Abstract
Background
Leukocyte telomere length (LTL) is regarded as a potential marker of biological aging. Oxidative stress plays a major role in the rate of telomeric DNA loss. The aim of this study was to explore whether the LTL was shorter in Chinese patients with premature coronary artery disease (PCAD) than in non-CAD controls and to determine the relationship between oxidative stress and LTL shortening in this population.
Material/Methods
Patients for coronary angiography were recruited. In total, 128 patients with PCAD and 128 non-CAD controls were enrolled. Samples of circulating leukocytes and plasma were collected. The mean LTL was measured using a polymerase chain reaction-based assay and expressed as the ratio of telomere repeat copies to single-copy gene (SCG) copies (T/S ratio). Reactive oxygen species (ROS) levels and total antioxidant capacity (T-AOC) were determined in plasma.
Results
Both the T/S ratio (0.88±0.86 vs. 1.10±0.57, P=0.015) and telomere base pairs (4.97±1.37 kb vs. 5.32±0.91 kb, P=0.015) were significantly shorter in the PCAD group than in non-CAD controls. The T-AOC levels of the PCAD group were significantly lower than those of the non-CAD controls (0.482 mM [0.279, 0.603 mM]) vs. 0.778 mM [0.421, 0.924 mM], P=0.000). The ratio of T-AOC to ROS in the PCAD patients was significantly decreased compared to that of the non-CAD controls (0.1026±0. 1587 [Mm*ml/ng] vs. 0.1435±0.1946 [Mm*ml/ng], P=0.013).
Conclusion
The results point to a potential link between reduced LTLs in patients with PCAD and early onset of atherosclerosis. The decline in antioxidant capacity may play an important role in accelerating the attrition of telomeres in PCAD patients.
MeSH Keywords: Coronary Artery Disease, Oxidative Stress, Telomere
Background
Telomeres are specialized DNA-protein structures found at the end of all chromosomes, which preserve chromosome stability and integrity [1]. In most proliferating cells, the telomere length is dynamic [2]. In human somatic cells, it decreases gradually (50–200 bp) in each cell division [2]. The leukocyte telomere length (LTL) has emerged as a potential biomarker of aging in the last decade [3]. Besides the end-replication problem caused by telomere attrition, oxidative stress and inflammation may play important roles in accelerating telomere loss [4,5]. According to some research, telomere length may be a promising marker of chronic oxidative stress [6].
Coronary artery disease is closely related to aging. Previous studies demonstrated that shorter LTLs were associated with CAD [7–10]. The West of Scotland Primary Prevention Study found that individuals with shorter LTLs were at more risk of developing coronary heart disease than individuals with longer LTLs, and that the increased risk was substantially attenuated by statin treatment [11]. An earlier study demonstrated that the LTL was associated with the risk of premature myocardial infarction [12]. In that study, the age- and sex-adjusted LTLs of patients with myocardial infarction were significantly shorter than those of controls. On average, the LTLs were equivalent to those of controls who were older (11.3 years) than the myocardial infarction patients, and other coronary risk factors did not account for the difference. Data from the Heart and Soul Study demonstrated that reduced LTLs was associated with all-cause mortality in patients with stable CAD [13]. In that study, the prognostic value of short telomeres in predicting death was not explained by existing clinical, inflammatory, and echocardiographic markers of risk. Generally, PCAD is defined as CAD in males less than 55 years old and females less than 65 years old. The study showed that the PCAD patients exhibited a higher rate of coronary artery occlusion than that of older patients. Traditional risk factors for oxidative stress, such as dyslipidemia, hypertension, diabetes, and smoking, are highly correlated with PCAD, suggesting that oxidative stress and LTL shortening may be associated with the disease. The aim of the present study was to measure LTLs in Chinese PCAD patients and controls and examine the contribution of oxidative stress to LTL shortening in this population.
Material and Methods
Subjects and treatments
Patients with PCAD presenting to Peking Union Medical College Hospital for coronary angiography between January 2013 and December 2014 were recruited into the study. PCAD was defined in males as CAD among those aged less than 55 years and in females as CAD among those aged less than 65 years. CAD was diagnosed according to the presence of ≥50% stenosis in the main coronary artery. Subjects with <20% stenosis in the main coronary artery were defined as non-CAD patients. Patients with chronic and current infection, malignancy, autoimmune disease, vasculitis, dermatitis, asthma, cirrhosis, severe renal failure (an estimated glomerular filtration rate of <30 mL/min), or shock were excluded from the study.
