Opinion Statement
There are no standardized treatment protocols for pediatric non-infectious uveitis. Topical corticosteroids are the typical first-line agent, although systemic corticosteroids are used in intermediate, posterior and panuveitic uveitis. Corticosteroids are not considered to be long-term therapy due to potential ocular and systemic side effects. In children with severe and/or refractory uveitis, timely management with higher dose disease-modifying antirheumatic drugs (DMARDs) and biologic agents is important. Increased doses earlier in the disease course may lead to improved disease control and better visual outcomes. In general, methotrexate is the usual first-line steroid-sparing agent and given as a subcutaneous weekly injection at >0.5 mg/kg/dose or 10–15 mg/m2 due to better bioavailability. Other DMARDs, for instance mycophenolate, azathioprine, and cyclosporine are less common treatments for pediatric uveitis. Anti-tumor necrosis factor-alpha agents, primarily infliximab and adalimumab are used as second line agents in children refractory to methotrexate, or as first-line treatment in those with severe complicated disease at presentation. Infliximab may be given at a minimum of 7.5 mg/kg/dose every 4 weeks after loading doses, up to 20 mg/kg/dose. Adalimumab may be given up to 20 or 40 mg weekly. In children who fail anti-tumor necrosis factor-alpha agents, develop anti-tumor necrosis factor-alpha antibodies, experience adverse effects, or have difficulty with tolerance, there is less data available regarding subsequent treatment. Promising results have been noted with tocilizumab infusions every 2–4 weeks, abatacept monthly infusions and rituximab.
Keywords: Uveitis, Pediatric uveitis, Methotrexate, Juvenile Idiopathic Arthritis, Infliximab, Adalimumab
Introduction
Uveitis is a broad term for inflammation involving the eye. It is classified according to the location of the inflammatory process, either anterior, intermediate, posterior or panuveitis (1, 2). Uveitis can be secondary to an infectious etiology, such as tuberculosis, toxocara canis, toxoplasmosis, herpes virus, lyme, and syphilis (3). Ocular inflammation can also be associated with an underlying systemic condition, including juvenile idiopathic arthritis (JIA), sarcoidosis, tubulointerstitial nephritis and uveitis (TINU), inflammatory bowel disease, Vogt-Koyonagi-Harada (VKH) and Behcet’s disease (4). Frequently, however, uveitis is not associated with an underlying condition and is termed, “idiopathic” (4).
In pediatric rheumatology, JIA is the most commonly associated disease, and uveitis is typically anterior and bilateral. Pediatric uveitis accounts for 5–10% of patients with uveitis (5). Thorne et al. reported a prevalence of pediatric uveitis of 31 per 100,000 patients. Of 291 pediatric cases in this study, nearly 95% were non-infectious uveitis, and JIA was associated with 26.2% of these cases (6).
Prolonged intraocular inflammation can lead to significant visual impairment and blindness. Uveitis is estimated to cause 30,000 new cases of legal blindness each year (6). The problem is compounded in pediatric patients where there is often a delay in presentation and diagnosis. Furthermore, children commonly experience a chronic course with frequent remission and relapse that can lead to significant ocular morbidity (7). Ocular complications such as cataract, glaucoma, posterior synechiae, and band keratopathy occur in up to 50% of children, vision loss (visual acuity 20/50 or worse) occurs in up to 50% of children, and legal blindness (visual acuity 20/200 or worse) occurs in up to 25% (8–11). Early diagnosis and treatment can mitigate these complications and potentially reduce the burden of visual impairment and blindness.
Evidence suggests an environmental trigger in a genetically susceptible individual leads to a release of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-alpha) and interleukins (IL) (12–15). With this understanding, an immunomodulatory therapy approach is a useful strategy for the management of non-infectious uveitis. However, there are a lack of randomized controlled studies in the treatment of pediatric uveitis. Most evidence is based on expert opinion or clinical experience and management remains non-standardized (16). As a result, multi-disciplinary panels have proposed treatment algorithms in an effort to standardize care (17–19). Despite the lack of level one and two evidence, immunomodulatory therapy remains the most effective approach to control ocular inflammation, reduce exposure to systemic corticosteroids, and decrease the incidence of vision loss and blindness.
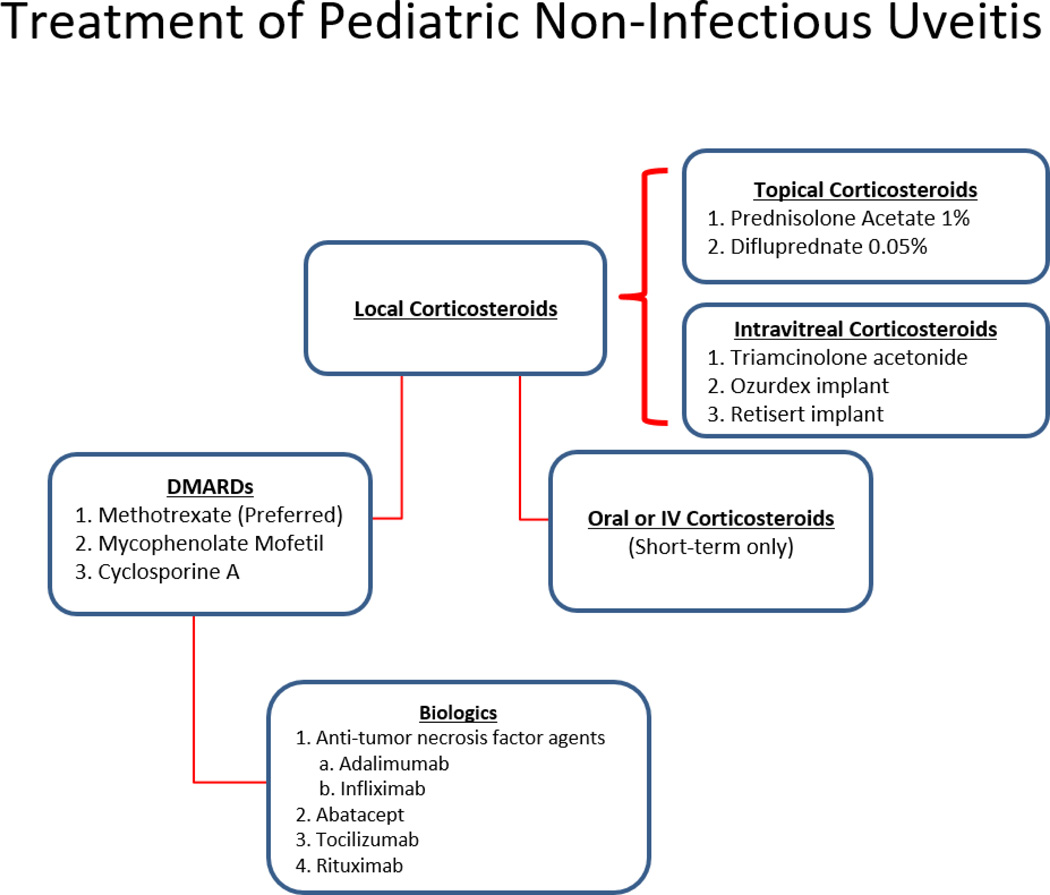
The focus of this review is to report the current treatment options for pediatric non-infectious uveitis. We will focus on medications described in pediatric uveitis patients enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRAnet) Registry, a large registry of North American pediatric rheumatology patients to examine practice patterns of pediatric rheumatologists (16). Furthermore, we suggest a stepwise approach to the use of immunomodulatory therapy, in particular JIA, which is the most common rheumatologic condition in childhood (Figure 1).
Figure 1.
Stepwise approach to the treatment of pediatric non-infectious uveitis. Topical corticosteroids are the typical first-line agent in anterior uveitis. Systemic corticosteroids may be used for rapid control of inflammation in intermediate, posterior or panuveitic uveitis. In children with severe and/or refractory uveitis, DMARDs are the next step in management with Methotrexate being the preferred agent. Anti TNF agents are used as second line agents in children refractory to methotrexate, or as a first-line treatment in children with severe and complicated disease at presentation. In patients who fail anti-TNF agents there is no agreement on subsequent therapy. Alternative biologics include abatacept, tocilizumab and rituximab. Many patients require a combination of medications or require short-term systemic corticosteroids for intermittent flares.
