Abstract
Background
Because TAFI (thrombin-activatable fibrinolysis inhibitor) antigen varies widely among different populations, we performed this case-control study to explore the relationship between TAFI levels and stroke in a Chinese population.
Material/Methods
Our population-based case-control study included 217 stroke patients and 218 healthy controls. The plasma TAFI level was measured by immune turbidimetry. Univariate and multivariate logistic regression analyses were used to analyze the association between different TAFI levels and stroke and its subtypes. Restricted cubic spline (RCS) combined with logistic regression analysis were used to explore the dose-response relationship between TAFI levels and stroke.
Results
The plasma TAFI levels of cases were much higher than in the control group (p=0.038) and this difference persisted even after adjustment (OR=2.2). In the elderly (aged over 60) and female subgroups, TAFI levels in stroke patients were higher than those in controls, and the results were also noted in ischemic stroke. The dose-response curve showed that, as a whole, with the increase of TAFI levels, the relative risk of stroke first increased and then decreased (p=0.0127). Similarly, in general, with the increase of TAFI levels, the curve showed that the relative risk of ischemic stroke first increased and then decreased (p=0.0110).
Conclusions
There was a definite correlation between TAFI levels and stroke in this Chinese population, and with the increase of TAFI levels, the relative risk of stroke or ischemic stroke first increased and then decreased.
MeSH Keywords: Carboxypeptidase U; Case-Control Studies; Dose-Response Relationship, Drug; Stroke
Background
Stroke is the second leading cause of death and is the most important cause of disability in the world [1]. As a major public health concern worldwide, it seriously harms human health [2] and imposes a heavy economic burden on society. The latest available estimates from China indicate that the total hospitalization expense of the 3 major cardiovascular diseases (ischemic stroke, myocardial infarction, and intracranial hemorrhage) is up to 46 billion yuan, and among them, the cost of ischemic stroke is more than 27 billion yuan [3,4]. Recently, a study [5] based on 480 000 people in China showed that the burden of stroke has gradually increased over the past 30 years, presenting an increasing trend from south to north, especially in rural areas. Compared to other similar surveys, China has the highest incidence and mortality rate of stroke in the world. Within China, compared with other regions, the northeast region has the highest stroke incidence (365.2/10 million) and the highest stroke mortality (158.5/10 million). Thus, stroke has become a serious problem that cannot be ignored in China, especially in the northeast region.
Thrombin-activatable fibrinolysis inhibitor (TAFI), composed of 423 amino acids, is an inactive precursor circulating in plasma and is activated by thrombin, plasmin, and thrombomodulin [6]. Activated TAFI effectively inhibits fibrinolysis by removing carboxyterminal lysine residues from partially degraded fibrin [7]. Because of its anti-fibrinolytic function, TAFI is believed to be involved in arterial thrombosis. Epidemiological studies have further shown that higher levels of TAFI are associated with an increased risk of several cardiovascular events, such as ischemic stroke (IS) [8–10], myocardial infarction (MI) [11], angina pectoris (AP) [12], acute coronary syndrome (ACS) [13], and coronary heart disease (CHD) [10]. Therefore, a discussion of changes in plasma level of TAFI will help identify stroke risk factors and the pathological process involved [14]. In addition, TAFI is expected to be a new biomarker and to be applied to clinical practice.
Despite numerous epidemiological studies of stroke in Western countries [15,16], little information is available from population-based studies in developing countries such as China. Because TAFI antigen varies widely among different populations [17,18], we performed the present case-control study to explore the relationship between TAFI levels and stroke in a Chinese population.
