Gram-negative and fungal infections are associated with substantial and early mortality.2,6 An understanding of the clinical progression of signs with rapid and fatal sepsis among infants is an important antecedent for the development of novel therapeutics. In addition, it is important to identify important indicators of clinical impact beyond mortality for future interventional trials.
Sepsis-associated multi-organ dysfunction is often described as a common terminal pathway in the pathophysiology of sepsis and may include respiratory, cardiovascular, central nervous system, adrenal, clotting, immune (leukopenia or neutropenia), and renal dysfunction.7,8 However, the timing of onset and frequency of these phenomena among hospitalized infants with fatal sepsis are unknown. In the current analysis, we examined a large cohort of infants to identify the presence and progression of specific signs of multi-organ dysfunction among infants with fatal sepsis.
METHODS
Data Source
Data were obtained from an electronic medical record that prospectively captured information from daily progress notes generated by clinicians using a computer-assisted tool in NICUs managed by the Pediatrix Medical Group from 1997 to 2012. During the study period, these data included 348 NICUs in the United States. Data on multiple aspects of patient care are available in this database, including demographics, medications, laboratory results, and diagnoses. This study was approved by the Duke University Institutional Review Board.
Study Population
We identified all infants who died within 3 days of the date of the first positive blood culture of an LOS episode. LOS was defined as growth of a bacterial or fungal pathogen from a blood culture obtained at postnatal age 4–120 days. We excluded likely bacterial contaminants including non-speciated streptococci, Bacillus spp., Corynebacterium spp., and Micrococcus spp. We divided coagulase-negative staphylococcus (CoNS) infections into three categories: definite, probable, and possible, as previously described.9 Only definite and probable CoNS infections were included. To examine the effect of organism type on measures of cardiorespiratory support and laboratory parameters, we excluded polymicrobial LOS episodes characterized by growth of more than one bacterial or fungal pathogen. We defined small for gestational age status as birth weight less than the 10th percentile for gestational age based on Olsen growth curves.10 We considered the following medications to be vasopressors: amrinone, dobutamine, dopamine, epinephrine, milrinone, and norepinephrine.
We defined the day of death as day 0 and extracted the following clinical and laboratory data on days −5 to 0: requirement for mechanical ventilation, fraction of inspired oxygen (FiO2), receipt of one or more vasopressor medications, serum creatinine, platelet count, and absolute neutrophil count (ANC). We used the maximum (creatinine) or minimum (platelet count, ANC) value recorded when multiple laboratory results were available on a given day. We defined neutropenia as ANC <1000 cells/μL,11 thrombocytopenia as platelet count <100,000/μL,12 and abnormal renal function as serum creatinine ≥1.3 mg/dL.13
Statistical Analysis
Baseline characteristics of the study population were described using percentages for categorical variables and median (with 25th and 75th percentiles) and means (with standard deviations) for continuous variables. We used chi-square tests or Wilcoxon signed rank tests to compare measures of cardiorespiratory support and laboratory parameters on consecutive days during the time period of interest (days −5 to 0). We categorized organisms from LOS episodes as being Gram-negative bacteria, Gram-positive bacteria, or fungi. Changes in cardiorespiratory support and the prevalence of laboratory abnormalities were compared by organism type using multilevel mixed-effects logistic regression. All statistical analyses were conducted using STATA version 13.1 (College Station, TX).
RESULTS
Patient Characteristics
We identified 679 infants from 124 NICUs who met the inclusion criteria (Table 1). Median gestational age and birth weight were 25 weeks (25th percentile, 75th percentile; 24–28) and 755 g (610–1040), respectively. Mean gestational age and birth weight were 26.6 weeks (standard deviation 3.7) and 957 g (600). The majority of infants were extremely low birth weight (<1000 g, 73%), male (59%), and non-white (61%). Eighty-two percent of LOS episodes occurred during the neonatal period (postnatal age 4–30 days), and only 5% of episodes occurred after 60 days postnatal age. Two (0.3%) infants received extracorporeal life support on 1 or more days during the time period of interest (days −5 to 0).
