Abstract
Background
The aim of this study was to investigate the safety and effectiveness of fan-shaped distribution and coaxial puncture technology for radioactive iodine 125 (125I) seed implantation in treatment of lung cancer patients with lung dysfunction.
Material/Methods
We enrolled and analyzed 33 lung cancer patients with lung dysfunction diagnosed in our hospital from 2013 to 2014 in this study, all of which were implanted with radioactive 125I seed with technology of fan-shaped distribution and coaxial puncture. The matched peripheral dose (MPD) range was 90–140GY. The brachytherapy planning system (TPS) was used to draw up a preoperative seed implantation plan. The fan-shaped distribution system was applied to simulate a surgery program, and seed implantation pitch was 0.5–1.0 cm. Real-time adjustment was necessary during surgery. Dose distributions were verified by TPS immediately after implantation. Intraoperative and postoperative surgery-related complications were analyzed. All patients were followed up for 6 months, and the local control rate of cancer was evaluated through CT scan.
Results
All patients were operated on successfully. The main surgery-related complications were pulmonary hemorrhage, pleural cavity hemorrhage, and pneumothorax. The local control rates of 2-month, 4-month, and 6-month were 29%, 73%, and 85%, respectively. The total complete remission rate was 18%, the partial response rate was 67%, the stable disease rate was 12%, and the disease progression rate was 3%.
Conclusions
The fan-shaped distribution and coaxial puncture technology for radioactive 125I seed implantation was safe and effective in treating lung cancer patients with lung dysfunction.
MeSH Keywords: Biomedical Technology, Brachytherapy, Lung Neoplasms
Background
In recent years, the morbidity and mortality of lung cancer have been rising annually. Due to the lack of medical examination and occult symptoms of disease, most lung cancer patients are at advanced stages when they are diagnosed, thereby losing the opportunities of surgical resection. Conventional chemoradiotherapy is usually characterized by serious adverse effects and limited efficacy. Image-guided minimally invasive interventional therapy has achieved good local effect [1–4]. However, it is difficult to implement the ablation technique in surgery because most patients with lung cancer are long-term smokers with chronic obstructive pulmonary disease (COPD) and severe pulmonary dysfunction. Radioactive iodine 125 (125I) seed interstitial implantation brachytherapy delivers a high dose within the tumor and a low dose outside the tumor, and, owing to its high effectiveness in tumor control, is widely used in minimally invasive therapy when ablation technique is impracticable. For seed implantation to satisfy the requirement of preoperative planning distribution, the implantation needs to use the template multipoint puncture method, which has been broadly applied in the treatment of prostate cancer and has achieved good results. Both template multipoint puncture and plane interpolation puncture without template can cause sieve-pore puncture points of organs when treating visceral tumors. Patients with lung dysfunction cannot tolerate hemorrhage and pneumothorax induced by the technology, which can be life-threatening. Radioactive seed implantation, based on the technology of fan-shaped distribution and coaxial trocar puncture, was performed in patients with lung dysfunction and good curative effects were obtained.
Material and Methods
Selection criteria
All patients undergoing radioactive seed implantation were pathologically diagnosed with lung cancer before surgery, and none of them received postoperative chemoradiotherapy or any other antineoplastic therapies during the follow-up period; they had at least 1 of the following: (1) a history of COPD; (2) emphysema or obvious fibrosis in both lungs detected by CT examination; and (3) shown by respiratory function tests to be unable to undergo surgery. The TNM status of the patients was determined according to the criteria of the IASLC staging system (seventh edition). Karnofsky performance status (KPS) was used and all patients had to score more than 60.
Information of patients
Thirty-three patients (23 males and 10 females) with lung cancer treated in our hospital from January 2013 to January 2014 were recruited for radioactive seed implantation. The age ranged from 41 to 84 years, and the median age was 67 years. Of a total of 34 lesions, the maximum diameter was 2.3–13 cm, and median was 8 cm. There were 13 cases of lung adenocarcinoma, 12 cases of lung squamous cell carcinoma, 3 cases of lung metastases, and 1 case each of small cell lung cancer, large cell lung cancer, alveolar soft tissue sarcoma, side lung squamous cell carcinoma, side lung adenocarcinoma, and lung cancer with unclear pathology. Prior to receiving radioactive seed implantation, 1 patient was still not diagnosed with a specific type of lung cancer despite 2 lung puncture biopsies, but the rest of the patients obtained clear pathological results via CT-guided lung puncture biopsy. Three patients suffered from postoperative recurrence or metastasis of lung cancer, but the others never underwent pulmonary surgery. Eight patients refused to receive chemoradiotherapy or any other antineoplastic therapies before radioactive seed implantation and in the follow-up period; the others underwent different degrees of chemoradiotherapy before seed implantation, but they quit as a result of poor efficacy or intolerance and had no treatment after seed implantation. Three patients had targeted therapy before seed implantation. KPS was used to access the general conditions of all patients. The scores were 70–90, and median score was 80. The specific characteristics of all patients are presented in Table 1.