Material and Methods
Participants
Our population-based case-control study included 217 stroke patients and 218 controls. All subjects were recruited from rural areas of Liaoning Province, which is located in northeast China. Cases were chosen by a convenience non-random sampling procedure. Inclusion criteria were: stroke patients examined by a neurologist, diagnosed by CT or magnetic resonance imaging, and certified by the hospital. Exclusion criteria were: patients with other types of cerebrovascular diseases (e.g., brain tumor, cerebrovascular malformation, and transient ischemic attack), hematologic disorders, and those taking anticoagulants. Controls were matched by age and sex with the case group and they were all “healthy” people who did not have a history of cerebrovascular diseases or neurological disorders. Controls with history of bleeding and those who had been taking anticoagulants were also excluded. Ethics approval was obtained from the China Medical University Ethics Committee. Informed consent was obtained from each participant and data analysis was conducted on the condition of anonymity.
Measurement of TAFI
The plasma TAFI level was measured by immune turbidimetry using kits provided by Liaoning Maidi Biological Technology Co. Ltd. The measurement principle is as follows: TAFI in plasma is specifically bound to the TAFI antibodies labeled on colloidal gold, and then colloidal gold is aggregated into an immune complex. The turbidity of the solution can be measured by a biochemical analyzer, and the turbidity is directly proportional to the content of the complex. Thus, the concentration of TAFI in plasma can be determined by establishing a standard curve of the standard sample.
Statistical methods
We used Kolmogorov-Smirnov test and Q-Q plots to check the normal distribution of variables. The median, upper, and lower quartiles, as well as frequency and percentage, were used to describe the variables. The baseline condition and plasma TAFI levels between the case group and the control group were compared by using the Mann-Whitney U test and chi-square test. Univariate and multivariate logistic regression were used to analyze the association between the different TAFI levels and stroke and its different subtypes. Restricted cubic spline (RCS) combined with logistic regression was used to explore the dose-response relationship between TAFI levels and stroke [19]. All data were analyzed using SPSS 19.0 and SAS 9.3.2, and the significance level was p<0.05 (2-sided).
Results
Our study included 217 cases and 218 controls. The case group consisted of 180 patients with ischemic stroke and 37 patients with hemorrhagic stroke. Among the 271 males in the study, 143 were in the case group and 128 were in the control group. The average age of all subjects was 62 years, range 30–82 years. The case group and control group were well-matched by age and sex.
BMI, total cholesterin (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), blood glucose (Glu), serum sodium (Na+), serum potassium (K+), hypertension history, family history of hypertension, family history of stroke, family history of coronary heart disease, smoking, drinking, and high-salt diet were compared between the 2 groups. The results showed statistically significant (p<0.05) differences between groups for BMI, triglyceride (TG), high-density lipoprotein (HDL), blood glucose (Glu), serum sodium (Na+), family history of hypertension, smoking, and high-salt diet (Table 1).
Table 1.
General characteristics of the participants based on case and control groups.
| Factors | All (n=435) | Cases (n=217) | Controls (n=218) | P |
|---|---|---|---|---|
| Age (years) | 62.00 (56.00–68.00) | 63.00 (55.00–69.00) | 60.00 (57.00–67.00) | 0.391 |
| Male | 271 (62.30%) | 143 (65.90%) | 128 (58.72%) | 0.122 |
| BMI (kg/m2) | 23.53 (21.10–26.15) | 24.69 (22.66–27.44) | 22.18 (20.04–24.77) | 0.000* |
| TC (mmol/L) | 5.16 (4.57–5.85) | 5.27 (4.60–5.98) | 5.06 (4.52–5.75) | 0.114 |
| TG (mmol/L) | 1.25 (0.88–1.89) | 1.36 (0.92–2.09) | 1.17 (0.81–1.77) | 0.033* |