TREATMENT
Pharmacologic treatment
The goal for treatment of pediatric non-infectious uveitis is three-fold: 1) resolve intraocular inflammation 2) achieve remission and prevent recurrences and 3) preserve vision and prevent ocular complications (8). Corticosteroids (CS) are the first-line treatment for non-infectious uveitis and are effective for acute inflammation(2). The mechanism of action involves modulating gene expression of pro-inflammatory cytokines. CS can achieve this effect either through local or systemic administration and the mode of administration typically depends on location of uveitis(15). Local treatments include topical drops or periocular and intraocular injections or implants.
Local Corticosteroids
Topical corticosteroids are first-line treatment in anterior segment inflammation and were used in 90% of patients with idiopathic uveitis enrolled in the CARRAnet Registry (16). The most commonly used topical CS is prednisolonate acetate 1%, which is administered at a frequency corresponding to the severity of inflammation. Topical difluprednate 0.05% (Durezol) is a more potent medication with superior intraocular penetration and requires less frequent dosing (20). However, long-term use of topical CS, in particular Durezol, is associated with corticosteroid induced ocular hypertension and cataracts (21, 22). Evidence has shown that the need for >2 drops a day leads to increased ocular complications.
Periocular or intraocular injections of triamcinolone acetonide (TA) have been effective at treating intermediate, posterior and pan-uveitis by providing a high concentration of drug at the site of inflammation (20). While systemic side-effects are minimized, local TA injections in the pediatric population often require general anesthesia, which poses its own risk to the patient. Moreover, the relatively short duration of action necessitates frequent repeat injections (23). Longer duration intraocular therapy includes Dexamethasone implant (Ozurdex) and 0.59 mg Fluocinolone acetonide implant (Retisert)(23–28). Ozurdex implantation lasts 6 months whereas Retisert implantation lasts for nearly 3 years, which reduces the overall risk associated with anesthesia compared to repeat TA injections (24, 26). An emerging option is a 0.19mg Fluocinolone acetonide injectable (Illuvien) that was FDA approved for the management of diabetic retinopathy. Reddy et al. reported good outcomes on the off-label use of Illuvien in the management of uveitis and macular edema (29). While more studies are needed, especially in the pediatric population, the Illuvien implant is a promising medication that lasts for nearly 3 years and is significantly less expensive than Retisert. Suprachoroidal TA administration is a novel route that is currently under investigation as another local treatment option for non-infectious uveitis (30). As with all forms of local therapy, in particular periocular and intraocular CS, there is a high risk for cataract formation, elevated intraocular pressure and worsening of pre-existing glaucoma (30–33).
Systemic Corticosteroids
Systemic steroids are excellent for acute control of inflammation that is refractory to local therapy, especially in children with intermediate, posterior or pan-uveitis. Approximately 94% of children with idiopathic uveitis in the CARRA registry were treated with systemic steroids during the disease course (16). Oral prednisone can be prescribed at a starting dose of 1–2mg/kg body weight and tapered according to the inflammatory response. Intravenous (IV) corticosteroids are occasionally used in cases of severe ocular inflammation, including Behcet’s disease, serpiginous choroiditis, or uveitis with optic nerve involvement (2). However, long-term use of systemic steroids has potential adverse effects in the pediatric patient that include growth retardation, hyperglycemia, weight gain, hypertension, osteoporosis, peptic ulcers, cataracts and glaucoma (23, 24).
Topical and systemic corticosteroids are not considered long-term treatment of chronic uveitis and there should not be a delay in initiating DMARDs or biologics.
Immunosuppressive Therapy
Due to steroid toxicity, it is prudent to initiate steroid sparing disease modifying anti-rheumatic drugs (DMARDs) and biologics early in the disease course to control ocular inflammation and prevent complications and vision loss (15). Options for long-term immunosuppression include first-line conventional immunotherapy with methotrexate (MTX), and less commonly, mycophenolate mofetil (MMF) and cyclosporine A (CsA).
Methotrexate
Methotrexate was used to treat 76% of patients with idiopathic juvenile uveitis enrolled in the CARRA Registry making it the most frequently used steroid-sparing agent (16). There are several reports of its use in pediatric patients with uveitis (34–38). MTX is a folic-acid analogue that inhibits DNA replication and RNA transcription in B and T lymphocytes (2). The recommended starting dose is 10–15 mg/m2 once a week orally or subcutaneously. Dosing can be increased to 30 mg/m2 if administered by the subcutaneous route. There is a lack of evidence for preferred administration route. However, parenteral administration achieved higher efficacy and bioavailability in adult patients with rheumatoid arthritis and pediatric patients with JIA (39–43). Foeldvari et al. reported good outcomes in JIA-associated uveitis (JIA-U) wherein 84% (21/25) of patients achieved remission after a mean treatment dose of 15.6 mg/m2 (range 10–25 mg/m2) for an average of 4.25 months (range 1–12) (37). A recent meta-analysis suggests MTX is effective in controlling inflammation in pediatric non-infectious uveitis wherein the proportion of responding subjects in nine eligible studies was 0.73 (95% Confidence interval 0.66 – 0.1)(44). Common side effects associated with MTX in children include gastrointestinal (GI) toxicity (oral ulcers, nausea, vomiting) and hepatorenal toxicity. Patients must have routine lab work to evaluate blood count and liver function (AST/ALT). In addition, folic acid should be supplemented on a daily basis to prevent GI related toxicity.
Methotrexate is considered first-line therapy in most patients with chronic non-infectious uveitis and can be effective treatment for ocular inflammation.
Mycophenolate Mofetil
MMF inhibits inosine monophosphate dehydrogenase, which is an enzyme critical to the de novo pathway of purine synthesis (45). Unlike other cell types, lymphocytes lack alternative pathways for purine synthesis and are therefore selectively suppressed by MMF (2). The optimal dose for uveitis is unknown, but most pediatric rheumatologists initiate therapy at dosages used in pediatric renal transplant recipients – 600mg/m2 twice daily or 2–3 grams/day in divided doses. To date, there are only two reports of MMF therapy in pediatric uveitis (46, 47). Chang et al. reported on 52 children with autoimmune uveitis treated with MMF monotherapy wherein 73% achieved uveitis control after 2 months. Approximately 60% (16/25) of patients with JIA-U achieved quiescence for at least 2 years of therapy. Furthermore, visual acuity was stable or improved in 94% (47). MMF may be useful in the management of pediatric non-infectious uveitis, although less in JIA-U since it may not be as effective for arthritis compared to other agents. Side-effects include hair loss, fatigue, gastrointestinal discomfort and leukopenia (2, 15).
Cyclosporine A
CsA belongs to the class of calcineurin inhibitors (CNIs) whose mechanism is to block the activity of calcineurin, an enzyme that facilitates gene expression of IL-2 and other cytokines (48). Down-regulation of these molecules impairs T-cell proliferation resulting in immunosuppression. There are only a few studies reporting outcomes with CsA in pediatric non-infectious uveitis (49–52). Tappeiner et al. reported on 82 patients with JIA-U, the largest retrospective series to date. CsA monotherapy had minimal efficacy in this study, with only 6/25 (24%) achieving inactivity. Efficacy increased to 48.6% (35/72) when combined with other immunosuppressive medications, suggesting the optimal role for CsA in treating pediatric non-infectious uveitis is as adjunct therapy (52). Pediatric dosing of CsA is recommended between 2.5 to 5mg/kg/day in divided doses to avoid systemic side-effects (53). Nephrotoxicity, hypertension, hepatotoxicity, anemia, nausea, vomiting, hirsutism and hypercholesterolemia are potential side effects (15). Routine testing should include renal and liver function, and blood pressure monitoring (2).
CsA is infrequently used to treat pediatric non-infectious uveitis, and other agents are preferred.