Table 1.
The specific characteristics of the patients.
| Age (years) | 67 (41–84) |
| Sex | |
| Male | 23 |
| Female | 10 |
| Pathological type | |
| Lung squamous cell carcinoma | 12 |
| Lung adenocarcinoma | 13 |
| Small cell lung cancer | 1 |
| Large cell lung cancer | 1 |
| Alveolar soft tissue sarcoma | 1 |
| Side lung squamous cell carcinoma and side lung adenocarcinoma | 1 |
| Lung cancer with unclear pathology | 1 |
| Lung metastases | 3 |
| TNM | |
| I | 1 |
| II | 13 |
| III | 9 |
| IV | 10 |
| History of treatment | |
| Chemotherapy/Radiotherapy | 24 |
| No treatment | 9 |
| Lung surgery history | |
| Have history | 0 |
| No history | 33 |
| KPS | |
| 70 score | 3 |
| 80 score | 19 |
| 90 score | 11 |
Equipment and medicine
We used a Philips large-aperture slice spiral CT device with scan parameter 5 mm for layer thickness, tube voltage 120 V, and tube current 250 mAs. The Radioactive Seed Implantation Brachytherapy Planning System (TPS) was manufactured by Beijing ASTRO Technology Co., Ltd. Radioactive 125I seed was produced by China National Nuclear Corporation, and seed activity was 0.7–0.8 mCi. The 18-G seed implantation needles were from the Japan Eight Light Company and were 15–20 cm long (Figure 1). The seed implantation gun and seed pushing rod were also made by Beijing ASTRO Technology Co. Ltd. The casing was a 17-G guide needle produced by Japanese TSK Corporation, and was 9–11 cm long (Figure 1). Gelatin sponges were from Jinling Pharmaceutical Co., Ltd. Thrombin for injection was supplied by Penglai Nuokang pharmaceutical Co., Ltd.
Figure 1.
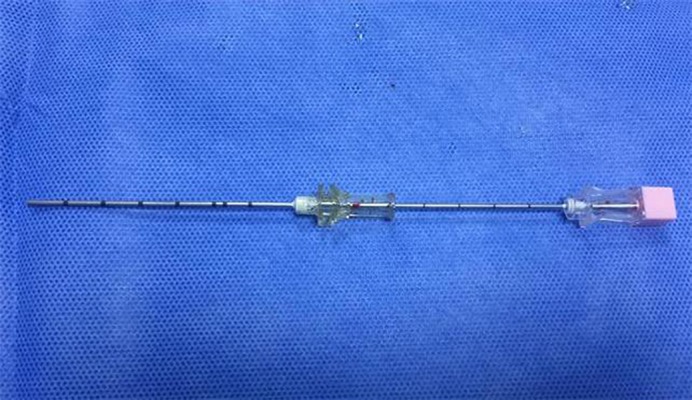
Seed implantation needle in work casing.
Radioactive seed implantation technique
All patients underwent a chest CT scan during the week before surgery, and enhanced examination was also performed if necessary. Image information was input into TPS, and a simulation program for lesions was carried out with fan-shaped distribution method. Clinical target volume (CTV) and planning target volume (PTV) of the tumors were delineated, with PTV expanded 0.5–1.0 cm compared to CTV. In accordance with the TPS fan-shaped distribution scheme, needle puncture was performed at each distribution level, so seed distribution was only simulated on the single plane of the fan-shaped distribution (Figure 2). In surgery, patients were in a position corresponding to TPS plan under local anesthesia. According to the preoperative plan, the work casing punctured the lesion surface or was inserted 0.5–1.0 cm into the lesion; the needle core was pulled out, and the seed implantation needle penetrated the lesions through the casing. After implantation of a row of seeds, the seed implantation needle was withdrawn into the work casing. Then, the casing direction was directly transformed based on the lever principle for the seed implantation needle to penetrate lesions again. Needle direction followed the preoperative TPS scheme as far as possible, with adjustment if deviation existed. Since seed implantation was completed on the same plane distribution, the direction of the work casing was adjusted for more seed implantation. For larger lesions, a single working casing could not meet demands of direction changes, so additional casings were sometimes needed in preoperative planning and during surgery (Figure 3). After seed implantation, 1 mg thrombin was injected by casing, and a gelatin sponge was used for hemostasis. After surgery, CT images were immediately guided into TPS for quality verification. If the dose was insufficient, more seeds were added (Figures 4, 5).