Coronary angiography was performed with a conventional angiography unit (Integris H; Philips Medical Systems, Amsterdam, the Netherlands). Coronary artery stenosis was imaged in the center of the field from multiple projections. Using the Quantity Coronary analysis system (Integris H; Philips Medical Systems, Amsterdam, the Netherlands), 2 interventional cardiologists with at least 5 years’ experience, who were blinded to the patients’ clinical information, evaluated the severity of coronary atherosclerotic lesions from at least 2 projections. The Gensini score was used to quantify the severity of the atherosclerosis, with each score for coronary stenosis calculated according to the degree of luminal narrowing and localization [14].
This study was approved by the Ethics Committee of Peking Union Medical College Hospital and conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants.
DNA isolation and telomere length detection
Blood samples were collected from the radial or femoral artery after sheath insertion prior to coronary angiography. After centrifugation at 3000 rpm for 10 min, the plasma was separated and placed in a new tube for biochemical measurements. Leukocyte samples were placed in new tubes for DNA analysis. The plasma and leukocytes were stored at −80°C until used. Genomic DNA was isolated from the leukocyte samples using a TIANamp Genomic DNA Kit (Beijing, China). The relative mean LTL was measured with the quantitative real-time PCR based technique, as previously described [15]. Briefly, the relative telomere length was calculated as the ratio of telomere repeat copies to single-copy gene (36B4, SCG) copies (T/S ratio, ratio of telomere repeat copy number [T] to single-copy gene copy number [S]). In each sample, the quantity of telomere repeats and SCG copies was determined in comparison to that of reference samples. A standard curve was not constructed. All the PCRs were performed on a CFX Touch real-time PCR detection system (Bio-Rad, CA). The data from each PCR were analyzed using comparative quantification analysis software (Bio-Rad CFX Manager, Bio-Rad, USA). The coefficient of variation in repeated measurements of the samples was 4.3%. The T/S values were converted to kilobases (kb) by the following formula: telomere base pairs (Kb)=1.585×T/S ratio +3.582 [16].
Measurement of the total antioxidant capacity (T-AOC) and reactive oxygen species (ROS)
The plasma T-AOC assay (Beyotime Institute of Biotechnology, China) was performed according to the instructions of the manufacturer. The T-AOC of plasma was determined by its capacity to inhibit peroxidase-mediated formation of the 2,2-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS+) radicals. In this assay, the relative inhibition of ABTS+ formation in the presence of plasma is proportional to the antioxidant capacity of the sample. The stock solutions include ABTS solution and oxidant solution. A working solution was prepared by mixing the 2 stock solutions in equal quantities and allowing them to react for 16 h at room temperature in the dark. The solution was then diluted by mixing 1 mL of the working solution with 90 ml of 80% ethanol to obtain an absorbance of 0.7±0.05 at 734 nm. A fresh ABTS solution was prepared for each assay. Plasma samples (10 μl), which were collected as described above, with a concentration range of 0.05–3.00 mM, were mixed with 200 μl of fresh ABTS solution, and the mixture was left at room temperature for 6 min. The absorbance was then measured at 734 nm. A standard curve was constructed using Trolox as a reference compound. The total antioxidant capacity of the sample was calculated as follows:
where A1 was the absorbance of the control (ABTS solution without test sample) and A2 was the absorbance in the presence of the test sample. The IC50 value was then determined (i.e., the concentration at which 50% of the ABTS radicals were scavenged).
A human ROS ELISA Kit (Bio-Swamp, China) was used for the quantitative determination of ROS concentrations in the plasma. This kit uses purified human ROS antibody to coat microtiter plate wells, and ROS and the antibody combine when the sample is added to the wells. Horseradish peroxidase-labeled anti-ROS antibody was added, resulting in the formation of an antibody-antigen-enzyme-antibody complex. After washing completely, 3,3′,5,5′-tetramethylbenzidine was added as substrate solution, turning the solution blue as a result of catalysis of the horseradish peroxidase. The reaction was terminated by the addition of a sulfuric acid solution. Absorbance was measured at 450 nm using a plate reader, and the plasma ROS concentration was calculated using associated software.
Determination of blood lipids
Each patient’s body mass index (BMI) was calculated as the weight (kg) divided by squared height (m2). Hypertension and diabetes were diagnosed using current guidelines [17,18]. Peripheral venous blood was drawn from the antecubital vein after a 12-h fasting period and used to determine the lipid profile (total cholesterol [TC], low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], triglycerides [TG], creatinine [Cr], and high-sensitivity C-reactive protein [hsCRP]).
