Biochemistry. In the article “The chromatin unfolding domain of chromosomal protein HMG-14 targets the N-terminal tail of histone H3 in nucleosomes” by Lothar Trieschmann, Brian Martin, and Michael Bustin, which appeared in number 10, May 12, 1998, of Proc. Natl. Acad. Sci. USA (95, pp. 5468–5473), the following correction should be noted. Figs. 3 and 6 as reproduced in the issue were of very poor quality, especially Fig. 3A, in which the protein bands appear to be washed. Both figures and their legends are reproduced below.
Figure 3.
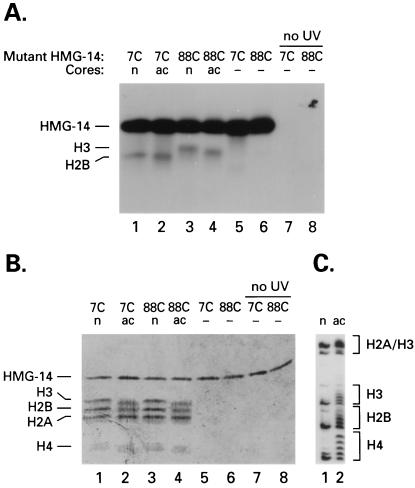
Identification of the targets of the HMG-14 point mutants. Acetylated (ac) or nonacetylated (n) cores containing the 125I-labeled HMG-14 point mutants indicated at the top of the lanes were exposed to UV, treated with 2-mercaptoethanol, and analyzed by SDS/PAGE. (A) Autoradiograph. (B) Coomassie blue stain. Lanes 5–8 contained only the labeled HMG-14 mutants. Lanes 7 and 8 were not exposed to UV and therefore the radioactive label runs with the ion front. (C) The degree of acetylation of the histones in the nucleosome cores was assessed by Coomassie blue staining of Triton/acid/urea polyacrylamide gels (33).