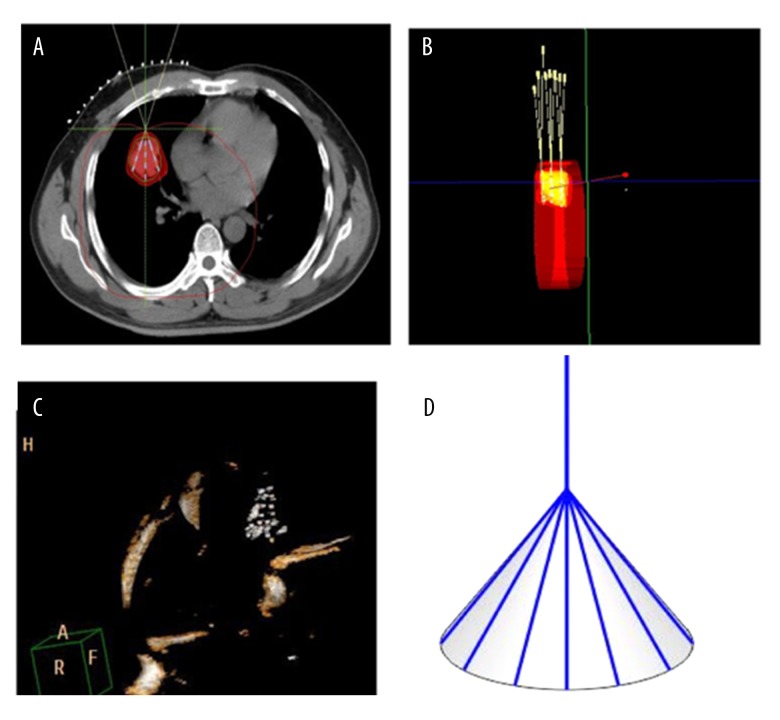
Figure 2.
(A) According to TPS preoperative planning, a simulation image displayed fan-shaped distribution of a right lung lesion, and the intersection point was located on the lesion surface. (B) Three-dimensional figure of multiple plane fan-shaped distribution is shown, and the fan-shaped distribution program was carried out on each plane. (C) After seed implantation, seeds revealed a cone-shape distribution in a three-dimensional figure. (D) Diagram showing cone-shaped distribution of implanted seeds; the top straight line indicated work casing, and distal end seeds were distributed in a cone shape.