| HDL (mmol/L) | 1.54 (1.23–1.79) | 1.37 (1.16–1.75) | 1.62 (1.34–1.85) | 0.000* |
| LDL (mmol/L) | 2.92 (2.38–3.55) | 2.90 (2.36–3.53) | 2.93 (2.43–3.55) | 0.630 |
| Glu (mmol/L) | 5.20 (4.60–6.00) | 5.50 (5.00–6.70) | 4.90 (4.40–5.60) | 0.000* |
| Na+ (mmol/L) | 142.80 (140.00–145.00) | 141.90 (139.70–143.70) | 143.80 (141.05–145.90) | 0.000* |
| K+ (mmol/L) | 4.18 (3.87–4.41) | 4.17 (3.85–4.44) | 4.19 (3.92–4.39) | 0.489 |
| HP history | 346 (79.54%) | 168 (77.42%) | 178 (81.65%) | 0.274 |
| Family HP | 165 (37.93%) | 103 (47.47%) | 62 (28.44%) | 0.000* |
| Family stroke | 96 (22.07%) | 56 (25.81%) | 40 (18.35%) | 0.085 |
| Family CHD | 47 (10.80%) | 19 (8.76%) | 28 (12.84%) | 0.131 |
| Smoking | 207 (47.59%) | 87 (40.09%) | 120 (55.05%) | 0.002* |
| Drinking | 141 (32.41%) | 68 (31.34%) | 73 (33.49%) | 0.632 |
| High-salt diet | 326 (74.94%) | 172 (79.26%) | 154 (70.64%) | 0.038* |
BMI – body mass index; TC – total cholesterin; TG – triglyceride; HDL – high density lipoprotein; LDL – low density lipoprotein; Glu – blood glucose; HP history – hypertension history; Family HP – family history of hypertension; Family stroke – family history of stroke; Family CHD – family history of coronary heart disease.
p<0.05.
By analysis, the median TAFI level of cases was 31.28 μg/ml, the upper quartile was 35.18 μg/ml, and the lower quartile was 27.23 μg/ml; while the median and upper and lower quartiles of TAFI levels in controls were 29.26 μg/ml, 34.97 μg/ml, and 25.32 μg/ml, respectively. The plasma TAFI level of cases was higher than in the control group (p=0.038). The level of TAFI in patients with ischemic stroke was also significantly higher than that in the control group (p=0.036), but the difference between hemorrhagic stroke patients and controls showed no significance (p=0.424).
When stratified by age and sex, the data showed that TAFI level of cases was significantly higher than in the control group (p=0.016 for age over 60, p=0.022 for females). In the elderly (aged over 60 years) and in females, plasma TAFI levels in ischemic stroke patients were also higher than in controls, whereas there were no differences in hemorrhagic stroke (Table 2).
Table 2.
Stratified analysis according to age and gender.
| Subgroup | Stroke | Ischemic stroke | Hemorrhagic stroke | Control group | |||
|---|---|---|---|---|---|---|---|
| TAFI (μg/mL) | P | TAFI (μg/mL) | P | TAFI (μg/mL) | P | TAFI (μg/mL) | |
| Age | |||||||
| ≤60 | 31.16 (27.10–34.92) | 0.570 | 31.22 (27.14–34.88) | 0.529 | 30.63 (27.10–35.96) | 0.886 | 30.55 (26.06–35.18) |
| >60 | 31.48 (27.33–35.25) | 0.016 | 31.73 (26.89–35.39) | 0.018 | 29.08 (28.19–32.97) | 0.321 | 28.00 (24.96–34.62) |
| Gender | |||||||
| Male | 30.48 (26.72–35.02) | 0.355 | 30.74 (26.70–35.02) | 0.394 | 30.05 (27.35–34.77) | 0.551 | 29.69 (25.03–34.89) |
| Female | 32.32 (28.43–36.29) | 0.022 | 32.50 (28.48–36.81) | 0.017 | 31.36 (27.36–33.06) | 0.586 | 28.78 (25.57–35.03) |
P value came from the compare between each case group (stroke/ischemic stroke/hemorrhagic stroke) and the control group.
Considering the results of univariate logistic regression (p<0.10 as the significance level) and the practical significance, we adjusted the elderly (age over 60 years), male, overweight (BMI >24), hyperglycemia (≥6.1mmol/L), high HDL (>1.2mmol/L), family history of hypertension, family history of stroke, high-salt diet (>180g/month), and TAFI as possible influencing factors. The TAFI was divided into 4 quartiles. Compared with the first quartile (<25.32 μg/ml), the adjusted OR of the third quartile (29.26~34.97 μg/ml) was 2.22, which means the risk of stroke was 2.22 times higher in the third quartile than in the first quartile. After adjusting for some possible influencing factors (age, male, overweight, family history of hypertension, hyperglycemia, high HDL, hypertriglyceridemia, and TAFI), the risk of ischemic stroke increased by 134% in the third quartile. However, the risk of hemorrhagic stroke was not statistically significant even after adjustment (Table 3).