Table 1.
Characteristics of the study population (n=679).
| Characteristic | % |
|---|---|
| Postnatal age at death, days | |
| 4–15 | 51% |
| 16–30 | 30% |
| 31–60 | 14% |
| 61–120 | 5% |
| Gestational age at birth, weeks | |
| ≤24 | 36% |
| 25–28 | 43% |
| 29–32 | 14% |
| ≥33 | 8% |
| Birth weight, g | |
| <750 | 49% |
| 750–999 | 24% |
| 1001–1499 | 15% |
| ≥1500 | 12% |
| Small for gestational age | 18% |
| Male | 59% |
| Race/ethnicity | |
| Caucasian | 39% |
| African-American | 34% |
| Hispanic | 22% |
| Other | 5% |
| Co-morbidities | |
| Medical or surgical NEC | 27% |
| PDA requiring surgical ligation | 7% |
| Grade III or IV IVH, either side | 24% |
| Day of first positive culture | |
| −3 | 18% |
| −2 | 30% |
| −1 | 38% |
| 0 | 15% |
NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; IVH, intraventricular hemorrhage
Microbiology
More than half (55%) of fatal LOS episodes were caused by Gram-negative bacteria, while Gram-positive bacteria and fungi accounted for 36% and 9% of episodes, respectively (Table 2). For the Gram-negative bacterial infections for which genus- or species-level data were available, Pseudomonas spp. and Escherichia coli were most frequently identified. Staphylococcus aureus and Group B Streptococcus were the most common Gram-positive bacteria isolated, while CoNS was isolated from only 2% of fatal LOS episodes. All fungal LOS episodes were caused by Candida spp.
Table 2.
Organisms identified from fatal late-onset sepsis episodes (n=679).
| n | % | |
|---|---|---|
| Bacteria | ||
| Gram-negative | 374 | 55 |
| Pseudomonas spp. | 53 | 8 |
| Escherichia coli | 42 | 6 |
| Klebsiella spp. | 20 | 3 |
| Enterobacter spp. | 20 | 3 |
| Serratia spp. | 12 | 2 |
| Citrobacter spp. | 3 | <1 |
| Stenotrophomonas spp. | 2 | <1 |
| Proteus spp. | 1 | <1 |
| Gram-negative bacilli (unspecified) | 221 | 33 |
| Gram-positive | 246 | 36 |
| Staphylococcus aureus | 60 | 9 |
| Group B Streptococcus | 17 | 3 |
| Coagulase-negative staphylococci | 14 | 2 |
| Enterococcus spp. | 9 | 1 |
| Clostridia spp. | 2 | <1 |
| Gram-positive cocci (unspecified) | 144 | 21 |
| Fungi | ||
| Yeast | 59 | 9 |
| Candida spp. | 59 | 9 |
Clinical and Laboratory Characteristics
Statistically significant changes were observed in all measures of cardiorespiratory support and laboratory parameters on 1 or more days preceding death (Table 3). Each measure of cardiorespiratory support and the proportion of infants with neutropenia increased significantly on day −2, while the most substantial changes in the proportions of infants with thrombocytopenia and abnormal renal function were observed on days −1 and 0, respectively. Compared with day −1, the day of death was characterized by an increased requirement for mechanical ventilation (96% vs. 91%, P<0.001) and higher mean FiO2 (80% vs. 59%, P<0.001), but no difference in requirement for vasopressor medications (42% vs. 40%, P=0.54). Thrombocytopenia and neutropenia were observed on the day of death in 71% and 28% of infants, respectively, while 41% of infants had abnormal renal function.
Table 3.