Biologics
Biologics represent a newer class of medications used to treat ocular inflammation. These agents target molecules in the immune system that play a key role in the ocular inflammatory process(54). For example, TNF alpha, interleukins (IL) and interferons are pro-inflammatory cytokines produced by immune cells and are consistently elevated in serum and aqueous fluid of patients with uveitis(55, 56). Experimental auto-immune uveitis studies in animals also confirm the role of TNF alpha in mediating intraocular inflammation (57–59). Therefore, targeting these molecules with biologic therapy appears to be an attractive approach for long-term control of uveitis refractory to corticosteroids or conventional immunotherapy. Therapeutic options within the class of biologics includes anti-TNF alpha agents, anti-IL agents and anti-B and T lymphocyte agents (19). Screening for hepatitis and tuberculosis before initiation is important. Anti-TNF alpha agents, specifically infliximab (IFX) and adalimumab (ADA) have been most commonly used with excellent success (60)
Anti-tumor necrosis factor agents
Infliximab
Infliximab is a chimeric (75% humanized, 25% mouse) monoclonal antibody that binds to and inhibits circulating and membrane bound TNF alpha (61). It is FDA approved for several auto-immune conditions, including rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis and inflammatory bowel disease (54). Numerous prospective and retrospective studies support IFX’s use for non-infectious uveitis, but dosage and interval vary (62–67). Kahn et al. reported on 17 patients with refractory uveitis (10 JIA-U, 2 VKH, 2 sarcoidosis, 3 idiopathic) treated with 10–20mg/kg of IFX every 4–8 weeks (64). Intraocular inflammation was undetectable after the first or second infusion in 76% (13/17). High-dose IFX was effective and well-tolerated in all patients in this series. Ardoin et al. reported on a cohort of 16 children with uveitis (5 JIA-U, 11 idiopathic) treated with 8.2mg/kg median dose (range 2.0 to 12.9) of IFX at median frequency of 5.6 weeks (range 4 to 10). At 1 year, 64% had no active inflammation, and increased to 79% if criteria was modified to include zero inflammation or a 2-step decrease in AC inflammation. Furthermore, all patients were on topical CS at baseline, whereas by 12 months approximately 69% (11/16) of patients discontinued use (67). Sukumaran et. al. compared varied doses of IFX: low-dose (< 10 mg/kg), moderate dose (≥ 10–15 mg/kg) and high dose (≥ 15–20 mg/kg) every 4 weeks in 34 children with refractory non-infectious uveitis (68). After one year, there was significant improvement in anterior chamber cells, flare, and VA in all groups. However, escalated doses may be necessary to achieve disease control since > 35% required an increase in dose to ≥ 10 mg/kg, and 94% were on high dose at end of study.
IFX is administered intravenously with a loading dose of 3–5 mg/kg at weeks 0, 2 and 6 followed by maintenance dosing at 3–10 mg/kg every 4–8 weeks. Additionally, methotrexate is recommended as an adjunct to prevent the formation of anti-TNF antibodies. In refractory uveitis, an increased dose of 20mg/kg has been used successfully (64, 68, 69). Potential side effects include, increased susceptibility to infections, reactivation of tuberculosis or histoplasmosis, malignancy and development of lupus-like syndrome (2, 54). Mild and moderate infusion reactions occurred in 17% of patients in one study (70).
IFX can be an effective treatment for uveitis but is often needed at high doses. There has been variability in the dosing and interval of IFX administration which may have led to the differences in patient outcomes.
Adalimumab
Adalimumab (ADA) is a fully humanized monoclonal antibody that also binds to and inhibits TNF alpha and is the only US Food and Drug Administration (FDA) approved non-corticosteroid medication for the treatment of non-infectious uveitis (71). Jaffe et al. recently published results from a multi-national phase 3 randomized controlled trial assessing the efficacy and safety of ADA for adults with non-infectious intermediate, posterior or panuveitic uveitis (72). All 217 patients had active, vision threatening, non-infectious uveitis and were randomly assigned to ADA 80-mg dose at baseline followed by 40-mg doses every 2 weeks (110 patients) or placebo (107 patients). They described a significantly lower risk of treatment failure compared to placebo wherein treatment failure occurred at 24 weeks in the ADA group and 13 weeks in the placebo group (HR, 0.50; 99% confidence interval, 0.36 to 0.70; P < 0.001).
Nguyen et. Al. reported on 229 patients with inactive uveitis on 10–35 mg/day of oral prednisone to maintain uveitis inactivity (73). They who were randomly assigned to ADA (115) or placebo (114 patients). Prednisone was tapered to 0 mg by week 19. Treatment failure was increased in the placebo group (61 (55%)) compared to the ADA group (45 (39%)), and there was significant improvement in time to treatment failure in patients receiving ADA.
Several prospective and retrospective studies support ADA treatment for pediatric uveitis (74–84). Diaz-Llopis et al. reported a prospective multi-center study of 131 patients (39 with JIA-U) treated with ADA for 6 months (75). Children received 40mg of ADA subcutaneously (SC) every other week (children between ages 4–12 received 24 mg/m2 body surface area up to maximum dose of 40mg SC every other week). There was a statistically significant decrease in anterior chamber and vitreous inflammation and overall gain in visual acuity (75).
There are reports on the development of antibodies against ADA (AAA) in other diseases, leading to decreased drug efficacy and adverse effects. Recently, Cordero-Coma described 25 patients (2 JIA-U) with active refractory uveitis who were followed prospectively for 6 months (85). At least 3 trough serum ADA and AAA were measured prior to ADA administration. Only 11 (44%) had a complete clinical response, 7 (28%) had a partial response, and 7 (25%) no response. There were increased ADA levels in responders, and an association between worse uveitis outcome in patients with permanent AAA (4 (14%)). Development of AAA was not associated with use of other immunosuppressive agents which is interesting since methotrexate in often used in conjunction with IFX for JIA. The relevance of measurement of ADA trough levels and AAA remains unclear.
ADA is administered subcutaneously at a dose of 24mg/m2 up to maximum dose of 40mg every 2 weeks. In children with severe uveitis, dose and interval of administration can be increased to 40 mg weekly to increase efficacy. Side effects are similar to IFX, including increased susceptibility to infections and reactivation of TB or histoplasmosis (2).
Reports on the successful use of ADA in pediatric uveitis and ease of administration at home versus infusions make it an excellent option for many families.
Golimumab
There have been limited reports on Golimumab, a fully humanized anti-TNF monoclonal antibody that is administered subcutaneously every 4 weeks. Miserocchi et. al. examined 13 patients with JIA-U and 4 with HLA-B27 spondyloarthropathy who had an inadequate response to other TNF-alpha blockers or other biologics such as rituximab and abatacept (86). Patients received golimumab 50 mg every 4 weeks as subcutaneous injections. They were all ≥ 18 years of age and had anterior uveitis or panuveitis. Response was evident in 14 patients, wherein 2 of the non-responders had JIA-U. At last visit, 12 of 17 had inactive disease. This is a promising anti-TNF therapy for uveitis patients.
Emerging therapies
Abatacept
Abatacept (ABA) is a soluble fusion protein composed of human cytotoxic T lymphocyte antigen 4 (CTLA-4) and modified FC domain of human IgG. It binds to CD80/CD86 on antigen-presenting cells and prevents activation of T-cells(87). Abatacept is FDA approved for JIA and RA, but off-label use in pediatric and adult uveitis has been reported (87–91). In 2008, we first reported on a 16-year-old female with psoriatic arthritis with long-standing uveitis that was refractory to conventional IMT and biologics, including trials of rituximab, IFX, etanercept and daclizumab. Abatacept infusions were started at 10mg/kg. Ocular inflammation rapidly improved and remained well-controlled 18 months after initiation of therapy (87). A recent case series described 3 children with idiopathic uveitis that was successfully controlled with Abatacept (89). However, Tappeiner et al. reported on the visual outcomes in 21 patients with JIA-U (90). All patients had severe and chronic uveitis with mean duration of 7.5 years and were treated with 10 mg/kg, max 750 mg every 4 weeks. Patients had previously failed tCS, systemic CS, methotrexate and several biologics (IFX, etanercept, ritixuimab, golimumab). Uveitis inactivity was achieved in 11 patients after starting ABA (median time of 6 months), but recurred in 8 of these patients. Abatacept failed to suppress inflammation in 10/21 (48%) of patients. In addition, new onset complications occurred in 3, and best corrected visual acuity (BCVA) did not significantly improve. Hence, ABA did not appear to be effective in this cohort compared to earlier studies. Birolo et al. compared ABA as a first-line or second-line biologic agent in 31 patients with severe JIA-U after 12 months of follow-up (92). Fourteen received ABA as first-line biologic therapy (at least 6 months of MTX) and 17 as a second-line biologic agent. Clinical remission was achieved in 54.8% of the total patients (17/31), but there was no significant difference in improvement in those who received ABA as first-line (57%) vs. second-line (52.9) (92). Dosing is 10mg/kg at 0 and 15 days followed by monthly administration (2). Side effects include infections, gastrointestinal disorders and low risk for malignancy (2, 20).
There are reports on the use of Abatacept in children with uveitis, but attainment of remission varied.