Table 3.
Multivariate logistic regression for stroke and different subtypes of stroke.
| Variate | Stroke | Ischemic stroke | Hemorrhagic stroke | |||
|---|---|---|---|---|---|---|
| P | OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | |
| TAFI | ||||||
| The first quartile | – | – | – | – | – | – |
| The second quartile | 0.110 | 1.79 (0.88–3.64) | 0.333 | 1.44 (0.69–2.99) | 0.351 | 1.90 (0.49–7.31) |
| The third quartile | 0.024 | 2.22 (1.11–4.44) | 0.018 | 2.34 (1.16–4.74) | 0.209 | 2.37 (0.62–9.12) |
| The fourth quartile | 0.304 | 1.45 (0.71–2.96) | 0.290 | 1.49 (0.71–3.09) | 0.993 | 1.01 (0.24–4.25) |
| Older | 0.000 | 2.69 (1.65–4.40) | 0.000 | 2.92 (1.75–4.88) | 0.590 | 0.77 (0.30–2.01) |
| Male | 0.059 | 1.62 (0.98–2.68) | 0.066 | 1.63 (0.97–2.73) | 0.615 | 0.74 (0.23–2.37) |
| Overweight | 0.000 | 2.46 (1.51–4.02) | 0.001 | 2.35 (1.41–3.93) | 0.003 | 4.16 (1.63–10.63) |
| Hyperglycemia | 0.065 | 1.68 (0.97–2.92) | 0.089 | 1.67 (0.93–3.01) | 0.013 | 3.22 (1.28–8.12) |
| Family HP | 0.018 | 1.86 (1.11–3.11) | 0.014 | 1.89 (1.14–3.13) | 0.467 | 1.38 (0.58–3.32) |
| High HDL | 0.074 | 0.59 (0.33–1.05) | 0.020 | 0.49 (0.27–0.89) | – | – |
| High-salt diet | 0.115 | 1.56 (0.90–2.70) | – | – | 0.267 | 1.88 (0.62–5.72) |
| Family stroke | 0.975 | 1.01 (0.55–1.85) | – | – | – | – |
| HTG | – | – | 0.325 | 0.75 (0.42–1.33) | – | – |
| Hypokalemia | – | – | – | – | 0.563 | 1.72 (0.28–10.68) |
| Drinking | – | – | – | – | 0.126 | 2.38 (0.78–7.23) |
Overweight: BMI >24; Hyperglycemia: blood glucose ≥6.1 mmol/L; High HDL: HDL >1.2mmol/L; High-salt diet: salt >180 g/month; HTG: hypertriglyceridemia, TG ≥1.7 mmol/L; hypokalemia: K+ <3.5 mmol/L.
Since the TAFI antigen level has a large variability in different populations [17,18], we used the median of the control group (29.26 μg/ml) as the reference concentration. We selected 5 knots (p5, p25, p50, p75, and p95) [19] of TAFI levels to explore the dose-response relationship between TAFI levels and stroke or ischemic stroke after adjustment. Because the number of patients with hemorrhagic stroke was too low and we found no association between TAFI and hemorrhagic stroke, hemorrhagic stroke was not analyzed in this section. Testing showed the association between TAFI levels and stroke was nonlinear (p=0.0065). The dose-response curve (Figure 1) showed that, as a whole, with the increase of TAFI levels, the risk of stroke first increased and then decreased (p=0.0127). Similarly, in general, with the increase of TAFI levels, the curve (Figure 2) showed that the risk of ischemic stroke first increases and then decreases (p=0.0110).