Changes in cardiorespiratory support and laboratory parameters preceding death among hospitalized infants with late-onset sepsis.
| Baseline Day −5 (n=650) | Change from Days −5 to −4 (n=660) | Change from Days −4 to −3 (n=665) | Change from Days −3 to −2 (n=671) | Change from Days −2 to −1 (n=677) | Change from Days −1 to 0a (n=679) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiorespiratory Support | n | % | nb | Δ% | P | nb | Δ% | P | nb | Δ% | P | nb | Δ% | P | nb | Δ% | P |
| Mechanical ventilation | 650 | 72 | 660 | 0 | 0.89 | 665 | +2 | 0.38 | 671 | +7 | 0.003 | 677 | +11 | <0.001 | 678 | +5 | <0.001 |
| Percentage FiO2, mean | 640 | 37 | 652 | −1 | 0.22 | 653 | +3 | <0.001 | 661 | +7 | <0.001 | 667 | +13 | <0.001 | 674 | +22 | <0.001 |
| Receipt of one or more vasopressors | 650 | 16 | 660 | −2 | 0.34 | 665 | 0 | 0.80 | 671 | +8 | <0.001 | 677 | +17 | <0.001 | 679 | +2 | 0.54 |
| Laboratory Parameters | |||||||||||||||||
| Creatinine ≥1.3 mg/dL | 240 | 18 | 258 | +3 | 0.46 | 257 | 0 | 0.90 | 277 | +6 | 0.08 | 329 | +3 | 0.47 | 281 | +11 | 0.01 |
| ANC <1000 cells/μL | 194 | 7 | 188 | 0 | 0.93 | 231 | +2 | 0.55 | 315 | +8 | 0.01 | 395 | +5 | 0.07 | 321 | +5 | 0.09 |
| Platelet count <100,000/μL | 257 | 30 | 250 | +4 | 0.29 | 289 | +5 | 0.20 | 368 | +7 | 0.06 | 460 | +18 | <0.001 | 384 | +6 | 0.06 |
Δ, change; FiO2, fraction of inspired oxygen; ANC, absolute neutrophil count.
P values estimated using chi square tests or Wilcoxon signed rank tests.
Day 0 corresponds to day of death.
n corresponds to the number of infants with available data on the second day in the time interval.
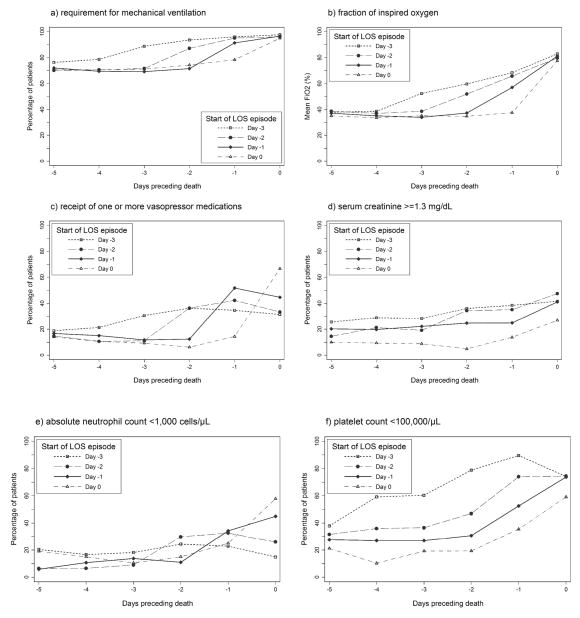
To better define the timing of changes in cardiorespiratory support and the prevalence of laboratory abnormalities in relation to the start of the LOS episode, we categorized infants by the day of first positive blood culture, relative to the day of death (Figure 1). Requirement for mechanical ventilation and mean FiO2 increased consistently on the day of the first positive blood culture and continued to rise through the day of death (day 0). Receipt of vasopressor medications tended to rise sharply on the start day of LOS episodes, although little additional increase in vasopressor support was observed on subsequent days. The prevalence of neutropenia also tended to increase on the day of the first positive blood culture. In contrast, changes in the prevalences of abnormal renal function and thrombocytopenia did not coincide closely with the start of the LOS episode.
Figure 1.