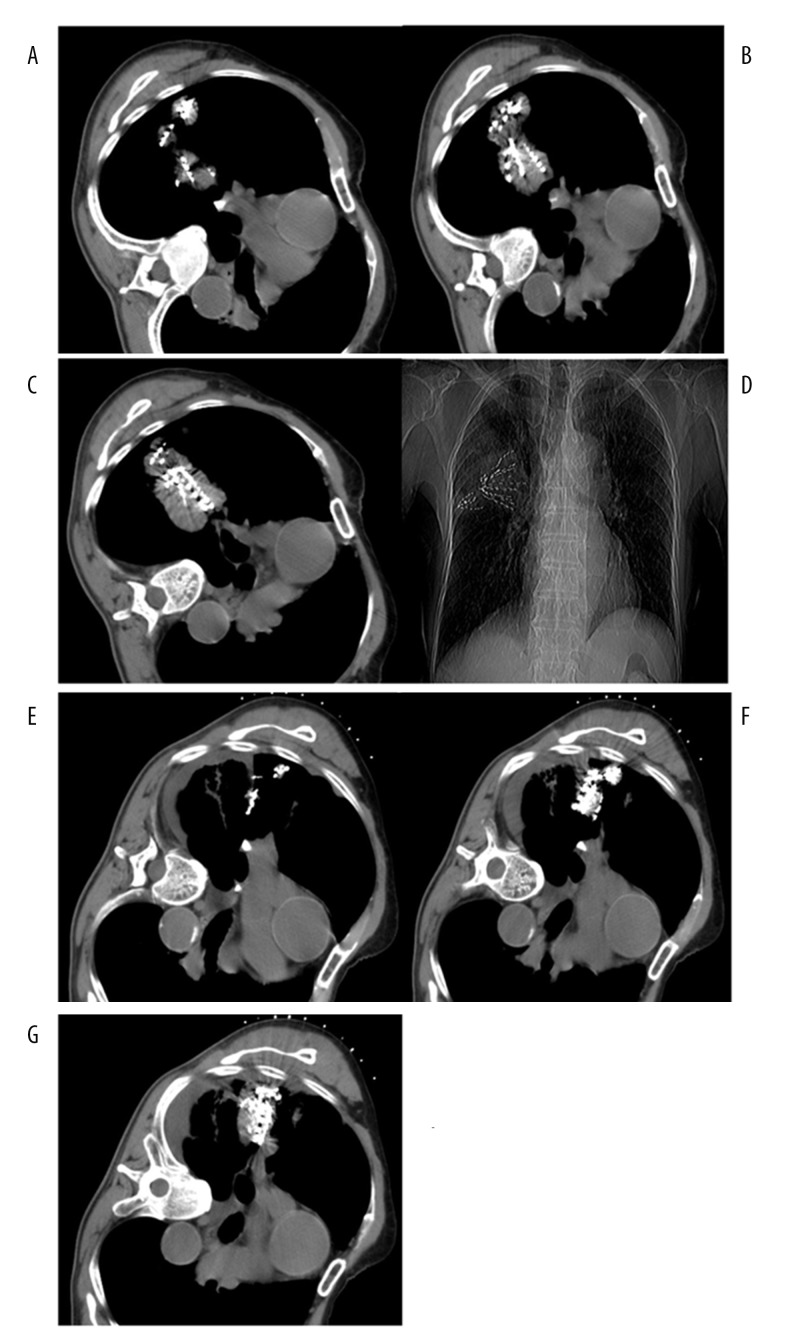
Figure 3.
(A–G) A 51-year-old male with poorly differentiated squamous carcinoma of the right lung. (A–C) Two work casings were used to implant seed from different directions in a large lesion; seed distribution was satisfactory on multiple planes. (D) X-ray radiography showed that there were 2 fan-shaped seed images in the lesion. (E, F) CT images at 6 months after seed implantation showed obviously reduced lesions and seed aggregation was found at the corresponding level.
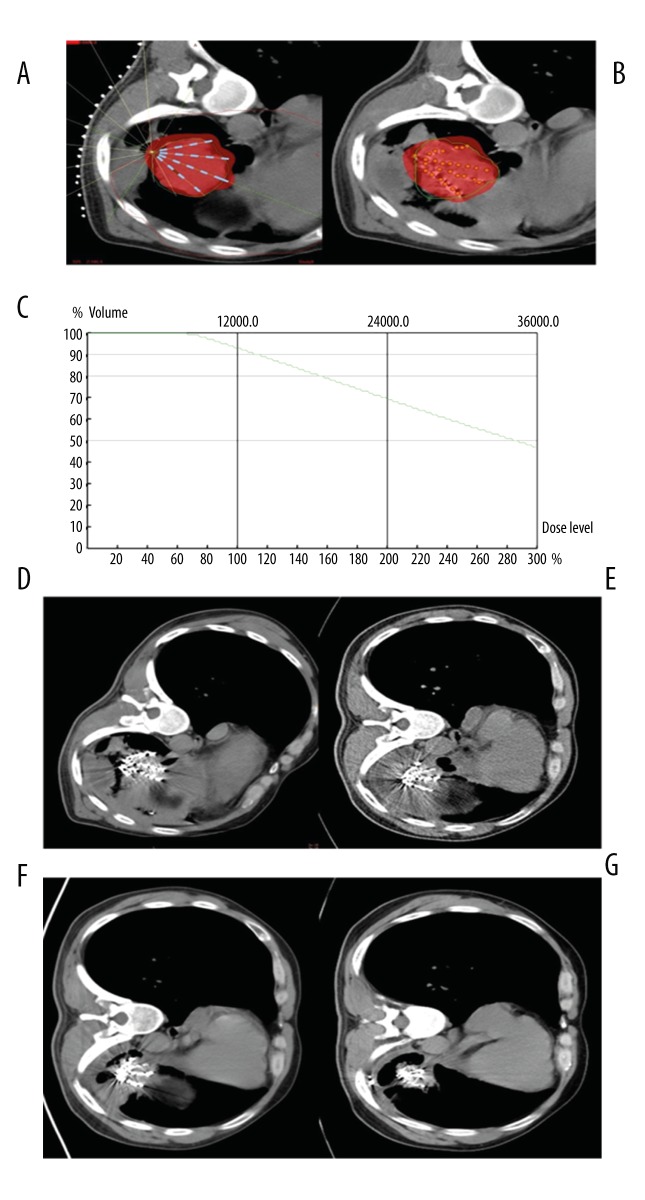
Figure 4.