Figure 1.
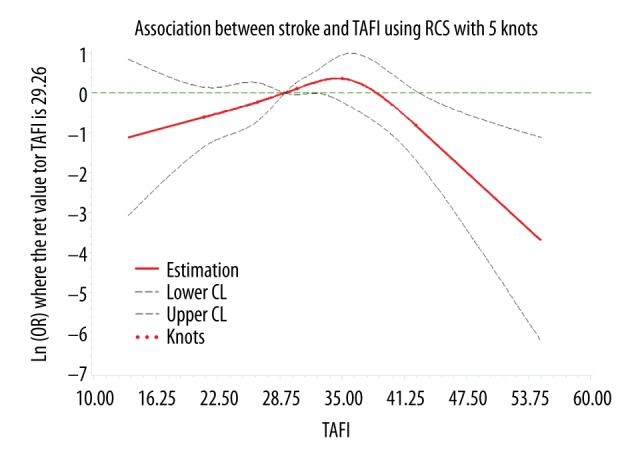
The dose-response curve of the relationship between TAFI and stroke.
Figure 2.
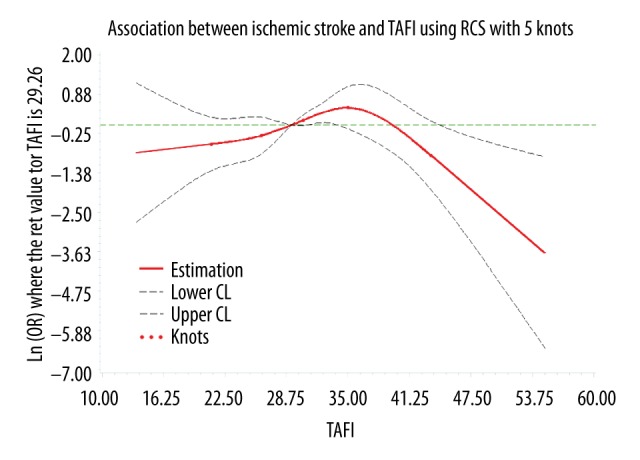
The dose-response curve of the relationship between TAFI and ischemic stroke.
Discussion
To the best of our knowledge, this is the first dose-response study of TAFI level and stroke in a Chinese population. The method of TAFI detection commonly used before was enzyme-linked immunosorbent assay (ELISA), but the complicated process limits clinical application to some extent. Different manufacturers use different antibodies and sites for detection, which makes it impossible to do a unified and accurate evaluation of the clinical significance of TAFI. In contrast, the TAFI kit for the test of TAFI antigen level used in our study, based on the immune turbidimetry, is simple and reliable. A previous study [20] has demonstrated that this method is feasible in clinical practice. Because detection methods were different and the TAFI antigen level has a large variability in different populations [17,18], quantitative results from different studies cannot be compared directly. However, our finding that TAFI level of stroke patients was higher than in the control group is similar to results reported by Leebeek et al. [8,21]; and our results regarding ischemic stroke are also consistent with results reported by Santamaría et al. [8,14,22,23]. After adjusting for some possible related factors, we found that a higher TAFI level (29.26~34.97μg/ml) can increase the risk of stroke by 122% and can increase the risk of ischemic stroke by 134%. The dose-response curve further shows that, as a whole, the risk of stroke first increases and then decreases with the increase in TAFI levels, and this tendency was also demonstrated in ischemic stroke. The mechanism involved is probably as follows: TAFI is mainly activated by thrombin, and the procoagulant activity of thrombin has been demonstrated. The coagulation system leads to the generation of thrombin and turns fibrinogen into fibrin, and then a fibrin clot forms at the vascular injury site, preventing blood loss from damaged vessels. If the coagulation system was over-activated and/or fibrinolysis function is reduced, the coagulation and fibrinolysis systems become imbalanced, which may lead to fibrin deposition and then thrombosis. The function of TAFI is not only limited to the regulation of thrombin, but also plays a widespread role in the regulation of fibrinolysis. Activated TAFI can effectively inhibit fibrinolysis by removing C-terminal lysine residues from the partially degraded fibrin. Therefore, it is speculated that the formation of thrombin and the activation of TAFI can lead to imbalance of the coagulation system and fibrinolysis system, which can lead to thrombotic diseases, especially ischemic stroke. In addition, a study has revealed that the activation and secretion of TAFI are dependent on the generation of thrombin in a dose-dependent manner [24]. In view of this opinion, in the coagulation process, increased thrombin generation is associated with increased TAFI secretion and activation, which can regulate the production of thrombin and reduce coagulation in a feedback loop. However, it is still impossible to distinguish whether the decrease in plasma TAFI concentration is due to reduced production, increased consumption, or some other reasons. Therefore, the trend of the dose-response curve may be explained, but the definite mechanism needs to be confirmed by more studies.