Statistical analysis
The normality of the data distribution was examined by the Kolmogorov-Smirnov normality test. Non-normally distributed data were log transformed. Data on the clinical and biological characteristics of patients that showed a normal distribution are expressed as the mean ± standard deviation. Skewed variables are expressed as the median and interquartile range. In the comparison of the patients and controls, independent-sample t-tests (unpaired) were used for continuous variables, and a chi-square test was used for categorical variables. The relation of telomere length with the quantitative variables was analyzed with Spearman’s partial correlations, adjusted separately for age and sex in the patient and control groups. A linear regression analysis was used to evaluate the association of clinical characteristics with LTL and oxidative stress. All analyses were performed using SPSS 19.0 (SPSS Inc.). A 2-sided P<0.05 value indicated statistical significance.
Results
General clinical data
The baseline demographics and laboratory values of the patients and controls are shown in Table 1. The study consisted of 256 participants: 128 patients and 128 age- and sex-matched controls. The average age of the patients and controls was 48 years. Compared with the controls, the PCAD patients had significantly higher BMIs; significantly higher ALT, TC, TG, and hs-CRP levels; significantly higher rates of smoking, dyslipidemia, hypertension, type 2 diabetes, and family history of CHD; and lower HDL-C levels.
Table 1.
Clinical characteristics of controls and patients with PCAD.
| Clinical characteristics | Controls (n=128) | PCAD patients (n=128) | P value |
|---|---|---|---|
| Age (years) | 48.5 (7.33) | 48.6 (7.26) | 0.539 |
| Male (%) | 74 (57.8%) | 74 (57.8%) | 1.000 |
| BMI(kg/m2) | 24.0 (2.95) | 26.2 (3.23) | 0.000 |
| ALT (U/L) | 19.7 (14,23) | 36.0 (18, 40.75) | 0.012 |
| Creatinine (μmol/L) | 73.5 (11.9) | 72.2 (21.6) | 0.550 |
| BUN (mmol/L) | 5.04 (1.73) | 5.48 (1.92) | 0.063 |
| Glucose (mg/dL) | 5.75 (1.21) | 6.12 (1.77) | 0.060 |
| HbA1C (%) | 5.87 (1.26) | 6.21 (1.40) | 0.152 |
| TC (mmol/L) | 4.24 (1.90) | 4.68 (1.06) | 0.001 |
| TG (mmol/L) | 1.10 (0.9, 1.36) | 1.98 (1.19, 2.35) | 0.020 |
| HDL-C (mmol/L) | 1.10 (0.25) | 1.00 (0.21) | 0.045 |
| LDL-C (mmol/L) | 2.64 (0.67) | 2.74 (0.77) | 0.558 |
| hs-CRP (ng/mL) | 0.88 (0.45, 1.02) | 9.30 (0.74, 5.925) | 0.035 |
| HTN (%) | 45 (35.2%) | 82 (64.1%) | 0.000 |
| T2D (%) | 30 (23.4%) | 43 (33.6%) | 0.096 |
| Dyslipidemia (%) | 47 (36.7%) | 78 (60.9%) | 0.000 |
| Family history of PCAD (%) | 29 (22.7%) | 48 (37.5%) | 0.014 |
| Smoking (%) | 37 (28.9%) | 64 (50.0%) | 0.001 |
Data are mean ±SD, number (%) or median and IQR. The bold – significant. BMI – body mass index; ALT – alanine transaminase; BUN – blood urea nitrogen; TC – total cholesterol; TG – triglycerides; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; hs-CRP – high-sensitivity C-reactive protein; HTN – hypertension; T2D – type 2 diabetes; PCAD – premature coronary artery disease.
The mean LTL was successfully measured in all 256 participants. Compared with the controls, the PCAD patients had significantly shorter LTLs, with a T/S ratio of 0.88±0.86 vs. 1.10±0.57 (P=0.015) and telomere base pairs of 4.97±1.37 kb vs. 5.32±0.91 kb (P=0.015).