(A–G) A 67-year-old male with poorly differentiated adenocarcinoma of the left lung. (A) According to TPS preoperative planning, seeds showed a fan-shaped distribution in single plane, and red color presented the coverage area of prescription dose. (B) TPS postoperative verification showed that seed distribution was similar to that of the preoperative plan, and the coverage area of the prescription dose covered the whole lesion. (C) Dose curve of TPS postoperative verification showed more than 90% of lesion volume was covered by 120GY seed dosage. (D) CT image immediately after seed implantation displayed a fan-shaped distribution of seeds. (E) CT image at 2 months after seed implantation showed no obvious reduction of the lesion. (F) CT image at 4 months after seed implantation showed slight reduction of the lesion. (G) CT image at 6 months after seed implantation showed obvious reduction of the lesion and seed aggregation.
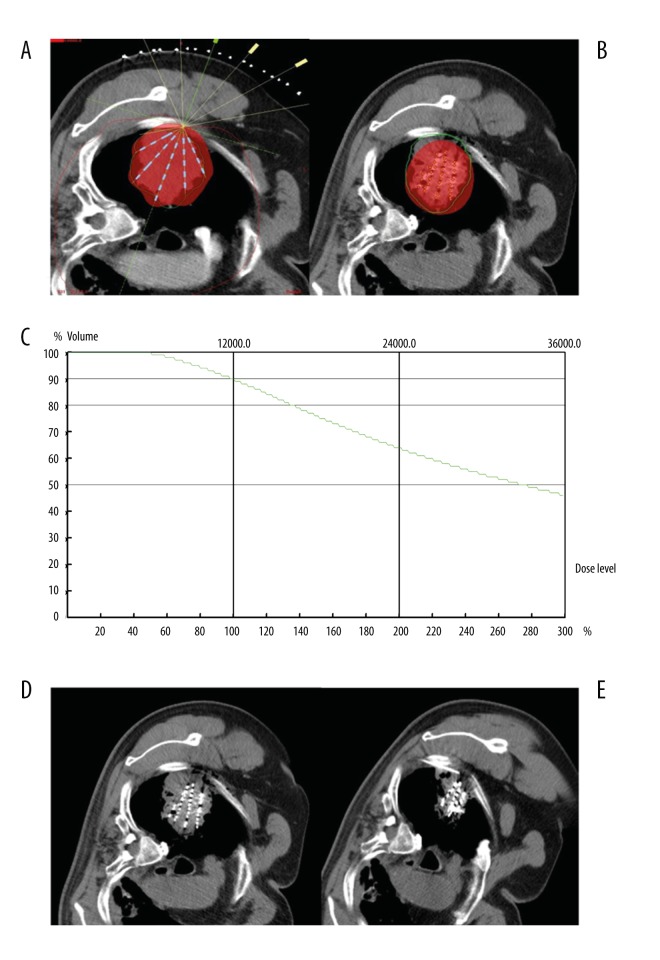
Figure 5.
(A–E) A 77-year-old male patient with poorly differentiated adenocarcinoma of the right lung. (A) According to TPS preoperative planning, seeds showed a fan-shaped distribution in single plane, and red color presented the coverage area of prescription dose. (B) TPS postoperative verification showed that seed distribution was different from that of preoperative plan; the prescription dose did not cover the whole lesion, and there was a dose cold-area near the pleura. (C) Dose curve of TPS postoperative verification showed 90% of lesion volume was covered by 120GY seed dosage. (D) CT image immediately after seed implantation displayed a fan-shaped distribution of seeds. (E) CT image at 2 months after seed implantation showed obvious reduction of the lesion and seed aggregation. Seeds were supplemented through implantation needle in the dose cold-area near the pleura.
Postoperative treatment and follow-up visit
Two patients had slight postoperative bleeding in pulmonary parenchyma, and 2 underwent pleural cavity hematocele and were given daily intramuscular injection with thrombin for 3 days. Two patients had a slight pneumothorax, treated by continuous low-flow oxygen. Postoperative pain occurred in some patients and was treated with Oxycodone and Acetaminophen tablets as symptomatic treatment. A minority of patients with postoperative fever given Xinhuang tablets. After seed implantation surgery, CT images were immediately guided into TPS for quality verification. Then, D90 (dose of 90% gross tumor volume) and V90 (target volume covered by 90% prescribed dose) were calculated. All patients underwent enhanced chest CT scans at 2, 4, and 6 months after the procedure for curative effect evaluation. Seeds were supplemented as needed.