Tocilizumab
Interleukin-6 (IL-6) is a cytokine produced by T-cells, B-cells and monocytes. Signaling through IL-6 helps appears to play a critical role in the immune system, including T-cell activation, immunoglobulin secretion and angiogenesis(93, 94). The importance of IL-6 in mediating ocular inflammation has also been demonstrated in animal models of experimental uveitis that show that treatment with anti-IL6 receptor antibody reduces uveitic inflammation (95). Furthermore, elevated IL-6 concentrations were noted in vitreous fluid of patients with VKH, Behcet’s, sarcoidosis, and idiopathic uveitis compared to controlled non-uveitic patients(95). Therefore, targeting IL-6 appears to be an attractive approach.
Tocilizumab (TCZ) is an IL-6 receptor antibody approved for RA and JIA and is an emerging treatment for uveitis and cystoid macular edema (93, 94, 96–99). It is a humanized recombinant antibody that binds the IL-6 receptor, leading to inhibition of the downstream signaling of IL-6 and proinflammatory effects. Tappeiner et al. reported on 17 patients treated with TCZ for chronic refractory JIA-U, including systemic corticosteroids, DMARDs and 1 TNF-alpha inhibitor (97). Patients were treated with intravenous TCZ 8mg/kg body weight at 4 week intervals and were followed for a mean of 8.5 months. Inactive uveitis was observed in 10/17 patients after at least 1 follow up, but there was a recurrence in 3. In the 7 patients who did not respond to TCZ, 4 had improved ocular activity, 2 had no change and 1 worsened. Additionally, 4 patients experienced new ocular complications during treatment, and there was no significant change in BCVA at the end of follow-up. There were no adverse events requiring discontinuation of TCZ in this study. In 2016, Silpa-archa et al. reported their experience with Tocilizumab in 17 patients with recalcitrant ocular inflammatory disease (10 uveitis, 6 scleritis, 1 orbital pseudotumor) (94). In the uveitis cohort, 5 patients (age range 14–50 years) had inflammation associated with JIA. After 9 months of treatment, 71% of uveitis and 50% of scleritis patients achieved the primary outcome: absence of inflammation and achievement of steroid sparing. However, serious side effects requiring cessation of therapy were reported in 24% of patients, including neutropenia, severe angioedema, dizziness and abdominal pain. Recently, Calvo-Rio et al. reported on the use of TCZ in 25 patients with JIA-U refractory to conventional IMT and at least one biologic agent including anti-TNF-alpha drugs, abatacept, rituximab, and anakinra (99). Dosing was 8 mg/kg IV every 2, 4 or 8 weeks or 2.9 mg/kg/subcutaneously every week. At 6 months of follow up in 23 patients, BCVA improved and anterior chamber cells decreased in 79%. This was consistent at 1 year in 15/17 patients (88%). Clinical trials evaluating the efficacy of TCZ in noninfectious intermediate, posterior or panuveitis (STOP-Uveitis) and JIA-U are on-going (www.clinicaltrials.gov)(93).
TCZ is a promising treatment for pediatric uveitis, but additional studies are needed to determine optimal dosing and intervals.
Rituximab
Ritixumab is a chimeric antibody directed against the B-cell marker CD20 thereby inducing B-cell apoptosis(100). Ritixumab initially gained FDA approval in 1997 for the treatment of lymphoma and leukemia(101). Since then, the medication has been approved for RA, microscopic polyangiitis and granulomatosis with polyangiitis(54). Rituximab’s use in ocular conditions has been limited, but several reports have shown efficacy for the management of scleritis, peripheral ulcerative keratitis, VKH and more recently, in JIA-U (102–105). Miserocchi et al. reported on a cohort of 8 patients (15 eyes) with severe JIA-U treated with rituximab who were previously resistant to IMT and TNF alpha inhibitors (106). The mean follow-up time on rituximab was 44.75 months with a mean number of 8.75 infusions. Uveitis was inactive in all patients at last follow-up, and improvement was noted 4 months after the first infusion. Mean systemic CS dose before initiating rituximab was 18mg/day and decreased to 1.8mg/day at final follow-up, with discontinuation in 6 patients. Similarly, conventional IMT was discontinued in 5/8 patients, and reduced from MTX + CSA to only MTX in 1 patient. The 2 remaining patients continued with baseline MTX throughout rituximab treatment. These are promising results for refractory uveitis associated with JIA, but further prospective trials need to be conducted to assess the efficacy of rituximab in pediatric uveitis.
Ritixumab is given intravenously and dosed at 1000 mg on days 1 and 14 and repeated after 6 months if needed. Effect on B-cells lasts 6 to 9 months making this an attractive biologic compared to anti-TNF alpha agents that require more frequent dosing (107). Side effects include infusion reactions, which can be mitigated with corticosteroids, as well as neutropenia, heart failure and rarely progressive multifocal leukoencephalopathy.
Future Directions
Several challenges exist in the timely and optimal treatment of children with uveitis. There are a lack of randomized controlled trials, and adalimumab is the only FDA approved drug for non-infectious intermediate, posterior and panuveitic uveitis in adults. Thus, treatment is usually guided by collective expertise, case reports/case series, retrospective studies, and panel guidelines (17–19).
Advances have emerged in the study of biomarkers for early detection of disease, indicators of active disease, and targeted therapy. Potential biomarkers associated with pediatric chronic anterior uveitis and JIA-associated uveitis were detected in the iris, serum, and aqueous humor, but most studies have been conducted in adults with varied involvement and associated diseases. Alterations in the inflammatory cells, autoantibodies, cytokines, chemokines and soluble adhesion molecules have been reported, most recently erythrocyte sedimentation rate (ESR), plasma cells, interleukin-29/interferon-λ1, S100A8/A9, and S100A12 (108–118). The analysis of tear fluid for biomarkers in chronic anterior non-infectious uveitis is a potential non-invasive collection method (119–121). With further developments in research and science, the potential exists to utilize markers to inform clinicians of optimal therapy choice as we gain more insight into promising drug targets.
Conclusions
Chronic pediatric non-infectious uveitis can lead to severe ocular complication and permanent vision loss. Timely and optimal management improves visual outcomes. There are no standardized treatments and few randomized controlled trials for pediatric uveitis. We report on conventional DMARDs and anti-tumor necrosis factor agents, but also discuss newer biologics for children with refractory uveitis. Additional studies are needed to determine optimal treatment and dose in this population.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Arjun B. Sood, MD and Sheila T. Angeles-Han, MD, MSc declare that they have no conflict of interest.