Cardiorespiratory support and laboratory abnormalities preceding death among infants with late-onset sepsis (LOS; n=679) according to start day of LOS episode.
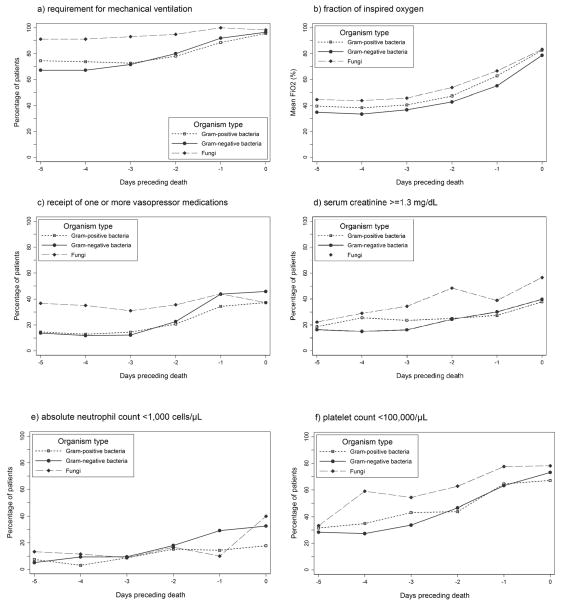
Measures of cardiorespiratory support and the prevalences of laboratory abnormalities on days −5 to 0 by organism type are shown in Figure 2. Requirement for mechanical ventilation differed at baseline (day −5) by organism type (P=0.001), with 91% of infants with fungal LOS episodes being mechanically ventilated compared with only 67% and 74% of infants with Gram-negative and Gram-positive bacterial LOS episodes, respectively. Infants with fungal LOS episodes were also more likely to be receiving vasopressor support at baseline than infants with bacterial LOS episodes (37% vs. 14%, P<0.001). Vasopressor support did not differ at baseline between infants with Gram-negative and Gram-positive bacterial LOS (P=0.78), but the proportion of infants receiving vasopressor medications increased more rapidly between days −3 and 0 for Gram-negative bacterial LOS (P=0.03). No significant differences in either the baseline prevalences of laboratory abnormalities or the rates of change of the proportion of infants with laboratory abnormalities between days −3 and 0 were observed between fungal and bacterial LOS episodes. The baseline prevalences of laboratory abnormalities also did not differ between infants with Gram-negative bacterial and Gram-positive bacterial LOS episodes. However, between days −3 and 0, the proportion of infants with thrombocytopenia (P=0.02) increased more rapidly in Gram-negative bacterial LOS compared with Gram-positive bacterial LOS.
Figure 2.
Cardiorespiratory support and laboratory parameters preceding death among infants with late-onset sepsis (n=679) by organism type.
DISCUSSION
We identified the presence and progression of multi-organ dysfunction in a large cohort of premature infants with fatal fulminant sepsis. The majority of fatal LOS episodes occurred in extremely low birth weight infants (<1000 g) during the first 30 days of life and with Gram-negative pathogens. Measures of cardiorespiratory support rose 2 days prior to death, while renal, immune, and hematologic dysfunction occurred most reliably on the day preceding death. Other investigations have identified risk factors for developing fatal sepsis during the birth hospitalization for premature infants. These studies focused on static infant and maternal associations at birth (gender, maternal age, 5-minute Apgar score4) and at the onset of sepsis (vasopressors and thrombocytopenia14). Our analysis of the dynamics of sepsis-associated organ dysfunction among hospitalized infants over the days preceding death adds another dimension to our understanding of the pathophysiologic response to sepsis in this population.
We could not find reports from any other patient population that chronicle the progression of organ failure with fatal sepsis as our study does. However, a comparison of the prevalence of organ dysfunction in our cohort with findings for adults and children with severe sepsis by point prevalence may reveal developmental differences in the host response to sepsis.7 The inaccuracy of the pediatric sepsis consensus definition in neonates15 and a lack of consensus on the definition of neonatal sepsis16 currently prevent a point-prevalence study in NICUs that would be highly informative.