At present, the common examination for stroke diagnosis is CT or MRI, but CT is usually not sensitive in the early stage, while MRI is expensive and requires much time. Since the detection of TAFI is simple, rapid, convenient, and low-cost, it is expected to become a biological marker and to assist in clinical diagnosis. However, there were no relevant published articles and the recommendations still need some well-designed and large-scale prospective studies.
Our study has certain limitations. First, the case-control design is a limit to determination of causation and prospective studies are needed. Second, since stroke is a complex heterogeneous disease, more details about subtypes are required. Third, our study included 435 patients but only 37 patients had hemorrhagic stroke; the sample size was thus a bit limited and the analysis in this part could not be performed effectively. Finally, due to limitations of the condition of detection, we did not test the TAFI activity. Therefore, the reliability of the conclusion needs to be further confirmed by high-quality studies.
Conclusions
There was a definite correlation between TAFI level and stroke in this Chinese population, and with the increase of TAFI levels, the relative risk of stroke or ischemic stroke first increased and then decreased.
Acknowledgments
We would like to acknowledge the entire team of the Cardiovascular Diseases Institute and Evidence Based Medicine Center of the First Affiliated Hospital of China Medical University.
Footnotes
Source of support: Departmental sources
References
- 1.WHO. The top 10 causes of death. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 2.Stroke – 1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20(10):1407–31. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Global Burden of Disease 2002: Deaths by age, sex and cause for the year 2002. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 4.National Cardiovascular Center. Cardiovascular diseases report of China 2012. Encyclopaedia of China Publishing House (ECPH); 2013. [Google Scholar]
- 5.Wang W, Jiang B, Sun H, et al. Prevalence, Incidence, and mortality of stroke in China: Results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135(8):759–71. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 6.Boffa MB, Koschinsky ML. Curiouser and curiouser: recent advances in measurement of thrombin-activatable fibrinolysis inhibitor (TAFI) and in understanding its molecular genetics, gene regulation, and biological roles. Clin Biochem. 2007;40(7):431–42. doi: 10.1016/j.clinbiochem.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Boffa MB, Bajzar L, et al. A study of the mechanism of inhibition of fibrinolysis by activated thrombin-activable fibrinolysis inhibitor. J Biol Chem. 1998;273(42):27176–81. doi: 10.1074/jbc.273.42.27176. [DOI] [PubMed] [Google Scholar]
- 8.Leebeek FW, Goor MP, Guimaraes AH, et al. High functional levels of thrombin-activatable fibrinolysis inhibitor are associated with an increased risk of first ischemic stroke. J Thromb Haemost. 2005;3(10):2211–18. doi: 10.1111/j.1538-7836.2005.01484.x. [DOI] [PubMed] [Google Scholar]
- 9.Ladenvall C, Gils A, Jood K, et al. Thrombin activatable fibrinolysis inhibitor activation peptide shows association with all major subtypes of ischemic stroke and with TAFI gene variation. Arterioscler Thromb Vasc Biol. 2007;27(4):955–62. doi: 10.1161/01.ATV.0000259354.93789.a6. [DOI] [PubMed] [Google Scholar]