Factors influencing the LTL
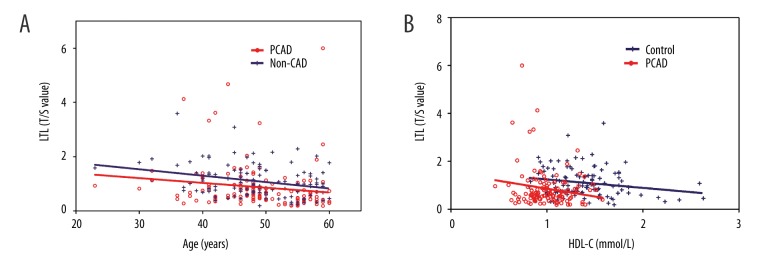
The results of the analysis of the relationship between LTLs and baseline demographics revealed that they did not differ between males and females. However, LTLs decreased steadily with age, at a mean rate of 0.085±0.027 (r=−0.316, P=0.000) per decade for the controls and a mean rate of 0.067±0.016 (r=−0.275, P=0.002) per decade for the PCAD patients (Figure 1). There was no significant difference in the rate of decline in the 2 groups. After adjustment for age and sex, the relative LTL decreased in accordance with HDL-C levels in the controls. Furthermore, the linear regression analysis revealed that ALT, glucose, and TG levels were negatively correlated with age- and sex-adjusted LTLs in all the subjects.
Figure 1.
Linear regression analysis of the association between leukocyte telomere length (LTLs) and age (A) and high-density lipoprotein cholesterol (HDL-C) levels, adjusted for age and sex (B) in the controls and PCAD patients. The telomere length is plotted as the T/S ratio.
Bivariate correlation of the association of clinical factors with LTLs
Table 2 presents the results of the bivariate correlation test of the relationship between clinical factors and LTLs. As shown in the table, the LTL was positively associated with age and sex-adjusted levels of T-AOC (P=0.046) and the ratio of T-AOC to ROS (P=0.002) but inversely associated with dyslipidemia (P=0.049) and type 2 diabetes (P=0.036) in PCAD patients. Unlike the PCAD patients, the LTL in the controls was negatively associated with the level of ROS (P=0.022), hypertension (P=0.043), and smoking (P=0.019).
Table 2.
Bivariate correlation for association of clinical factors and LTL.
| Clinical factors | LTL | |||
|---|---|---|---|---|
| pCAD patients | Non-CAD controls | |||
| Spearman coefficient | P value | Spearman coefficient | P value | |
| BMI (kg/m2) | −0.185 | 0.436 | −0.018 | 0.909 |
| ALT (U/L) | −0.142 | 0.551 | 0.008 | 0.958 |
| Creatinine (μmol/L) | 0.018 | 0.941 | −0.210 | 0.167 |
| BUN (mmol/L) | −0.146 | 0.538 | −0.280 | 0.063 |
| Glucose (mmol/L) | 0.002 | 0.992 | −0.109 | 0.474 |
| HGB (g/L) | −0.197 | 0.405 | −0.194 | 0.201 |
| TC (mmol/L) | −0.069 | 0.773 | −0.132 | 0.386 |
| TG (mmol/L) | −0.051 | 0.830 | −0.267 | 0.076 |
| HDL-C (mmol/L) | 0.224 | 0.342 | −0.209 | 0.168 |
| LDL-C (mmol/L) | −0.212 | 0.370 | −0.021 | 0.891 |
| hs-CRP (ng/ml) | −0.062 | 0.796 | 0.254 | 0.092 |
| HbA1C (%) | 0.027 | 0.909 | −0.116 | 0.449 |
| Gensini score | 0.087 | 0.716 | —— | —— |
| history of MI | −0.297 | 0.203 | —— | —— |
| family history of PCAD (%) | −0.371 | 0.107 | −0.039 | 0.798 |
| HTN | −0.111 | 0.642 | −0.303 | 0.043 |
| dyslipidemia | −0.415 | 0.049 | −0.207 | 0.173 |
| T2D | −0.472 | 0.036 | −0.065 | 0.669 |
| smoking | 0.077 | 0.748 | −0.348 | 0.039 |
| level of ROS (ng/ml) | −0.050 | 0.834 | −0.034 | 0.022 |
| T-AOC (mM) | 0.451 | 0.046 | −0.161 | 0.290 |
| T-AOC/ROS value | 0.641 | 0.002 | 0.122 | 0.424 |
The bold – significant difference. LTL – leukocyte telomere length; PCAD – premature coronary artery disease; BMI – body mass index; ALT – alanine transaminase; BUN – blood urea nitrogen; HGB – hemoglobin; TC – total cholesterol; TG – triglycerides; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; hs-CRP – high-sensitivity C-reactive protein; MI – myocardial infarction; HTN – hypertension; T2D – type 2 diabetes; ROS – reactive oxygen species; T-AOC – total antioxidant capacity.