Adjuvant treatments for sepsis have largely targeted enhancement of immune system function.17 Our results suggest that progressive impairment of respiratory function is very common with fatal LOS among hospitalized infants. Sepsis (or pneumonia) is the second most common cause of respiratory failure in premature infants after respiratory distress syndrome.18 Secondary surfactant deficiency contributes to acute respiratory morbidity in late-preterm and term neonates with pneumonia or sepsis, and it has been suggested that surfactant replacement may be beneficial for these infants.19,20 A pilot study of 20 premature infants (24–29 weeks’ gestational age) from 7 days to 3 months of age with respiratory decompensation and associated pneumonia or confirmed sepsis showed improvements in short-term respiratory parameters (FiO2, mean airway pressure, and respiratory severity scores) after surfactant administration. While appropriately powered studies are warranted, these studies suggest that respiratory failure associated with sepsis among hospitalized infants may be attenuated with surfactant replacement.21 It is also unknown if other respiratory rescue interventions such as inhaled nitric oxide or high-frequency ventilation can reduce the severity of respiratory morbidity in the setting of sepsis.
To our knowledge, this is the first published study that specifically chronicles the progression of clinical and laboratory characteristics of hospitalized infants in the NICU with fatal sepsis. Strengths of the study include the comprehensive accuracy of the electronic medical database, the large number of infants with fatal sepsis, and the number of NICUs represented. We did not have genus- and species-level identification for all recovered pathogens, which, if known, might have permitted a more complete analysis on pathogen-specific effects. We speculate that genus and species were likely to be missing in these cases because many infants may have died before these data were made available to the clinician. Previous reports have shown a higher rate of mortality with fungal and Gram-negative bacterial sepsis as compared to Gram-positive bacterial sepsis.3,6 Laboratory studies were performed at the discretion of the local clinicians, not on each day for each patient, which may mean that changes in parameters we identified may have occurred earlier. Inflammatory biomarkers that would further substantiate the diagnosis of sepsis or identify infants with fatal culture-negative sepsis for inclusion were not available. We cannot prove that the multi-organ dysfunction and death were the result of sepsis; however, the infants in the cohort manifested significant clinical and laboratory signs of sepsis including mortality within 3 days of a positive blood culture.
CONCLUSIONS
This is the first report that chronicles organ dysfunction among hospitalized infants with fatal fulminant LOS. Knowledge of the prevalence and timing of these signs may be important for the development and testing of novel therapeutics for sepsis in infants.
Acknowledgments
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR001117 and by the Best Pharmaceuticals for Children Act –Pediatric Trials Network (Government Contract HHSN275201000003I). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Dr. Benjamin Jr. receives support from the NIH (award 2K24HD058735-06, National Center for Advancing Translational Sciences award UL1TR001117, National Institute of Child Health and Human Development contract HHSN275201000003I, and National Institute of Allergy and Infectious Diseases contract HHSN272201500006I). Dr. Smith receives salary support for research from the NIH and the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the National Institute of Child Health and Human Development (HHSN275201000003I and 1R01-HD081044-01), and the Food and Drug Administration (1R18-FD005292-01). Dr. Wynn receives research support from the National Institute of General Medical Sciences (GM106143). The other authors have no financial disclosures relevant to this article.
Abbreviations
- ANC
absolute neutrophil count
- CoNS
coagulase-negative staphylococcus
- FiO2
fraction of inspired oxygen
- LOS
late-onset sepsis
- NICUs
neonatal intensive care units
Footnotes
Conflict of Interest
Drs. Benjamin Jr. and Smith receive research support from Cempra Pharmaceuticals (subaward to HHSO100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp).