Curative effect judgment
According to the RECIST solid tumor evaluation criteria, complete response (CR) means that the tumor disappeared completely for more than 4 weeks; partial response (PR) means that the sum of tumor maximum diameter was decreased by more than 30% after 4 weeks; progressive disease (PD) means that the sum of tumor maximum diameter was increased by more than 20%, or new lesions occurred; stable disease (SD) was defined as being between PR and PD. Local control rate is the ratio of CR and PR. Postoperative complications and adverse reactions were evaluated using radiation injury classification scheme from Radiation Therapy Oncology Group (RTOG) or European Organization for Research and Treatment of Cancer (EORTC).
Results
Radioactive seed implantation
In this study, 33 patients were operated on successfully. The number of seeds implanted in each patient ranged from 28 to 150, with a median of 71, and seed activity was 0.7–0.8 mCi. Due to the differences in lesion size and location, 22 lesions needed 1 work casing, 10 lesions needed 2 casings, and 2 lesions needed 3 casings. The median matched peripheral dose (MPD) was 120GY (range 90–140GY) after surgery. The median D90 was 118GY (range 95–146GY) and the median V90 was 94% (range 92%–97%).
CT scan results
Postoperative CT scans demonstrated that 1 patient had seed migration in the pleura (Figure 4G). The local control rates at 2, 4, and 6 month were 29%, 73%, and 85%, respectively. The overall CR rate was 18%, the PR rate was 67%, the SD rate was 12%, and the PD rate was 3% (Table 2).
Table 2.
The result distribution of local control rate.
| Follow-up time | CR | PR | PD | SD |
|---|---|---|---|---|
| 2 months | 0 | 10 | 1 | 23 |
| 4 months | 4 | 21 | 2 | 7 |
| 6 months | 6 | 23 | 1 | 4 |
Surgery-related complications
Surgical complications included pulmonary hemorrhage (2 cases), pleural cavity hematocele (2 cases), slight pneumothorax (2 cases), acute radiation pneumonia (grade 0, 29 cases; grade I, 4 cases), and late radiation pneumonia (grade 0, 30 cases; grade I, 3 cases). No radioactive damage above grade II occurred in any patients.
Discussion
Lung cancer has the highest incidence of all malignancies, seriously threatening human life and health. Most patients are in the late stage at diagnosis and cannot undergo surgical resection. Chemoradiotherapy is the standard treatment for patients with locally advanced lung cancer, but the efficacy is still unsatisfactory. Due to the toxic and adverse effects of traditional chemoradiotherapy, many patients cannot tolerate these effects and thus quit therapy. In recent years, many scholars have obtained good results through applying local minimally invasive physical ablations (such as argon-helium cryosurgery and radiofrequency ablation) in the treatment of a single lesion or a small number of multiple lesions in the lung. However, some patients have poor pulmonary function and lesions may adjoin important organization structures, which contribute to incomplete ablation in lesions and poor local control rate of tumors. Radioactive 125I seed interstitial implantation is a brachytherapy technology featuring a small radiation radius. After implantation, the tumor itself has a high therapeutic dose, and surrounding normal tissues receives less radiation, so it has been widely used in treatment of various solid tumors [5–12]. Furthermore, this technology has high conformality because seeds are implanted on the basis of tumor shape, which was first applied in prostate cancer therapy and achieved great effects.
Radioactive seed implantation in lung cancer is mainly conducted by CT guidance, and the most common method is parallel needle distribution [13–19]. Preoperative planning and postoperative verification can be carried out based on TPS. In parallel distribution, multiple needles puncture pleurae and lung tissues, easily resulting in intraoperative and postoperative pneumothorax and pulmonary hemorrhage. Intraoperative pneumothorax may lead to oxygen reduction, lesion translocation, and even an unsuccessful operation in patients with poor pulmonary function. If the location of the lesion is deeper, pulmonary hemorrhage may cause intraoperative hemoptysis and operation failure. Parallel needle distribution is quite easily occluded by ribs and other bones due to multiple needle points and large range, which make it difficult for seeds to reach preoperative planning distribution, thus affecting the efficacy. In this study, the coaxial puncture-based fan-shaped distribution technology was used to implant radioactive seeds in malignant lung tumors. The single puncture of the work trocar bridges the lesion and external environment. It is different from the general fan-shaped distribution and therefore greatly reduces complications.