- 10.de Bruijne EL, Gils A, Guimarães AH, et al. The role of thrombin activatable fibrinolysis inhibitor in arterial thrombosis at a young age: The ATTAC study. J Thromb Haemost. 2009;7(6):919–27. doi: 10.1111/j.1538-7836.2009.03350.x. [DOI] [PubMed] [Google Scholar]
- 11.Zorio E, Castelló R, Falcó C, et al. Thrombin-activatable fibrinolysis inhibitor in young patients with myocardial infarction and its relationship with the fibrinolytic function and the protein C system. Br J Haematol. 2003;122(6):958–65. doi: 10.1046/j.1365-2141.2003.04549.x. [DOI] [PubMed] [Google Scholar]
- 12.Morange PE, Juhan-Vague I, Scarabin PY, et al. Association between TAFI antigen and Ala147Thr polymorphism of the TAFI gene and the angina pectoris incidence. The PRIME Study (Prospective Epidemiological Study of MI) Thromb Haemost. 2003;89(3):554–60. [PubMed] [Google Scholar]
- 13.Santamaría A, Martínez-Rubio A, Borrell M, et al. Risk of acute coronary artery disease associated with functional thrombin activatable fibrinolysis inhibitor plasma level. Haematologica. 2004;89(7):880–81. [PubMed] [Google Scholar]
- 14.Santamaría A, Oliver A, Borrell M, et al. Risk of ischemic stroke associated with functional thrombin-activatable fibrinolysis inhibitor plasma levels. Stroke. 2003;34(10):2387–91. doi: 10.1161/01.STR.0000088642.07691.15. [DOI] [PubMed] [Google Scholar]
- 15.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2011 update: A report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith S, Horgan F, Sexton E, et al. The cost of stroke and transient ischemic attack in Ireland: a prevalence-based estimate. Age Ageing. 2012;41(3):332–38. doi: 10.1093/ageing/afr141. [DOI] [PubMed] [Google Scholar]
- 17.Xinming Y. TAFI and thrombotic diseases: A systematic review. Practical Clinical Medicine. 2010;11(5):123–25. [Google Scholar]
- 18.Schneider M, Boffa M, Stewart R, et al. Two naturally occurring variants of TAFI (Thr-325 and Ile-325) differ substantially with respect to thermal stability and antifibrinolytic activity of the enzyme. J Biol Chem. 2002;277(2):1021–30. doi: 10.1074/jbc.M104444200. [DOI] [PubMed] [Google Scholar]
- 19.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–57. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 20.Guiyan L, Ping L, Mingli J. The feasibility of clinical application for plasma TAFI determined by rate nephelometry assay. Clin J Med Offic. 2016;44(10):1059–61. [Google Scholar]
- 21.Guiping C, Ping Y, Jingyi H. The clinical study of plasma thrombin activatable fibrinolysis inhibitor in the patients with stroke. Laboratory Medicine. 2009;24(1):1–4. [Google Scholar]
- 22.Rooth E, Wallen H, Antovic A, et al. Thrombin activatable fibrinolysis inhibitor and its relationship to fibrinolysis and inflammation during the acute and convalescent phase of ischemic stroke. Blood Coagul Fibrinolysis. 2007;18(4):365–70. doi: 10.1097/MBC.0b013e3281139c34. [DOI] [PubMed] [Google Scholar]
- 23.Montaner J, Ribó M, Monasterio J, et al. Thrombin-activable fibrinolysis inhibitor levels in the acute phase of ischemic stroke. Stroke. 2003;34(4):1038–40. doi: 10.1161/01.STR.0000063139.06585.45. [DOI] [PubMed] [Google Scholar]
- 24.Mosnier LO, Buijtenhuijs P, Marx PF, et al. Identification of thrombin activatable fibrinolysis inhibitor (TAFI) in human platelets. Blood. 2003;101(12):4844–46. doi: 10.1182/blood-2002-09-2944. [DOI] [PubMed] [Google Scholar]