References
- 1.Brocklehurst P, Farrell B, King A, et al. Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med. 2011;365(13):1201–1211. doi: 10.1056/NEJMoa1100441. [DOI] [PubMed] [Google Scholar]
- 2.Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88(Suppl 2):S69–S74. doi: 10.1016/S0378-3782(12)70019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitha A, Foix-L’Helias L, Arnaud C, et al. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics. 2013;132(2):E372–E380. doi: 10.1542/peds.2012-3979. [DOI] [PubMed] [Google Scholar]
- 4.Person MK, Esposito DH, Holman RC, Mehal JM, Stoll BJ. Risk factors for infectious disease death among infants in the United States. Pediatr Infect Dis J. 2014;33(11):e280–e285. doi: 10.1097/INF.0000000000000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 7.Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics. 2010;125(5):1031–1041. doi: 10.1542/peds.2009-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin Perinatol. 2010;37(2):439–479. doi: 10.1016/j.clp.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornik CP, Benjamin DK, Becker KC, et al. Use of the complete blood cell count in early-onset neonatal sepsis. Pediatr Infect Dis J. 2012;31(8):799–802. doi: 10.1097/INF.0b013e318256905c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):E214–E224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 11.Maheshwari A. Neutropenia in the newborn. Curr Opin Hematol. 2014;21(1):43–49. doi: 10.1097/MOH.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiedmeier SE, Henry E, Sola-Visner MC, Christensen RD. Platelet reference ranges for neonates, defined using data from over 47,000 patients in a multihospital healthcare system. J Perinatol. 2009;29(2):130–136. doi: 10.1038/jp.2008.141. [DOI] [PubMed] [Google Scholar]
- 13.Bateman DA, Thomas W, Parravicini E, Polesana E, Locatelli C, Lorenz JM. Serum creatinine concentration in very-low-birth-weight infants from birth to 34–36 wk postmenstrual age. Pediatr Res. 2015;77(5):696–702. doi: 10.1038/pr.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levit O, Bhandari V, Li FY, Shabanova V, Gallagher PG, Bizzarro MJ. Clinical and laboratory factors that predict death in very low birth weight infants presenting with late-onset sepsis. Pediatr Infect Dis J. 2014;33(2):143–146. doi: 10.1097/INF.0000000000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofer N, Zacharias E, Muller W, Resch B. Performance of the definitions of the systemic inflammatory response syndrome and sepsis in neonates. J Perinat Med. 2012;40(5):587–590. doi: 10.1515/jpm-2011-0308. [DOI] [PubMed] [Google Scholar]
- 16.Wynn JL, Wong HR, Shanley TP, Bizzarro MJ, Saiman L, Polin RA. Time for a neonatal-specific consensus definition for sepsis. Pediatr Crit Care Med. 2014;15(6):523–528. doi: 10.1097/PCC.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn JL, Neu J, Moldawer LL, Levy O. Potential of immunomodulatory agents for prevention and treatment of neonatal sepsis. J Perinatol. 2009;29(2):79–88. doi: 10.1038/jp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian L, Liu C, Zhuang W, et al. Neonatal respiratory failure: a 12-month clinical epidemiologic study from 2004 to 2005 in China. Pediatrics. 2008;121(5):E1115–E1124. doi: 10.1542/peds.2006-2426. [DOI] [PubMed] [Google Scholar]
- 19.Tan K, Lai NM, Sharma A. Surfactant for bacterial pneumonia in late preterm and term infants. Cochrane Database Syst Rev. 2012;2:CD008155. doi: 10.1002/14651858.CD008155.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Alkan S, Ozer EA, Ilhan O, Sutcuoglu S, Tatli M. Surfactant treatment for neonatal respiratory disorders other than respiratory distress syndrome. J Matern Fetal Neonatal Med. 2015;28(2):131–133. doi: 10.3109/14767058.2014.906575. [DOI] [PubMed] [Google Scholar]
- 21.Bissinger R, Carlson C, Michel Y, Dooley C, Hulsey T, Jenkins D. Secondary surfactant administration in neonates with respiratory decompensation. J Perinatol. 2008;28(3):192–198. doi: 10.1038/sj.jp.7211909. [DOI] [PubMed] [Google Scholar]