Dr. Angeles-Han was supported by Award Number K23EY021760 from the National Eye Institute and also by a grant from the American College of Rheumatology Research and Education Foundation Career Development Bridge Funding Award. However, these did not support this study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. American journal of ophthalmology. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonini G, Cantarini L, Bresci C, Lorusso M, Galeazzi M, Cimaz R. Current therapeutic approaches to autoimmune chronic uveitis in children. Autoimmunity reviews. 2010;9(10):674–683. doi: 10.1016/j.autrev.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Dhoot DS, Martin DF, Srivastava SK. Pediatric infectious posterior uveitis. International ophthalmology clinics. 2011;51(1):113–128. doi: 10.1097/IIO.0b013e318200e0ed. [DOI] [PubMed] [Google Scholar]
- 4.Kim SJ. Diagnosis and management of noninfectious pediatric uveitis. International ophthalmology clinics. 2011;51(1):129–145. doi: 10.1097/IIO.0b013e318200e01b. [DOI] [PubMed] [Google Scholar]
- 5.Levy-Clarke GA, Nussenblatt RB, Smith JA. Management of chronic pediatric uveitis. Current opinion in ophthalmology. 2005;16(5):281–288. doi: 10.1097/01.icu.0000177414.79030.32. [DOI] [PubMed] [Google Scholar]
- 6. Thorne JE, Suhler E, Skup M, Tari S, Macaulay D, Chao J, et al. Prevalence of Noninfectious Uveitis in the United States: A Claims-Based Analysis. JAMA ophthalmology. 2016 doi: 10.1001/jamaophthalmol.2016.3229. Provides information of the epidemiology of uveitis in the United States
- 7.Holland GN, Denove CS, Yu F. Chronic anterior uveitis in children: clinical characteristics and complications. American journal of ophthalmology. 2009;147(4):667–678.e5. doi: 10.1016/j.ajo.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Gregory AC, 2nd, Kempen JH, Daniel E, Kacmaz RO, Foster CS, Jabs DA, et al. Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the Systemic Immunosuppressive Therapy for Eye Diseases Study. Ophthalmology. 2013;120(1):186–192. doi: 10.1016/j.ophtha.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angeles-Han ST, Rabinovich CE. Uveitis in children. Current opinion in rheumatology. 2016;28(5):544–549. doi: 10.1097/BOR.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitale AT, Graham E, de Boer JH. Juvenile idiopathic arthritis-associated uveitis: clinical features and complications, risk factors for severe course, and visual outcome. Ocular immunology and inflammation. 2013;21(6):478–485. doi: 10.3109/09273948.2013.815785. [DOI] [PubMed] [Google Scholar]
- 11.Edelsten C, Lee V, Bentley CR, Kanski JJ, Graham EM. An evaluation of baseline risk factors predicting severity in juvenile idiopathic arthritis associated uveitis and other chronic anterior uveitis in early childhood. The British journal of ophthalmology. 2002;86(1):51–56. doi: 10.1136/bjo.86.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hersh AO, Prahalad S. Immunogenetics of juvenile idiopathic arthritis: A comprehensive review. Journal of autoimmunity. 2015;64:113–124. doi: 10.1016/j.jaut.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angeles-Han S, Prahalad S. The genetics of juvenile idiopathic arthritis: what is new in 2010? Current rheumatology reports. 2010;12(2):87–93. doi: 10.1007/s11926-010-0087-0. [DOI] [PubMed] [Google Scholar]
- 14.Glass DN, Giannini EH. Juvenile rheumatoid arthritis as a complex genetic trait. Arthritis and rheumatism. 1999;42(11):2261–2268. doi: 10.1002/1529-0131(199911)42:11<2261::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Cantarini L, Simonini G, Frediani B, Pagnini I, Galeazzi M, Cimaz R. Treatment strategies for childhood noninfectious chronic uveitis: an update. Expert opinion on investigational drugs. 2012;21(1):1–6. doi: 10.1517/13543784.2012.636350. [DOI] [PubMed] [Google Scholar]
- 16. Henderson LA, Zurakowski D, Angeles-Han ST, Lasky A, Rabinovich CE, Lo MS. Medication use in juvenile uveitis patients enrolled in the Childhood Arthritis and Rheumatology Research Alliance Registry. Pediatric rheumatology online journal. 2016;14(1):9. doi: 10.1186/s12969-016-0069-5. Provides information on the practice patterns of the majority of pediatric rheumatologists in North America for pediatric non-infectious uveitis
- 17.Heiligenhaus A, Michels H, Schumacher C, Kopp I, Neudorf U, Niehues T, et al. Evidence-based, interdisciplinary guidelines for anti-inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Rheumatology international. 2012;32(5):1121–1133. doi: 10.1007/s00296-011-2126-1. [DOI] [PubMed] [Google Scholar]
- 18.Bou R, Adan A, Borras F, Bravo B, Calvo I, De Inocencio J, et al. Clinical management algorithm of uveitis associated with juvenile idiopathic arthritis: interdisciplinary panel consensus. Rheumatology international. 2015;35(5):777–785. doi: 10.1007/s00296-015-3231-3. [DOI] [PubMed] [Google Scholar]
- 19.Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, Van Gelder RN. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology. 2014;121(3):785–796.e3. doi: 10.1016/j.ophtha.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 20.Mehta PJ, Alexander JL, Sen HN. Pediatric uveitis: new and future treatments. Current opinion in ophthalmology. 2013;24(5):453–462. doi: 10.1097/ICU.0b013e3283641ede. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorne JE, Woreta FA, Dunn JP, Jabs DA. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology. 2010;117(7):1436–1441. doi: 10.1016/j.ophtha.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slabaugh MA, Herlihy E, Ongchin S, van Gelder RN. Efficacy and potential complications of difluprednate use for pediatric uveitis. American journal of ophthalmology. 2012;153(5):932–938. doi: 10.1016/j.ajo.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Tomkins-Netzer O, Talat L, Seguin-Greenstein S, Bar A, Lightman S. Outcome of Treating Pediatric Uveitis With Dexamethasone Implants. American journal of ophthalmology. 2016;161:110–115.e1-2. doi: 10.1016/j.ajo.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 24.Bratton ML, He YG, Weakley DR. Dexamethasone intravitreal implant (Ozurdex) for the treatment of pediatric uveitis. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus. 2014;18(2):110–113. doi: 10.1016/j.jaapos.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Patel CC, Mandava N, Oliver SC, Braverman R, Quiroz-Mercado H, Olson JL. Treatment of intractable posterior uveitis in pediatric patients with the fluocinolone acetonide intravitreal implant (Retisert) Retina (Philadelphia, Pa) 2012;32(3):537–542. doi: 10.1097/IAE.0b013e31822058bb. [DOI] [PubMed] [Google Scholar]
- 26.Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Louis TA, Sugar EA, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011;118(10):1916–1926. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sella R, Oray M, Friling R, Umar L, Tugal-Tutkun I, Kramer M. Dexamethasone intravitreal implant (Ozurdex(R)) for pediatric uveitis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2015;253(10):1777–1782. doi: 10.1007/s00417-015-3124-x. [DOI] [PubMed] [Google Scholar]
- 28.Taylor SR, Tomkins-Netzer O, Joshi L, Morarji J, McLoone E, Lightman S. Dexamethasone implant in pediatric uveitis. Ophthalmology. 2012;119(11):2412-.e2. doi: 10.1016/j.ophtha.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 29.Reddy AK, Burkholder BM, Khan IR, Thorne JE. Iluvien Implantation for Uveitis and Uveitic Macular Edema. Ocular immunology and inflammation. 2016:1–2. doi: 10.1080/09273948.2016.1215472. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein DA, Do D, Noronha G, Kissner JM, Srivastava SK, Nguyen QD. Suprachoroidal Corticosteroid Administration: A Novel Route for Local Treatment of Noninfectious Uveitis. Translational vision science & technology. 2016;5(6):14. doi: 10.1167/tvst.5.6.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bollinger KE, Smith SD. Prevalence and management of elevated intraocular pressure after placement of an intravitreal sustained-release steroid implant. Current opinion in ophthalmology. 2009;20(2):99–103. doi: 10.1097/icu.0b013e32831d7f3a. [DOI] [PubMed] [Google Scholar]