Levels of oxidative stress markers in the PCAD patients
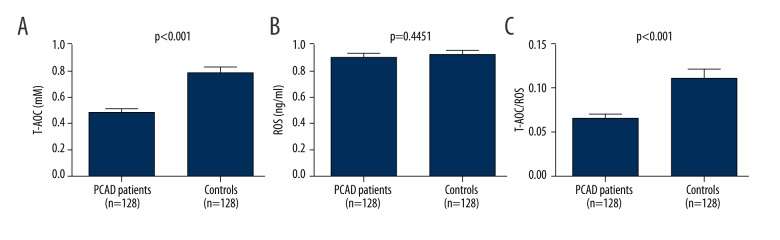
Figure 2 presents the levels of oxidative stress markers in the 2 groups. Compared with the controls, the PCAD patients had significantly lower T-AOC levels (0.482 mM (0.279 mM, 0.603 mM) vs. 0.778 mM (0.421 mM, 0.924 mM), P=0.000). However, the plasma ROS levels were similar in the 2 groups (8.88 ng/ml [5.79 ng/ml, 11.73 ng/ml] vs. 9.16 ng/ml [6.39 ng/ml, 11.52 ng/ml]). The mean ratio of T-AOC to ROS, a reflection of the oxidant-antioxidant status, in the PCAD patients was decreased by 40.4% compared to that of the controls (0.1026±0.1587 [Mm*ml/ng] vs. 0.1435±0.1946 [Mm*ml/ng], P=0.013), pointing to increased oxidative stress in the PCAD patients. As shown in Figure 3, the LTL was positively associated with T-AOC/ROS levels in the PCAD patients, showing that longer LTLs were associated with more severe oxidative stress.
Figure 2.
Comparison of the level of oxidative stress markers in the 2 groups. (A) T-AOC; (B) level of ROS; (C) T-AOC/ROS value. Histogram represents mean ±SD.
Figure 3.
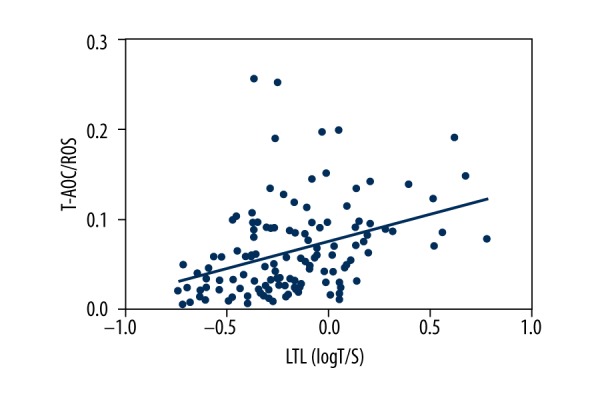
The relationship between LTLs and T-AOC/ROS levels in the PCAD patients.
Multiple linear regression analysis
Table 3 shows the results of the multiple linear regression analyses performed to determine which parameters were crucial in determining the LTL in both groups. The enter method was used in the regression analysis. For PCAD patients, the presence of type 2 diabetes (P=0.006) and T-AOC/ROS values (P=0.002) were independently associated with LTLs. In the controls, the level of ROS (P=0.003) was independently associated with LTLs.
Table 3.
Multiple linear regression model for association between related factors and LTL.
| Variables | PCAD patients | Controls | ||||
|---|---|---|---|---|---|---|
| B | 95%CI | P | B | 95%CI | P | |
| Age (years) | −0.041 | −0.015~0.011 | 0.798 | −0.097 | −0.011~0.005 | 0.451 |
| Sex (Male=1, Female=0) | 0.150 | −0.115~0.297 | 0.385 | 0.153 | −0.038~0.185 | 0.195 |
| HTN (Yes=1, No=0) | 0.044 | −0.872~0.844 | 0.975 | −0.017 | −0.108~0.073 | 0.704 |
| Dyslipidemia (Yes=1, No=0) | −0.088 | −0.039~0.539 | 0.558 | 0.009 | −0.098~0.117 | 0.863 |
| T2D (Yes=1, No=0) | −0.219 | −0.069~−0.369 | 0.006 | −0.109 | −0.249~0.031 | 0.124 |
| Smoking (Yes=1, No=0) | 0.082 | −0.206~0.369 | 0.575 | −0.093 | −0.180~−0.006 | 0.066 |
| Level of ROS (ng/ml) | −0.250 | −0.871~0.371 | 0.427 | −0.337 | −0.553~−0.120 | 0.003 |
| T-AOC/ROS value | 0.273 | 0.110~0.436 | 0.002 | 0.002 | −0.001~0.006 | 0.172 |
The bold – significant difference. 95% CI – 95% confidence interval. Other abbreviations as in Table 2. B – values are not standardized.