TPS was used for preoperative planning in the present study, and the fan-shaped distribution method was applied to simulate seed distribution in the axial level in each plan. Surgical position conformed to the TPS program. Generally, only 1 casing was used for lesions less than 3 cm in diameter, and additional casings were used for lesions more than 3 cm in diameter. If the intraoperative puncture pathway was involved in more muscle tissues, the directional transformation of the work casing was more difficult, thereby requiring more casings. A total of 34 lesions were found in the patients: 22 lesions needed 1 work casing and the rest needed more than 2 casings each. In principle, work casings needed to go 5–10 mm inside the lesions to prevent repeated puncturing of lung tissues from implanted needles, and also removed intratumoral hemorrhage, thus protecting patients from hemoptysis. The coaxial puncture technique differs from the general fan-shaped distribution method, and was used in surgery. After the casing reached its desired location, the seed implantation needle punctured the casing repeatedly for direction adjustment. Seeds were centered at the end of the casing and then scattered inside the lesion. The preoperative TPS fan-shaped distribution plan provides a reference for single-plane seed distribution. The adjustment of each puncture direction was based on the previous puncture direction and initial position of the work casing. Due to the fan-shape distribution, the distance between 2 seeds gradually increased in the distal end of the lesion, so seed density needs to be increased to reduce the cold-area of the dose, and seed density in the near end needed to be decreased inversely to control the hot-area of the dose. According to the different pathological types of lesions, postoperative MPD was 90–140 GY with the median value 120 GY. The vast majority of patients reached the prescription dose after postoperative TPS verification; the cold-area of the dose occurred in 2 cases and seeds were added later. No other treatment was carried out in the patients within 6 months after seed implantation, which eliminated the influence on the short-term curative effect. MPD was adjusted according to pathological type and differentiation degree of the lesion. In principle, the prescription dose was larger in the lesion which was not sensitive to radiotherapy pathologically and had lower degree of differentiation. In the present study, the local control rates at 2, 4, and 6 months were 29%, 73%, and 85% respectively; the total complete remission rate was 18%; the partial response rate was 67%; the stable disease rate was 12%; and the progression disease rate was 3%. The findings were slightly different from those in previous studies [15–17], which might be due to differences in lesion size and follow-up time. Two patients had a slight pulmonary hemorrhage and 1 had a slight hemoptysis during the surgery, but the operation was not noticeably affected. The main reason was that the work casing failed to go inside the lesion through direction adjustment, thus resulting in intratumoral hemorrhage and further seeping into the lungs. After surgery, 2 patients suffered from pleural cavity hematocele and another 2 had a slight pneumothorax. The major causes of pleural cavity hematocele were: (1) if the lesion was rich in blood supply, repeated puncture caused severe intratumoral hemorrhage, and blood might infiltrate the pleural crevasse along the edge of the casing; and (2) each directional adjustment of the casing contributed to tearing the pleural crevasse, and the blood could enter the pleural cavity. No radioactive damage above grade II occurred in patients postoperatively, and normal lung tissues around the lesion were effectively protected. Compared with the traditional method, the incidence rate of complications was lower in this group of patients. The use of work casings for intratumoral puncture required accurate puncture technique and a three-dimensional sense of space, so that every puncture correctly placed and the number of punctures was reduced as much as possible. In addition, full preoperative anesthesia was necessary to reduce intraoperative stimulation of the pleura induced by the adjusting casing direction, which not only relieved the pain, but also ensured a successful operation.
Conclusions
Fan-shaped distribution technology based on coaxial trocar puncture has high safety and effectiveness. Compared with the traditional distribution method, use of the work casing effectively protected normal lung tissue, reduced lung injury caused by repeated punctures, and lowered the occurrence rate of intraoperative and postoperative complications while ensuring efficacy. The operation planning could be adjusted flexibly during surgery to avoid bone blocking the puncture path. However, due to the lack of a well-matched TPS preoperative plan, as well as the influence of intraoperative puncture technique, seed distribution may be uneven and fail to completely conform to the TPS simulation program, thereby leading to inaccurate estimation of dosimetry. Therefore, postoperative TPS quality verification was particularly important; if there was a cold-area of dose, seeds needed to be supplemented quickly.