- 32.Rhee DJ, Peck RE, Belmont J, Martidis A, Liu M, Chang J, et al. Intraocular pressure alterations following intravitreal triamcinolone acetonide. The British journal of ophthalmology. 2006;90(8):999–1003. doi: 10.1136/bjo.2006.090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye (London, England) 2006;20(4):407–416. doi: 10.1038/sj.eye.6701895. [DOI] [PubMed] [Google Scholar]
- 34.Chan AY, Liu DT. Methotrexate and chronic uveitis associated with juvenile idiopathic arthritis. The Journal of rheumatology. 2006;33(1):198. author reply. [PubMed] [Google Scholar]
- 35.Weiss AH, Wallace CA, Sherry DD. Methotrexate for resistant chronic uveitis in children with juvenile rheumatoid arthritis. The Journal of pediatrics. 1998;133(2):266–268. doi: 10.1016/s0022-3476(98)70232-x. [DOI] [PubMed] [Google Scholar]
- 36.Heiligenhaus A, Mingels A, Heinz C, Ganser G. Methotrexate for uveitis associated with juvenile idiopathic arthritis: value and requirement for additional anti-inflammatory medication. European journal of ophthalmology. 2007;17(5):743–748. doi: 10.1177/112067210701700509. [DOI] [PubMed] [Google Scholar]
- 37.Foeldvari I, Wierk A. Methotrexate is an effective treatment for chronic uveitis associated with juvenile idiopathic arthritis. The Journal of rheumatology. 2005;32(2):362–365. [PubMed] [Google Scholar]
- 38.Malik AR, Pavesio C. The use of low dose methotrexate in children with chronic anterior and intermediate uveitis. The British journal of ophthalmology. 2005;89(7):806–808. doi: 10.1136/bjo.2004.054239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braun J, Kastner P, Flaxenberg P, Wahrisch J, Hanke P, Demary W, et al. Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis: results of a six-month, multicenter, randomized, double-blind, controlled, phase IV trial. Arthritis and rheumatism. 2008;58(1):73–81. doi: 10.1002/art.23144. [DOI] [PubMed] [Google Scholar]
- 40.Hoekstra M, Haagsma C, Neef C, Proost J, Knuif A, van de Laar M. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31(4):645–648. [PubMed] [Google Scholar]
- 41.Herman RA, Veng-Pedersen P, Hoffman J, Koehnke R, Furst DE. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989;78(2):165–171. doi: 10.1002/jps.2600780219. [DOI] [PubMed] [Google Scholar]
- 42.Zuber Z, Turowska-Heydel D, Sobczyk M, Banach-Gornicka M, Rusnak K, Piszczek A, et al. Methotrexate efficacy and tolerability after switching from oral to subcutaneous route of administration in juvenile idiopathic arthritis. Reumatologia. 2016;54(1):19–23. doi: 10.5114/reum.2016.58757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franova J, Fingerhutova S, Kobrova K, Srp R, Nemcova D, Hoza J, et al. Methotrexate efficacy, but not its intolerance, is associated with the dose and route of administration. Pediatric rheumatology online journal. 2016;14(1):36. doi: 10.1186/s12969-016-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simonini G, Paudyal P, Jones GT, Cimaz R, Macfarlane GJ. Current evidence of methotrexate efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach. Rheumatology (Oxford, England) 2013;52(5):825–831. doi: 10.1093/rheumatology/kes186. Reviews the literature and provides information on the use of methotrexate
- 45.Budde K, Glander P, Bauer S, Braun K, Waiser J, Fritsche L, et al. Pharmacodynamic monitoring of mycophenolate mofetil. Clinical chemistry and laboratory medicine. 2000;38(11):1213–1216. doi: 10.1515/CCLM.2000.191. [DOI] [PubMed] [Google Scholar]
- 46.Doycheva D, Deuter C, Stuebiger N, Biester S, Zierhut M. Mycophenolate mofetil in the treatment of uveitis in children. The British journal of ophthalmology. 2007;91(2):180–184. doi: 10.1136/bjo.2006.094698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang PY, Giuliari GP, Shaikh M, Thakuria P, Makhoul D, Foster CS. Mycophenolate mofetil monotherapy in the management of paediatric uveitis. Eye (London, England) 2011;25(4):427–435. doi: 10.1038/eye.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anglade E, Yatscoff R, Foster R, Grau U. Next-generation calcineurin inhibitors for ophthalmic indications. Expert opinion on investigational drugs. 2007;16(10):1525–1540. doi: 10.1517/13543784.16.10.1525. [DOI] [PubMed] [Google Scholar]
- 49.Walton RC, Nussenblatt RB, Whitcup SM. Cyclosporine therapy for severe sight-threatening uveitis in children and adolescents. Ophthalmology. 1998;105(11):2028–2034. doi: 10.1016/S0161-6420(98)91120-4. [DOI] [PubMed] [Google Scholar]
- 50.Kilmartin DJ, Forrester JV, Dick AD. Cyclosporin A therapy in refractory non-infectious childhood uveitis. The British journal of ophthalmology. 1998;82(7):737–742. doi: 10.1136/bjo.82.7.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotaniemi K. Late onset uveitis in juvenile-type chronic polyarthritis controlled with prednisolone, cyclosporin A and methotrexate. Clinical and experimental rheumatology. 1998;16(4):469–471. [PubMed] [Google Scholar]
- 52.Tappeiner C, Roesel M, Heinz C, Michels H, Ganser G, Heiligenhaus A. Limited value of cyclosporine A for the treatment of patients with uveitis associated with juvenile idiopathic arthritis. Eye (London, England) 2009;23(5):1192–1198. doi: 10.1038/eye.2008.174. [DOI] [PubMed] [Google Scholar]
- 53.Feutren G, Mihatsch MJ. Risk factors for cyclosporine-induced nephropathy in patients with autoimmune diseases. International Kidney Biopsy Registry of Cyclosporine in Autoimmune Diseases. The New England journal of medicine. 1992;326(25):1654–1660. doi: 10.1056/NEJM199206183262502. [DOI] [PubMed] [Google Scholar]
- 54.Pasadhika S, Rosenbaum JT. Update on the use of systemic biologic agents in the treatment of noninfectious uveitis. Biologics : targets & therapy. 2014;8:67–81. doi: 10.2147/BTT.S41477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santos Lacomba M, Marcos Martin C, Gallardo Galera JM, Gomez Vidal MA, Collantes Estevez E, Ramirez Chamond R, et al. Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic research. 2001;33(5):251–255. doi: 10.1159/000055677. [DOI] [PubMed] [Google Scholar]
- 56.Perez-Guijo V, Santos-Lacomba M, Sanchez-Hernandez M, Castro-Villegas Mdel C, Gallardo-Galera JM, Collantes-Estevez E. Tumour necrosis factor-alpha levels in aqueous humour and serum from patients with uveitis: the involvement of HLA-B27. Current medical research and opinion. 2004;20(2):155–157. doi: 10.1185/030079903125002847. [DOI] [PubMed] [Google Scholar]
- 57.Dick AD, Forrester JV, Liversidge J, Cope AP. The role of tumour necrosis factor (TNF-alpha) in experimental autoimmune uveoretinitis (EAU) Progress in retinal and eye research. 2004;23(6):617–637. doi: 10.1016/j.preteyeres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Nakamura S, Yamakawa T, Sugita M, Kijima M, Ishioka M, Tanaka S, et al. The role of tumor necrosis factor-alpha in the induction of experimental autoimmune uveoretinitis in mice. Investigative ophthalmology & visual science. 1994;35(11):3884–3889. [PubMed] [Google Scholar]
- 59.Sartani G, Silver PB, Rizzo LV, Chan CC, Wiggert B, Mastorakos G, et al. Anti-tumor necrosis factor alpha therapy suppresses the induction of experimental autoimmune uveoretinitis in mice by inhibiting antigen priming. Investigative ophthalmology & visual science. 1996;37(11):2211–2218. [PubMed] [Google Scholar]
- 60.Lerman MA, Burnham JM, Chang PY, Daniel E, Foster CS, Hennessy S, et al. Response of pediatric uveitis to tumor necrosis factor-alpha inhibitors. The Journal of rheumatology. 2013;40(8):1394–1403. doi: 10.3899/jrheum.121180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cordero-Coma M, Sobrin L. Anti-tumor necrosis factor-alpha therapy in uveitis. Survey of ophthalmology. 2015;60(6):575–589. doi: 10.1016/j.survophthal.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 62.Richards JC, Tay-Kearney ML, Murray K, Manners P. Infliximab for juvenile idiopathic arthritis-associated uveitis. Clin Exp Ophthalmol. 2005;33(5):461–468. doi: 10.1111/j.1442-9071.2005.01062.x. [DOI] [PubMed] [Google Scholar]
- 63.Rajaraman RT, Kimura Y, Li S, Haines K, Chu DS. Retrospective case review of pediatric patients with uveitis treated with infliximab. Ophthalmology. 2006;113(2):308–314. doi: 10.1016/j.ophtha.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 64. Kahn P, Weiss M, Imundo LF, Levy DM. Favorable response to high-dose infliximab for refractory childhood uveitis. Ophthalmology. 2006;113(5):860–864.e2. doi: 10.1016/j.ophtha.2006.01.005. This study describes the successful use of increased doses of inflixmab in pediatric uveitis