As shown by the multiple linear regression model of the relationship between oxidative stress and biochemical characteristics, the level of ROS showed a significant positive correlation with age in the PCAD group (P=0.043). In addition, in the PCAD patients, T-AOC displayed a significant positive correlation with age (P=0.014) and LDL-C levels (P=0.033), and the ratio of T-AOC to ROS showed a significant positive correlation with age (P<0.001) and a history of myocardial infarction MI (P=0.001). In contrast, in the controls, only age was correlated with ROS (P=0.001) and the ratio of T-AOC to ROS (P=0.025). None of the other clinical factors were independently associated with T-AOC in the controls (Table 4).
Table 4.
Linear regression of association between clinical factors and oxidative stress.
| Oxidative stress | Variables | PCAD patients | Controls | ||||
|---|---|---|---|---|---|---|---|
| B | 95%CI | P | B | 95%CI | P | ||
| ROS | Age | 0.088 | 0.003~0.173 | 0.043 | 0.140 | 0.127~0.153 | 0.001 |
| Sex | −1.251 | −4.750~2.248 | 0.477 | −0.343 | −6.440~1.053 | 0.155 | |
| BMI | 0.197 | 0.012~0.382 | 0.598 | −0.020 | 0.061~0.220 | 0.825 | |
| Creatinine | −0.230 | −0.328~−0.132 | 0.087 | −0.195 | −0.397~0.007 | 0.158 | |
| HGB | −0.197 | −0.206~−0.188 | 0.375 | −0.268 | −0.331~−0.205 | 0.097 | |
| hs-CRP | 0.213 | −0.017~0.443 | 0.067 | 0.306 | 0.104~0.508 | 0.324 | |
| MI | 0.219 | 0.165~0.273 | 0.112 | — | — | — | |
| T-AOC | Age | −0.013 | −0.022~−0.004 | 0.014 | −0.052 | −0.107~0.003 | 0.561 |
| Sex | −0.096 | −0.275~0.084 | 0.292 | 0.106 | −0.195~0.448 | 0.438 | |
| Glucose | −0.009 | −0.425~0.407 | 0.925 | −0.206 | −0.278~−0.134 | 0.689 | |
| LDL-C | −0.088 | −0.168~−0.007 | 0.033 | −0.067 | −0.089~−0.045 | 0.459 | |
| HDL-C | 0.181 | 0.013~0.349 | 0.768 | −0.011 | −0.783~−0.761 | 0.906 | |
| T-AOC/ROS | Age | −4.621 | −6.767~−2.475 | <0.001 | −0.125 | −0.283~0.035 | 0.025 |
| Sex | −0.025 | −0.051~0.001 | 0.060 | −0.033 | −0.126~0.061 | 0.487 | |
| BMI | −0.196 | −0.234~−0.158 | 0.097 | 0.071 | −0.234~−0.016 | 0.438 | |
| Creatinine | 0.141 | 0.089~0.193 | 0.113 | 0.273 | 0.078~0.465 | 0.098 | |
| HGB | 0.080 | −0.125~0.285 | 0.383 | 0.194 | 0.166~0.222 | 0.383 | |
| TC | −0.200 | −0.017~0.417 | 0.375 | −0.108 | −0.198~−0.018 | 0.521 | |
| MI | −11.346 | −17.853~4.838 | 0.001 | — | — | — | |
The bold – significant difference. 95% CI – 95% confidence interval. Other abbreviations as in Table 2. B – values are not standardized.
Discussion
LTL is regarded as a potential marker of biological aging because it usually shortens in a predictable way with age. LTL shortening is a systemic factor induced by accumulated environmental injury and genetic predisposition, and might be a better predictor than other more conventional risk markers that reflect only the patient’s current risk status [4].
In the present case-control study, we measured the LTLs in young patients to reduce the influence of traditional risk factors such as age, hypertension, and diabetes. The results further confirmed the association between PCAD and shorter LTLs. The correlation analyses confirmed that LTLs were negatively correlated with age in both PCAD patients and controls, which was consistent with the findings of previous studies [8,19]. The mean rate of LTL decrease with age was somewhat lower in the PCAD patients than in the controls, but the difference was not significant. This finding might be explained by the youngest patients having a specific genetic make-up that resulted in shorter LTLs. As a result, the rate of LTL shortening in PCAD patients slowed.