Footnotes
Source of support: Departmental sources
References
- 1.Kodama H, Yamakado K, Hasegawa T, et al. Radiofrequency ablation using a multiple-electrode switching system for lung tumors with 2.0–5.0-cm maximum diameter: Phase II clinical study. Radiology. 2015;277(3):895–902. doi: 10.1148/radiol.2015141153. [DOI] [PubMed] [Google Scholar]
- 2.Sun YH, Song PY, Guo Y, Sheng LJ. Computed tomography-guided percutaneous microwave ablation therapy for lung cancer. Genet Mol Res. 2015;14(2):4858–64. doi: 10.4238/2015.May.11.18. [DOI] [PubMed] [Google Scholar]
- 3.Tavares E, Castro A, Freitas S, Portilha A, et al. Efficacy and safety of percutaneous radiofrequency thermal ablation in the treatment of lung cancer lesions. Acta Med Port. 2015;28(1):63–69. doi: 10.20344/amp.5620. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Tian J, Zhao L, et al. CT-guided conformal cryoablation for peripheral NSCLC: Initial experience. Eur J Radiol. 2012;81(11):3354–62. doi: 10.1016/j.ejrad.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 5.Qin QH, Huang BS, Tan QX, et al. Radiobiological effect induced by different activities of (125)I seed brachytherapy in a hepatocellular carcinoma model. Int J Clin Exp Med. 2014;7(12):5260–67. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang FJ, Li CX, Wu PH, et al. [Radioactive seed 125I implantation in treating recurrence and metastasis after liver transplantation in hepatoma]. Zhonghua Yi Xue Za Zhi. 2007;87(14):956–59. [in Chinese] [PubMed] [Google Scholar]
- 7.Zhang FJ, Li CX, Jiao DC, et al. CT guided 125iodine seed implantation for portal vein tumor thrombus in primary hepatocellular carcinoma. Chin Med J (Engl) 2008;121(23):2410–14. [PubMed] [Google Scholar]
- 8.Nag S, DeHaan M, Scruggs G, et al. Long-term follow-up of patients of intrahepatic malignancies treated with iodine-125 brachytherapy. Int J Radiat Oncol Biol Phys. 2006;64(3):736–44. doi: 10.1016/j.ijrobp.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 9.van der Noordaa ME, Pengel KE, Groen E, et al. The use of radioactive iodine-125 seed localization in patients with non-palpable breast cancer: A comparison with the radioguided occult lesion localization with 99m technetium. Eur J Surg Oncol. 2015;41(4):553–58. doi: 10.1016/j.ejso.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Shen X, Li Y, Zhang Y, et al. An analysis of brachytherapy with computed tomography-guided permanent implantation of Iodine-125 seeds for recurrent nonkeratin nasopharyngeal carcinoma. Onco Targets Ther. 2015;8:991–97. doi: 10.2147/OTT.S83140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherian S, Kittel JA, Reddy CA, et al. Safety and efficacy of iodine-125 permanent prostate brachytherapy in patients with J-pouch anastomosis after total colectomy for ulcerative colitis. Pract Radiat Oncol. 2015;5(5):e437–42. doi: 10.1016/j.prro.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Liu YH, Xu L, Liu LH. Efficacy of permanent iodine-125 seed implants and gemcitabine chemotherapy in patients with platinum – resistant recurrent ovarian carcinoma. Asian Pac J Cancer Prev. 2014;15(20):9009–13. doi: 10.7314/apjcp.2014.15.20.9009. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y, Xiao YY, Li SD, et al. The treatment of non-small cell lung cancer by interstitial I-125 seeds implantation combined with chemotherapy and Chinese medicine. Chin J Integr Med. 2012;18(9):663–69. doi: 10.1007/s11655-012-1203-y. [DOI] [PubMed] [Google Scholar]
- 14.Johnson M, Colonias A, Parda D, et al. Dosimetric and technical aspects of intraoperative I-125 brachytherapy for stage I non-small cell lung cancer. Phys Med Biol. 2007;52(5):1237–45. doi: 10.1088/0031-9155/52/5/002. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Guan J, Yang L, et al. Iodine-125 brachytherapy improved overall survival of patients with inoperable stage III/IV non-small cell lung cancer versus the conventional radiotherapy. Med Oncol. 2015;32(1):395. doi: 10.1007/s12032-014-0395-8. [DOI] [PubMed] [Google Scholar]
- 16.Jiang G, Li Z, Ding A, et al. Computed tomography-guided iodine-125 interstitial implantation as an alternative treatment option for lung cancer. Indian J Cancer. 2015;51(Suppl 2):e9–12. doi: 10.4103/0019-509X.151999. [DOI] [PubMed] [Google Scholar]
- 17.Huang Q, Chen J, Chen Q, et al. Computed tomographic-guided iodine-125 interstitial implants for malignant thoracic tumors. Eur J Radiol. 2013;82(11):2061–66. doi: 10.1016/j.ejrad.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 18.Niu L, Zhou L, Xu K, Mu F. Combination of cryosurgery and Iodine-125 seeds brachytherapy for lung cancer. J Thorac Dis. 2012;4(5):504–7. doi: 10.3978/j.issn.2072-1439.2012.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu L, Chen J, Yao F, et al. Percutaneous cryoablation for stage IV lung cancer: A retrospective analysis. Cryobiology. 2013;67(2):151–55. doi: 10.1016/j.cryobiol.2013.06.005. [DOI] [PubMed] [Google Scholar]