- 65.Sobrin L, Kim EC, Christen W, Papadaki T, Letko E, Foster CS. Infliximab therapy for the treatment of refractory ocular inflammatory disease. Archives of ophthalmology (Chicago, Ill : 1960) 2007;125(7):895–900. doi: 10.1001/archopht.125.7.895. [DOI] [PubMed] [Google Scholar]
- 66.Tugal-Tutkun I, Ayranci O, Kasapcopur O, Kir N. Retrospective analysis of children with uveitis treated with infliximab. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus. 2008;12(6):611–613. doi: 10.1016/j.jaapos.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 67.Ardoin SP, Kredich D, Rabinovich E, Schanberg LE, Jaffe GJ. Infliximab to treat chronic noninfectious uveitis in children: retrospective case series with long-term follow-up. American journal of ophthalmology. 2007;144(6):844–849. doi: 10.1016/j.ajo.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sukumaran S, Marzan K, Shaham B, Reiff A. High dose infliximab in the treatment of refractory uveitis: does dose matter? ISRN rheumatology. 2012;2012:765380. doi: 10.5402/2012/765380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tambralli A, Beukelman T, Weiser P, Atkinson TP, Cron RQ, Stoll ML. High doses of infliximab in the management of juvenile idiopathic arthritis. The Journal of rheumatology. 2013;40(10):1749–1755. doi: 10.3899/jrheum.130133. [DOI] [PubMed] [Google Scholar]
- 70.Aeschlimann FA, Hofer KD, Cannizzaro Schneider E, Schroeder S, Lauener R, Saurenmann RK. Infliximab in pediatric rheumatology patients: a retrospective analysis of infusion reactions and severe adverse events during 2246 infusions over 12 years. The Journal of rheumatology. 2014;41(7):1409–1415. doi: 10.3899/jrheum.131231. [DOI] [PubMed] [Google Scholar]
- 71.Balevic SJ, Rabinovich CE. Profile of adalimumab and its potential in the treatment of uveitis. Drug design, development and therapy. 2016;10:2997–3003. doi: 10.2147/DDDT.S94188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jaffe GJ, Dick AD, Brezin AP, Nguyen QD, Thorne JE, Kestelyn P, et al. Adalimumab in Patients with Active Noninfectious Uveitis. The New England journal of medicine. 2016;375(10):932–943. doi: 10.1056/NEJMoa1509852. Important study that describes the efficacy of adalimumab in adult uveitis patients
- 73. Nguyen QD, Merrill PT, Jaffe GJ, Dick AD, Kurup SK, Sheppard J, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet (London, England) 2016;388(10050):1183–1192. doi: 10.1016/S0140-6736(16)31339-3. Another important study that describves the efficacy of adalimumab in adult uveitis using RCT
- 74.Castiblanco C, Meese H, Foster CS. Treatment of pediatric uveitis with adalimumab: the MERSI experience. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus. 2016;20(2):145–147. doi: 10.1016/j.jaapos.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 75.Diaz-Llopis M, Salom D, Garcia-de-Vicuna C, Cordero-Coma M, Ortega G, Ortego N, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology. 2012;119(8):1575–1581. doi: 10.1016/j.ophtha.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 76.Vazquez-Cobian LB, Flynn T, Lehman TJ. Adalimumab therapy for childhood uveitis. The Journal of pediatrics. 2006;149(4):572–575. doi: 10.1016/j.jpeds.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 77.Bravo-Ljubetic L, Peralta-Calvo J, Noval S, Pastora-Salvador N, Abelairas-Gomez J, Merino R. Adalimumab therapy for refractory childhood uveitis. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus. 2013;17(5):456–459. doi: 10.1016/j.jaapos.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 78.Garcia-De-Vicuna C, Diaz-Llopis M, Salom D, Bou R, Diaz-Cascajosa J, Cordero-Coma M, et al. Usefulness of adalimumab in the treatment of refractory uveitis associated with juvenile idiopathic arthritis. Mediators of inflammation. 2013;2013:560632. doi: 10.1155/2013/560632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Magli A, Forte R, Navarro P, Russo G, Orlando F, Latanza L, et al. Adalimumab for juvenile idiopathic arthritis-associated uveitis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2013;251(6):1601–1606. doi: 10.1007/s00417-013-2275-x. [DOI] [PubMed] [Google Scholar]
- 80. Simonini G, Taddio A, Cattalini M, Caputo R, de Libero C, Parentin F, et al. Superior efficacy of Adalimumab in treating childhood refractory chronic uveitis when used as first biologic modifier drug: Adalimumab as starting anti-TNF-alpha therapy in childhood chronic uveitis. Pediatric rheumatology online journal. 2013;11:16. doi: 10.1186/1546-0096-11-16. The authors provide a review of adalimumab for pediatric uveitis
- 81.Jeroudi A, Angeles-Han ST, Yeh S. Efficacy of adalimumab for pediatric Vogt-Koyanagi-Harada syndrome. Ophthalmic surgery, lasers & imaging retina. 2014;45(4):332–334. doi: 10.3928/23258160-20140709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim JB, Jeroudi A, Angeles-Han ST, Grossniklaus HE, Yeh S. Adalimumab for pediatric sympathetic ophthalmia. JAMA ophthalmology. 2014;132(8):1022–1024. doi: 10.1001/jamaophthalmol.2014.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zannin ME, Birolo C, Gerloni VM, Miserocchi E, Pontikaki I, Paroli MP, et al. Safety and efficacy of infliximab and adalimumab for refractory uveitis in juvenile idiopathic arthritis: 1-year followup data from the Italian Registry. The Journal of rheumatology. 2013;40(1):74–79. doi: 10.3899/jrheum.120583. [DOI] [PubMed] [Google Scholar]
- 84.Tynjala P, Kotaniemi K, Lindahl P, Latva K, Aalto K, Honkanen V, et al. Adalimumab in juvenile idiopathic arthritis-associated chronic anterior uveitis. Rheumatology (Oxford, England) 2008;47(3):339–344. doi: 10.1093/rheumatology/kem356. [DOI] [PubMed] [Google Scholar]
- 85.Cordero-Coma M, Calleja-Antolin S, Garzo-Garcia I, Nunez-Garnes AM, Alvarez-Castro C, Franco-Benito M, et al. Adalimumab for Treatment of Noninfectious Uveitis: Immunogenicity and Clinical Relevance of Measuring Serum Drug Levels and Antidrug Antibodies. Ophthalmology. 2016;123(12):2618–2625. doi: 10.1016/j.ophtha.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 86.Miserocchi E, Modorati G, Pontikaki I, Meroni PL, Gerloni V. Long-term treatment with golimumab for severe uveitis. Ocular immunology and inflammation. 2014;22(2):90–95. doi: 10.3109/09273948.2013.844265. [DOI] [PubMed] [Google Scholar]
- 87.Angeles-Han S, Flynn T, Lehman T. Abatacept for refractory juvenile idiopathic arthritis-associated uveitis- a case report. The Journal of rheumatology. 2008;35(9):1897–1898. [PubMed] [Google Scholar]
- 88.Kenawy N, Cleary G, Mewar D, Beare N, Chandna A, Pearce I. Abatacept: a potential therapy in refractory cases of juvenile idiopathic arthritis-associated uveitis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2011;249(2):297–300. doi: 10.1007/s00417-010-1523-6. [DOI] [PubMed] [Google Scholar]
- 89.Marrani E, Paganelli V, de Libero C, Cimaz R, Simonini G. Long-term efficacy of abatacept in pediatric patients with idiopathic uveitis: a case series. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2015;253(10):1813–1816. doi: 10.1007/s00417-015-3140-x. [DOI] [PubMed] [Google Scholar]
- 90. Tappeiner C, Miserocchi E, Bodaghi B, Kotaniemi K, Mackensen F, Gerloni V, et al. Abatacept in the treatment of severe, longstanding, and refractory uveitis associated with juvenile idiopathic arthritis. The Journal of rheumatology. 2015;42(4):706–711. doi: 10.3899/jrheum.140410. The authors provide data on the unsuccessful use of abatacept in a pediatric uveitis population
- 91.Zulian F, Balzarin M, Falcini F, Martini G, Alessio M, Cimaz R, et al. Abatacept for severe anti-tumor necrosis factor alpha refractory juvenile idiopathic arthritis-related uveitis. Arthritis care & research. 2010;62(6):821–825. doi: 10.1002/acr.20115. [DOI] [PubMed] [Google Scholar]
- 92. Birolo C, Zannin ME, Arsenyeva S, Cimaz R, Miserocchi E, Dubko M, et al. Comparable Efficacy of Abatacept Used as First-line or Second-line Biological Agent for Severe Juvenile Idiopathic Arthritis-related Uveitis. The Journal of rheumatology. 2016 doi: 10.3899/jrheum.151389. Interesting study that compares the use of abatacept as first line therapy vs. second-line therapy