A large number of free radicals are generated by oxidative stress. Thus, the level of ROS can reflect the degree of oxidative stress. In the present study, the level of ROS was similar in the patients and controls, and increased steadily with age, indicating that oxidative stress-induced injury accumulated in the aging process.
The rates of cellular aging and telomere shortening in bulk culture depend on the balance between oxidative stress and antioxidative defense [20]. Antioxidants can remove various ROS and protect the body against damage caused by oxidative stress. The plasma T-AOC, a measure of the T-AOC of antioxidases and nonenzymatic antioxidants [21], represents the net effect of many different compounds and systemic interactions. Therefore, it is possible that the plasma T-AOC provides more biologically relevant information than that obtained from measuring plasma concentrations of individual antioxidants.
In the present study, the comparison of the plasma T-AOC and ratio of T-AOC to ROS indicated that the antioxidant capacity of PCAD patients was decreased, which adversely affected the balance between oxidative stress and antioxidative defense. Drug treatment, which was probably higher in the PCAD patients, may explain the absence of a significant difference in the plasma ROS levels. Increased levels of LDL-C and a history of past myocardial infarctions were significantly correlated with the decline in the T-AOC and T-AOC/ROS of the PCAD patients. Previous research suggested that oxidized LDL cholesterol may be an important contributory factor in vascular senescence [22]. In a life-course study, a high serum level of total cholesterol in midlife (even with a subsequently low level of cholesterol in old age) was associated with reduced LTLs in older men (mean age 76 years) [20]. A study of patients with chronic kidney disease provided evidence that carbamylated low-density lipoproteins induced oxidative stress and accelerated senescence in human endothelial progenitor cells [23]. Other research suggested that oxidized LDL cholesterol may play an important role in accelerating oxidative stress, as it induces a chain reaction of free radicals, resulting in increased levels of ROS and decreased antioxidant enzyme activity. In addition, a study indicated that myocardial infarctions may contribute to a breakdown in the oxidative-antioxidative balance because ischemia-reperfusion injury after myocardial infarction produced large amounts of free radicals [24]. The same study reported that after myocardial infarctions, the body tended to be in an oxidation state as a result of a decline in the ratio of T-AOC to ROS.
In an effort to reveal the factors determining the observed shorter telomere length in patients with PCAD, we examined the correlation of LTL with classical risk factors and plasma oxidative stress, as reflected by ROS and T-AOC values. As we hypothesized, other than a history of type 2 diabetes, telomere shortening was independently correlated with a decline in the ratio of T-AOC to ROS. This finding confirms the relationship between LTLs and the oxidative stress-antioxidative defense balance for the first time. Moreover, telomere shortening in the controls was independently correlated with increased ROS levels.
Our study has some potential limitations. First, as this was a case-control study, selection bias of the controls and PCAD patients cannot be excluded. Second, we did not measure the mean telomere length in specific leukocyte subpopulations. However, a previous study showed that LTL shortening in patients with CAD affected all blood cell populations equally, including granulocytes and monocytes, B or T lymphocytes, peripheral blood stem cells, and progenitor cells [25]. Finally, as this was a case-control study, no definite conclusions can be made about whether the observed shorter telomeres in patients were a cause or consequence of PCAD. Future large clinical studies should be performed to elucidate the mechanistic link between LTL shortening, oxidative stress, and PCAD in humans.
Conclusions
In conclusion, this study revealed age-associated increases in LTL shortening and decreased antioxidant capacity associated with clinical PCAD. The results establish a link between LTL shortening and oxidative stress in PCAD. Factors associated with PCAD, especially increased LDL-C levels, myocardial infarction-induced increases in oxidative stress, and breakdown of the oxidative-antioxidative balance, may result in further LTL shortening. LTL primarily reflects the burden of increased oxidative stress.
Footnotes
Conflict of interest
All authors declare that there is no conflict of interest.