- 93.Lin P. Targeting interleukin-6 for noninfectious uveitis. Clinical ophthalmology (Auckland, NZ) 2015;9:1697–1702. doi: 10.2147/OPTH.S68595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Silpa-Archa S, Oray M, Preble JM, Foster CS. Outcome of tocilizumab treatment in refractory ocular inflammatory diseases. Acta ophthalmologica. 2016;94(6):e400–e406. doi: 10.1111/aos.13015. [DOI] [PubMed] [Google Scholar]
- 95.Yoshimura T, Sonoda KH, Ohguro N, Ohsugi Y, Ishibashi T, Cua DJ, et al. Involvement of Th17 cells and the effect of anti-IL-6 therapy in autoimmune uveitis. Rheumatology (Oxford, England) 2009;48(4):347–354. doi: 10.1093/rheumatology/ken489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tappeiner C, Heinz C, Ganser G, Heiligenhaus A. Is tocilizumab an effective option for treatment of refractory uveitis associated with juvenile idiopathic arthritis? The Journal of rheumatology. 2012;39(6):1294–1295. doi: 10.3899/jrheum.120010. This is an important study that reports on tocilizumab as treatment for refractory uveitis
- 97.Tappeiner C, Mesquida M, Adan A, Anton J, Ramanan AV, Carreno E, et al. Evidence for Tocilizumab as a Treatment Option in Refractory Uveitis Associated with Juvenile Idiopathic Arthritis. The Journal of rheumatology. 2016 doi: 10.3899/jrheum.160231. [DOI] [PubMed] [Google Scholar]
- 98.Tsang AC, Roth J, Gottlieb C. Tocilizumab for severe chronic anterior uveitis associated with juvenile idiopathic arthritis in a pediatric patient. Ocular immunology and inflammation. 2014;22(2):155–157. doi: 10.3109/09273948.2013.866254. [DOI] [PubMed] [Google Scholar]
- 99. Calvo-Rio V, Santos-Gomez M, Calvo I, Gonzalez-Fernandez MI, Lopez Montesinos B, Mesquida M, et al. Anti-IL6-R Tocilizumab for Severe Juvenile Idiopathic Arthritis-Associated Uveitis Refractory to anti-TNF therapy. A multicenter study of 25 patients. Arthritis & rheumatology (Hoboken, NJ) 2016 doi: 10.1002/art.39940. This is another study that reports on tocilizumb as an emerging therapy for refractory pediatric uveitis
- 100. Miserocchi E, Pontikaki I, Modorati G, Gattinara M, Meroni PL, Gerloni V. Anti-CD 20 monoclonal antibody (rituximab) treatment for inflammatory ocular diseases. Autoimmunity reviews. 2011;11(1):35–39. doi: 10.1016/j.autrev.2011.07.001. This is an important article that describes the use of rituximab for pediatric uveitis
- 101.Lerman MA, Rabinovich CE. The Future Is Now: Biologics for Non-Infectious Pediatric Anterior Uveitis. Paediatric drugs. 2015;17(4):283–301. doi: 10.1007/s40272-015-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freidlin J, Wong IG, Acharya N. Rituximab treatment for peripheral ulcerative keratitis associated with Wegener's granulomatosis. The British journal of ophthalmology. 2007;91(10):1414. doi: 10.1136/bjo.2006.113316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheung CM, Murray PI, Savage CO. Successful treatment of Wegener's granulomatosis associated scleritis with rituximab. The British journal of ophthalmology. 2005;89(11):1542. doi: 10.1136/bjo.2005.075689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Umran RM, Shukur ZY. Rituximab for sight-threatening refractory pediatric Vogt-Koyanagi-Harada disease. Modern rheumatology. 2015:1–3. doi: 10.3109/14397595.2015.1071234. [DOI] [PubMed] [Google Scholar]
- 105.Caso F, Rigante D, Vitale A, Costa L, Bascherini V, Latronico E, et al. Long-lasting uveitis remission and hearing loss recovery after rituximab in Vogt-Koyanagi-Harada disease. Clinical rheumatology. 2015;34(10):1817–1820. doi: 10.1007/s10067-014-2781-1. [DOI] [PubMed] [Google Scholar]
- 106.Miserocchi E, Modorati G, Berchicci L, Pontikaki I, Meroni P, Gerloni V. Long-term treatment with rituximab in severe juvenile idiopathic arthritis-associated uveitis. The British journal of ophthalmology. 2016;100(6):782–786. doi: 10.1136/bjophthalmol-2015-306790. [DOI] [PubMed] [Google Scholar]
- 107.Heiligenhaus A, Miserocchi E, Heinz C, Gerloni V, Kotaniemi K. Treatment of severe uveitis associated with juvenile idiopathic arthritis with anti-CD20 monoclonal antibody (rituximab) Rheumatology (Oxford, England) 2011;50(8):1390–1394. doi: 10.1093/rheumatology/ker107. [DOI] [PubMed] [Google Scholar]
- 108.Kalinina Ayuso V, de Boer JH, Byers HL, Coulton GR, Dekkers J, de Visser L, et al. Intraocular biomarker identification in uveitis associated with juvenile idiopathic arthritis. Investigative ophthalmology & visual science. 2013;54(5):3709–3720. doi: 10.1167/iovs.12-10865. [DOI] [PubMed] [Google Scholar]
- 109.Haasnoot AJ, van Tent-Hoeve M, Wulffraat NM, Schalij-Delfos NE, Los LI, Armbrust W, et al. Erythrocyte sedimentation rate as baseline predictor for the development of uveitis in children with juvenile idiopathic arthritis. American journal of ophthalmology. 2015;159(2):372–377.e1. doi: 10.1016/j.ajo.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 110.Kalinina Ayuso V, van Dijk MR, de Boer JH. Infiltration of Plasma Cells in the Iris of Children With ANA-Positive Anterior Uveitis. Investigative ophthalmology & visual science. 2015;56(11):6770–6778. doi: 10.1167/iovs.15-17351. [DOI] [PubMed] [Google Scholar]
- 111.Godfrey WA, Lindsley CB, Cuppage FE. Localization of IgM in plasma cells in the iris of a patient with iridocyclitis and juvenile rheumatoid arthritis. Arthritis and rheumatism. 1981;24(9):1195–1198. doi: 10.1002/art.1780240914. [DOI] [PubMed] [Google Scholar]
- 112.Parikh JG, Tawansy KA, Rao NA. Immunohistochemical study of chronic nongranulomatous anterior uveitis in juvenile idiopathic arthritis. Ophthalmology. 2008;115(10):1833–1836. doi: 10.1016/j.ophtha.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 113.Sabates R, Smith T, Apple D. Ocular histopathology in juvenile rheumatoid arthritis. Annals of ophthalmology. 1979;11(5):733–737. [PubMed] [Google Scholar]
- 114.Walscheid K, Heiligenhaus A, Holzinger D, Roth J, Heinz C, Tappeiner C, et al. Elevated S100A8/A9 and S100A12 Serum Levels Reflect Intraocular Inflammation in Juvenile Idiopathic Arthritis-Associated Uveitis: Results From a Pilot Study. Investigative ophthalmology & visual science. 2015;56(13):7653–7660. doi: 10.1167/iovs.15-17066. [DOI] [PubMed] [Google Scholar]
- 115.Haasnoot AM, Kuiper JJ, Hiddingh S, Schellekens PA, de Jager W, Imhof SM, et al. Ocular Fluid Analysis in Children Reveals Interleukin-29/Interferon-lambda1 as a Biomarker for Juvenile Idiopathic Arthritis-Associated Uveitis. Arthritis & rheumatology (Hoboken, NJ) 2016;68(7):1769–1779. doi: 10.1002/art.39621. [DOI] [PubMed] [Google Scholar]
- 116.Schmeling H, Mahler M, Levy DM, Moore K, Stevens AM, Wick J, et al. Autoantibodies to Dense Fine Speckles in Pediatric Diseases and Controls. The Journal of rheumatology. 2015;42(12):2419–2426. doi: 10.3899/jrheum.150567. [DOI] [PubMed] [Google Scholar]
- 117.Nordal EB, Songstad NT, Berntson L, Moen T, Straume B, Rygg M. Biomarkers of chronic uveitis in juvenile idiopathic arthritis: predictive value of antihistone antibodies and antinuclear antibodies. The Journal of rheumatology. 2009;36(8):1737–1743. doi: 10.3899/jrheum.081318. [DOI] [PubMed] [Google Scholar]
- 118.Sijssens KM, Rijkers GT, Rothova A, Stilma JS, de Boer JH. Distinct cytokine patterns in the aqueous humor of children, adolescents and adults with uveitis. Ocular immunology and inflammation. 2008;16(5):211–216. doi: 10.1080/09273940802409969. [DOI] [PubMed] [Google Scholar]
- 119.Velez G, Roybal CN, Colgan D, Tsang SH, Bassuk AG, Mahajan VB. Precision Medicine: Personalized Proteomics for the Diagnosis and Treatment of Idiopathic Inflammatory Disease. JAMA ophthalmology. 2016;134(4):444–448. doi: 10.1001/jamaophthalmol.2015.5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nekhai SHA, Ammosova T, Obukhov Y, Ekaterina G, Kononov A, Dubko M, Nikitina T, Serogodskaia E, Kostik M, Kalashnikova O, Masalova V, Snegireva L, Chasnyk V. A128: Hierarchical Clustering Methodology for Exploration of Proteomic Profile in Tears: Seeking for Markers of Uveitis Associated With Juvenile Idiopathic Arthritis. Arthritis & Rheumatology. 2014;66(S3):S168. [Google Scholar]
- 121.Angeles-Hans SDD, Yeh S, Patel P, Jenkins K, Prahalad S, Holand G. Identification of Biomarkers Using Tear Proteomics in Children with Chronis Anterior Uveitis. Arthritis & Rheumatology. 2016;2016(68)(suppl 10) [Google Scholar]