Source of support: This study was supported by a Peking Union Medical College Hospital Young Investigator Grant (PUMCH-2013-073), and the Doctoral Scientific Research Fund Project of the Ministry of Education in China (20131106110007)
References
- 1.Blackburn EH. Structure and function of telomeres. Nature. 1991;350(6319):569–73. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 2.Huffman KE, Levene SD, Tesmer VM, et al. Telomere shortening is proportional to the size of the G-rich Telomeric 3′-overhang. J Biol Chem. 2000;275(26):19719–22. doi: 10.1074/jbc.M002843200. [DOI] [PubMed] [Google Scholar]
- 3.Fairlie J, Holland R, Pilkington JG, et al. Lifelong leukocyte telomere dynamics and survival in a free-living mammal. Aging Cell. 2016;15(1):140–48. doi: 10.1111/acel.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fyhrquist F, Saijonmaa O, Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol. 2013;10(5):274–83. doi: 10.1038/nrcardio.2013.30. [DOI] [PubMed] [Google Scholar]
- 5.Papapetrou A, Moris D, Patelis N, et al. Oxidative stress and total antioxidant status during internal carotid artery clamping with or without shunting: An experimental pilot study. Med Sci Monit Basic Res. 2014;21:200–5. doi: 10.12659/MSMBR.894756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houben JM, Moonen HJ, van Schooten FJ, Hageman GJ. Telomere length assessment: Biomarker of chronic oxidative stress? Free Radic Biol Med. 2008;44(3):235–46. doi: 10.1016/j.freeradbiomed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 7.De Meyer T, Rietzschel ER, De Buyzere ML, et al. Telomere length and cardiovascular aging: The means to the ends? Ageing Res Rev. 2011;10(2):297–303. doi: 10.1016/j.arr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Saliques S, Zeller M, Lorin J, et al. Telomere length and cardiovascular disease. Arch Cardiovasc Dis. 2010;103(8–9):454–59. doi: 10.1016/j.acvd.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Huzen J, de Boer RA, van Veldhuisen DJ, et al. The emerging role of telomere biology in cardiovascular disease. Front Biosci (Landmark Ed) 2010;15:35–45. doi: 10.2741/3604. [DOI] [PubMed] [Google Scholar]
- 10.Wen D. Association of serum irisin concentrations with presence and severity of coronary artery disease. Med Sci Monit. 2016;22:4193–97. doi: 10.12659/MSM.897376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: A nested case-control study. Lancet. 2007;369(9556):107–14. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 12.Brouilette S, Singh RK, Thompson JR, et al. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23(5):842–46. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 13.Farzaneh-Far R, Cawthon RM, Na B, et al. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: Data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2008;28(7):1379–84. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 15.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye S, Shaffer JA, Kang MS, et al. Relation between leukocyte telomere length and incident coronary heart disease events (from the 1995 Canadian Nova Scotia Health Survey) Am J Cardiol. 2013;111(7):962–67. doi: 10.1016/j.amjcard.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mancia G, De Backer G, Dominiczak A, et al. The task force for the management of arterial hypertension of the European Society of Hypertension, The task force for the management of arterial hypertension of the European Society of Cardiology. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28(12):1462–536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YY, Chen AF, Wang HZ, et al. Association of shorter mean telomere length with large artery stiffness in patients with coronary heart disease. Aging Male. 2011;14(1):27–32. doi: 10.3109/13685538.2010.529196. [DOI] [PubMed] [Google Scholar]
- 20.Strandberg TE, Saijonmaa O, Fyhrquist F, et al. Telomere length in old age and cholesterol across the life course. J Am Geriatr Soc. 2011;59(10):1979–81. doi: 10.1111/j.1532-5415.2011.03610_13.x. [DOI] [PubMed] [Google Scholar]
- 21.Salpea KD, Talmud PJ, Cooper JA, et al. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis. 2010;209(1):42–50. doi: 10.1016/j.atherosclerosis.2009.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bekaert S, De Meyer T, Rietzschel ER, et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6(5):639–47. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 23.Carracedo J, Merino A, Briceño C, et al. Carbamylated low-density lipoprotein induces oxidative stress and accelerated senescence in human endothelial progenitor cells. FASEB J. 2011;25(4):1314–22. doi: 10.1096/fj.10-173377. [DOI] [PubMed] [Google Scholar]
- 24.Inserte J, Barrabés JA, Hernando V, Garcia-Dorado D. Orphan targets for reperfusion injury. Cardiovasc Res. 2009;83(2):169–78. doi: 10.1093/cvr/cvp109. [DOI] [PubMed] [Google Scholar]
- 25.Spyridopoulos I, Hoffmann J, Aicher A, et al. Accelerated telomere shortening in leukocyte subpopulations of patients with coronary heart disease: role of cytomegalovirus seropositivity. Circulation. 2009;120(14):1364–72. doi: 10.1161/CIRCULATIONAHA.109.854299. [DOI] [PubMed] [Google Scholar]